Abstract
The causes for malignant progression of disseminated tumors and why recurrence rates differ in women with different breast cancer subtypes are unknown. Here, we report novel mechanisms of tumor plasticity that are mandated by microenvironmental factors and demonstrate that recurrence rates are not strictly due to cell intrinsic properties. Specifically, outgrowth of the same population of incipient tumors is accelerated in mice with triple-negative breast cancer (TNBC) relative to those with luminal breast cancer (LBC). Systemic signals provided by overt TNBCs cause formation of a tumor-supportive microenvironment enriched for EGF and IGF-1 at distant indolent tumor sites. Bioavailability of EGF and IGF-1 enhances expression of transcription factors associated with pluripotency, proliferation, and epithelial-mesenchymal transition. Combinatorial therapy with EGFR and IGF1R inhibitors prevents malignant progression. These results suggest that plasticity and recurrence rates can be dictated by host systemic factors and offer novel therapeutic potential for patients with TNBC.
Keywords: Systemic Instigation, Dormancy, Disseminated Tumor Cells, Triple-negative Breast Cancer, Tumor Microenvironment
INTRODUCTION
Breast cancer is categorized into histopathological subtypes based on estrogen (ER) and progesterone (PR) hormone receptor status and HER2/ERBB2 expression levels. Triple-negative breast cancer (TNBC), which is considered the most malignant form of breast cancer, does not express ER or PR and lacks HER2/ERBB2 amplification. Women with TNBC are at the greatest risk of early recurrence compared, for instance, to women with ER-positive or luminal breast cancer (LBC) (1), but the reasons for these differences in recurrence rates are unclear. Patients who present with distant metastases at the time their primary tumor is detected are diagnosed with Stage IV disease. Other patients who do not have detectable metastases at the time of diagnosis will eventually recur with disease in distant organs. For women with metastatic TNBC, intensive cytotoxic chemotherapy is currently the only treatment approach, even though it is not curative. Furthermore, therapies designed to target primary tumors are not as successful against recurrent disease (2).
The fact that disease recurs after primary breast tumor removal indicates that tumor cells were disseminated prior to surgical resection, but remained indolent and undetected before progressing to symptomatic disease (3, 4). Hence, in women with recurrent or Stage IV disease, the primary tumor and a number of disseminated tumors co-exist for an indefinite period of time. A growing body of clinical and experimental evidence supports the concept that co-existing tumors in a patient with clinically silent metastases can interact with the host environment to modulate overall disease progression [reviewed in (5)]. These interactions arise from a host response involving circulating cytokines, immune cells, and bone marrow-derived cells that instruct formation of tumor-supportive microenvironments [reviewed in (6)]. The tumor microenvironment regulates primary tumor growth, homeostasis, and progression (7); however, the means by which systemic and microenvironmental processes facilitate malignancy of otherwise indolent disseminated tumors have been unclear. We report here that bioavailability of epidermal growth factor (EGF) and insulin-like growth factor-1 (IGF-1), provided by the tumor microenvironment, modulates phenotypic plasticity, gene expression, and the recurrence rate of certain TNBC tumors. Combinatorial therapy with EGFR and IGF1R inhibitors prevents disease progression by interrupting paracrine interactions between TNBC tumor cells and their microenvironment.
RESULTS
Malignancy of Indolent Tumors is Accelerated in Hosts with TNBC
To understand if systemic processes might explain the differences in relapse rates associated with different breast cancers, we used a human tumor xenograft model that represents situations in which a patient either has co-existing primary and distant metastases (i.e., stage IV disease) or multiple disseminated metastatic foci (i.e., recurrent disease) and allows us to precisely trace the growth kinetics of individual tumors (Fig. 1A). Based on previously defined functional properties of various tumor cells in this xenograft system (8, 9), we use the term “instigator” to define tumors that elicit a pro-tumorigenic host systemic response; we use the term “responder” to define tumors that are otherwise indolent, but can respond to systemic stimuli to form overt tumors. We injected responding and instigating TNBC cells into anatomically distinct sites in Nude mice, using Matrigel as a vehicle control for the instigators in another group of mice. We also injected the same responder cell population into hosts bearing LBC tumors, which we previously determined can stimulate responding tumor growth (8).
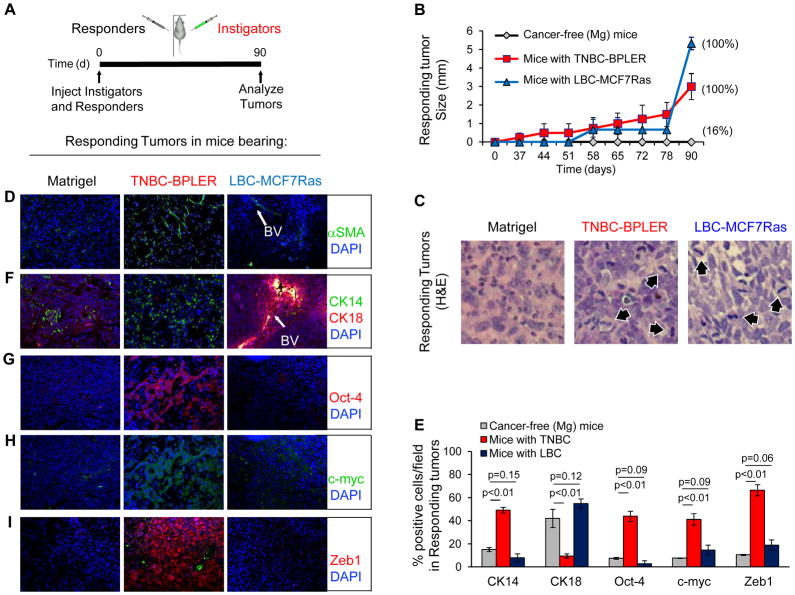
Figure 1. Systemic Environment Determines Growth Kinetics and Histopathology of Responsive Tumors.
(A) Scheme of bilateral human tumor xenograft implantation system used for data represented in figure. For these experiments, we used triple-negative HMLER hygro-H-rasV12 (HMLER-HR) tumor cells (46) as responders, oncotype-matched BPLER tumor cells (47) as TNBC instigators, and MCF7Ras tumor cells (Orimo, et al, Cell, 2005) as LBC instigators. (B) Growth kinetics of responding tumors in mice bearing Matrigel (n=6), triple-negative breast cancer (TNBC-BPLER; n=4), or luminal breast cancer (LBC-MCF7Ras; n=3). Data are represented only for cases in which the contralateral instigating tumors grew; incidence of responding tumor formation is indicated on graph. (C) Hematoxylin and eosin (H&E) stains of responding tumors from indicated cohorts. 60x magnification; arrows indicate mitotic tumor cells. (D) Merged photomicrographs of responding tumors stained for alpha-smooth muscle actin (αSMA). (E) Quantification of responding tumors stained for the indicated malignancy profile factors. The number of positively stained cells is represented as percentage of the total number of DAPI-positive cells per random field; 3 fields per tumor (n=3 Matrigel; n=12 TNBC; n=9 LBC). (F–I) Merged photomicrographs of responding tumors stained for the indicated malignancy profile factors. BV denotes blood vessels.
