Abstract
Rationale
Convergent evidence shows that alcohol exerts its effects on social behavior via modulation of amygdala reactivity to affective stimuli. Given that affective processing involves dynamic interactions between the amygdala and the prefrontal cortex (PFC), alcohol's effects are likely to extend beyond regional changes in brain activity to changes that manifest on a broader functional circuit level.
Objective
The current study examines alcohol's effects on functional connectivity (i.e., ‘coupling’) between the amygdala and the PFC during the processing of socio-emotional stimuli using functional magnetic resonance imaging (fMRI).
Methods
In a randomized, double blind, placebo-controlled, within-subjects cross-over design, twelve heavy, social drinkers performed an fMRI task designed to probe amygdala response to socio-emotional stimuli (angry, fearful, and happy faces) following acute ingestion of alcohol or placebo. Functional connectivity between the amygdala and PFC was examined and compared between alcohol and placebo sessions using a conventional generalized psychophysiological interaction (gPPI) analysis.
Results
Relative to placebo, alcohol reduced functional coupling between the amygdala and the right orbitofrontal cortex (OFC) during processing of both angry and fearful faces. Alcohol also reduced functional coupling between the amygdala and left OFC during processing of happy faces.
Conclusions
These preliminary findings suggest that alcohol's effects on social behavior may be mediated by alternations in functional connectivity between the amygdala and OFC during processing of emotional faces.
Keywords: alcohol, amygdala, functional connectivity, social threat
Introduction
Alcohol is known to affect both affective states and social behavior (Armeli et al. 2003; Giancola et al. 2009; Kushner et al. 1996). Moreover, alcohol's ability to modulate affective states is widely considered a key motivational factor underlying drinking behavior (Baker et al. 2004; Cooper et al. 1995; Khantzian 1997; Levenson et al. 1980). Numerous theoretical models indicate that alcohol use brings perceived and/or actual relief from negative affective states (e.g., stress, anxiety), thereby reinforcing drinking behavior and increasing the likelihood of future alcohol use (Baker et al. 2004; Khantizan 1997). Acutely, alcohol intoxication reduces subjective and physiological responses to stress (Hefner and Curtin 2012; Kushner et al. 1996; Moberg and Curtin 2009; Sayette et al. 1992), reduces social inhibition, and increases the propensity to act aggressively towards others (Bushman and Cooper 1990; Chermack and Giancola 1997). Given these findings, identifying mechanisms that underlie modulation of negative affective states and social behavior by alcohol use is of the utmost public health significance. Yet, relatively little is known regarding the neural processes that mediate this association.
Functional magnetic resonance imaging (fMRI) studies have begun to examine the acute effects of alcohol on processing social stimuli with negative valence. Initial work by Gilman and colleagues (2008, 2011) examining the effects of alcohol on neural response to fearful and neutral faces indicates that intravenous alcohol administration results in attenuated BOLD activity in the amygdala during the viewing of fearful faces and also enhanced activity in striatal reward circuits to neutral faces in social drinkers. Unexpectedly, these authors also found that alcohol increased amygdala activity to neutral faces, concluding that alcohol may exert its anxiolytic effects by reducing the amygdala's ability to detect threatening information and/or by attenuating amygdala reactivity to threat (Gilman et al. 2008). Using the Emotional Face Assessment Task (EFAT) to probe amygdala activity to threat (angry and fearful faces), a prior study by our group also demonstrated that alcohol attenuated amygdala reactivity to threat-related faces (but not happy faces) in heavy, social drinkers (Sripada et al. 2011). These findings suggest that alcohol may mediate its anxiolytic effects by down-regulating the brain's response to signals of threat, consistent with a large body of human and animal literature noting the importance of the amygdala in negative affective processing (Adolphs 2002; LeDoux 2000; Phan et al. 2002; Phelps 2004).
While the amygdala is clearly a viable target region for alcohol's anxiolytic effects, the generation, expression and modulation of emotion involves dynamic interactions between emotion-evoking regions such as the amygdala and its relation to other brain areas such as the prefrontal cortex (PFC), in the context of mediating change in affect regulation and in social behavior (Goldin et al. 2008; Harenski and Hamann, 2006; Kanske et al. 2011; Ohira et al. 2006). Existing neuroimaging research has shown that frontal control regions such as the orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), dorsal medial prefrontal cortex (DMPFC), and ventrolateral prefrontal cortex (VLPFC) are engaged during the recognition and regulation of emotion (Beauregard et al. 2001; Ochsner et al. 2002, 2004; Levesque et al. 2003; Phan et al. 2005) and during social cognition and social interaction (Beer et al. 2003; Gallager and Frith 2003; Mitchell et al. 2006). Importantly, engagement of these regions during processing of affective stimuli is associated with the modulation of amygdala activity (Beauregard et al. 2001; Ochsner et al. 2002; Phan et al. 2005; Urry et al. 2006). Data from our lab has shown that in response to negative affect, there is increased connectivity between the amygdala and the PFC, specifically the anterior cingulate cortex (ACC), medial prefrontal cortex (MPFC), inferior frontal gyrus (IFG), OFC, and DLPFC (Banks et al. 2007; Prater et al. 2012). These findings are consistent with other studies which have also reported increased connectivity between the amygdala and PFC during social decision-making and interpretation of facial cues (Adolphs 2002; Blair et al. 1999; Iidaka et al. 2011; Nomura et al. 2004). Anatomical tracing studies have also shown that the amygdala has strong reciprocal connections with frontal regions, including the OFC, VLPFC, and DMPFC (Amaral and Price 1984; Ghashghaei and Barbas 2002; Ghashghaei et al. 2007). Taken together, these data suggest that alcohol may affect socio-emotional processing and behavior by altering the functional interactions between the amygdala and PFC (Hariri et al. 2003; Forbes and Grafman 2010; Frith and Frith 2007; Meyer-Lindenberg et al. 2005; Ochsner et al. 2004; Stein et al. 2007).
Consistent with this speculation, studies have demonstrated that individuals with alcohol use disorders have disrupted patterns of functional connectivity (Chanraud et al. 2011; Courtney et al. 2012; O'Daly et al. 2012; Rogers et al. 2012). For example, Courtney et al. (2012) found that greater alcohol dependence severity was associated with weaker functional connectivity within fronto-striatal pathways during a response inhibition task, while Pitel et al. (2012) demonstrated aberrant hippocampal and cerebellar connectivity during a social associative learning task. Relevant to the current study, in response to fearful faces alcoholic patients with multiple detoxifications have been shown to exhibit decreased connectivity between the amygdala and globus pallidus, and the insula and prefrontal regions including the ACC, OFC, and VLPFC (O'Daly et al. 2012). These data lend support to the hypothesis that alcohol may disrupt functional interactions between neural regions; however, the acute effects of alcohol on functional connectivity during socio-emotional processing are still unknown.
