Abstract
Background
With the growing numbers of liver transplant recipients, it is increasingly important to understand the risks of de novo malignancy after liver transplantation.
Aim
To characterize the incidence of de novo malignancy after liver transplantation compared to a control non-transplant population.
Methods
We studied 534 Indiana state residents undergoing liver transplantation at our center between 1997 and 2004, followed through August 2010 The incidence and predictors of malignancy were determined. The standardized incidence ratio (SIR) of cancer in our cohort was compared to age, gender and period matched state population using the Indiana State Cancer Registry.
Results
After a mean follow up of 5.7 ± 3.2 years, 73 patients (13.7%) developed 80 cancers, with 5 and 10 year incidence rates of 11.7%, and 24.8%, respectively. These included 24 (30%) skin, 16 (20%) hematologic and 40 (50%) solid tumors. The most common solid cancers were aerodigestive. Compared to matched state population, liver transplant recipients had significantly higher incidence of all cancers (SIR:3.1, 95%CI:2.9–3.2), skin (melanoma) (SIR:5.8, 95%CI:4.7–7.0), hematologic (SIR:7.1, 95%CI:6.3–8.0), and solid (SIR:2.7, 95%CI:2.5–2.8) tumors.
Conclusion
There is a significantly increased risk of de novo malignancies after liver transplantation, highlighting the need for surveillance strategies in this population.
Keywords: Malignancy, de novo, liver transplantation, standardized incidence ratio, population control study
Introduction
Liver transplantation outcomes have improved over the last two decades due to improved surgical and medical expertise and advances in immunosuppression. Liver transplantation is also increasingly performed in older recipients.1 Patient and graft survival rates exceeding 90% at one year have been achieved in many transplant centers. With improved long term survival and an aging transplant population, there is increased likelihood of long-term complications in organ transplant recipients. Late deaths and graft loss are less commonly related to rejection or technical complications, and more to cardiovascular complications, infections, disease recurrence and de novo malignancies.2–3
It is believed that the increased incidence of de novo cancers in liver transplant patients occurs primarily as a consequence of immunosuppression which may affect tumor development and progression by a variety of mechanisms. These include; providing a permissive environment for malignant cells to proliferate, infection or reactivation of oncogenic viruses within the host, chronic antigen stimulation leading to a cytokine-rich milieu, and impairing immune surveillance.4–6 Some immunosuppressive drugs may also influence the behavior of the malignant cells through intrinsic oncogenic properties.7 Other risk factors include exposure to antigenic stimulation by viral agents, alcohol and tobacco use before and after transplantation, acute rejection episodes, type and doses of immunosuppressive drugs and treatment with monoclonal antibody OKT3. 7
Previous registry based studies reported an overall incidence of de novo malignancies of only 5.6 to 6.1% for cancers after solid organ and liver transplantation. 8–9 The reported incidence after liver transplantation from individual centers varies from 2.6% to 21.7%. 10–14 The variation in rates of de novo malignancy in different studies may be accounted for by differences in the duration of follow-up, geographic variation and differences in methods of identifying and reporting of de novo malignancies.10–16
While there have been a number of U.S. centers reporting incidence of de novo malignancy post liver transplantation, population controlled studies to define the relative risk of de novo malignancy have been limited. A few, mainly European studies have analyzed the standardized incidence ratio (SIR) for de novo malignancy post liver transplantation by comparing the incidence of cancers in single center or national liver transplant cohorts with those of the general population from which the transplant cohorts are derived using national cancer registries.9, 16–17
The aim of this study was to describe the incidence of and the risk factors for de novo malignancy after liver transplantation at our center, and to characterize the standardized incidence ratio of de novo malignancy in comparison to age and gender matched general population in the state of Indiana.
Materials and Methods
Patients and transplant factors
The study was approved by the institutional review board of Indiana University (#1011004298). A retrospective analysis was performed of all Indiana state residents undergoing liver transplantation at Indiana University centers between the years of 1997 and 2004. Liver transplant recipients have at least annual clinical visits at our center after the first year post transplant. Electronic medical records were reviewed to confirm their Indiana state residence and to collect the following demographic and clinical characteristics: age, gender, race, co-morbidities, personal and family history (first degree) of cancer, history of significant alcohol use,18 tobacco use (current or previous), etiology of liver disease and immunosuppression. The incidence of de novo malignancy was determined through review of the institutional medical records and from the Indiana State Cancer Registry.
