Abstract
Very small embryonic-like cells (VSELs), found in murine bone marrow and other adult tissues, are small, non-hematopoietic cells expressing markers of pluripotent embryonic and primordial germ cells. A similar cell type in humans has begun to be characterized, though with a slightly different phenotype and surface markers. Consistent with expression of pluripotency genes, murine VSELs differentiate into cell types from three germ layer lineages in vitro, though pluripotency has yet to be shown at the single cell level or in vivo. VSELs appear to be quiescent under steady state conditions, apparently due to partially erased imprinting and overexpression of cell cycle inhibitory genes. In vivo, VSELs can enter the cell cycle under stress conditions, but which factors regulate quiescence versus proliferation and self-renewal versus differentiation are as yet unknown, and in vitro conditions that induce proliferation and self renewal have yet to be defined. Future experiments are needed to address whether a VSEL niche actively regulates quiescence in vivo or quiescence is cell autonomous under steady state conditions. Insights into these mechanisms may help to address whether or not VSELs could play a role in regenerative medicine in the future.
Keywords: Stem Cells, Pluripotency, Adult stem cells, regeneration
Introduction
Mammalian tissues that undergo constant turnover (such as the blood and epithelia of the skin, lung, and intestine) are maintained through stem cells, which are characterized by the ability to self-renew and differentiate. Self-renewal can occur when cells divide symmetrically to produce two stem cells or asymmetrically, in which case they give rise to an identical stem cell and a more differentiated, tissue-specific cell type. Adult stem cells are either uni- or multipotent. Examples of multipotent stem cells include hematopoietic stem cells (HSCs) residing in the bone marrow, which give rise to all blood cell types (Worton et al. 1969); mesenchymal stem cells (MSCs), which give rise to bone, cartilage, and adipose cells (Prockop 1997); neural stem cells in the brain, which give rise to neurons and glia (Reynolds and Weiss 1992); and broncho-alveolar stem cells in the lung, which give rise to alveolar epithelial cells as well as epithelial cells of the bronchus (Kim et al. 2005). Spermatogonial stem cells are an example of unipotent stem cells.
The view that adult stem cells are restricted to contribute to cell lineages within one particular tissue type has been challenged by several reports describing stem cells from one tissue giving rise to cell types of another, a phenomenon termed “plasticity.” Different subsets of bone marrow cells, for example, have been reported to give rise to lung epithelial cells, hepatocytes, neurons, and/or skins in vitro or in vivo. An absence of proper controls, low image quality, lack of evidence to rule out fusion, poor proof of differentiation, and/or insufficient specificity of molecular markers render many of these studies inconclusive; however, a few studies do provide definitive evidence of bone marrow derived cells giving rise to lung epithelial cells (reviewed in Kassmer and Krause 2010). Overall, these findings could be explained by multiple scenarios, such as by transdifferentiation of a committed cell type in the bone marrow into an epithelial or neuronal cell type. Recently, our group has demonstrated that hematopoietic cell types do not transdifferentiate to give rise to lung epithelial cells, and that all bone marrow derived lung epithelial cells arise from non-hematopoietic cell types (Kassmer et al. 2012). Instead, this finding could be explained by the differentiation of mesenchymal stromal cells into epithelial cells, the presence of a rare population of lung stem cells in the marrow, or by the presence of a primitive, pluripotent cell type in the adult bone marrow.
Pluripotency, defined as the ability to give rise to cell types from the three primary germ layer lineages (meso-, ecto-, and endoderm) is an inherent property of embryonic stem cells (ESCs), which are derived from the inner cell mass of the blastocyst of murine embryos and can be maintained in vitro (Tremml et al. 2008) as cell lines that retain the ability to generate all cells of the body, including the germ line. An alternative source for pluripotent stem cells can be found in the epiblast stage of the embryo. When isolated from a mouse embryo, these ‘epiblast stem cells’ (EpiSCs) share features with human embryonic stem cells, which are isolated from the epiblast stage of the human embryo (Brons et al. 2007; Tesar et al. 2007). Murine EpiSC can be manipulated in culture to exhibit a more primitive phenotype, similar to murine ESC (Bao et al. 2009). Also, primordial germ cells (PGCs), the embryonic precursor cells that give rise to cells of the germline, express pluripotency markers and can be isolated and cultured as pluripotent cell lines (Labosky et al. 1994a; Matsui et al. 1992). Genes that are required for pluripotency and are expressed in pluripotent cells include Oct4 and Nanog. Other genes involved in maintaining pluripotency include cMyc, Lin28, Klf4, Rex1 and Dppa2.
The concept of adult stem cells with pluripotent characteristics is highly controversial. Several reports demonstrate expression of pluripotency genes such as Oct4 in adult stem cell populations (summarized in Lengner et al. 2007), however. It can therefore be hypothesized that demonstrations of apparent developmental plasticity of lineage-committed adult stem cell types may be related to the presence of rare, pluripotent cells residing in adult tissues. In 2006, a population of small cells expressing pluripotency genes, including Oct4 and Nanog, was first identified in the bone marrow of mice (Kucia et al. 2006), and have since been demonstrated to reside in all adult tissues studied (Zuba-Surma et al. 2008b). In vitro, these very small embryonic-like cells (VSELs) can give rise to the three germ layer lineages (Kucia 2006). Yet, self-renewal has not yet been demonstrated for VSELs, thus it is not yet appropriate to use the term “stem cell” in association with VSELs.
Here, we provide an overview of the known biology of VSELs, including their isolation, gene expression profile, and evidence for pluripotent differentiation potential, as well as a critical analysis of published findings. Human VSELs have been less thoroughly characterized than murine VSELs, and thus we focus mostly on data obtained for murine VSELs.
Very small embryonic-like cells: Definition
In murine bone marrow, VSELs are defined as Lineage-negative, Sca-1-positive and CD45-negative cells of small size (less than 6 micrometers in diameter) (Kucia et al. 2006). VSELs have a high nuclear-to-cytoplasmic ratio, and high expression of pluripotency core factors. All of these criteria, described below in detail, need to be fulfilled in order for a cohort to be termed a “VSEL” population. Therefore, we are not discussing a number of publications that used the term VSELs in appropriately (Bhartiya et al.; Danova-Alt et al. 2012; Sovalat et al. 2011), namely because the cell populations studied in those publications do not fulfill all of the above criteria and thus the term VSELs is not appropriate.
Isolation of VSELs by flow cytometric cell sorting
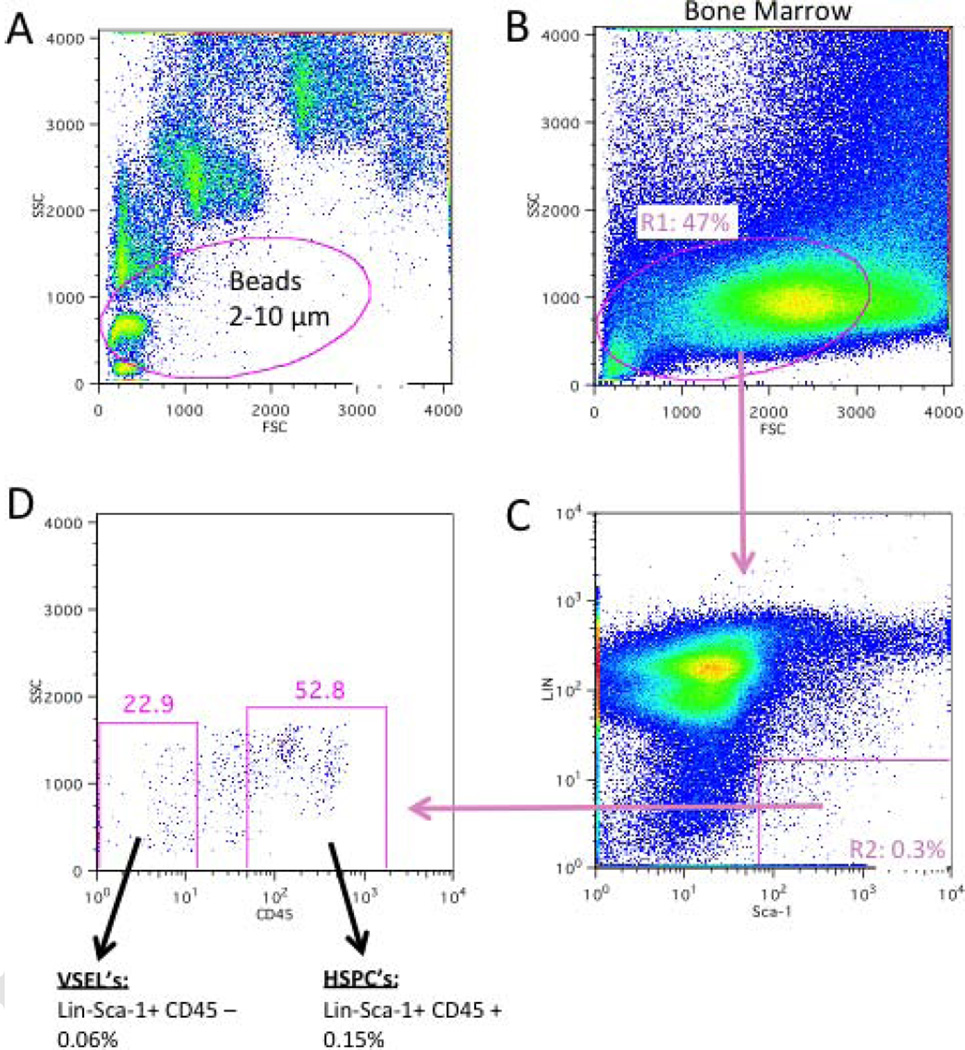
The VSEL population comprises roughly 0.03% of total bone marrow cells, depending on the mouse strain and the age of the mouse. The isolation strategy has been published in detail elsewhere (Zuba-Surma and Ratajczak 2010), but we briefly cover it here for clarity. To obtain a pure population of VSELs by flow cytometry, it is important to make sure that very small events, including those of cells of less than 3 micrometers in diameter, can be resolved in the forward and side scatter. Size beads need to be used to create a gate that includes events between 2–10 micrometers in diameter (Figure 1A and B) when comparing the VSEL population with hematopoietic stem and progenitor cells, which fall into the larger range. Only lineage marker (CD45R/B220, Gr-1, CD11b, Ter119, TCRαβ, TCRγδ)-negative cells that are also positive for Sca-1 are included in a second gate (Figure 1D). It is important to include the Sca-1-dim cells, as this population contains most of the VSELs. In a third gate, only cells that are truly negative for CD45 are included, omitting CD45-dim cells (Figure 1C). Due to the rarity of the target population, slow sorting speeds (less than 18,000 events per second) are also important for obtaining high purity.