The current study aimed to test this hypothesis with a novel analysis of fMRI data collected during our previous study (Sripada et al. 2011). The study utilized a two-session (placebo vs. alcohol), double blind, within-subjects cross-over design. Our analyses indicated that alcohol consumption diminished amygdala reactivity to social signals of threat (angry and fearful faces), without affecting amygdala reactivity to non-threat signals (happy faces; Sripada et al. 2011). In the present study, we employed a generalized form of context-dependent physiological interaction analyses (gPPI; McLaren et al. 2008), which allows for more than two conditions to be modeled independently and has greater sensitivity and specificity than standard PPI methods (McLaren et al. 2012). Given these above results, we examined functional connectivity between the amygdala and the PFC as a function of emotional stimuli (angry, fearful, and happy faces) and condition (placebo and alcohol), using the amygdala as the seed region. We hypothesized that alcohol would reduce amygdala connectivity to these broad areas within the PFC during processing of angry and fearful faces, relative to placebo.
Methods
Participants
Sample descriptive information are provided in Table 1. The sample consisted of twelve healthy, right-handed volunteers. Individuals were selected to meet criteria for heavy social drinking consistent with our prior studies (King et al. 2002, 2009), defined as consuming ≥10 standard alcoholic drinks per week with one to five weekly “binge” drinking episodes (5+ drinks per occasion for men; 4+ drinks for women; NIAAA 2005a, SAMHSA 2005). The rationale for recruiting a group of heavy social drinkers included both ethical concerns (e.g., avoid potential alcohol withdrawal) and scientific considerations (e.g., non-dependent social drinkers exhibit heightened alcohol reward without excessive sedative effects; King et al. 2002, 2011). Most notably, Gilman et al. (2011) recently reported that heavy problematic drinkers demonstrate amygdala hypoactivation in response to threatening faces across both placebo and alcohol conditions and thus, exhibit differences in alcohol-induced neural responding relative to social drinkers. Approximately 50% of interested participants screened by phone were eligible for the in-laboratory assessment; all candidates attending the in-person screening were accepted into the study, one of whom withdrew prior to the first session.
Table 1.
Participant Demographics and Clinical Characteristics
| Demographics | Mean (SD) or % |
| Age (years) | 23.2 (1.8) |
| Sex (% male) | 83.3% |
| Race (% Caucasian) | 66.7% |
| Education (years) | 15.7 (1.2) |
| Alcohol Use | |
| Lifetime diagnosis of DSM-IV alcohol abuse | 91.7% |
| Lifetime diagnosis of DSM-IV alcohol dependence | 0% |
| AUDIT total score | 12.3 (4.5) |
| Alcohol drinking days per month | 13.2 (7.1) |
| Drinks per drinking day | 6.7 (3.5) |
| Bingesa in last month | 7.8 (3.4) |
| Maximum number of drinks on one occasion in last month | 13.5(7.7) |
| Days since last alcohol use | 3.8 (1.7) |
| Range of days since last alcohol use | 1.6 - 6.7 |
| Cigarette Use | |
| Daily smokers | 0.0% |
| FTND total score | 0.08 (0.1) |
| Cigarettes smoked in last month | 14.7 (7.1) |
| Cigarettes smoked per smoking day | 4.3 (2.8) |
| Illicit Drug Use | |
| Used cannabis within the past 30 days | 8.3% |
| Used any other illicit drug in past 30 days | 0.0% |
| Psychiatric Symptoms | |
| Depressive Symptoms- BDI total score | 3.6 (4.2) |
| Anxiety Symptoms- STAI-Trait total score | 52.8 (3.1) |
Note.
A binge is defined as consuming 5 or more drinks for men, 4 or more for women, on one occasion.
AUDIT = Alcohol Use Disorders Identification Test (Babor et al. 1989); BDI = Beck Depression Inventory (Beck et al. 1961); STAI-Trait = State-Trait Anxiety Scale, Trait Anxiety subscale (Spielberger et al. 1970)
Participants were excluded from the study if they met lifetime criteria for any major Axis I (except alcohol abuse) or Axis II psychiatric disorders according to the Structured Clinical Interview for DSM-IV (SCID-IV Patient Edition; First et al. 1995). Individuals were also excluded if they were taking medications, had a history of neurological or medical illness as confirmed by medical examination, or had liver enzyme tests out of the normal range for aspartate aminotransferase and alanine transaminase. All participants provided written informed consent, as approved by the Institutional Review Board. All baseline breathalyzer tests were negative for alcohol upon arrival to the sessions, confirming compliance with study abstinence instructions on testing days.
Procedure
The study protocol has been described in detail elsewhere (see Sripada et al. 2011). In brief, the current study was a two-session, double-blind, placebo-controlled, within-subjects design. Prior to each session, participants were instructed to abstain from alcohol, recreational drugs, and any psychoactive medications for at least 48 hours, as well as caffeine, food, and cigarette smoking for 3 hours. Upon arrival, the subject underwent abstinence verification, consumed a low-fat snack (20% daily calories), and acclimated to the laboratory.
Approximately 45-minutes after arrival, participants were served a beverage and a small placebo gel capsule containing dextrose. To reduce alcohol expectancies, the Alternative Substance Paradigm (Conrad et al. 2012) was employed in which participants were told that the beverage or capsule might contain alcohol, a stimulant, a sedative, a placebo, or some combination of those substances. In actuality, the beverage contained either a high dose of alcohol (ALC; 0.8 g/kg; 16% volume alcohol) or placebo (PBO; 0.0 g/kg; 1% volume ethanol as a taste mask).
The beverages were prepared with flavored drink mix, a sucralose-based sugar substitute, water, and the appropriate dose of 190-proof ethanol based on body weight and were consumed through a straw in a lidded, opaque cup to conceal potential scent cues. Women received an 85% dose to adjust for total body water differences (Frezza et al. 1990; Sutker et al. 1983). Total beverage volumes for placebo and alcohol were identical (mean 472 mL; range 297-581 mL) and based on the participants’ body weight. Consumption of the beverage occurred within a 13-minute interval and was timed such that the upcoming fMRI task would concur with expected peak breath alcohol concentration (Epstein et al. 2007; King et al. 2002, 2011). Immediately following beverage consumption, the participant was escorted into the scanning room, underwent fMRI preparation and structural MRI, and completed the EFAT (described below). The end of the task occurred approximately 65-minutes after alcohol ingestion. Prior to consumption of the alcohol or placebo beverage, participants completed the Biphasic Alcohol Effects Scale (BAES; Martin et al. 1993; Rueger et al. 2009), which was repeated 75-minutes later. Notably, the EFAT followed a visual stimulus processing task involving smoking and non-smoking images as part of a larger experiment, the results of which have previously been published (King et al. 2010). BrAC levels for the session have been previously published (Sripada et al. 2011) and followed the expected time-course (average BrAC at 75-min, 90-min, 120-min, 150-min, and 180-min post alcohol ingestion = 0.090, 0.085, 0.076, 0.069, 0.060). Of note, we did not assess BrAC while participants were in the scanner and therefore we do not have exact BrAC values during the EFAT task. Alcohol ingestion increased to an average of 0.091 (± 0.014) % 75-minutes after initiation of beverage consumption with a slow elimination phase over the next few hours. Once participants’ BrAC was below 0.04% and they displayed no overt signs of intoxication (NIAAAb), they were discharged from the study and given a vehicle service ride to their home and instructed not to drive or operate machinery for at least 12-hours.