Only 1 of the 6 mice injected with Matrigel formed a distant responding tumor, which was predominantly necrotic (Fig. 1B, C and not shown). In contrast, responding tumors formed after a latency period of ~50 days in 100% of the mice with LBC (Fig. 1B). These responders were highly mitotic without forming αSMA-rich desmoplastic stroma (Fig. 1C, D). In mice with TNBC, responding tumors formed with 100% penetrance following a latency period of only ~35 days, after which they maintained a constant rate of growth (Fig. 1B). These responders showed a spectrum of pathological grades from atypical/high grade to differentiated/low grade, were moderately mitotic with no observable necrosis, and were highly desmoplastic (Fig. 1C, D). Importantly, responding tumors were devoid of instigating tumor cells and were comprised exclusively of the responding tumor cells (Supplementary Figs. S1A, S1B, S1C). In both cases, responding tumor histopathology was consistent with breast adenocarcinomas observed in the clinic (10). Moreover, the latency and growth kinetics with which responding tumors formed in hosts bearing different breast cancer subtypes reflected the relative rates of recurrence that are observed in patients with the respective breast cancer subtype (1).
Specific cytokeratin expression is frequently used to stratify normal epithelium and for tumor diagnosis (11). Responding tumors that formed in mice with Matrigel or LBC expressed both the luminal cytokeratin, CK18, (~42% and 55%, respectively) and the basal cytokeratin, CK14 (~15% and 8%, respectively) (Fig. 1E, F). Conversely, in mice with TNBC, responding tumors were significantly enriched for CK14 (50%), while only ~10% were CK18 positive (Fig. 1E, F).
Expression of transcription factors that modulate proliferation and pluripotency has been correlated with high-grade breast cancers and poor clinical outcome (12–15). In particular, Oct4 maintains pluripotency and self-renewal (16), while c-myc, which is often amplified in lethal metastases of unamplified primary tumors (17), regulates proliferation, differentiation, apoptosis, and self-renewal (18). Tumor forming capacity has also been associated with cells that undergo an epithelial-mesenchymal transition (EMT) (19). Specifically, expression of the EMT-inducing transcription factor, Zeb1, is correlated with early relapse (20–22). Immunohistochemical analysis revealed that in mice with TNBC, responding tumors were enriched with cells expressing Oct4 (~47%) and c-myc (~42%) relative to counterpart tumors in control Matrigel bearing mice (~8% each) (Figs. 1E, G–I). In responding tumors from mice with TNBC, the majority of c-Myc was localized to the cytoplasm, in agreement with clinical studies demonstrating that ~95% of myc-amplified breast tumors display cytoplasmic c-myc localization (23). Zeb1 was expressed in the nucleus of ~67% of TNBC-responsive tumor cells, as opposed to only ~10% of responder cells in the cancer-free mice (Figs. 1E, I). We obtained similar results using another responding cell line, BT549 (Supplementary Fig. S2) and another TNBC instigator cell line, MDA-MB-231 (data not shown), demonstrating that response to the TNBC-induced systemic environment was not oncotype dependent. In contrast, only 2% of the responding tumor cells in mice with LBC expressed Oct4, ~17% expressed c-myc, and ~19% expressed nuclear Zeb1 (Figs. 1E, G–I). These expression levels were significantly lower than those of responding tumors in the mice with TNBC and not significantly different from those in the control mice (Fig. 1E), supporting our earlier report that LBC systemic tumor promotion operates via different mechanisms than that of TNBC (8).
Together, these results established for the first time that the same population of otherwise indolent xenografted tumor cells forms tumors with dramatically different growth kinetics and resultant tumor cell phenotypes depending on the breast tumor subtype borne by the host. In particular, enhanced expression of the same pluripotency and EMT-inducing transcription factors that we observed in the mice with TNBC has been observed in tumors from women with TNBC (12–20).
Brief exposure to TNBC is sufficient for responder tumors to progress independently
To understand if malignant conversion – defined by enhanced proliferation and the collective expression of Oct-4, c-myc, and Zeb1 – was an early event in hosts with TNBC, we injected GFP+ responding tumor cells into mice that were either cancer-free or bearing TNBC intigator tumors and recovered tumor tissues after 8 days (Fig. 2A). At this time point, tissue plugs from cancer-free and TNBC mice were comparable in size and contained responding tumor cells (Figs. 2B and Supplementary Fig. S1C).
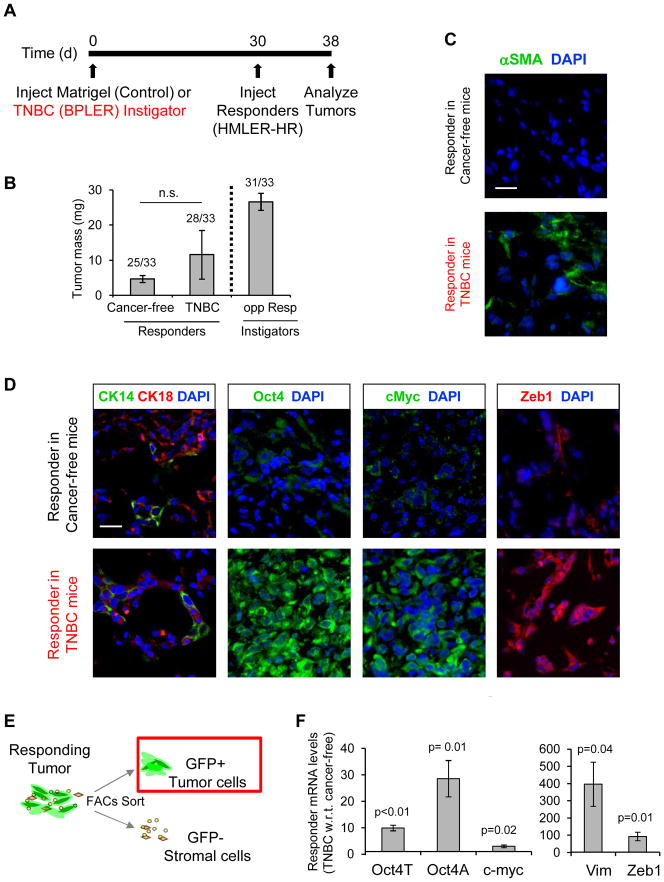
Figure 2. Systemic Modulation of Malignancy is an Early Event in TNBC Progression.