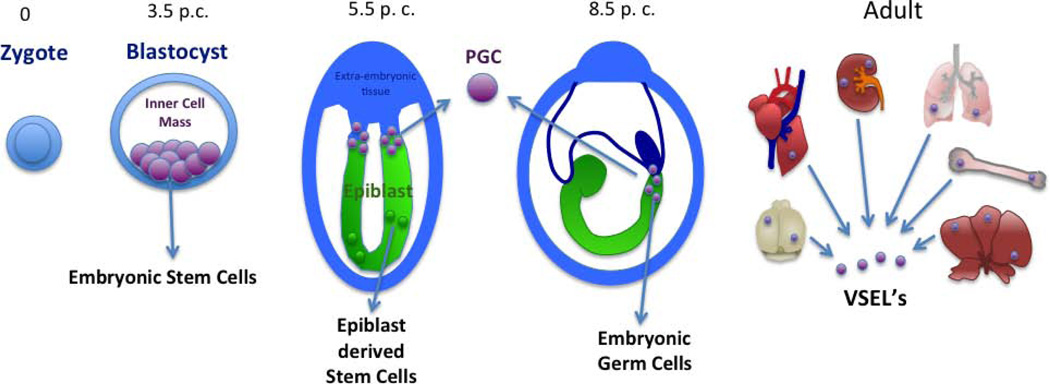
Figure 1. Murine pluripotent stem cells can be derived from different developmental stages.
At Day 3.5 of embryonic development (Day 3.5 post coitum, p.c.), embryonic stem cells can be isolated from the inner cell mass of the blastocyst. At the epiblast stage on Day 5.5, epiblast stem cells (EpiSCs) or primordial germ cells (PGCs) can be isolated from the embryo and propagated in different culture conditions. For derivation of embryonic germ cell lines (EGCs), primordial germ cells (PGCs) are isolated from the base of the allantois of Day-8.5 embryos or from the genital ridges of Day-10.5 embryos (not shown). Very small embryonic like cells (VSELs) are present in all adult tissues, including bone marrow, liver, heart, kidney, brain, and lung. VSELs are also present in the fetal liver (not shown).
Expression of core pluripotency factors
VSELs express very high levels of pluripotent stem cell marker genes, such as Oct4, Nanog, Rex-1, Dppa3, and the telomerase protein Rif1. RNA expression levels of these genes can be as much as twofold higher in VSELs than in murine ESCs, and about 200-fold higher than in Lin− CD45+ Sca-1+ cells (Kucia et al. 2006). (In a later paper, the same group reported that VSELs express Oct4 and Nanog at 50% and 20% of the level of murine ESCs, indicating that either populations isolated were not 100% pure or that the expression levels in VSELs can vary (Shin et al. 2012)). By immunofluorescence, expression of Oct4 and Nanog protein was confirmed, as well as expression of the embryonic surface molecule SSEA-1 (Kucia et al. 2006). We have since confirmed expression of Oct4, Nanog, Sox2, Rex1, and Dppa3 at the mRNA level, and Oct4 and Nanog at the protein level in our laboratory. These findings suggest that VSELs are pluripotent.
Microscopic characterization and analysis of cell migration in vitro
Transmission electron microscopy confirmed that VSELs contain a high nuclear-to-cytoplasmic ratio and revealed that their nuclei contain decondensed euchromatin, indicative of the primitive nature of these cells (Kucia et al. 2006). VSELs express CXCR4, c-met, and LIF-R. In migration assays, VSELs respond robustly to stromal cell-derived factor-1 (SDF-1), hepatocyte growth factor (HGF), and leukemia inhibitory factor (LIF) (Kucia et al. 2006). In mice, VSELs can be mobilized into peripheral blood in response to granulocyte colony-stimulating factor (G-CSF) treatment; in a model of toxic liver or skeletal muscle damage induced by injection of carbon tetrachloride or cardiotoxin, respectively (Kucia et al. 2008b); as well as after myocardial infarction (Zuba-Surma et al. 2008a) or after exposure to intermittent hypoxia (Gharib et al.).
In vitro differentiation and pluripotency
VSELs can undergo multi-lineage differentiation into cells from all three germ layers in vitro, and thereby fulfill the criteria for pluripotency. Specifically, when GFP-positive VSELs were co-cultured with non-GFP bone marrow cells in cardiac-, neural- and pancreatic differentiation media, GFP-positive cells differentiated into the respective cardiomyocytes, neuronal cells (including glial cells), and pancreatic cells (Kucia et al. 2006). Changes in the expression of mRNA for markers of myocardium, neural cells, and pancreatic cells were upregulated in the differentiation cultures. GFP-negative controls, however, were not shown in the paper, leaving the possibility that the GFP signal detected in cells staining positive for differentiated cell markers (labeled by red fluorescence) is due to autofluorescence rather than differentiation. Since mRNA expression levels were not analyzed specifically for GFP+ cells, it remains unknown to what extent VSELs differentiated into neurons, pancreatic cells, and myotubes in this study.
Presence of VSELs in adult tissues and organs
VSEL populations sorted by flow cytometry frequently contain debris and dead cells. In contrast, image stream technology allows visual exclusion of dead cells from analysis based on bright field morphology. In addition, nucleated cells can be identified using DNA dyes making it possible to specifically analyze only nucleated events, and cells can be fixed and stained for Oct4. VSELs, identified as Lin− CD45− Sca-1+ Oct4+ cells, have been identified by image stream technology in several adult murine organs, including brain, heart, skeletal muscle, pancreas, lungs, testis, kidneys, spleen thymus, and liver, with pancreas and brain containing the highest percentages of VSELs (Zuba-Surma et al. 2008b). Even though the cells for these experiments were harvested from organs after whole-body vascular perfusion, it cannot be excluded that detection of VSELs in adult organs was due to the presence of mobilized VSELs in the blood. For example, the efficiency of removing blood from the brain strongly depends on the cardiac perfusion technique and the site of saline injection (right versus left ventricle). Even after perfusion, most organs still contain residual blood cells. Therefore, the presence of extravascular VSELs in adult tissues needs to be confirmed by microscopy of tissue sections stained for Sca-1, Oct4, and CD45; however, this has not been feasible due to the extremely low frequency and small size of VSELs. Strikingly, these experiments also showed that VSELs comprise a heterogeneous population. In the spleen, 41% of VSELs stained for nuclear Oct4, while only 11% of VSELs in the brain were Oct4-positive. Bone marrow-derived VSELs contain 29% Oct4-positive cells. In addition, the size of VSELs differs between organs. The smallest VSELs are found in the bone marrow (average size 3.6 micrometers), whereas the largest VSELs are in the liver (8.4 micrometers). It will be important to document variations in expression of functionally important genes, such as Nanog, between different VSEL sub-populations. Most importantly, as we will discuss later, VSEL heterogeneity needs to be assessed on a functional level. The apparent difference in size of VSELs from different organs and the differences in the percentage of Oct4-positive cells suggests that VSEL-populations from different organs may differ in their phenotype, possibly because they reside in different niches. Functional testing (differentiation assays) will be needed to establish whether or not VSEL populations from different adult organs are functionally similar.