Emotional Face Assessment Task (EFAT)
The EFAT task has previously been described in detail (see Phan et al. 2008). The EFAT and variants of the task have been shown to reliably and robustly engage the amygdala (Hariri et al. 2002; Kirsch et al. 2005; Paulus et al. 2005). In brief, participants viewed three faces (one on the top of the screen and two on the bottom) and were asked to select one of the two faces on the bottom of the screen that expressed the same emotion as the target face on the top of the screen. The identity of all three faces was always different and an equal number of male and female faces were presented. The target face and the congruent probe face always displayed one of three expressions (angry, fearful, or happy) and the other (i.e., incongruent) probe face always displayed a neutral expression. The face photographs were selected from the validated stimulus set from Gur et al. (2002). The angry, fearful, and happy target faces were presented in separate blocks and a total of three blocks of each target expression were presented. Importantly, no target stimuli were repeated within or across blocks. In addition, there were also blocks in which participants similarly matched simple geometric shapes (i.e., circles, rectangles, or triangles). This ‘control’ task was included to maintain attention and allow limbic brain responses to return to baseline. The paradigm consisted of eighteen 20-seconds blocks: 9 blocks of matching emotional faces, interleaved with 9 blocks of matching shapes (blocks occurred back-to-back without intervening fixation), counterbalanced across two runs for a total task time of 6-minutes. Each task block contained four sequential matching trials, 5-seconds each. Participants made responses by pressing the left or right response buttons with their dominant hand. These responses also provided a measure of participants’ response accuracy and reaction time.
Brain Imaging
Functional MRI was performed on a 3T GE magnetic resonance scanner which acquired functional images (i.e., blood oxygenated level-dependent [BOLD]) from 30 axial, 5-mm-thick slices using a T2*-sensitive gradient echo reserve spiral acquisition sequences (repetition time, 2000 ms; echo time, 25 ms; 64 × 64 matrix; 24 cm field of view; flip angle, 77), optimized to minimize susceptibility artifacts in the amygdala (Stenger et al. 2000). This was followed by a high-resolution, T1-weighted volumetric anatomical scan (three-dimensional magnetization-prepared rapid gradient echo) for anatomical localization.
Functional MRI Data Analyses
Data from all 12 participants met criteria for high quality and scan stability with minimum motion correction (i.e., 3 mm or less displacement in any one direction) and thus were included in subsequent analyses. Functional data were analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK) using similar previously published methods (e.g., Rabinak et al. 2011). Images were spatially realigned to correct for head motion, warped to standardized Montreal Neurological Institute (MNI) space using the participant's mean functional image, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model (GLM) was applied to the time series, convolved with the canonical hemodynamic response function (HRF; Friston et al. 1995) and with a 128 second high-pass filter. Condition effects were modeled with box-car regressors representing the occurrence of each block type. Effects were estimated at each voxel, and for each subject. Individual contrast maps (statistical parametric maps [SPMs]) were then analyzed at the second level in a random-effects statistical model (Holmes and Friston 1998).
The current analysis intended to examine angry and fearful faces separately given that prior work has suggested that different facial expressions may convey different messages about the ‘source’ of threat (e.g., direct threat from angry faces, indirect threat from fearful faces) and may differentially engage the amygdala, insula, ACC and MPFC (Fusar-Poli et al. 2009) and elicit qualitatively different behavioral responses (Pichon et al. 2009; Whalen et al. 2001). Angry faces often signal to the observer to modify his/her own behavior, while fearful faces typically signal danger in the environment (Fridlund 1994; Whalen 1998), which may be differentially sensitive to alcohol's effect on amygdala-PFC connectivity. Of note, earlier studies examining the effects of alcohol on neural responses to threat had either used fearful faces only (Gilman et al. 2008, 2011) or had collapsed across fearful and angry faces (Sripada et al. 2011). Therefore, examining the threat conditions separately will extended these previous findings.
To examine functional coupling between the amygdala and other areas of the brain during placebo (PBO) vs. alcohol (ALC) in the context of processing social signals of threat, we used a generalized form of context-dependent psychophysiological interaction analyses (gPPI; http://brainmap.wisc.edu/PPI, McLaren et al. 2008). The use of gPPI allowed us to model each condition (i.e., angry, fearful, happy, and shapes) independently. We chose to define functionally-derived amygdala seeds of interest (SOIs) as activation clusters that exhibited a significant modulation by alcohol. In other words, in order to facilitate interpretations, we only chose to examine the effects of alcohol on amygdala functional connectivity if there was a main effect of ALC (vs. PBO) on amygdala activation to angry/fearful faces in the primary analysis, as shown by a paired-sample t-test contrasting PBO against ALC. We considered activations that survived p < 0.005 (uncorrected; Lieberman and Cunningham, 2009) for between-session contrast t-maps as significant. In-order to clarify the direction of differences (or lack thereof), we also extracted BOLD signal responses (i.e., parameter estimates, β weights [arbitrary units, a.u.]) from 10mm spheres surrounding peak activations within the amygdala from each session (ALC, PBO) and emotion (angry, fearful, and happy) separately. Using conventional gPPI methods, the de-convolved time series from these functionally-derived spherical SOIs around the group peak activation voxel within the amygdala (identified in the above analyses) was extracted for each subject to create the physiological variable. Next, the condition onset times for angry faces, fearful faces, happy faces, and shapes were separately convolved with the canonical HRF for each condition, creating the psychological regressors. The interaction terms (PPIs) were then computed by multiplying the time series from the psychological regressors (angry/fearful/happy) with the physiological variable. To examine the effect of the interaction terms, activity within the amygdala was regressed on a voxel-wide basis against the interaction, with the physiological and psychological variables serving as regressors of interest. The individual contrast images were then entered into a 2nd-level random effects analysis in which drug effects (ALC > PBO; PBO > ALC) were investigated using paired-sample t-tests, in order to identify which areas, if any, exhibited a difference in functional connectivity with the amygdala between PBO and ALC sessions. For a whole-brain analysis, we set the significance at p < 0 .005 (uncorrected) with a cluster extent threshold of greater than 20 contiguous voxels (volume > 160mm3) to balance between Type I and Type II errors consistent with prior fMRI studies of drug effects on amygdala-frontal connectivity (Kobiella et al. 2010; Labuschagne et al. 2010; Lieberman and Cunningham 2009; Parent et al. 2011).
Results
Behavioral Results
The behavioral results from the EFAT task have been previously published (Sripada et al. 2011). Results (n = 7; 5 participants missing button-press data) indicated that participants were more accurate (F(1,6) = 12.79, p = 0.012) and responded quicker (F(1,6) = 27.44, p = 0.002) during the non-threat conditions compared with the threat conditions. There were no differences between the ALC and PBO sessions on task accuracy or response time [(beverage: accuracy: F(1,6) = 2.54, p = 0.162), response time: F(1,6) = 0.65, p = 0.452) beverage × condition: accuracy: F(1,6) = 0.89, p = 0.383, response time: F(1,6) = 0.81, p = 0.403)].