(A) Experimental scheme for “short-term instigation” used for data represented in figure. Since only one responding tumor was recoverable from the control cohort after 90 days (Fig. 1), short term instigation allows us to recover all tissue plugs for analysis. (B) Final mass of responder tissues after 8 days in Matrigel or TNBC bearing mice. Differences were not significant (n.s.). Data to right of dashed line represent average mass of TNBC-BPLER instigators after 5 weeks of growth. Incidence of tumor formation is shown above data bars (n=33 mice per group). (C, D) Merged immunofluorescent images of responding tumors from each cohort that were stained as indicated. Scale bar = 25 μm. (E) Scheme of responding tumor subfractionation into GFP+ responding tumor cell and GFP-negative stromal cell constituents. (F) qPCR expression levels of indicated malignancy profile genes in GFP+ responder tumor cells that had grown in mice with TNBC relative to those from control Matrigel mice (n=3).
Immunoflourescence analysis of resulting tissues revealed that αSMA-positive myofibroblasts were abundant in the responding tumors in mice with TNBC but not in those with Matrigel, confirming that a hallmark of systemic tumor promotion – stromal desmoplasia – had already been initiated (Fig. 2C). Luminal and basal cytokeratins were equivalently expressed in responding tumors from both cohorts (Fig. 2D), indicating that enrichment for CK14, which occured after 90 days, was not an early event in the TNBC-mediated response. However, responding tumors from mice with TNBC were significantly enriched for expression of malignancy profile factors after only 8 days (Fig. 2D).
To validate these observations, we sorted GFP-positive tumor cells from the tissues by FACS (Fig. 2E) and analyzed expression by qPCR. All 3 malignancy factors were elevated in responding tumor cells exposed to TNBC: Oct-4A ~29-fold, c-myc ~3-fold, and Zeb1 ~93-fold (Fig. 2F). Expression of the EMT marker, vimentin (VIM), was also significantly elevated (~397-fold) in the responding tumors from mice with TNBC (Fig. 2F). We confirmed these results using another responding TNBC cell line, BT549, which acquired a proliferative advantage and displayed the malignant profile after only 8 days in mice with TNBC as compared with cancer-free controls (Supplementary Fig. S2A–C).
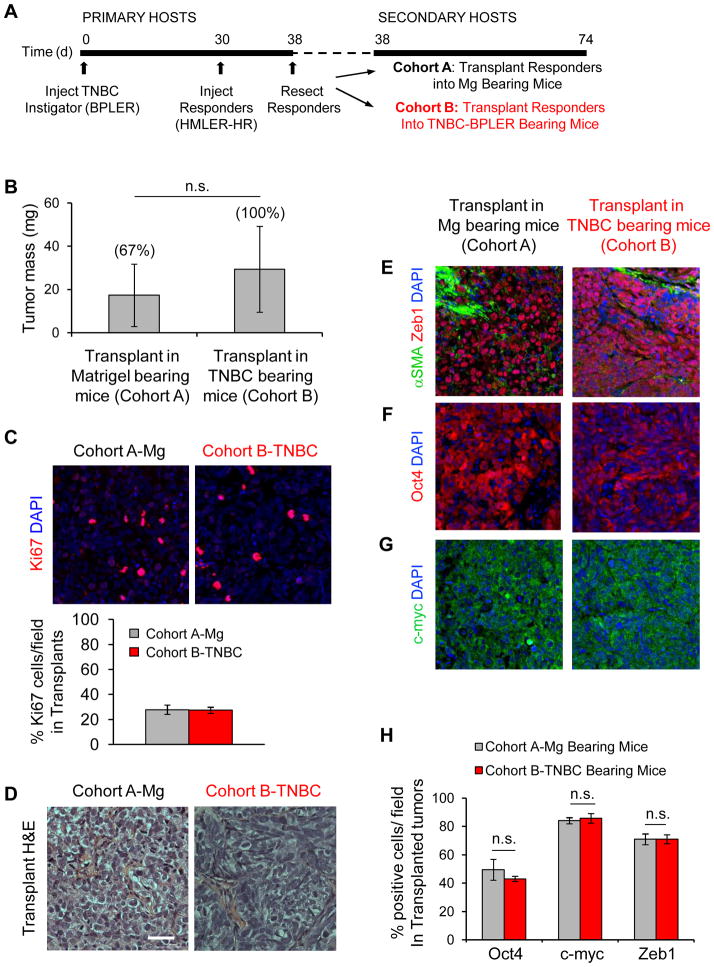
To determine whether early acquisition of the malignant phenotype was relevant to disease progression, we surgically removed responder plugs after 8 days of exposure to the TNBC-induced environment, immediately transplanted them into secondary hosts bearing either Matrigel or a TNBC instigator tumor, and allowed them to progress (Fig. 3A). After 5 weeks, the percentage of Ki67+ proliferative cells and the average mass of responding tumors was the same in both secondary cohorts (Fig. 3B, C). Responding tumors from both cohorts were also nearly identical on the histopathological level (Fig. 3D) and maintained an activated stroma, characterized by the presence of αSMA+ myofibroblasts (Fig. 3E). Likewise, transplanted responding tumors maintained equivalent expression of Oct4, cmyc, and Zeb1 in both cohorts (Fig. 3F–H). Hence, the tumor microenvironment and the malignant coversion that occurred during the initial phases of instigation by TNBC was sufficient to maintain malignancy, even in the absence of the initiating TNBC tumor.
Figure 3. Brief Exposure to the TNBC Environment is Sufficient for Responding Tumors to Progress Independently.
(A) Scheme of responding tumor transplantation system used for data represented in figure. (B) Mass of responding tumors from mice with TNBC-BPLER 36 days following their surgical transplantation into secondary hosts bearing either Matrigel or TNBC-BPLER. Incidence of tumor formation is shown above data bars (n=3 mice per group); differences were not statistically significant (n.s.). (C) Transplants from indicated cohorts stained for the proliferation marker Ki67 (red), and cell nuclei (blue) under indicated conditions. Graph represents number of Ki67+ cells as a percentage of the total number of cells per field; n=6 Matrigel images; n=9 TNBC images. (D) Hematoxylin and eosin (H&E) stains of responder tumors that had been transplanted into indicated secondary hosts; scale bar = 50 μm. (E–H) Merged immunofluorescent images (E–G) and corresponding quantification (H) of transplanted responding tumors stained for the indicated malignancy profile factors (n=6 Matrigel images; n=9 TNBC images).
Microenvironmental Factors that Mediate TNBC Progression
We and others have demonstrated that systemic signals impinge upon the microenvironment of disseminated tumors to facilitate their outgrowth [reviewed in (6)]. To identify candidate fators that mediated malignant conversion in hosts with TNBC, we interrogated our gene expression data that had been generated from different components of the responding tumor microenvironment in hosts with TNBC or Matrigel control (9), including pro-tumorigenic bone marrow derived cells (GEO GSE25620) and two different types of cancer-asociated fibroblasts (GEO GSE25619). From each dataset, we generated a list of the most differentially expressed genes that met the following criteria: 1) protein products that are secreted; 2) cytokines known to regulate self-renewal, transdifferentiation, and EMT; and 3) factors that mediate recruitment of BMDCs. This resulted in a combined list of genes representing the collective responding tumor microenvironment (Supplementary Fig. S3A). We then sorted the GFP-negative stromal cells from responding tumors recovered from cancer-free or TNBC-bearing mice (Fig. 4A) and analyzed relative expression of 13 of these genes. In this analysis, we included IGF-1 due to the high expression levels of activating IGF binding proteins and reduced expression of IGF-inactivating binding proteins (24) revealed from our meta-analysis (Supplementary Fig. S3A).