A 2009 study reported that the neonatal retina contains VSELs that express high levels of Oct4 and Nanog (Liu et al. 2009), consistent with the notion that VSELs are present in organs other than the bone marrow. VSELs isolated from neonatal retina expressed Pax6 and Otx2 genes, which are important for establishing the early eye field, at much higher levels than murine ESCs or VSELs from the bone marrow, and even at higher levels than retinal cells that are not VSELs. This suggests that the niche might influence differentiation of VSELs. By immunofluorescence, Oct4-positive cells were found in the retina in the ganglion cell layer, suggesting that this area may be the niche for VSELs in this organ. These cells were non-proliferative, consistent with the finding that bone marrow-derived VSELs are quiescent. The size of Oct4-positive cells in the retina was not assessed, however, so it is not clear whether all of these cells were VSELs or there is another population of Oct4-positive cells. Furthermore, not all of the Oct4 staining appeared to be nuclear, and confocal microscopy was not used, making definitive analysis of these findings difficult.
Another study reported the presence of small, Sca-1+ CD45− cells of round morphology in the adult murine kidney. These cells lacked markers of hematopoietic cells, and were distinct from MSCs or epithelial cells (Dekel et al. 2006). Amongst these cells, Sca-1-dim cells were smaller and nonadherent, whereas Sca-1-bright cells were larger, adhered to plastic, and proliferated in serum-containing media. In our hands, VSELs are contained within the Sca-1-dim fraction of bone marrow cells, suggesting that the Sca-1-dim cells in this study represent VSELs that reside in the kidney. Expression of pluripotency marker genes and differentiation assays would need to be performed to be certain that Sca-1-dim cells from the kidney are truly VSELs.
Ratajczak et al. proposed the hypothesis that VSELs seed organs and tissues during early development, and that this explains their presence in adult organs (Ratajczak et al. 2007). Future experiments need to clarify if this process is truly occurring and, if so, whether those VSELs become resident in the tissue after seeding or they can actively migrate throughout the body in the adult. The fact that VSELs can mobilize into peripheral blood as well as their migratory phenotype in vitro together suggest that VSELs are highly motile cells that travel throughout the body at all times. This issue needs thorough experimental evaluation.
Global gene expression analysis: Comparison to ESCs
Global gene expression was analyzed in VSELs and compared to ESCs and HSCs (Shin et al. 2012). cDNA libraries were produced from 20 freshly isolated VSELs, 20 hematopoietic progenitor cells, and 20 murine ESCs and expression assessed using microarrays. Global gene expression analysis was performed, and the VSEL transcriptome clustered tightly with that of ESCs, but was distant from HSCs. Consistent with their quiescent status, VSELs expressed low levels of genes involved in protein turnover and growth factor or mitogen signaling, while expressing high levels of cell-cycle checkpoint genes. Of note, VSELs from only one sort were assessed using the microarray after prescreening 3 VSEL sorts for the batch with the best enrichment for Oct4 and Stella expression. This suggests that not all VSEL sorts give precisely the same enrichment for ESC-like cells.
VSELs also express genes characteristic of EpiSCs, such as Gbx2, Fgf5, and Nodal, at much higher levels than murine ESCs. In contrast, a gene specific for the inner cell mass (Rex-1) is expressed at lower levels in VSELs, suggesting a relationship between VSELs and epiblast-derived cells (Shin et al. 2010b).
Expression of PGC-specific genes, hypothesis of developmental origin
Early in embryonic development, PGCs become specified in the proximal epiblast and migrate into extra-embryonic tissues, where instructive signals regulate their differentiation. Subsequently, they re-enter the embryo and migrate to the genital ridges, where they ultimately give rise to gametes.
Pluripotency core factors, such as Oct4, Nanog, and Sox-2, are decreased during differentiation of somatic cells, but continue to be expressed in PGCs. The specification of PGCs is tightly regulated by expression of genes such as Vasa, Blimp1, Stella, Prdm14, Nanos3, and Fragilis (Saga 2008). Based on quantitative PCR, all of these genes are more highly expressed in VSELs than murine ESCs, suggesting a close relationship of VSELs to PGCs. VSELs also express high levels of Dppa2, Dppa4, and Vasa, which are characteristic of late migratory PGC. As PGCs were not used as a positive control for direct comparison in this study, it is not clear how the expression level of germline-specific genes in VSELs compares to expression levels in PGCs. Nevertheless, these data support the hypothesis that VSELs are developmentally related to PGCs, and possibly are derived from the same cell population during development. Instead of migrating to the genital ridges as do PGCs, however, VSELs become distributed in adult tissues. Related to this hypothesis, fetal porcine skin has been reported to contain PGC-like cells that are similar to murine VSELs (Linher et al. 2009).
Imprinting and quiescence
Genomic imprinting at specific loci leads to the differential gene expression that is dependent upon the parent of origin, resulting in monoallelic expression. Amongst the imprinted genes, there are growth-promoting and growth-inhibitory genes, the proper balance of which is key for normal cell growth, proliferation, and development. In contrast to the genomic imprints that are maintained in somatic cells throughout the body, VSELs exhibit modified methylation of imprinted genes, leading to low expression of growth-promoting imprinted genes and high expression of growth-inhibitory genes (Shin et al. 2009). Specifically, VSELs show erased methylation of the H19 and Rasgrf1 loci, whereas Kcnq1 and IGF2R are hypermethylated. As a result, VSELs express low levels of the growth-promoting genes IGF2 and Rasgrf1, while upregulating the growth-inhibitory genes H19, p57KIP, and IGF2R. The ratio of expression of H19-to-IGF2, which is 400 in VSELs and 1 in ESCs, may be responsible for the low VSEL proliferation in vitro, and may be a mechanism to prevent VSELs from uncontrolled proliferation, thus underlying their quiescent state in adult tissues (Shin et al. 2010a; Shin et al. 2009). A direct functional relationship between the imprinting status and quiescence in VSELs has not been proven, however. Of note, induced pluripotent stem cells (iPSCs) as well as embryonic germ cells (EGCs) erase parent-of-origin-specific imprinting of H19, Kcnq1, CDKn1c, and IGF2 over time in culture, while remaining pluripotent and self-renewing (Sun et al.; Surani et al. 2008). In fact, no correlation between imprinting of the IGF2R gene and germline competence was found for EGCs (Labosky et al. 1994b). This suggests that erased imprinting is compatible with proliferation and pluripotency, as long as the correct ratio of growth-promoting and growth-inhibitory genes is maintained. In VSELs, quiescence is likely to be caused by high expression of the cell cycle inhibitor p57KIP, as well as high expression of the transcription factor E2F2 (Shin et al. 2012), which represses cell cycle genes to establish the G0 state. Knockdown experiments would help to address whether or not the high expression of p57KIP is functionally related to VSEL quiescence. For instance, TGF-beta can induce expression of p57KIP in HSCs, inducing cell cycle arrest, while knockdown of p57KIP blocks this cytostatic effect on hematopoietic cells (Scandura et al. 2004). VSELs express high levels of TGF-beta (Shin et al. 2012), suggesting a possible link to their high expression of p57KIP and quiescence.
Despite their propensity to exist in quiescence, VSELs can proliferate in vivo. VSEL numbers increase in vivo in murine bone marrow upon treatment with 5-FU (Taichman et al. 2010). In another study, 12% of VSELs were reported to incorporate BrdU and their numbers increased after total body irradiation with 1000 – 1500 cGy in mice (Ratajczak et al. 2011b). Together, these experiments suggest that in vivo, certain extracellular signals can induce cell cycle entry in VSELs, a process that probably occurs primarily under stress conditions.
We have no been able to obtain VSEL proliferation in culture, despite extensive efforts using a variety of different conditions and various different combinations of mitogens and growth factors (Table 3). Of note, TGF-beta inhibitory antibody did not allow the cells to overcome quiescence in culture (Table 3), suggesting that other, as-yet-unknown factors are needed to induce cell cycle entry in culture.
Table 3.