Imaging Results
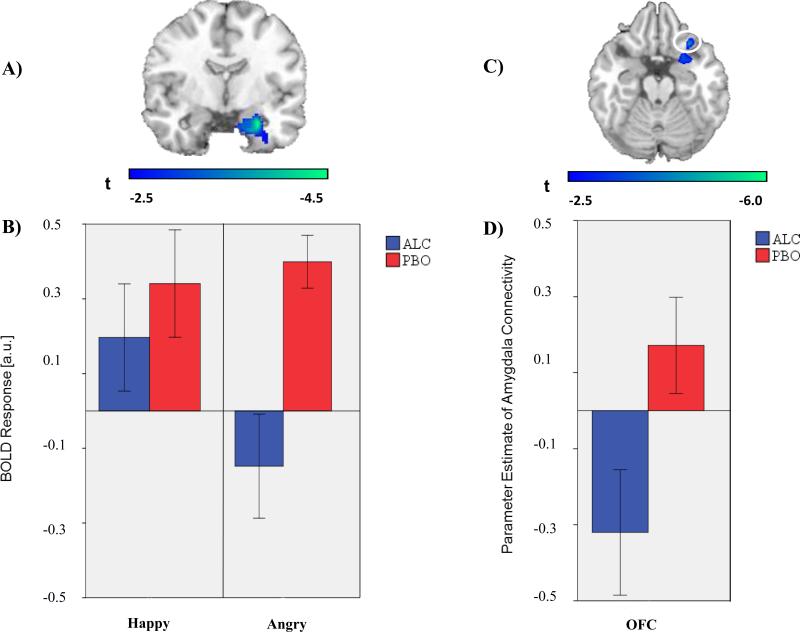
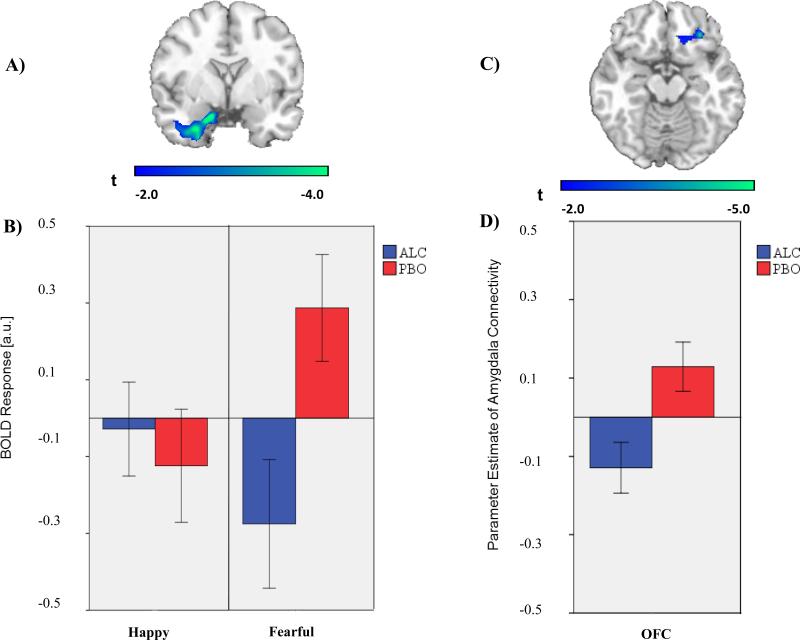
We observed two main effects of alcohol on amygdala activation. First, activation of right amygdala to angry faces ([22, -4, -26]; Z = 3.67; p < 0.005) was greater on PBO than ALC (Figure 1a). Post hoc extraction showed that the amygdala activation to angry faces on PBO was absent on ALC (Figure 1b) and that there were no differences in activation to happy faces on PBO and ALC in this same region. Second, activation of left amygdala to fearful faces ([-24, -4, -18]; Z = 3.33; p < 0.005) was greater on PBO than ALC (Figure 2a). Post hoc extraction showed that amygdala activation to fearful faces on PBO was deactivated on ALC (Figure 2b) and that there were no differences in activation to happy faces on PBO and ALC in this same region. From these main effects, we chose a right amygdala SOI for angry faces and a left amygdala SOI for fearful faces to conduct our planned gPPI analyses.
Fig. 1.
(A) Voxel-wise statistical t-map displayed on a canonical brain; Activation difference shown in relation to the amygdala anatomically derived region of interest (ROI); Color scale reflects t-score. (B) Bar graphs illustrating extracted BOLD responses from the anatomical right amygdala ROI during happy faces (> shapes) and angry faces (> shapes) for the alcohol and placebo conditions; ALC = alcohol condition; PBO = placebo condition (C) Voxel-wise statistical t-map displayed on a canonical brain; Color scale reflects t-score. (D) Bar graph illustrating extracted parameter estimates of right amygdala-right OFC connectivity during angry > shapes for the alcohol and placebo condition.
Fig. 2.
(A) Voxel-wise statistical t-map displayed on a canonical brain; Activation difference shown in relation to the amygdala anatomically derived region of interest (ROI); Color scale reflects t-score. (B) Bar graphs illustrating extracted BOLD responses from the anatomical left amygdala ROI during happy faces (> shapes) and fearful faces (> shapes) for the alcohol and placebo condition; ALC = alcohol condition; PBO = placebo condition (C) Voxel-wise statistical t-map displayed on a canonical brain; Color scale reflects t-score. (D) Bar graph illustrating extracted parameter estimates of left amygdala-right OFC connectivity during fearful > shapes for the alcohol and placebo condition.
gPPI analyses during angry faces (> shapes) indicated that activity in the right amygdala exhibited greater connectivity with right orbitofrontal cortex (OFC; [30, 22, -22]; Z = 3.60, p < 0.001, uncorrected) during PBO relative to ALC (Figure 1c). Analyses during fearful faces (> shapes) indicated that activity in the left amygdala exhibited greater connectivity with a similar area localized to the right OFC ([30, 34, -16], Z = 3.42, p < 0.001) during PBO relative to ALC (Figure 2c). In both instances, post hoc comparisons of extracted gPPI parameter estimates (a.u.) indicated greater amygdala-OFC connectivity on PBO than on ALC, albeit with trend level significance from left amygdala seed (right amygdala: t(11) = 3.22, p < .01; left amygdala: t(11) = 2.06, p = .06; Figure 1d and 2d).
Post hoc analyses showed that alcohol-induced changes in amygdala activation were not significantly correlated with changes in amygdala-OFC functional connectivity (PBO vs. ALC) when processing fearful faces (r = .24, p = .46) or angry faces (r = -0.07, p = .84).
Results also indicated during angry faces (> shapes), activity in the right amygdala exhibited greater connectivity with the right superior frontal gyrus (SFG, [18, 20, 62], Z = 3.04, p < 0.005) and the left middle frontal gyrus (MFG, [-32, -10, 66], Z = 3.03, p < 0.005) during ALC relative to PBO. Other brain regions outside the PFC that demonstrated effects of ALC (>PBO) on amygdala connectivity are shown for completeness in Table 2. In addition, although there were no main effects of alcohol on amygdala activation to happy faces, we conducted exploratory post-hoc gPPI analyses during happy faces (> shapes) using the same left and right amygdala seed regions. These results are displayed in Table 3.
Table 2.