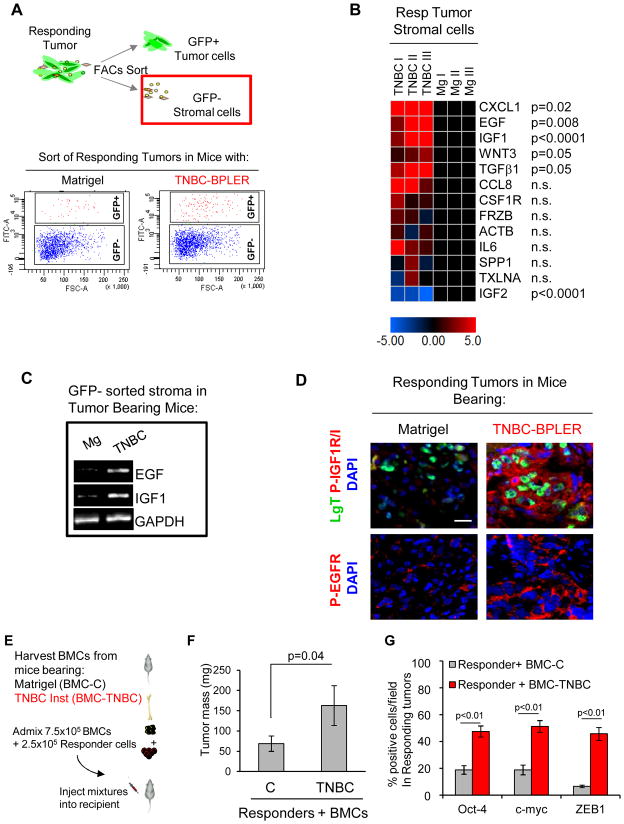
Figure 4. Identification of Responding Tumor Stromal-Derived Factors.
(A) Top panel: Scheme of tumor subfractionation into GFP+ HMLER-HR responding tumor cell and GFP-negative stromal cell constituents after 8 days of exposure to mice with either Mg or TNBC-BPLER. Bottom panel: FACS plot of dissociated responding tumors that were separated into GFP+ (responder tumor cells) and GFP- (tumor stroma) subfractions. (B) Heat map representing expression levels of indicated genes in the GFP-negative stromal cells from the responding tumors of mice with TNBC-BPLER relative to those with Matrigel. (C) Photomicrograph of agarose gel to visualize indicated qPCR products; RNA was prepared from GFP-negative tumor-associated stromal cells after 8 days of exposure to mice with either Matrigel (Mg) or TNBC-BPLER. (D) Merged immunofluorescent images showing activated forms of EGF (phospho-Tyr1068; P-EGFR) and IGF/Ins (phospho-Tyr1161/Tyr1185; P-IGF1R/IR) receptors on responding tumor cells that had grown for 8 days in indicated mice. Responding HMLER-HR tumor cells express the simian virus 40 large-T oncoprotein (LgT; green). Scale bar = 25 μm. (E) Experimental scheme for implantig bone marrow cells (BMCs) with responding tumor cells. (F) Mass of responding HMLER-HR tumors 12 weeks following injection of indicated BMC admixtures (n=10 tumors per group). (G) Quantification of malignancy profile factors in responding HMLER-HR tumor cells under indicated conditions. The number of cells stained positively for each factor is represented as a percentage of DAPI-positive tumor cells per random field (n=9 fields per group; 3 images quantified from each of 3 tumors per group).
CXCL1, EGF, IGF-1, Wnt3, and TGFβ1 were significantly up-regulated in the responding tumor microenvironment of mice with TNBC relative to that of control mice (Fig. 4B). Expression levels of CCL8, CSF1R, FRZB, ACTB, IL6, SPP1, and TXLNA (IL14) were not significantly different, and IGF-2 expression was significantly down-regulated in the responding tumor microenvironment of mice with TNBC relative to controls (Fig. 4B).
We concentrated on two growth factors that were highly up-regulated in the TNBC-induced microenvironment, EGF and IGF-1 (Fig. 4B, C). EGF ligands are found in 50–90% of tumors from patients with poor prognosis and the majority of TNBCs express the EGF receptor (1, 25, 26). In certain contexts, EGF induces c-myc expression to reduce breast tumor latency (27) and has been shown to enhance Zeb1 expression in breast tumor cells (28). High levels of phosphorylated IGF1R receptor and its ligands are present in malignant human breast tissues and are associated with poor patient prognosis (29–31). IGF-1 signaling has been shown to induce Oct4 expression during cellular reprogramming (32).
The responding HMLER-HR tumor cells expressed both the EGF and IGF receptors (EGFR and IGF1R) in vitro and did not express either of the ligands (Supplemental Fig. S3B, C, D). Moreover, in the absence of growth factor supplements, EGF and IGF-1 receptors were not activated (Supplemental Fig. S3D), indicating that these cells would depend on paracrine sources of EGF and IGF1 to activate the cognate receptors. Responding BT-549 cells also expressed both receptors and expression of the EGF ligand was similar to that of the responding HMLER-HR cells; however, IGF-1 levels were ~3000-fold higher in BT-549 relative to HMLER-HR (Supplemental Fig. S2E, F), and IGF1R was activated in vitro (Supplemental Fig. S3D). Therefore, BT-549 cells active IGF1R in an autocrine manner and rely on exogenous sources of EGF to activate the EGF receptor.
Using phospho-specific antibodies to activated EGFR and IGF1R/IR (33, 34), we found that at both early (8 days) and late (60 days) time points in mice with TNBC, responding tumor cells, as well as some stromal cells, expressed the active forms of EGFR and IGF1R/IR (Fig. 4D and Supplementary Fig. S4A). Receptor activation was not observed to any significant extent in the responders from control mice (Fig. 4D and Supplementary Fig. S4A). Strikingly, the early stage HMLER-HR responding tumors that had been transplanted into secondary recipient hosts (Fig. 3) also displayed EGF and IGF receptor activation (Supplementary Fig S4A). Although the majority of the BT-549 responder cells displayed activated IGF1R in the control cohort, it was not sufficient to drive malignant growth; only when EGFR was concomitantly activated in the mice with TNBC did these cells form aggressively growing tumors (Supplementary Fig. S2B–D).