Conditions tested for Culture of VSEL’s
| Culture Condition | Result |
|---|---|
| SCF, LIF, bFGF, BMP4, SDF1, Forskolin, RA on fibronectin and laminin | Larger cells, Vasa positive, no proliferation |
| LIF, Activin A, bFGF, SCF, EGF on fibronectin and laminin | Negative |
| PDGFbb, LIF, EGF, Dexamethasone1 | Negative |
| PDGFbb, LIF, EGF, Dexamethasone + anti TGFbeta1 | Negative |
| PDGFbb, LIF, EGF, Dexamethasone, IGF21 | Negative |
| PDGFbb, LIF, EGF, Dexamethasone, Activin A1 | Negative |
| PDGFbb, LIF, EGF, Dexamethasone, IGF2, Activin A1 | Negative |
| PDGFbb, LIF, EGF, Dexamethasone, IGF2, Activin A, IL-6, bFGF1 | Negative |
| IGF2, IL-6, Activin A, PDGFbb2 | Negative |
| IGF2, IGF1, Activin A, PDGFbb2 | Negative |
| IGF2, IGF1, LIF, PDGFbb2 | Negative |
| IGF2, IGF1, LIF, PDGFbb, Activin A, LIF, EGF, FGF2 | Negative |
| IGF1,b FGF, EGF3 | Negative |
| IGF1, bFGF, EGF, LIF3 | Negative |
| bFGF, EGF, LIF3 | Negative |
| bFGF, Activin A3 | Negative |
| Transwell over C2C12 cell, serum free | Spheres affer 3 weeks – Oct4 not detected by PCR |
Negative = No Proliferation, cells gradually lost. Culture medium: DMEM/F12 + B27+N2 supplements. Density: 1000 cells/100µl/well.
2–10% knockout serum replacement. Tested both fibronectin or uncoated plastic, hyopxia and normoxia
tested both laminin or fibronectin
Tested both hypoxia or normoxia, hydrogel or laminin.
Interestingly, it has been reported that in co-culture with C2C12 cells, 5–10% of VSELs form spheres consisting of cells that express Oct4, Nanog, and SSEA-1 (Kucia et al. 2008a), suggesting that quiescence can be overcome under certain conditions. But, VSEL proliferation was not proven to occur in these experiments. Also, expression of Oct4 was gradually lost in the VSEL-derived spheres, suggesting that under these conditions, VSELs start to differentiate; in fact, these VSEL spheres gradually acquire somatic imprinting methylation patterns on a number of genes, including IGF2 and H19 (Shin et al. 2009). Thus, the methylation status of imprinted genes in VSELs can be modified upon exposure to extracellular stimuli.
In vivo pluripotency
In vitro, VSELs have several phenotypic features that are characteristic of pluripotent stem cells, including expression of pluripotency core factors such as Oct4, an undifferentiated morphology, and the ability to differentiate along the three germ layer lineages. More stringent criteria for pluripotency were defined for murine ESCs and later for murine iPSCs, however. By these criteria, a stem cell can only be classified as pluripotent if it is able contribute to formation of chimeric embryos when injected into a blastocyst. Contribution to the germline is particularly critical to fully establish pluripotency of murine ESCs and iPSCs. For human iPSCs, pluripotency is assessed by testing formation of teratomas after injection into mice. Unlike murine ESCs and iPSCs, VSELs do not participate in the formation of chimeras when injected into blastocysts (Ratajczak et al. 2012), and they do not form teratomas. Thus, VSELs do not fulfill all functional criteria for pluripotency.
EpiSCs, which are derived in culture from primary epiblasts and expanded in the presence of Activin-A and fibroblast growth factor 2 (FGF-2), also do not contribute to chimeras (Brons et al. 2007). After exposure to LIF and induction of STAT3 signaling, EpiSCs are reprogrammed to ESC-like cells, which are then able to contribute to the germline in chimeras (Bao et al. 2009). Similarly, PGCs do not participate in the formation of chimeric embryos when injected into blastocysts, but when reprogrammed in vitro by exposure to LIF, FGF-2, and stem cell factor (SCF), PGCs acquire the ability to self renew and become very similar to ESCs. These EGCs contribute to chimeras when injected into blastocysts, and exhibit germline transmission (Labosky et al. 1994b).
Could exposure to certain, thus far unknown, extracellular signals allow VSELs to overcome the quiescent status and “reprogram” them to full pluripotency? Identifying the nature of these signals requires further investigation. These signals are likely going to be different than those for EpiSCs and EGCs since, in our hands, VSELs do not grow in culture in the presence of Activin-A, FGF-2 with or without LIF, or in the presence of LIF, FGF-2, and SCF (Table 3). Different signals may be required to overcome the inherent quiescent status of VSELs, regulate self-renewal, and induce “reprogramming” to an ESC-like state. If it should become possible to expand VSELs in culture as a pluripotent cell type, which still lacks the potential to form teratomas, this cell type would hold great promise for clinical applications, not only for safety reasons but also for the lack of ethical considerations and for the potential of obtaining autologous cells from the blood of patients.
In vivo differentiation of VSELs into tissue specific cell types
Differentiation of VSELs into tissue-specific cell types has been demonstrated by transplantation of purified VSELs into recipient mice and subsequent detection based on expression of donor-specific and tissue-specific markers. In one study, mesenchymal differentiation of VSELs was assessed. VSELs were isolated from mice expressing GFP under control of the ubiquitous beta-actin promoter, incorporated into gelatin sponge implants, and transplanted under the skin of immunodeficient mice (Taichman et al. 2010). Sponges containing VSELs showed the formation of mineralized tissue, whereas sponges containing HSCs did not. GFP expression co-localized with expression of the osteoblastic marker Runx-2 or with expression of PPARγ in the VSEL sponges that contained ossicles. Confocal microscopy was not performed and negative controls were not shown, however, so it is possible that signal overlap in this case is due to autofluorescence. Furthermore, fusion of GFP-positive VSELs with recipient-derived cells migrating into the sponge, such as MSCs or osteoblasts, was not ruled out. Still, differentiation of VSELs into mesenchymal lineages under these conditions is not surprising, since there may be a close relationship between bone marrow-derived VSELs and bone marrow-derived MSCs, which like VSELs are CD45− Lin−.
Differentiation of VSELs into cardiomyocytes was assessed in mice after transplantation of GFP-positive VSELs into recipients after myocardial infarction (Zuba-Surma et al. 2011a). GFP-positive cells were found in the infarcted area that co-expressed α-sarcomeric actin, but fusion of VSELs with α-sarcomeric actin-positive cells was not ruled out.
No study to date has addressed differentiation of VSELs into ectodermal tissue in vivo. In our studies to date, VSELs have not given rise to Keratin-14-positive cells in the skin after intravascular transplantation followed by full-thickness skin injury, using a expression of H2B–GFP from the keratin-14 promoter as a reporter (Kassmer et al, unpublished observations). Bone marrow cells have been previously shown to give rise to epithelial cells in the skin under similar conditions (Borue et al. 2004). It is possible that VSELs are not capable of giving rise to epithelial cells, and that instead, another cell type exists in the bone marrow that is capable of differentiating into skin epithelial cells. For example, skin epithelial cells can be derived from platelet-derived growth factor receptor (PDGFR)-positive bone marrow cells with a stromal cell-like phenotype (Tamai et al. 2011). Stringent lineage tracing experiments using a VSEL-specific promoter will be required to definitively confirm whether VSELs give rise to any tissue specific cell type in vivo under normal, unmanipulated conditions.
Hematopoietic potential
Freshly isolated VSELs do not contain hematopoietic potential. No hematopoietic engraftment occurs from VSELs after intravenous transplantation into lethally irradiated recipients, either with or without co-transplantation of whole bone marrow (Kucia et al. 2006; Ratajczak et al. 2011b). If VSELs are injected into lethally irradiated mice without supporting cells, the mice die, confirming lack of hematopoietic engraftment by VSELs (Ratajczak et al. 2011b). In vitro, VSELs do not form hematopoietic colonies in methylcellulose assays after 5 days. When cultured on OP9 stromal cells for 5 days prior to being switched to methylcellulose, however, VSELs do form a small number of hematopoietic colonies that increase over time as VSELs are passaged in methylcellulose for a total of 15 days. When injected into recipient mice, VSELs primed over OP9 stromal cells and subsequently cultured in methylcellulose provide hematopoietic engraftment in lethally irradiated recipient mice (Ratajczak et al. 2011b). It is possible that a small number of hematopoietic stem cells were co-purified with VSELs, and expanded in cytokine-containing methylcellulose, thus giving rise to hematopoietic colonies and providing engraftment after transplantation. When VSELs are isolated by fluorescence-activated cell sorting, contamination from abundant hematopoietic cell types cannot be completely excluded. The purity of the sorted VSEL population is generally between 94–97%. Thus it is possible that, in small numbers, primitive HSCs may have provided engraftment when co-transplanted with VSELs into lethally irradiated recipients, despite their inability to generate colonies in methylcellulose after short periods of culture. Therefore, stringent lineage tracing experiments using a VSEL-specific promoter will be helpful to more definitively address the question of whether or not VSELs give rise to any hematopoietic cell types in vivo.