Results of Whole-brain gPPI Analyses with the Amygdala as the Seed Region for Angry and Fearful Faces (>Shapes)
| Seed Region | Emotion | Contrast | Region Name | MNI Coordinates | Voxels | Z-score | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Right Amygdala | Angry | PBO > ALC | R Orbitofrontal Cortex | 30 | 22 | -22 | 26 | 3.60 |
| R Olfactory Region | 24 | 10 | -20 | 49 | 3.18 | |||
| ALC > PBO | L Postcentral Gyral | -2 | -38 | 80 | 275 | 3.98 | ||
| R Sub-Gyral | 34 | -48 | 4 | 92 | 3.94 | |||
| L Lateral Ventricle | -30 | -58 | 6 | 378 | 3.84 | |||
| L Extra-Nuclear | -20 | -50 | 22 | 36 | 3.74 | |||
| L Middle Temporal Gyrus | -62 | -64 | 8 | 45 | 3.55 | |||
| L Cuneus | 0 | -98 | -2 | 51 | 3.46 | |||
| L Insula | -32 | -40 | 20 | 58 | 3.44 | |||
| L Lingual Gyrus | -12 | -84 | -20 | 465 | 3.36 | |||
| R Middle Temporal Gyrus | 60 | -32 | -18 | 37 | 3.30 | |||
| R Middle Occipital Gyrus | 34 | -96 | 10 | 73 | 3.26 | |||
| R Declive | 18 | -66 | -20 | 120 | 3.10 | |||
| R Superior Frontal Gyrus | 18 | 20 | 62 | 32 | 3.04 | |||
| L Middle Frontal Gyrus | -32 | -10 | 66 | 77 | 3.03 | |||
| L Culmen | -8 | -42 | -28 | 39 | 2.98 | |||
| L Precuneus | -2 | -48 | 52 | 27 | 2.80 | |||
| Left Amygdala | Fear | PBO > ALC | L Precentral Gyrus | -22 | -26 | 74 | 151 | 3.75 |
| R Superior Frontal Gyrus | 14 | -14 | 80 | 59 | 3.69 | |||
| L Fastigium | -8 | -50 | -28 | 47 | 3.44 | |||
| R Orbitofrontal Cortex | 30 | 34 | -16 | 22 | 3.42 | |||
| R Temp Inferior Cortex | 66 | -40 | -18 | 34 | 3.11 | |||
| R Sub-Gyral | 52 | -32 | -14 | 33 | 3.10 | |||
| L Supp Motor Area | -6 | -14 | 58 | 225 | 3.05 | |||
| ALC > PBO | None | |||||||
Note. gPPI= generalized psychophysiological interaction; MNI = Montreal Neurological Institute; results at p < .005, uncorrected; >20 voxel minimum.
Table 3.
Results of Exploratory Whole-brain gPPI Analyses with the Amygdala as the Seed Region for Happy Faces (>Shapes)
| Seed Region | Emotion | Contrast | Region Name | MNI Coordinates | Voxels | Z-score | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Right Amygdala | Happy | PBO > ALC | L Inferior Frontal Gyrus | -48 | 42 | 14 | 108 | 3.05 |
| R Cerebellar Tonsil | 18 | -40 | -48 | 32 | 3.02 | |||
| L Cerebellar Tonsil | -46 | -52 | -42 | 20 | 3.00 | |||
| ALC > PBO | L Calcarine | 4 | -98 | -2 | 602 | 4.31 | ||
| L Sub-lobar | -18 | -30 | 16 | 132 | 3.94 | |||
| L Cerebelum Crus II | -34 | -80 | -34 | 252 | 3.29 | |||
| L Cuneus | -24 | -78 | 10 | 100 | 3.28 | |||
| R Paracentral Lobule | 6 | -44 | 80 | 69 | 3.25 | |||
| R Cerebelum | 8 | -48 | -16 | 31 | 3.17 | |||
| R Fusiform | 50 | -66 | -18 | 76 | 3.12 | |||
| L Sub-Gyral | -32 | -36 | 22 | 22 | 2.93 | |||
| R Inferior Temporal Gyrus | 62 | -34 | -18 | 25 | 2.85 | |||
| Left Amygdala | Happy | PBO > ALC | R Fusiform Gyrus | 18 | -94 | -22 | 38 | 3.60 |
| L Precuneus | 0 | -50 | 58 | 50 | 3.56 | |||
| L Orbitofrontal Cortex | -42 | 48 | -12 | 59 | 3.31 | |||
| L Medial Frontal Gyrus | -8 | -10 | 54 | 42 | 3.08 | |||
| L Inferior Occipital Gyrus | -32 | -86 | -18 | 35 | 3.02 | |||
| R Cerebelum | 30 | -50 | -30 | 87 | 3.02 | |||
| L Cerebelum | -16 | -36 | -46 | 29 | 2.97 | |||
| ALC > PBO | None | |||||||
Note. gPPI= generalized psychophysiological interaction; MNI = Montreal Neurological Institute; results at p < .005, uncorrected; >20 voxel minimum.
Discussion
Recently, it has been postulated that alcohol's ability to modulate affect may be mediated by attenuation of threat processing in the amygdala (Gilman et al. 2008, 2011; Sripada et al. 2011). Given that emotional processes involve dynamic interactions between the amygdala and regions of the PFC (Ochsner et al. 2004; Stein et al. 2007) there are likely important alterations in functional interactions between these regions that underlie alcohol's effects. The current study was the first to our knowledge to examine alcohol's effects on functional connectivity between the amygdala and the PFC during the viewing of socio-emotional stimuli using gPPI analyses (McLaren et al. 2012). Our results indicated that during the processing of angry and fearful faces (examined separately), alcohol significantly reduced functional coupling between the amygdala and the right OFC relative to placebo. However, these preliminary findings are not specific to threatening faces, as results also indicated that alcohol reduced connectivity between the amygdala and left OFC during processing of happy faces. Alcohol's effects on amygdala-OFC connectivity may therefore be broad and extend to non-threatening social stimuli. These findings are noteworthy given that previous research has demonstrated that the OFC has direct, dense projections to the amygdala (Ghashghaei et al. 2007; Porrino et al. 1981; Amaral and Price 1984), and that the amygdala-OFC network is implicated in the expression and modulation of anxiety (Blackmon et al. 2011; Rauch et al. 2006).
Reduced amygdala-OFC connectivity during alcohol intoxication likely has important influences on processing of socio-emotional signals. A large body of evidence indicates that the amygdala and OFC work together to decode and represent affective information (see Murrary and Izquierdo 2007 for a review). Specifically, the amygdala is thought to detect and recognize the valence of affective stimuli and then feed forward this information to the OFC to guide goal-directed behavior (Bechara et al. 2000; Blair 2007). Animal and human research suggests that these functional interactions are reciprocal and necessary to process the threat value of a stimulus and subsequently generate an emotional response (Ghashghaei et al. 2007; Price 2003). In the present study, alcohol significantly attenuated amygdala reactivity to threat signals and reduced amygdala-OFC connectivity. Moreover, our results indicated that these alcohol-induced effects are not be dependent, as changes in amygdala activity were not correlated with changes in amygdala-OFC connectivity. Thus, it is plausible that during acute alcohol intoxication, stimuli that typically signal threat are not perceived as salient due to dampened amygdala reactivity and/or reduced interactions between the amygdala and OFC. This diminished perception of threat salience may then further lead to reduced negative affect and/or decreased scanning of the environment for threatening cues.