Bone marrow cells (BMCs) play an important role in breast tumor progression, systemic instigation, and resistance to chemotherapy (9, 35, 36). Hence, we tested BMCs as a potential source of ligands that drive malignancy of responding tumor cells. Ex vivo, EGF and IGF-1 expression levels were both ~2-fold higher in BMCs harvested from mice with TNBC than in control mice (Supplementary Fig. S4B). We therefore tested BMCs from cancer-free or TNBC-bearing mice for their ability to promote malignancy in vivo using a functional test of BMC activity (36) (Fig. 4E). Only BMCs from mice with TNBC were capable of inducing responder growth (Fig. 4F) and expression of Oct-4, c-myc, and Zeb1 (Fig. 4G, and Supplementary Fig. S4C–E). These results indicated that in hosts with TNBC, bone marrow derived cells, either directly or in cooperation with other stromal components, provided a source of EGF and IGF-1 and modulated malignant conversion of otherwise indolent tumors.
EGF and IGF1 Bioavailability Modulates Responder Tumor Cell Plasticity and Malignancy
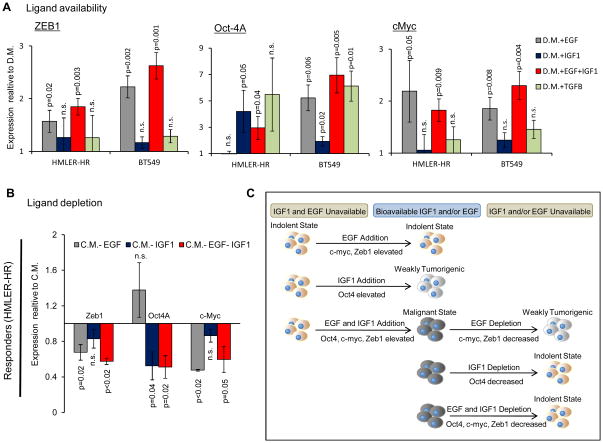
We conducted a series of in vitro experiments to determine the effects of EGF and IGF1 on malignancy of both HMLER-HR and BT549 responder cells. When HMLER-HR responder cells were maintained in medium deprived of ligands (see Methods and Supplementary Fig. S5A), EGF induced expression of Zeb1 and cMyc (Fig. 5A), but was not sufficient to convert responder cells to a malignant state, as determined by colony formation in vitro (Supplementary Fig. S5B). EGF was sufficient for malignant conversion of BT549 cells (Fig. 5A and S5B), which express IGF1 in an autocrine fashion. Addition of IGF-1 induced expression of Oct4 in both cell lines (Fig. 5A), but only promoted modest colony formation (Supplementary Fig. S5B). When both EGF and IGF-1 were bioavailable, responder cells converted to malignancy and Oct4, cMyc, and Zeb1 were significantly upregulated (Fig. 5A and S5B). In all cases, we monitored cell proliferation and apoptosis (Supplementary Figs. S6A, B) and confirmed that cognate receptors were activated (Fig. S3D).
Figure 5. EGF and IGF-1 Modulate Indolent and Malignant States in vitro.
(A) Expression levels of indicated malignancy profile genes in responder tumor cells (HMLER-HR and BT549) after 4 days in indicated culture conditions relative to those in depleted medium (D.M.). See Supplementary Fig. S5A for experimental scheme. n=6; where each sample was run in duplicate in 3 separate experiments. (B) Expression levels of indicated malignancy profile genes in responder tumor cells (HMLER-HR) after 4 days in indicated culture conditions relative to those maintained in completed media (C.M.). n=6; each sample run in duplicate in 3 separate experiments. (C) Model representing interconversion of responsive tumor cells between malignant and indolent states depending upon EGF and IGF1 ligand availability.
As a control, we tested the effects of TGFβ, another growth factor we identified in the TNBC instigated stroma. TGFβ induced Oct4 expression in BT549 responder cells but otherwise did not induce expression of the other malignancy profile genes (cMyc and Zeb1) in either responder tumor cell line (Figure 5A). In both cell lines, proliferation was moderately but significantly reduced upon TGFβ treatment (Supplementary Fig. S6A, B).
Under conditions in which responder cells were deprived of EGFR and IGF1R ligands (see Methods and Supplementary Fig. S5A), malignancy profile factor expression was significantly reduced in vitro and in vivo and cells failed to form colonies in vitro (Fig. 5B and Supplementary Figs. S5B, S6C). Loss of EGF resulted in a reduction of Zeb1 and cMyc expression while IGF1 loss modulated a reduction in Oct4 expression (Fig. 5B).
Collectively, these results suggest a model in which both EGFR and IGF1R activation together modulate inter-conversion of responsive tumor cell populations between indolent and malignant states (Fig. 5C).
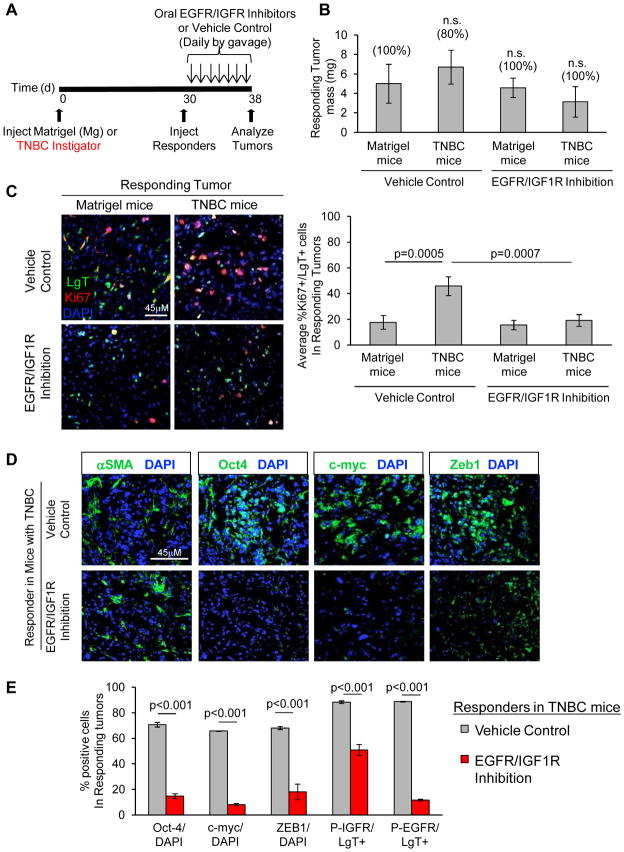
EGFR and IGF1R Inhibition Prevents Malignant Conversion of Responder Tumor Cells in Hosts with TNBC
Prompted by these results and clinical findings indicating that EGFR and IGFR activation are both associated with poor prognosis (29–31, 37–39), we tested whether EGFR and IGFR dual inhibition would prevent outgrowth of responding tumors in mice with TNBC. Mice bearing responding tumors in the context of TNBC or Matrigel control were treated with either DMSO control or a combination therapy of the EGFR inhibitor, erlotinib, plus the IGFR inhibitor, BMS-754807. Treatment was administered once per day for 8 days after which tissues of equivalent mass were recovered from all cohorts (Fig. 6A and 6B). We confirmed that activation of EGFR and IGF1R/IR were both significantly attenuated in the drug-treated cohorts, but not in control cohorts (Supplemental Fig. S7A, B). Instigating TNBC tumor mass was not affected by dual inhibitor treatment during the course of this dosing regimen (Supplementary Fig. S7C).