Loss of VSELs with aging, potential role of insulin-like growth factor signaling
The number of VSELs in the bone marrow declines dramatically with age. In mice over 3 years old, the number of VSELs in the bone marrow was reduced 17 times compared to young mice (2 months old) from the same strain (Kucia et al. 2006; Zuba-Surma et al. 2009). VSELs from older mice had a reduced ability to form spheres over C2C12 cells (Zuba-Surma et al. 2009) as well as reduced expression of pluripotency factors and decreased pluripotency (Kucia et al. 2006). Likewise, the number of VSELs was greatly reduced in adult retina compared to neonatal retina, and expression of embryonic marker genes such as Oct4 and Nanog was drastically reduced (Liu et al. 2009). It would be interesting to assess if all neonatal organs contain higher numbers of VSELs expressing high levels of pluripotency markers, and how these are different from VSELs in adult animals.
In another study, the number of VSELs was assessed in different mouse models with extended or shortened lifespan. Long-lived Laron dwarf mice, which are small due to deficiency of growth hormone (GH), have higher numbers of circulating VSELs compared to age-matched wildtype littermates (Ratajczak et al. 2011a). In line with these findings, short-lived transgenic mice overexpressing bovine growth hormone (BGH) display a reduced number of VSELs (Kucia et al. 2012). It has been hypothesized that exposure to insulin-like growth factor 1 (IGF-1) leads to depletion of VSELs, since levels of IGF-1 are low in Laron dwarf mice, while high in BGH mice. Supporting this hypothesis, GH or IGF-1 treatment reduces the number of VSELs in Laron Dwarf or wildtype mice, and increased methylation of the H19-IGF2 imprinted locus was detected in aged mice, BGH mice, and Laron Dwarf mice treated with IGF-1 (Kucia et al. 2012). The epigenetic changes correlated with increased expression of IGF-2, H19, and RasGrf1, a small GTP exchange factor involved in signaling downstream from the IGF-receptor. Differences in expression of Oct4 levels between VSELs from Laron Dwarf, BGH, or wildtype mice were not assessed, although it was observed that the Oct4 promoter was hypermethylated in VSELs from old BGH mice, versus being hypomethylated in VSELs from old Laron Dwarf mice (Ratajczak et al. 2011a). This suggested that VSELs in BGH-overexpressing mice lose their pluripotent character, although this was not assessed functionally.
Although these data strongly suggest a role for IGF and signaling from the IGF-receptor in VSEL-depletion during aging, this causative link has not yet been fully established. Differences in the level of growth hormone and IGF-1 could potentially have other physiologic and molecular effects in young and aging mice, which may indirectly affect the numbers of VSELs. Such changes may include differences in the levels of oxidative stress or expression of sirtuins, which are mitochondrial molecules that may inhibit cellular aging. It is also not clear if exposure to IGF would cause VSELs to die, differentiate, or divide and then differentiate. Injections of IGF-1 into wildtype mice followed by assessment of cell cycle status in VSELs, analysis of pluripotency and expression of pluripotency markers, as well as analysis of signaling pathways downstream of the IGF-receptor could help to address these questions. An ideal experiment would be removal of the IGF-1-receptor gene specifically in VSELs using a floxed allele and an inducible Cre-recombinase.
VSELs in humans
VSELs have been identified in human umbilical cord blood as CXCR4+ CD34+ CD133+ Lin− CD45− cells enriched for Oct4 and SSEA-4 (Kucia et al. 2007; Zuba-Surma et al. 2011b). VSELs have also been isolated from umbilical cord blood as CD45-GlyA-CD13+ ALDHlow cells (Ratajczak et al. 2011c). The fact that umbilical cord blood-derived VSELs express only fivefold higher levels of Oct4 than corresponding CD45-positive hematopoietic stem cells suggests that these VSELs might not be equivalent to murine bone marrow-derived VSELs.
Danova-Alt et al. (2012) isolated a cell population of CXCR4+ CD45− Lin− cells from umbilical cord blood that they perhaps incorrectly termed “VSELs.” The authors reported that this population does not express genes of pluripotent cells. But, the cells were not selected by size, which is key for VSEL isolation, and they were CD133-negative, again inconsistent with these cells being human VSELs.
In another study, VSELs were purified from normal human bone marrow and peripheral blood (Sovalat et al. 2011). Small (2–6 micrometers) CD45− Lin− CD133+ CD34+ CXCR4+ cells were isolated, which expressed SSEA-4, Oct4, and Nanog by immunofluorescence and PCR. Immunofluorescence images showed somewhat questionable expression of Oct4 and Nanog in the nucleus, however, because the staining only partially overlapped with the DAPI signal. Confocal microscopy would have clarified this, and negative controls should have been shown. Additionally, no positive control (ESCs) was included for expression of Oct4 and Nanog by PCR, and expression of Oct4 was only 4–6-fold higher than in unpurified bone marrow or peripheral blood cells.
In humans, as in mice, VSELs are motile cells. VSELs have been detected in peripheral blood after myocardial infarction (Abdel-Latif et al. 2010; Wojakowski et al. 2010a), skin burn injury (Drukala et al. 2012), G-CSF treatment (Havens et al. 2012) stroke (Paczkowska et al. 2009; Wojakowski et al. 2010b), and in patients with Crohn’s disease (Marlicz et al. 2012).
Other studies identifying VSEL-like or Oct4-positive cells in adult tissues
Multiple studies have reported expression of pluripotency genes such as Oct4 in stem cells from multiple adult tissues, most frequently in the bone marrow (D’Ippolito et al. 2004; Jiang et al. 2002; Kuroda et al. 2010; Sauerzweig et al. 2009; Tsai et al. 2012; Wei and Shen), but also in the lung (Kajstura et al.), hair follicles (Yu et al. 2006), testes (Conrad et al. 2008; Huang et al. 2009), and others (summarized in Lengner et al. 2007). It seems likely that some of these studies are describing cell populations that are similar to or contain VSELs, while others are not. Together, these studies suggest that besides VSELs, other Oct4-positive cell populations exist in the adult body, or that expression of Oct4 can be induced by certain culture conditions. Below, we discuss some of the cell populations described in these studies as well as their possible relationship to VSELs.
One group describes the appearance of dividing pluripotent cells from murine bone marrow cells after several weeks of culture in the presence of LIF, epidermal growth factor (EGF), and platelet-derived growth factor (PDGF) (Jiang et al. 2002). The resulting clonogenic cells were termed multipotent adult progenitor cells (MAPCs). Oct4 is expressed at 1000-fold lower levels in murine MAPCs than in murine ESCs. MAPCs can give rise to endothelium, neuroectoderm, and endoderm at the single cell level, and to contribute to most somatic tissues when injected into blastocysts. In contrast to VSELs, which are small and round, these cells have a fibroblast-like morphology and proliferate and self-renew in culture. We found that VSELs do not proliferate in culture under MAPC culture conditions (table 3), and thus a direct relationship of MAPC to VSELs seems unlikely. VSELs can attach to and migrate beneath bone marrow-derived stromal cells (Kucia et al. 2005), and it has been suggested that populations of MAPC could be contaminated with tiny VSELs attached to these stromal/fibroblast-like cells, explaining their pluripotency (Zuba-Surma et al. 2009). MAPC-clones, on the other hand, were derived by seeding 10 cells per well, and the expanded culture was found to contain only 1 retroviral insert, demonstrating that it was derived from a single cell (Jiang et al. 2002). Therefore, MAPCs and VSELs are likely separate and phenotypically different bone marrow cell populations exhibiting pluripotency.
SSEA-1+ Lin− CD45− CD31− cells were identified in murine bone marrow, which express low levels of Sca-1 (Anjos-Afonso and Bonnet 2007). This population was found to decrease with age. Expression of Oct4 and Nanog was detected. SSEA-1-positive cells were found to give rise to the three germ layer lineages in differentiation assays. SSEA-1-positive cells isolated from passage-1 MSC cultures were able to expand in MAPC culture conditions. Clones were derived in culture, and one clone was selected for further study based on its expression of pluripotency factors, and was shown to give rise to the three germ layer lineages in vitro. The fact that other clones did not show the same pluripotency suggests that the starting cell population of SSEA-1-positive cells was heterogeneous or that this clone of cells changed over time in culture. In our hands, VSELs isolated from passage-1 MSC cultures did not grow in MAPC medium (Table 3). One likely explanation could be that the SSEA-1-positive fraction contains another cell population that differs from VSELs, and is able to grow under these conditions and subsequently displays a pluripotent phenotype. This population might be identical to the previously described MAPC population. Interestingly, SSEA-1-positive, non-hematopoietic cells were located by immunofluorescence in the endochondral bone region close to CD45+ CD11b+ Ter119+ CD31+ cells, supporting the idea that VSELs and SSEA-1-positive MSC might occupy a similar niche near the endosteum.