Importantly, the aforementioned interpretation of the present findings is consistent with numerous theoretical models of alcohol use, which broadly posit that alcohol dampens negative affect via disruption of attention and appraisal processes (Curtin et al. 2001; Hull 1987; Steele and Josephs 1990; Sayette 1993). Although there have been some mixed findings within the literature, accumulating evidence over the past several decades have supported aspects of these theories. For instance, Sayette and colleagues (2001) have demonstrated that alcohol's anxiolytic effects are more robust when alcohol consumption occurs prior to stimulus presentation and thus, prior to threat appraisal, rather than when drinking occurs in response to a stressor. In addition, a series of studies by Curtin and colleagues (Moberg and Curtin 2009; Hefner and Curtin 2012), provides evidence to suggest that when threat stimuli are well-defined and unambiguous (i.e., temporally predictable cues that reliably signal threat), alcohol does not modulate affective responding. Thus, extant theoretical and empirical evidence indicate that alcohol exerts its anxiolytic effects by disrupting the perception of threat salience, which the current study suggests may be mediated by reduced functional amygdala-OFC connectivity.
Reduced amygdala-OFC activity during alcohol intoxication may also have important implications for emotion regulation processes. In addition to determining the affective salience of threat stimuli, converging lines of evidence indicate that interactions between the amygdala and OFC are instrumental in the modulation and expression of emotion (Ochsner et al. 2002; Levesque et al. 2003; Ochsner et al. 2004; Phan et al. 2005). For instance, emotion regulation strategies such as appraisal and suppression have repeatedly been shown to be associated with amygdala-OFC interactions (Schaefer et al. 2002; Urry et al. 2006), and evidence indicates that the extent of the functional coupling between these regions predicts successful emotion modulation (Banks et al. 2007). Therefore, reduced amygdala-OFC coupling in the context of affective stimuli may contribute to many of the well-known dysregulated emotional and behavioral consequences of alcohol use including increased risk-taking (Burian et al. 2002; Morris and Albery 2001), aggression (Bushman and Cooper 1990; Parrott et al. 2003), and impaired inhibitory control (Marczinski et al. 2005; de Wit 2000). This is a potentially important avenue for future research and studies are needed to delineate the consequences of alcohol's effects on amygdala-OFC connectivity.
Functional connectivity between the amygdala and two other PFC regions involved in emotion regulation processes were also found to be affected by alcohol in the present study (Banks et al. 2007; Kim et al. 2007; Oshner et al. 2002). More specifically, our findings indicated that alcohol enhanced coactivation between the right amygdala and the right SFG and left MFG during the processing of angry faces. This increase in functional connectivity may reflect compensatory processes such that the SFG and MFG were recruited to help the amygdala decode socio-emotional information during alcohol intoxication. The MFG and SFG have been shown to play a role in attentional influences on visual processing (Barceló et al. 2000; Chawla et al. 1999) and threat detection and evaluation (Han et al. 2008), which may have been used to compensate for alcohol's deleterious effects on amygdala reactivity and/or amygdala-OFC connectivity. However, because these frontal regions have indirect connections to the amygdala (Ghashghaei et al. 2007) it is difficult to interpret these patterns of coactivation and future studies are needed to delineate the consequences of these findings.
One of the secondary aims of the present study was to explore whether alcohol had similar effects on amygdala-PFC connectivity during angry and fearful faces. Interestingly, there is a growing literature suggesting that the OFC and amygdala play critical roles in regulating aggressive emotions (Davidson et al. 2001; Saddoris et al. 2005), and that clinical populations characterized by high levels of aggression (e.g., psychopathy, borderline personality disorder) exhibit abnormal amygdala-OFC connectivity (Blair 2003; Tebartz van Elst et al. 2003). Further, a meta-analysis by Murphy and colleagues (2003) indicates that the OFC may be more activated during the processing of anger stimuli relative to other emotions. Our current findings indicate that alcohol was associated with reduced amygdala-OFC coactivation during processing of socio-emotional stimuli . However, the strength of this effect (i.e., Z-score value) appears to be slightly more robust in response to angry faces in a qualitative comparison to fearful and happy faces
Given that alcohol affects multiple neurotransmitter systems in the brain including glutamate, GABA, dopamine, serotonin, and acetylcholine (Chastain 2006; Nutt and Peters 1994), it is likely that there are key neurochemical mechanisms underlying processing of socio-emotional stimuli during alcohol intoxication. For instance, research suggests that many of alcohol's anxiolytic effects are primarily mediated via direct and indirect increases in GABAergic synaptic transmission, particularly in the extended amygdala (Buck 1996; Criswell and Breese, 2005; Hyytia and Koob 1995; Koob 2003, 2004; Kumar et al., 2009; Weiner and Valenzuela, 2006), and that administration of benzodiazepines (drugs with similar neurochemical effects as alcohol on GABA neurotransmission) dampens amygdala reactivity (Arce et al. 2006; Paulus et al. 2005). Importantly, the OFC has projections to several alcohol-induced pro-GABAerigc regions including the amygdala and the nucleus accumbens (Nie et al. 2004; Ray and Price 1993; Roberto et al. 2004), suggesting that alcohol may affect the transmission of GABA in the amygdala and OFC individually and the amygdala-OFC circuit. Evidence also suggests that increased dopamine release during alcohol intoxication may relate to the present findings, as a large body of evidence indicates that the mesolimbic dopamine system is implicated in anxiety (de la Mora et al. 2005; Diaz et al. 2011; Talalenko et al. 1994), and that dopamine levels in the amygdala have been shown to be associated with amygdala and amygdala-PFC processing of aversive stimuli (Kineast et al. 2008).
While the results extend prior findings on alcohol's acute disruption of neurocognitive circuits during processing of emotional stimuli, there are a few limitations worth noting. First, the current findings come from a small sample and also would not withstand correction for multiple comparisons and thus, are preliminary. Second, although a within-subjects study, the sample size was small, which likely reduced statistical power to detect gPPI effects elsewhere in the PFC and limited our ability to conduct sub-analyses on individual differences (e.g., gender). Similarly, the EFAT task included a low number of trials which may have also limited statistical power. Future research is needed to replicate the present findings in the OFC and investigate effects in other areas. Third, all participants in the current study were heavy social drinkers and it is unclear whether the current pattern of results apply to individuals that are less and/or more frequent (e.g., dependent) drinkers. Fourth, gPPI analyses are correlational and therefore directionality between the amygdala and OFC cannot be inferred. Future studies on dose-dependent effects of alcohol are needed to make causal inferences. Likewise, future studies are needed to determine whether alcohol's affective effects are mediated by attenuating amygdala reactivity and/or disrupting amygdala-OFC functional connectivity. The specificity of the present findings to threat stimuli or socio-emotional more broadly also requires further investigation. Lastly, the functional relevance of alcohol's effects on amygdala-OFC connectivity remain unclear and future tests to link these effects on functional (e.g., behavioral, affective, cognitive) outcomes are needed.
In sum, the results of the present study extend the literature on the acute effects of alcohol on human processing of affective stimuli and suggest that alcohol attenuates amygdala reactivity and reduces amygdala-OFC connectivity during the processing of emotional faces. These neural effects of alcohol may serve to impair the perception and appraisal of the salience of social signals of threat, and contribute to alcohol-associated harm during intoxicated states. Given this pattern of results, psychological and pharmacological treatments targeting this pathway may be a valuable for alcohol prevention or harm-reduction interventions.