Figure 6. EGFR/IGFR Dual Inhibition Prevents Malignant Progression.
(A) Scheme of pharmacological targeting of TNBC progression for data represented in figure. Mice were treated with vehicle DMSO or both the EGFR inhibitor (erlotinib; 100 mg/kg) and IGFR inhibitor (BMS-754807; 50 mg/kg) once per day for eight days by oral gavage. (B) Mass of responder HMLER-HR tumor/tissue plugs after 8 days in indicated mice, with indicated drug or control treatment. Incidence of tumor formation is shown above data bars (n=5 mice per group). Differences were not significant (n.s.). (C) Indicated responding HMLER-HR tumors stained for LgT antigen (green; expressed only by responder cells), and Ki67 (red), and cell nuclei (blue). Scale bar = 45 μm. Quantification of Ki67+ cells as a percentage of the total number of LgT+ responder cells per field (n = 9; 3 random fields for each of 3 tumors per group). (D, E) Merged photomicrographs (D) and quantification (E) of malignancy profile factors in responding tumor cells under indicated conditions. The number of cells stained positively for each of the indicated factors is represented as a percentage of the total number of DAPI-positive nuclei or LgT-positive (indicated) tumor cells (n = 9; 3 random fields for each of 3 tumors per group).
With respect to cancer-free mice, responding tumor cells in the vehicle-treated mice with TNBC were significantly more proliferative (~46% vs. ~18% Ki67-positive, respectively), formed with a desmoplastic stroma, and maintained expression of the malignancy profile factors, Oct-4, c-myc, and Zeb1 (Fig. 6C–E). Dual EGFR/IGFR inhibition resulted in a ~60% decrease in Ki67-positive responder cells in mice with TNBC (Fig. 6C). These responders showed evidence of a myofibroblast-rich, reactive stroma; however, the percentage of cells expressing Oct-4, c-myc, and Zeb1, were significantly reduced relative to the vehicle-treated controls (~79%, ~87%, and ~73% reductions, respectively) (Figs. 6D, E). Responding tumor cell expression profile and proliferation in the Matrigel bearing control mice (~18%) was unaffected by drug treatment (~19%) (Fig. 6C and Supplemental Fig. S7D). The fact that recruitment of reactive stroma was not affected in hosts with TNBC under this treatment regimen suggested that EGFR/IGFR dual inhibition prevented paracrine interactions between responding tumor cells and their systemically mandated microenvironment.
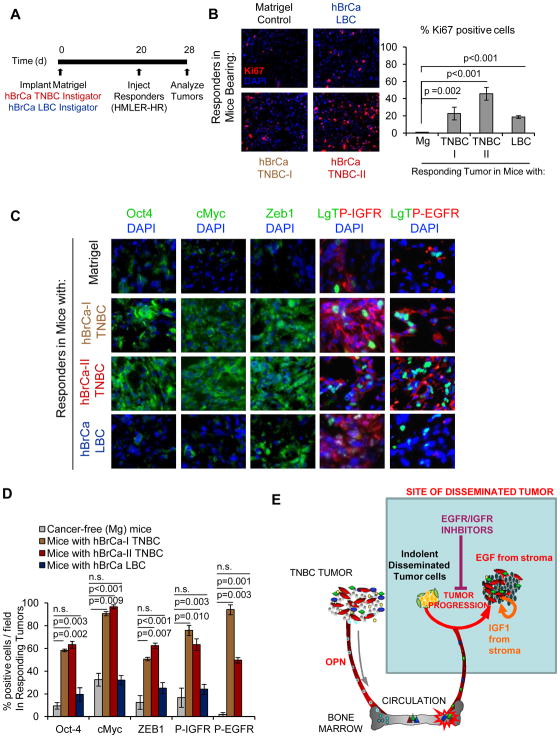
Primary Tumors from Patients with TNBC Accelerate Malignant Conversion of Otherwise Indolent Tumors
In an effort to understand whether primary tumors from breast cancer patients establish similar pro-tumorigenic environments, we analyzed the effects of two different TNBC primary tumor specimens (designated as: hBrCa TNBC-I, and hBrCa TNBC-II) on responding tumor outgrowth. For comparison, we tested a tumor from a woman with LBC (designated as hBrCa-LBC) (Fig. 7A). After a 20-day period of equivalent tumor growth of primary tumor specimens (Supplementary Fig. S8A, B), responding tumor cells recovered from the mice with hBrCa TNBC-I, hBrCa-TNBC-II, and hBrCa-LBC were significantly more proliferative than those from the control Matrigel bearing mice (Fig. 7B). Responding tumors from the hBrCa TNBC-I and hBrCa TNBC-II cohorts were significantly enriched for Oct4, c-Myc, and Zeb1 relative to those from cancer-free and hBrCa-LBC cohorts (Fig. 7C). Correspondingly, positive staining for activated EGFR and IGFR were highest in the two hBrCa-TNBC cohorts (Fig. 7C, D).
Figure 7. Human Tumor Specimens Establish Tumor-Supportive Systemic Environments that Influence Disease Malignancy.
(A) Implantation scheme of human breast cancer surgical specimens from 2 different patients with TNBC (hBrCa TNBC-I and hBrCa TNBC-II) and 1 patient with LBC (hBrCa LBC). Each tumor specimen was minced and divided into equal portions that were surgically implanted with Matrigel beneath the skin of 3 different Nude mice per cohort. Mice injected with Matrigel were used as a control. HMLER-HR responder cells were injected contralateraly 20 days later and analyzed after 8 days. (B) Responding tumors stained for the proliferation marker Ki67 (red), and cell nuclei (blue) under indicated conditions. Graph represents number of Ki67+ cells as a percentage of the total number of cells per field; n=6 for Matrigel controls; n=6 for the LBC cohort; n=9 each for TNBC-I and TNBC-II cohorts. (C) Representative merged immunofluorescent images of responding tumors from indicated cohorts. (D) Quantification of malignancy profile factor expression in responding tumor cells under indicated conditions. The number of cells stained positively for each of the indicated factors is represented as a percentage of the total number of DAPI-positive nuclei or LgT-positive (indicated) responder tumor cells per field. A minimum of 3 fields were quantified per responder tumor for each group; n=6 for Mg controls, n=9 for TNBC-I, n=9 TNBC-II, n=6 LBC. (E) TNBC Systemic Instigation Model. The host reaction to certain TNBC tumors (“Instigators”) establishes a systemic environment that supports the outgrowth of otherwise indolent disseminated tumors (“Responders”). Instigating TNBC tumors secrete OPN to mobilize tumor-supportive bone marrow cells (BMCs) which are subsequently recruited to responding tumor sites, creating a pro-tumorogenic tumor microenvironment (9, 36). This microenvironment is enriched for the growth factors EGF and IGF1. Combinatorial treatment with EGFR and IGF1R inhibitors prevents malignant conversion of the incipient TNBCs.