One study reports pluripotent cells isolated from cultures of human skin fibroblasts, human MSCs, or naïve human bone marrow cells by their ability to survive after in vitro exposure to trypsin for 16 hours (Kuroda et al. 2010). Surviving cells were suspended in methylcellulose medium, where they formed clusters that stained positive for Oct4, Nanog, SSEA-1, Sox2, and alkaline phosphatase (ALP). These cells were termed “Muse” cells, for multilineage stress enduring. The conclusions from this study are problematic. Expression of pluripotency markers was not assessed by PCR, and negative controls for immunofluorescence were not shown. Cells from clusters were shown to differentiate into the three germ layer lineages in vitro, but cell morphology and negative controls were not shown. SSEA-3+ CD105+ Muse cells were sorted directly from bone marrow (0.04%). Expression of Oct4, Sox2, and Nanog was detected in freshly sorted cells by PCR, and expression levels were lower than in ESCs. Of the sorted cells, 1.2% (0.003% of total bone marrow MNC) formed ALP+ Muse cell clusters. Expression of endodermal, ectodermal, and mesodermal genes was detected by PCR after differentiation. It seems possible that the SSEA-3+ CD105+ population from human bone marrow may contain VSELs. Yet, VSELs do not grow under the conditions used (data not shown). Since it was not definitively proven in the described study that cells remained pluripotent in culture and retained expression of pluripotency markers, no conclusions can yet be made regarding the nature of the “Muse” cell population.
A recent study reports expression of Oct4 and Nanog in early passage, bone marrow-derived MSC or in MSCs cultured long-term under hypoxic conditions (Tsai et al. 2012). Expression of these two transcription factors maintained self-renewal in the MSC as well as the undifferentiated state, and differentiation potential was achieved by regulating Dnmt1. Knockdown of Oct4 and Nanog decreased proliferation and enhanced spontaneous differentiation in early passage or hypoxic-cultured MSCs. Interestingly, knockdown of p21 increased expression of Oct4 and Nanog in MSCs.
None of these studies examined expression of Oct4 or Nanog in freshly isolated bone marrow cells, however. It is therefore possible that Oct4 expression was induced by the culture conditions, and would thus not be an indication of VSELs in these cultures.
The role of Oct4 in adult stem cells is unknown
While Oct4 has been used as a ‘marker’ of VSELs and other potential pluripotent cell populations in the adult, it may not be required for maintaining this pluripotency. Lengner et al (2007) reported that Oct4 is not required for somatic stem cell self-renewal (Lengner et al. 2007). Cre-recombinase-dependent removal of the Oct4 gene in adult tissues did not have any effect on intestinal epithelial architecture, MSC proliferation, HSC formation, liver regeneration, brain morphology, proliferation of cells in the hair follicle, or wound healing. Thus, it was concluded that Oct4 does not play a role in adult stem cell function. Yet, these data do not address whether or not VSELs are critical for certain forms of tissue repair. In addition, it is unlikely that rare Oct4-positive VSELs would significantly participate in tissue regeneration under the conditions tested. The contribution of VSELs to tissue repair is highly dependent upon the injury and the availability of local tissue stem cells for repair. Thus, potentially perturbing VSEL function by removing Oct4 would not be expected to cause obvious defects in tissue architecture. Furthermore, the promoters used in this study for inducing Cre-recombinase expression might not be activated in VSELs. The investigators also reported that Oct4 is not expressed in adult tissues, as GFP- positive cells could not be detected in mice expressing GFP under the control of the Oct4 promoter. However, VSELs are rare, small cells with a large nucleus and a very narrow ring of cytoplasm, so they might be missed even if they had some GFP. We have found that cytoplasmic GFP, whether expressed from a ubiquitous promoter or the Oct4-promoter, cannot be detected in VSELs, even with the use of an antibody (Kassmer, unpublished observations). Thus, a histone-fused nuclear GFP may be required in order to detect expression of the transgene in VSELs, Therefore, the results presented in this study do not exclude the presence of rare Oct4-positive cells or a possible function for VSELs in adult tissues.
Remaining questions and future directions
The most fundamental question regarding the biology of VSELs concerns their functional role in vivo. The presence of a pluripotent, quiescent cell in adult tissues raises the possibility that these cells may participate in tissue repair in response to damage, and/or that they may serve as a reservoir or precursor to lineage-restricted adult stem cells. Lineage tracing experiments using VSEL-specific promoters may address these issues. The available evidence regarding the pluripotency of VSELs suggests that these cells may be able to differentiate into cells from the three different germ layer lineages, not only in vitro but also in vivo. But experimental evidence to confirm this hypothesis is lacking, as ectodermal differentiation has not yet been demonstrated in vivo.
Most importantly, the cell population defined as VSELs shows considerable heterogeneity. As described above, only 11–41% of VSELs (depending on the organ of origin) express Oct4 (Zuba-Surma et al. 2008b), and gene expression analysis of libraries from 20 VSELs revealed three different types of libraries: ESC-like, Epiblast-like, and Oct4-enriched (Shin et al. 2012). Thus, it is possible that there are different subpopulations of VSELs with different potentials, and it is likely that only a subset or possibly none of them are capable of giving rise to cells from the three germ layers on a single-cell level. This can only be tested if one single VSEL is challenged to give rise to cells from the three germ layers within the same experiment in vivo (e.g. by single cell transplantation); however, this process would require cell proliferation, and it is not yet known whether VSELs are able to undergo proliferation without losing their differentiation potential.
Based on the evidence for expression of germline specific genes in VSELs, it would be interesting to investigate whether or not VSELs have germline potential and are able to give rise to gametes in vivo. As described above, gene expression analysis suggests that VSELs contain a sub-population that is highly enriched for germline-specific genes such as Stella and Prdm14 (Shin et al. 2012). Presumably, this sub-population is more closely related to PGCs than other VSEL sub-populations, and it would be informative to investigate whether VSELs or a subpopulation of VSELs may be able to give rise to germ cells in vitro or in vivo. Testing this ability would be interesting with respect to the presumptive developmental origin of VSELs from PGCs.
It is not yet known if VSELs in adult tissues reside in a specific niche, and if so, which cell types form this niche, and how does this niche might differ from tissue to tissue. Using collagenase digestion of bone fragments, 200-fold higher numbers of VSELs can be obtained from the bone marrow (Taichman et al. 2010), although it has not yet been assessed if VSELs from the subendosteal niche are functionally equivalent to VSELs from the central bone marrow and whether or not they express equal levels of Oct4. A nuclear-localized fluorescent reporter for a VSEL-specific promoter (e.g. Stella H2B–GFP or Oct4-H2B–GFP) would help to microscopically identify the location of VSELs in bone marrow and other tissues.
The quiescent phenotype of VSELs and the apparent block in cell cycle that cannot be easily overcome in culture by exposure to growth factors requires further investigation. Quiescence in vivo could either be due to exposure to inhibitory factors, the absence of stimulatory factors, or both. In vivo, VSELs enter the cell cycle after exposure to irradiation or 5-FU, suggesting that at least in the bone marrow, stress-induced signals can induce proliferation of VSELs. Whether this proliferation is true self-renewal or it involves differentiation and ultimately leads to loss of VSELs was not assessed. The nature of factors able to induce proliferation and self-renewal of VSELs is difficult to assess experimentally. Tissue culture experiments in the presence of absence of stromal cells, stress conditions, and various combinations of growth factors and cytokines may help identify soluble factors or requirement for cell-cell contact between VSELs and niche cells. For instance, PGCs can be induced to self renew and form pluripotent cell lines in culture in the presence of stromal cells expressing a membrane bound form of SCF, whereas the soluble form of SCF is ineffective (Matsui et al. 1992). Similar conditions may apply for self-renewal of VSELs.
Another important field of investigation is the developmental origin of the VSEL-populations. Due to the methylation pattern of imprinted genes as well as gene expression, it has been hypothesized that VSELs are developmentally related to PGCs, and that a sub-population of EpiSCs gives rise to VSELs during development. Lineage tracing experiments using inducible, PGC-specific promoters will be necessary to provide conclusive evidence for the developmental origin of VSELs. This will help to clarify their developmental relationship to PGCs and EpiSCs. Since both these cell types can be induced to form self-renewing, pluripotent cell lines in vitro, global analysis of gene expression as well as epigenetic marks in direct comparison to PGCs, EpiSCs, and their cell culture derivatives may increase our understanding of the mechanisms that govern quiescence, self-renewal and pluripotency in VSELs.