Acknowledgments
This study was supported by a Brain Research Foundation Grant awarded to ACK and KLP.
Footnotes
All authors declare that they have no conflicts of interest.
References
- Arce E, Miller DA, Feinstein JS, Stein MB, Paulus MP. Lorazepam dose-dependently decreases risk-taking related activation in limbic areas. Psychopharmacology (Berl) 2006;189:105–116. doi: 10.1007/s00213-006-0519-8. doi: 10.1007/s00213-006-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. J Neurosci. 2002;12:169–77. doi: 10.1016/s0959-4388(02)00301-x. doi: 10.1016/S0959-4388(02)00301-X. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdala-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984;230:465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Armeli S, Tennen H, Todd M, Carney MA, Mohr C, Affleck G, Hromi A. A daily process examination of the stress-response dampening effects of alcohol consumption. Psychol Addict Behav. 2003;17:266–276. doi: 10.1037/0893-164X.17.4.266. doi: 10.1037/0893-164X.17.4.266. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Soc Cog Affect Neur. 2007;2:303–312. doi: 10.1093/scan/nsm029. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, de la Fuente JR, Saunders J, Grant M. The Alcohol Use Identification Test: Guidelines for use in primary health care. World Health Organization; Geneva, Switzerland: 1989. [Google Scholar]
- Barceló F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:6993–7000. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio A. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beck AX, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EH, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. J Pers Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Blackmon K, Barr WB, Carlson C, Devinsky O, Dubois J, Pogash D, et al. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiat Res. 2011;194:296–303. doi: 10.1016/j.pscychresns.2011.05.007. doi:10.1016/j.pscychresns.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Neurobiological basis of psychopathy. Brit J Psychiat. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Buck KJ. Molecular genetic analysis of the role of GABAergic systems in the behavioral and cellular actions of alcohol. Behav Genet. 1996;26:313–323. doi: 10.1007/BF02359387. [DOI] [PubMed] [Google Scholar]
- Burian SE, Liguori A, Robinson JH. Effects of alcohol on risk-taking during simulated driving. Hum Psychopharm Clin. 2002;17:141–150. doi: 10.1002/hup.384. doi: 10.1002/hup.384. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Cooper HM. Effects of alcohol on human aggression: An integrative research review. Psychol Bull. 1990;107:341–354. doi: 10.1037/0033-2909.107.3.341. doi: 10.1037/0033-2909.107.3.341. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain G. Alcohol, neurotransmitter systems, and behavior. J Gen Psychol. 2006;133:329–335. doi: 10.3200/GENP.133.4.329-335. doi: 10.3200/GENP.133.4.329-335. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2:671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Chermack S, Giancola P. The relationship between alcohol and aggression: An integrated biopsychosocial approach. Clin Psychol Rev. 1997;6:621–649. doi: 10.1016/s0272-7358(97)00038-x. doi: 10.1016/S0272-7358(97)00038-X. [DOI] [PubMed] [Google Scholar]
- Conrad M, McNamara P, King A. The Alternative Substance Paradigm: Effectiveness of beverage blinding and effects on acute alcohol responses. Exp Clin Psychopharm. 2012;20:382–389. doi: 10.1037/a0029261. doi: 10.1037/a0029261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. doi:10.1037/0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol. 2012 doi: 10.1111/adb.12013. doi: 10.1111/adb.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacol. 2005;30(8):1407–1425. doi: 10.1038/sj.npp.1300750. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Patrick CJ, Lang AR, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychol Sci. 2001;12:527–531. doi: 10.1111/1467-9280.00397. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation-A possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Cardenas-Cachon L, Vazquez-Garcia M, Crespo-Ramirez M, Jacobsen K, Hoistad M, et al. Anxiolytic effects of intra-amygdaloid injection of the D1 antagonist SCH23390 in the rat. Neurosci Lett. 2005;377:101–105. doi: 10.1016/j.neulet.2004.11.079. doi: 10.1016/j.neulet.2004.11.079. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacol. 2011;36:1090–1103. doi: 10.1038/npp.2010.246. doi: 10.1038/npp.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl.) 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. doi: 0.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. New Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ. Human facial expression: An evolutionary view. Academic Press; San Diego, CA: 1994. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time–series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:724–732. doi: 10.1016/j.cub.2007.05.068. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiat Neurosci. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind.”. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. doi: 10.1016/S1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. doi: 10.1016/S0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. doi: 10.1016%2Fj.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J, Ramchandani V, Crouss T, Hommer D. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacol. 2011;37:467–477. doi: 10.1038/npp.2011.206. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J, Ramchandani V, Davis M, Bjork J, Hommer D. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Levinson CA, Corman MD, Godlaski AJ, Morris DH, Phillips JP, Holt JC. Men and women, alcohol and aggression. Exp Clin Psychopharm. 2009;17:154–164. doi: 10.1037/a0016385. doi: 10.1037/a0016385. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiat. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. doi: 10.1016%2Fj.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Meth. 2002;115:137–143. doi: 10.1016/s0165-0270(02)00006-7. doi:10.1016/S0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Han S, Gao X, Humphreys GW, Ge J. Neural processing of threat cues in social environments. Hum Brain Mapp. 2008;29(8):945–957. doi: 10.1002/hbm.20439. doi: 10.1002/hbm.20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. NeuroImage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacol. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. doi:10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of amygdala response to fearful stimuli. Biol Psychiat. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. doi: 10.1016/S0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hefner KR, Curtin JJ. Alcohol stress response dampening: Selective reduction of anxiety in the face of uncertain threat. J Psychopharmacol. 2012;26:232–244. doi: 10.1177/0269881111416691. doi: 10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage: Abstracts of the Fourth International Conference on Functional Mapping of the Human Brain. 1998;7:S754. [Google Scholar]
- Hull JG. Self-awareness model. In: Blane HT, Leonard KE, editors. Psychological theories of drinking and alcoholism. Guilford Press; New York: 1987. pp. 272–304. [Google Scholar]
- Hyytiä P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283(1):151–159. doi: 10.1016/0014-2999(95)00314-b. doi:10.1016/0014-2999(95)00314-B. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Harada T, Sadato N. Forming a negative impression of another person correlates with activation in medial prefrontal cortex and amygdala. Soc Cogn Affect Neurosci. 2011;6:516–525. doi: 10.1093/scan/nsq072. doi: 10.1093/scan/nsq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: Reconsideration and recent applications. Harvard Rev Psychiat. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19(5):776–98. doi: 10.1162/jocn.2007.19.5.776. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. doi: 10.1111/j.1530-0277.2002.tb02611.x. [PubMed] [Google Scholar]