Collectively, these results support a model in which the body’s response to an overt triple-negative breast cancer creates a cascade of systemic events that impinge upon plasticity and the recurrence rate of responsive disseminated tumors (Fig. 7E).
DISCUSSION
Previous ideas about why patients with TNBC relapse earlier than patients with other types of breast cancer focused on tumor cell intrinsic properties. Our results support a novel idea that the host systemic environment also determines recurrence rates and the phenotype of the resultant tumors. We do not yet know if systemically induced tumor plasticity is a consequence of a selection process (i.e., cell subpopulations that are able to respond to instigating tumors), or whether systemic signaling cascades serve to reprogram individual responding tumor cells. Nevertheless, in hosts with TNBC, distant tumor cell populations convert between indolent and malignant states depending on the bioavailablity of EGF and IGF-1. This observed plasticity also suggests that the state in which tumor cell populations metastasize from a primary tumor, or the state in which they exist during a period of indolence in a foreign tissue, might not be reflected in the resulting tumor once it is detected. Indeed, striking clinical findings indicate that the molecular and histopathological characteristics of recurrent breast cancer often does not reflect that of the primary tumor from which it was derived (17, 40).
Targeting features of malignancy and the signaling pathways that drive them appears to be a strategy that could prevent recurrence of certain TNBCs. A recent study demonstrated that pre-treatment of various TNBC cell lines in vitro with the EGFR inhibitor, erlotinib, rendered a subset of these lines more sensitive to DNA damage-mediated cell death by rewiring cell signaling networks (41). Interestingly, in their study, EGFR inhibition did not synergize with cytotoxic agents in some tumor cell populations, including BT549. In fact, in phase II clinical trials of breast cancer patients with advanced disease, fewer than 10% of patients responded to EGFR-targeted therapy and resistance to treatment appeared to be a primary contributor to patient demise. It has been suggested that signaling through other tyrosine kinase receptors may confer resistance to EGFR inhibition. Our results support this conclusion and provide an explanation for why EGFR inhibition alone does not program some tumor cells to the indolent state; i.e., tandem inhibition of IGFR is also required.
A number of experimental studies have highlighted the importance of EGF and IGF-1 during tumor evolution (42, 43). Our results expand upon these earlier findings by addressing the source of bioavailable ligands in vivo – the tumor-associated stroma. Bone marrow derived cells, at least in part, provide these growth factors in hosts with TNBC. Indeed, xenografting human breast cancer cells and primary tumor specimens into Nude mice revealed important information about malignant progression that are independent of mature lymphocytes. How lymphocytes play a role in the process will be an important aspect of future studies.
Our studies also reveal the somewhat surprising fact that, ultimately, availability of EGF and IGF-1 is determined by systemic processes that confer a malignant phenotype upon cells that would otherwise remain indolent in a foreign environment. While it may seem easier to target tyrosine kinase receptor signaling pathways that program malignancy rather than the transcription factors that do so, a recent study of pancreatic cancer revealed that resveratrol inhibited pluripotency and EMT factors, including Oct-4, c-Myc, and Zeb1 (44). Therefore, valuable insights might also be gained by testing resveratrol in our preclinical model or others like it.
Our results suggest that an appropriately selected subset of breast cancer patients would benefit from dual EGFR/IGFR inhibition and emphasize the need for focused preclinical and clinical trials. A phase II clinical trial using dual inhibition for both IGF1R (BMS-754807) and EGFR (Cetuximab) in patients with advanced colorectal cancer and squamous cell cancers is currently underway (Clinical Trials.gov Identifier NCT00908024). Our results reveal novel aspects of simultaneously targeting both EGFR and IGFR and advocate for similar trials in patients diganosed with TNBC, for which the mainstay of current therapy is cytotoxic chemotherapy. Continued understanding of systemic processes that promote disease progression and the identity of otherwise indolent disseminated tumor cells that are capable of responding to systemic and microenvironmental cues should make it possible to treat TNBC patients at a time when recurrent disease might yet be prevented.
METHODS
Cell lines
HMLER hygro-H-rasV12 (HMLER-HR), BPLER, and MCF7-Ras human breast tumor cells were a generous gift from Dr. Robert A. Weinberg’s Lab and have been previously described (45–48). Expression of cytokeratins and introduced oncogenes was validated for these studies. All cell lines were validated as mycoplasma-negative. No additional authentication was conducted by the authors.
Animals and Tumor Xenografts
Female Nude mice were purchased from Taconic (Hudson, NY). All experiments were performed in accordance with the regulations of Harvard Medical School on Animal Care (protocol #09-12-1566). Tumor cells were prepared in 20% growth factor reduced Matrigel (BD Biosciences) in their respective growth medium. For BPLER cells, 5 × 104 cells in 0.1 ml Matrigel were injected per mouse, and 2 × 106 cells in 0.1 ml Matrigel were injected per mouse for HMLER-HR or BT549 cells. In all cases, cells were injected subcutaneously into nonirradiated mice. Tumor diameter was measured on the flanks of live Nude mice using digital calipers; volume was caculated as ½(length(width2)).
Real time PCR
RNA was extracted from cells or snap-frozen tissues using Trizol reagent following manufacturer’s instructions (Invitrogen). RNA was retrotranscribed with ProtoScript AMV First Strand cDNA Synthesis Kit (New England BioLabs). PCR amplification was performed on a ABI Prism 7900 sequence detector using SYBR-Green (Applied Biosystems). Analysis was done using delta-delta Ct method, normalizing first to GAPDH. See Table S1 for primer sequences.
Immunohistochemistry and Image Analysis
Dissected tissues were fixed in 4% (wt/vol) paraformaldehyde for 24 hr, stored in 70% ethanol for 24 hr, embedded in paraffin, and sectioned onto ProbeOn Plus slides (Fisher Scientific, Pittsburgh, PA) for immunohistochemistry using Vectastain Elite ABC kits (Vector Laboratories, Burlingame, CA) as previously described (36). See Table S2 for antibodies and dilutions. Images were captured under indicated magnification with identical exposure and gain for any given experiment, using a Nikon Eclipse 90i microscope. Staining was quantified using ImageJ software.
Human Breast Tumor Specimens
Primary breast tumors were collected in compliance with a protocol approved by the Brigham and Women’s Hospital (IRB 93-085). Each tumor was analyzed for receptor (ER/PR/HER2) status and used for these studies without any patient identifiers. Shortly after resection, tumor specimens were cut into 3–4mm pieces, washed in RPMI, and frozen in RPMI + 10% DMSO. For xenografts, tumor specimens were quickly thawed at 37°C, washed 3 times in RPMI, and minced finely into <1mm organoids to ensure homogeneity of viable tumor tissue or non-tumor areas. Organoids were divided into equal portions, transferred to individual wells of a 96-well plate, covered with 50% Matrigel in RPMI media, and incubated for 10 minutes at 37°C. Organoids were surgically implanted beneath the skin of Nude mice following sterile surgical procedure.