In future studies, efforts need to concentrate on defining VSEL-subsets and assessing their gene expression signature as well as their function in vitro and in vivo. Defining conditions and mechanisms regulating quiescence versus proliferation and self renewal versus differentiation in VSELs will be important towards developing cell culture techniques that may allow expansion of VSELs for regenerative medicine. Understanding the developmental origin of VSELs and their function in vivo will provide important insight related to this goal.
Figure 2. Isolation strategy for murine very small embryonic like cells from bone marrow.
A: Flow cytometry size-beads (1, 2, 4, 6, 8, and 10µm) are used to calibrate the forward scatter (FSC) and gate on events between 2 and 10 µm in diameter. B: The same gate is then applied to whole bone marrow. C: From this gate (R1), expression of lineage markers (Lin = CD45R/B220, Gr-1, CD11b, Ter119, TCRαβ, TCRγδ) and Sca-1 are displayed; events negative for Lin and positive for Sca-1 are gated (R2). D: From the events included in R2, expression of CD45 is displayed. CD45-negative events are VSELs, whereas CD45-positive events are hematopoietic stem and progenitor cells.
Table 1.
Phenotype of different murine stem cell populations
| Oct4 | Nanog | Stella (DPPA3) |
Vasa | SSEA-1 | CXCR4 | CD45 | Sca-1 | c-kit | LIFR | |
|---|---|---|---|---|---|---|---|---|---|---|
| VSEL | + | + | + | + | + | + | − | + | +/− | + |
| ESC | + | + | + | + | + | − | − | − | + | + |
| PGC | + | + | + | + | + | + | − | ? | + | + |
| HSPC | − | − | − | − | − | + | + | + | + | ? |
Gene expression levels (Oct-4, Nanog, Stella, Vasa) or expression levels of cell surface markers (SSEA-1, CXCR4, CD45, Sca-1, c-kit, LIFR) on murine cell populations. VSEL = very small embryonic like cells, ESC= embryonic stem cells, PGC = primordial germ cells, HSPC = hematopoietic stem and progenitor cells. + = positive, − = low or negative, +−= expressed on a fraction of the cells, ?= No literature available. References: (Kucia et al. 2006), (Gu et al. 2010; Muramatsu and Muramatsu 2004; Saga 2008). C-Kit on VSEL’s: Ratajczak and Kassmer, unpublished observations.
Table 2.
In vitro culture conditions for different murine stem cell populations
| ESC | EpiSC | EG cells | MAPC | VSEL | |
|---|---|---|---|---|---|
| Tissue of Origin | Blastocyst: Inner cell mass | Epiblast, 5.5dpc | PGC, 8.5 dpc, base of allantois | Adult bone marrow, plastic adherent | Adult bone marrow, Lin− Sca-1+ CD45− |
| Culture conditions/Cytokines | LIF (BMP4) | Activin A, bFGF | LIF, bFGF, mSCF (BMP4) | LIF, EGF, PDGFbb, 5%O2 | None defined |
| Culture Conditions/Feeder Cells | MEF | sl4-m220 cells | C2C12 Self renewal not demonstrated |
ESC = Embryonic Stem Cells, EpiSC = Epiblast stem cells, EG cells = Embryonic germ cells, MAPC = Multipotent adult ptogenitor cells, VSEL= very small embryonic like cells. LIF: Leukemia inhibitory factor. MEF: Murine Embryonic Fibroblasts. D.p.c.: day post coitum. (BMP4): BMP4 is secreted by feeder cells, not added to culture media. References: Tremml et al 2008, Tesar et al. 2007, Brons et al. 2007, Jiang et al. 2002; Matsui et al. 1992, Kucia 2008a.
Acknowledgments
This work was supported by the following funding: NIH R01 HL073742, NIH R01 DK61846, NIH P30 CA-16359, and NIH P30 DK 072442.
Abbreviations
- EGC
embryonic germ cell
- EpiSC
epiblast stem cell
- ESC
embryonic stem cell
- G-CSF
granulocyte colony stimulating factor
- HSC
hematopoietic stem cell
- IGF
insulin-like growth factor
- LIF
leukemia inhibitory factor
- MSC
mesenchymal stem cell
- PGC
primordial germ cell
- SCF
stem cell factor
- VSEL
very small embryonic-like cell
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/mrd.22168]
References
- Abdel-Latif A, Zuba-Surma EK, Ziada KM, Kucia M, Cohen DA, Kaplan AM, Van Zant G, Selim S, Smyth SS, Ratajczak MZ. Evidence of mobilization of pluripotent stem cells into peripheral blood of patients with myocardial ischemia. Exp Hematol. 2010;38(12):1131–1142. e1131. doi: 10.1016/j.exphem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109(3):1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461(7268):1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhartiya D, Kasiviswananthan S, Shaikh A. Cellular origin of testis-derived pluripotent stem cells: a case for very small embryonic-like stem cells. Stem Cells Dev. 21(5):670–674. doi: 10.1089/scd.2011.0554. [DOI] [PubMed] [Google Scholar]
- Borue X, Lee S, Grove J, Herzog EL, Harris R, Diflo T, Glusac E, Hyman K, Theise ND, Krause DS. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165(5):1767–1772. doi: 10.1016/S0002-9440(10)63431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448(7150):191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, Wagner HJ, Minger S, Matzkies M, Reppel M, Hescheler J, Sievert KD, Stenzl A, Skutella T. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456(7220):344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117(Pt 14):2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- Danova-Alt R, Heider A, Egger D, Cross M, Alt R. Very small embryonic-like stem cells purified from umbilical cord blood lack stem cell characteristics. PLoS One. 2012;7(4):e34899. doi: 10.1371/journal.pone.0034899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel B, Zangi L, Shezen E, Reich-Zeliger S, Eventov-Friedman S, Katchman H, Jacob-Hirsch J, Amariglio N, Rechavi G, Margalit R, Reisner Y. Isolation and characterization of nontubular sca-1+lin− multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17(12):3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- Drukala J, Paczkowska E, Kucia M, Mlynska E, Krajewski A, Machalinski B, Madeja Z, Ratajczak MZ. Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev. 2012;8(1):184–194. doi: 10.1007/s12015-011-9272-4. [DOI] [PubMed] [Google Scholar]
- Drusenheimer N, Wulf G, Nolte J, Lee JH, Dev A, Dressel R, Gromoll J, Schmidtke J, Engel W, Nayernia K. Putative human male germ cells from bone marrow stem cells. Soc Reprod Fertil Suppl. 2007;63:69–76. [PubMed] [Google Scholar]
- Gharib SA, Dayyat EA, Khalyfa A, Kim J, Clair HB, Kucia M, Gozal D. Intermittent hypoxia mobilizes bone marrow-derived very small embryonic-like stem cells and activates developmental transcriptional programs in mice. Sleep. 33(11):1439–1446. doi: 10.1093/sleep/33.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B, Zhang J, Wang W, Mo L, Zhou Y, Chen L, Liu Y, Zhang M. Global expression of cell surface proteins in embryonic stem cells. PLoS One. 2010;5(12):e15795. doi: 10.1371/journal.pone.0015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens AM, Shiozawa Y, Jung Y, Sun H, Wang J, McGee S, Mishra A, Taichman LS, Danciu T, Jiang Y, Yavanian G, Leary E, Krebsbach PH, Rodgerson D, Taichman RS. Human Very Small Embryonic-Like Cells Generate Skeletal Structures. In VivoStem Cells Dev. 2012 doi: 10.1089/scd.2012.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JC, Lee WH, Morrison P, Zhong J, Yoder MC, Srour EF. Pluripotent stem cells identified in multiple murine tissues. Ann N Y Acad Sci. 2003;996:158–173. doi: 10.1111/j.1749-6632.2003.tb03244.x. [DOI] [PubMed] [Google Scholar]
- Huang YH, Chin CC, Ho HN, Chou CK, Shen CN, Kuo HC, Wu TJ, Wu YC, Hung YC, Chang CC, Ling TY. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1- dependent pathway. FASEB J. 2009;23(7):2076–2087. doi: 10.1096/fj.08-121939. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30(8):896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogorek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P. Evidence for human lung stem. cells. N Engl J Med. 364(19):1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kassmer SH, Bruscia EM, Zhang PX, Krause DS. Nonhematopoietic cells are the primary source of bone marrow-derived lung epithelial cells. Stem Cells. 2012;30(3):491–499. doi: 10.1002/stem.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassmer SH, Krause DS. Detection of bone marrow-derived lung epithelial cells. Exp Hematol. 2010;38(7):564–573. doi: 10.1016/j.exphem.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21(2):297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- Kucia M, Masternak M, Liu R, Shin DM, Ratajczak J, Mierzejewska K, Spong A, Kopchick JJ, Bartke A, Ratajczak MZ. The negative effect of prolonged somatotrophic/insulin signaling on an adult bone marrow-residing population of pluripotent very small embryonic-like stem cells (VSELs) Age (Dordr) 2012 doi: 10.1007/s11357-011-9364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19(7):1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- Kucia M, Wysoczynski M, Ratajczak J, Ratajczak MZ. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008a;331(1):125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- Kucia MJ, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells. 2008b;26(8):2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Fujiyoshi Y, Dezawa M. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 2010;107(19):8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labosky PA, Barlow DP, Hogan BL. Embryonic germ cell lines and their derivation from mouse primordial germ cells. Ciba Found Symp. 1994a;182:157–168. doi: 10.1002/9780470514573.ch9. discussion 168–178. [DOI] [PubMed] [Google Scholar]
- Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994b;120(11):3197–3204. doi: 10.1242/dev.120.11.3197. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1(4):403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linher K, Dyce P, Li J. Primordial germ cell-like cells differentiated in vitro from skin-derived stem cells. PLoS One. 2009;4(12):e8263. doi: 10.1371/journal.pone.0008263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gao L, Zuba-Surma EK, Peng X, Kucia M, Ratajczak MZ, Wang W, Enzmann V, Kaplan HJ, Dean DC. Identification of small Sca-1(+), Lin(−), CD45(−) multipotential cells in the neonatal murine retina. Exp Hematol. 2009;37(9):1096–1107. doi: 10.1016/j.exphem.2009.05.014. 1107 e1091. [DOI] [PubMed] [Google Scholar]
- Marlicz W, Zuba-Surma E, Kucia M, Blogowski W, Starzynska T, Ratajczak MZ. Various types of stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients with Crohn's disease. Inflamm Bowel Dis. 2012;18(9):1711–1722. doi: 10.1002/ibd.22875. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70(5):841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Paczkowska E, Kucia M, Koziarska D, Halasa M, Safranow K, Masiuk M, Karbicka A, Nowik M, Nowacki P, Ratajczak MZ, Machalinski B. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40(4):1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A, Ratajczak MZ. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice--novel view on Igf-1, stem cells and aging. Leukemia. 2011a;25(4):729–733. doi: 10.1038/leu.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC, Ratajczak MZ. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol. 2011b;39(2):225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Zuba-Surma E, Klich I, Liu R, Wysoczynski M, Greco N, Kucia M, Laughlin MJ, Ratajczak MZ. Hematopoietic differentiation of umbilical cord blood-derived very small embryonic/epiblast-like stem cells. Leukemia. 2011c doi: 10.1038/leu.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21(5):860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Shin DM, Liu R, Mierzejewska K, Ratajczak J, Kucia M, Zuba-Surma EK. Very small embryonic/epiblast-like stem cells (VSELs) and their potential role in aging and organ rejuvenation--an update and comparison to other primitive small stem cells isolated from adult tissues. Aging (Albany NY) 2012;4(4):235–246. doi: 10.18632/aging.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Saga Y. Mouse germ cell development during embryogenesis. Curr Opin Genet Dev. 2008;18(4):337–341. doi: 10.1016/j.gde.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Sauerzweig S, Munsch T, Lessmann V, Reymann KG, Braun H. A population of serum deprivation-induced bone marrow stem cells (SD-BMSC) expresses marker typical for embryonic and neural stem cells. Exp Cell Res. 2009;315(1):50–66. doi: 10.1016/j.yexcr.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Scandura JM, Boccuni P, Massague J, Nimer SD. Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc Natl Acad Sci U S A. 2004;101(42):15231–15236. doi: 10.1073/pnas.0406771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Liu R, Klich I, Ratajczak J, Kucia M, Ratajczak MZ. Molecular characterization of isolated from murine adult tissues very small embryonic/epiblast like stem cells (VSELs) Mol Cells. 2010a;29(6):533–538. doi: 10.1007/s10059-010-0081-4. [DOI] [PubMed] [Google Scholar]
- Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010b;24(8):1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- Shin DM, Liu R, Wu W, Waigel SJ, Zacharias W, Ratajczak MZ, Kucia M. Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells Dev. 2012;21(10):1639–1652. doi: 10.1089/scd.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ, Kucia M. Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia. 2009;23(11):2042–2051. doi: 10.1038/leu.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, Ragerdi Kashani I. BMP4 can generate primordial germ cells from bone marrow-derived pluripotent stem cells. Cell Biol Int. doi: 10.1042/CBI20110651. [DOI] [PubMed] [Google Scholar]
- Sovalat H, Scrofani M, Eidenschenk A, Pasquet S, Rimelen V, Henon P. Identification and isolation from either adult human bone marrow or G-CSF-mobilized peripheral blood of CD34(+)/CD133(+)/CXCR4(+)/ Lin(−)CD45(−) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp Hematol. 2011;39(4):495–505. doi: 10.1016/j.exphem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Sun B, Ito M, Mendjan S, Ito Y, Brons IG, Murrell A, Vallier L, Ferguson-Smith AC, Pedersen RA. Status of genomic imprinting in epigenetically distinct pluripotent. stem cells. 30(2):161–168. doi: 10.1002/stem.793. [DOI] [PubMed] [Google Scholar]
- Surani MA, Durcova-Hills G, Hajkova P, Hayashi K, Tee WW. Germ line, stem cells, and epigenetic reprogramming. Cold Spring Harb Symp Quant Biol. 2008;73:9–15. doi: 10.1101/sqb.2008.73.015. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Wang Z, Shiozawa Y, Jung Y, Song J, Balduino A, Wang J, Patel LR, Havens AM, Kucia M, Ratajczak MZ, Krebsbach PH. Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells Dev. 2010;19(10):1557–1570. doi: 10.1089/scd.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K, Yamazaki T, Chino T, Ishii M, Otsuru S, Kikuchi Y, Iinuma S, Saga K, Nimura K, Shimbo T, Umegaki N, Katayama I, Miyazaki J, Takeda J, McGrath JA, Uitto J, Kaneda Y. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A. 2011;108(16):6609–6614. doi: 10.1073/pnas.1016753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448(7150):196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Tremml G, Singer M, Malavarca R. Culture of mouse embryonic stem cells. Chapter 1: Unit 1C 4. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc01c04s5. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47(2):169–182. doi: 10.1016/j.molcel.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Wei X, Shen CY. Transcriptional regulation of oct4 in human bone marrow mesenchymal stem cells. Stem Cells Dev. 20(3):441–449. doi: 10.1089/scd.2010.0069. [DOI] [PubMed] [Google Scholar]
- Wojakowski W, Kucia M, Liu R, Zuba-Surma E, Jadczyk T, Bachowski R, Nabialek E, Kazmierski M, Ratajczak MZ, Tendera M. Circulating very small embryonic-like stem cells in cardiovascular disease. J Cardiovasc Transl Res. 2010a;4(2):138–144. doi: 10.1007/s12265-010-9254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojakowski W, Ratajczak MZ, Tendera M. Mobilization of very small embryonic-like stem cells in acute coronary syndromes and stroke. Herz. 2010b;35(7):467–472. doi: 10.1007/s00059-010-3389-0. [DOI] [PubMed] [Google Scholar]
- Worton RG, McCulloch EA, Till JE. Physical separation of hemopoietic stem cells differing in their capacity for self-renewal. J Exp Med. 1969;130(1):91–103. doi: 10.1084/jem.130.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168(6):1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, Kucia M, Abdel-Latif A, Tang XL, Ratajczak MZ, Dawn B, Bolli R. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med. 2011a;15(6):1319–1328. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Klich I, Greco N, Laughlin MJ, Ratajczak J, Ratajczak MZ. Optimization of isolation and further characterization of umbilical-cord-blood-derived very small embryonic/ epiblast-like stem cells (VSELs) Eur J Haematol. 2011b;84(1):34–46. doi: 10.1111/j.1600-0609.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008a;44(5):865–873. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr., Ratajczak J, Ratajczak MZ. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A. 2008b;73A(12):1116–1127. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba-Surma EK, Ratajczak MZ. Overview of very small embryonic-like stem cells (VSELs) and methodology of their identification and isolation by flow cytometric methods. Chapter 9: Unit9 29. Curr Protoc Cytom. 2010 doi: 10.1002/0471142956.cy0929s51. [DOI] [PubMed] [Google Scholar]
- Zuba-Surma EK, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Very small embryonic-like stem cells in adult tissues-potential implications for aging. Mech Ageing Dev. 2009;130(1–2):58–66. doi: 10.1016/j.mad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]