- King AC, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharmacology (Berl) 2009;207:107–117. doi: 10.1007/s00213-009-1638-9. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, McNamara P, Angstadt M, Phan KL. Neural substrates of alcohol induced smoking urge in heavy drinking nondaily smokers. Neuropsychopharmacol. 2010;35:692–701. doi: 10.1038/npp.2009.177. doi: 10.1038/npp.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiat. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer–Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiella A, Ulshöfer DE, Vollmert C, Vollstädt-Klein S, Bühler M, Esslinger C, Smolka MN. Nicotine increases neural response to unpleasant stimuli and anxiety in non-smokers. Addict Biol. 2010;16:285–295. doi: 10.1111/j.1369-1600.2010.00237.x. doi: 10.1111/j.1369-1600.2010.00237.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharm. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. doi:10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanism in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA A receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Mackenzie TB, Fiszdon J, Valentiner DP, Foa E, Wangensteen D. The effects of alcohol consumption on laboratory induced panic and state anxiety. Arch Gen Psychiat. 1996;53:264–270. doi: 10.1001/archpsyc.1996.01830030086013. doi: 10.1001/archpsyc.1996.01830030086013. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacol. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Sher KJ, Grossman LM, Newman J, Newlin DB. Alcohol and stress response dampening: Pharmacological effects, expectancy, and tension reduction. J Abnorm Psychol. 1980;89:528–538. doi: 10.1037//0021-843x.89.4.528. doi: 10.1037/0021-843X.89.4.528. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Rebalancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljequist S, Engel JA. The effects of GABA and benzodiazepine receptor antagonists on the anti-conflict actions of diazepam or ethanol. Pharmacol Biochem Be. 1984;21:521–525. doi: 10.1016/s0091-3057(84)80033-7. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Abroms BD, Van Selst M, Fillmore MT. Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacology. 2005;182:452–459. doi: 10.1007/s00213-005-0116-2. doi: 10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McLaren D, Ries M, Xu G, Fitzgerald M, Kastman E, Gliori G, Jabbar B, Johnson S. A method for improved sensitivity and flexibility of psychophysiological interactions in event-related fMRI experiments.. Annual Meeting of the Organzization for Human Brain Mapping.2008. [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–93. doi: 10.1038/nn1494. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Mason MF, Macrae CN, Banaji MR. Thinking about others: The neural substrates of social cognition. In: Cacioppo JT, Visser PS, Pickett CL, editors. Social Neuroscience: people thinking about thinking people. MIT Press; Cambridge, MA: 2006. pp. 63–82. [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable vs. predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. doi:10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AB, Albery IP. Alcohol consumption and HIV risk behaviours: Integrating the theories of alcohol myopia and outcome expectancies. Addict Res Theory. 2001;9:73–86. doi: 10.3109/16066350109141773. [Google Scholar]
- Murray EA, Izquierdo AD. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann NY Acad Sci. 2007;1121:273–296. doi: 10.1196/annals.1401.021. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA . Helping patients with alcohol problems: A clinician's guide. NIH Pub No. 05–3769. Bethesda, MD: 2005a. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA . Recommended council guidelines on ethyl alcohol administration in human experimentation. National Advisory Council on Alcohol Abuse and Alcoholism; Bethesda, MD: 2005b. [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, Yonekura Y. Functional association of the amygdale and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: An event-related fMRI study. Neuroimage. 2004;21:352–363. doi: 10.1016/j.neuroimage.2003.09.021. doi: :10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Peters TJ. Alcohol: the drug. Br Med Bull. 1994;50:5–17. doi: 10.1093/oxfordjournals.bmb.a072883. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- O'Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SC, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacol. 2012;37:2267–2276. doi: 10.1038/npp.2012.77. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Association of neural and physiological responses during voluntary emotion suppression. NeuroImage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Parent MB, Krebs-Kraft DL, Ryan JP, Wilson JS, Harenski C, Hamann S. Glucose administration enhances fMRI brain activation and connectivity related to episodic memory encoding for neutral and emotional stimuli. Neuropsychologia. 2011;49:1052–1066. doi: 10.1016/j.neuropsychologia.2011.02.013. doi: 10.1016/j.neuropsychologia.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Parrott DJ, Zeichner A, Stephens D. Effects of alcohol, personality, and provocation on the expression of anger in men: a facial coding analysis. Alcohol Clin Exp Res. 2003;27:937–945. doi: 10.1097/01.ALC.0000071923.78618.5B. doi: 10.1111/j.1530-0277.2003.tb04418.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiat. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenui I, Povpovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiat. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Pichon S, de Gelder B, Grezes J. Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. Neuroimage. 2009;47:1873–1883. doi: 10.1016/j.neuroimage.2009.03.084. doi: 10.1016/j.neuroimage.2009.03.084. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Chanraud S, Müller-Oehring EM, Pfefferbaum A, Sullivan EV. Modulation of limbic-cerebellar functional connectivity enables alcoholics to recognize who is who. Brain Struct Funct. 2012:1–13. doi: 10.1007/s00429-012-0421-6. doi: 10.1007/s00429-012-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdale-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2012;00:1–8. doi: 10.1002/da.22014. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers Psychiat. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. doi: 10.3389%2Ffpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiat. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993;337:1–31. doi: 10.1002/cne.903370102. doi: 10.1002/cne.903370102. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR. Reduced Fronto-Cerebellar Functional Connectivity in Chronic Alcoholic Patients. Alcohol Clin Exp Res. 2012;36:294–301. doi: 10.1111/j.1530-0277.2011.01614.x. doi: 10.1111/j.1530-0277.2011.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger SY, McNamara PJ, King AC. Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES). Alcohol Clin Exp Res. 2009;33:916–924. doi: 10.1111/j.1530-0277.2009.00914.x. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA . National survey on drug use and health. Office of Applied Studies; Bethesda, MD: 2005. [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol's effectiveness on stress responses in social drinkers. Psychol Bull. 1993;114:459–476. doi: 10.1037/0033-2909.114.3.459. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Perrott MA, Wertz JM, Hufford MR. A test of the appraisal-disruption model of alcohol and stress. J Stud Alcohol. 2001;62:247–256. doi: 10.15288/jsa.2001.62.247. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Smith DW, Breiner MJ, Wilson GT. The effect of alcohol on emotional response to a social stressor. J Stud Alcohol. 1992;53:541–545. doi: 10.15288/jsa.1992.53.541. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol timeline follow-back users’ manual. Addiction Research Foundation; Toronto, Canada: 1995. [Google Scholar]
- Spielberger CD, Gorsuch RL, Luschene R. Test Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Sripada CS, Angstadt M, McNamara P, King AC, Phan KL. Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage. 2011;55:371–380. doi: 10.1016/j.neuroimage.2010.11.062. doi: 10.1016/j.neuroimage.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM, Josephs RA. Alcohol myopia: Its prized and dangerous effects. Amer Psychol. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. doi: 10.1037/0003-066X.45.8.921. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stenger VA, Boada FE, Noll DC. Three–dimensional tailored RF pulses for the reduction of susceptibility artifacts in T(*)(2)–weighted functional MRI. Mag Reson Med. 2000;44:525–531. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. doi: 10.1002/1522-2594(200010)44:4<525::AIDMRM5>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutker PB, Tabakoff B, Goist KC, Jr, Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Be. 1983;18(Suppl 1):349–354. doi: 10.1016/0091-3057(83)90198-3. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- Talalaenko AN, Abramets IA, Stakhovskii Yu V, Shekhovtsov AA, Chernikov AV, Shevchenko SL. The role of dopaminergic mechanisms on the brain in various models of anxious states. Neurosci Behav Physiol. 1994;24:284–288. doi: 10.1007/BF02362037. doi: 10.1007/BF02362037. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Henning J, Ebert D. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiat. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. doi: 10.1016/S0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol therapeut. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:117–188. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. doi:10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. doi: 10.1037/0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]