EGFR/IGFR Inhibitors
For each administration, the EGFR inhibitor, erlotinib (LC Laboratories, 100mg/kg) and IGFR inhibitor, BMS-754807 (Activebiochem, 50mg/kg), were freshly dissolved in 80% DMSO in PBS. Drugs or DMSO/PBS vehicle control were administered by oral gavage once daily for a period of 7 days. All mice were monitored on a daily basis during the course of drug treatment and were found to be healthy. Of note, the mice developed a skin rash, which has also been reported for patients treated with erlotinib (49).
Statistical Analysis
Data are expressed as mean ± SEM. Data were analyzed by Student’s t test and were considered statistically significant if p ≤ 0.05.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Currently, processes that mediate progression of otherwise indolent tumors are not well understood, making it difficult to accurately predict which cancer patients are likely to relapse. Our findings reveal novel mechanisms of tumor phenotypic and gene expression plasticity that are mandated by microenvironmental factors, identifying novel therapeutic targets for patients with TNBC.
Acknowledgments
Financial support: This work was supported by funds from the Harvard Stem Cell Institute, the American Cancer Society, and NIH RO1 CA166284-01 (SSM), and partially supported by the Dana-Farber/Harvard SPORE in breast cancer (N.C.I., 2 P50 CA89393-06, ALR).
The authors would like to thank Amy Li, Victor F. Hevia, Sarah Harney, Ronald Mathieu, Dr. Ann Mullally and Esther Baena for technical assistance, Dr. Tan Ince for histopathological assessment, Drs. George Daley and Ben Ebert for equipment use, and members of the McAllister lab for helpful discussion. We thank Drs. Nancy Berliner, Rubén Pío, Ann Mullaly, and Mandy Redig for helpful reading of the manuscript and Jessica Hughes and Jamie Brien, Hematology Division, Brigham and Women’s Hospital, for help in preparation of the manuscript.
Footnotes
Authors report no conflict of interest.
References
- 1.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 2.Guarneri V, Conte P. Metastatic breast cancer: therapeutic options according to molecular subtypes and prior adjuvant therapy. Oncologist. 2009;14:645–56. doi: 10.1634/theoncologist.2009-0078. [DOI] [PubMed] [Google Scholar]
- 3.Demicheli R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol. 2001;11:297–306. doi: 10.1006/scbi.2001.0385. [DOI] [PubMed] [Google Scholar]
- 4.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 5.McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. J Clin Oncol. 2010;28:4022–8. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- 6.Castano Z, Tracy K, McAllister SS. The tumor macroenvironment and systemic regulation of breast cancer progression. Int J Dev Biol. 2011;55:889–97. doi: 10.1387/ijdb.113366zc. [DOI] [PubMed] [Google Scholar]
- 7.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuznetsov HS, Marsh T, Markens BA, Castaño Z, Greene-Colozzi A, Hay SA, et al. Identification of Luminal Breast Cancers that Establish a Tumor Supportive Macroenvironment Defined by Pro-Angiogenic Platelets and Bone Marrow Derived Cells. Cancer Discov. 2012 doi: 10.1158/2159-8290.CD-12-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, et al. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest. 2011;121:784–99. doi: 10.1172/JCI43757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–44. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–77. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 16.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 17.Singhi AD, Cimino-Mathews A, Jenkins RB, Lan F, Fink SR, Nassar H, et al. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Mod Pathol. 2012;25:378–87. doi: 10.1038/modpathol.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer. 2010;10:301–9. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 19.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–69. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 21.Sarrio D, Franklin CK, Mackay A, Reis-Filho JS, Isacke CM. Epithelial and mesenchymal subpopulations within normal basal breast cell lines exhibit distinct stem cell/progenitor properties. Stem Cells. 2012;30:292–303. doi: 10.1002/stem.791. [DOI] [PubMed] [Google Scholar]
- 22.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao DJ, Dickson RB. c-Myc in breast cancer. Endocr Relat Cancer. 2000;7:143–64. doi: 10.1677/erc.0.0070143. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian A, Sharma A, Mokbel K. Insulin-like growth factor binding proteins and breast cancer. Breast Cancer Res Treat. 2008;107:181–94. doi: 10.1007/s10549-007-9549-0. [DOI] [PubMed] [Google Scholar]
- 25.Saeki T, Salomon DS, Johnson GR, Gullick WJ, Mandai K, Yamagami K, et al. Association of epidermal growth factor-related peptides and type I receptor tyrosine kinase receptors with prognosis of human colorectal carcinomas. Jpn J Clin Oncol. 1995;25:240–9. [PubMed] [Google Scholar]
- 26.Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–36. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987;49:465–75. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 28.Vergara D, Valente CM, Tinelli A, Siciliano C, Lorusso V, Acierno R, et al. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 2011;310:1–8. doi: 10.1016/j.canlet.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–70. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 30.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 31.Resnik JL, Reichart DB, Huey K, Webster NJ, Seely BL. Elevated insulin-like growth factor I receptor autophosphorylation and kinase activity in human breast cancer. Cancer Res. 1998;58:1159–64. [PubMed] [Google Scholar]
- 32.Li Y, Geng YJ. A potential role for insulin-like growth factor signaling in induction of pluripotent stem cell formation. Growth Horm IGF Res. 2010;20:391–8. doi: 10.1016/j.ghir.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 34.Litzenburger BC, Creighton CJ, Tsimelzon A, Chan BT, Hilsenbeck SG, Wang T, et al. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin Cancer Res. 2011;17:2314–27. doi: 10.1158/1078-0432.CCR-10-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgillo F, Bareschino MA, Bianco R, Tortora G, Ciardiello F. Primary and acquired resistance to anti-EGFR targeted drugs in cancer therapy. Differentiation. 2007;75:788–99. doi: 10.1111/j.1432-0436.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- 38.Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 39.Jones HE, Goddard L, Gee JM, Hiscox S, Rubini M, Barrow D, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 40.Lindstrom LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–8. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Ye AS, Gardino AK, Heijink AM, Sorger PK, MacBeath G, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–94. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–9. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 43.Rowinsky EK, Schwartz JD, Zojwalla N, Youssoufian H, Fox F, Pultar P, et al. Blockade of Insulin-Like Growth Factor Type-1 Receptor With Cixutumumab (IMC-A12): A Novel Approach to Treatment for Multiple Cancers. Curr Drug Targets. 2011 doi: 10.2174/138945011798829401. [DOI] [PubMed] [Google Scholar]
- 44.Shankar S, Nall D, Tang SN, Meeker D, Passarini J, Sharma J, et al. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 2011;6:e16530. doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 46.Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, Karnoub AE, et al. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12:160–70. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 49.Numico G, Silvestris N, Grazioso Russi E. Advances in EGFR-directed therapy in head and neck cancer. Front Biosci (Schol Ed) 2011;3:454–66. doi: 10.2741/s164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.