To avoid inclusion of undiagnosed cancers in recipients at the time of liver transplant, de novo malignancies were defined as cancers diagnosed more than 6 months post transplant. Since post transplant lymphoproliferative disorders (PTLD) could develop within 6 months of transplant all cases of PTLD were included as de novo malignancies.19 Recurrences of known pre-transplant malignancies including hepatocellular carcinoma were also classified as pre-existing and not de novo tumors. Colonic high grade dysplasia developing in patients with underlying inflammatory bowel disease was also classified as de novo malignancy.
Immunosuppression from 1997 through 2001 was based on cyclosporin with or without prednisone, and since 2002 included induction with anti-thymocyte globulin and steroid free maintenance with tacrolimus. 20 The calcineurin inhibitors were changed to sirolimus in cases of renal dysfunction not responding to dose reductions and/or addition of second-line immunosuppressive agents (azathioprine, mycophenolate mofetil or prednisone). Discontinuation of calcineurin inhibitors in favor of sirolimus in patients with de novo malignancy may have occurred in some cases but was not uniform. Immunosuppression was analyzed according to the primary immunosuppressive agent used (cyclosporin vs. tacrolimus), and by the use of multiple agents (primary agent plus second-line agents,) beyond the first year post transplant.
Analysis of the Standardized Incidence Ratio
The Indiana State Cancer Registry (ISCR) was used to identify de novo cancer in the transplant cohort as well as matched control population. Controls were defined as age and gender, matched individuals residing in the state of Indiana, with cancers reported during the study period. As previously described, the ISCR identifies cases of malignancy (excluding non-melanoma skin cancers not involving mucous membranes) through a variety of methods including histology/pathology and radiology reports, diagnostic codes and death certificates, with review and confirmation of all cases by a certified death registrar. 21 The ISCR maintains a capture rate of approximately 95% of cancer patients in the state of Indiana.
The Indiana University liver transplant recipient study cohort was cross referenced with the ISCR database to identify all reported cancers in the cohort between 1997 and August 2010. Computerized probabilistic matching was performed by the ISCR to maximize sensitivity of cancer reporting, and included the date of the registered malignancy, anatomic site, histology and age at diagnosis. This process allowed for multiple tumors occurring in the same individual to be reported by the ISCR. The development of de novo malignancy was also determined by careful review of the medical record in all patients. All ISCR reported cancers in the study cohort were confirmed by cross-referencing with the medical records.
Only cancers identified by the ISCR were used to analysis the SIR of de novo cancers in the transplant cohort. Cancers were analyzed according to four main categories- all cancers combined, hematologic malignancies, solid tumors and skin cancers (melanoma only). Squamous cell cancer of mucous membranes were reported and included in organ system by site e.g. squamous cell cancer of the lip would be included as oral cancer. Non-melanoma skin cancers are not captured by the ISCR. The ISCR also determined the incidence of cancers reported for the state population during the same period.
Cancer incidence in the study cohort was reported by the ISCR by age in increments of 5 years. Age-adjusted rates of de novo malignancy were calculated as the number of cases divided by the population in each cohort multiplied by 100,000. Cancer incidence was similarly adjusted according to gender. The SIR was calculated as the number of observed cases of de novo malignancy divided by the expected number of cases based on the ISCR incidence rate of cancers and person years of study population at risk. To calculate the SIRs and corresponding 95% confidence intervals, we used the Breslow-Day method and assume a Poisson distribution and take the 97.5th, 50th, and 2.5th percentiles. P-values show whether the ratio (comparing overall vs. Indiana rate) is significantly different from 1. SIR analyses were performed using SAS v9.2 (SAS Institute, Cary, NC, USA).22
Statistical analysis
In the analysis of risk factors for the development of de novo malignancy and the effect on patient survival, the endpoint was all cancers combined. Descriptive statistics were used to characterize demographics and clinical data. Results are expressed as mean ± standard deviation or median and range. Comparisons between patients with and without de novo malignancy were made using the Mann–Whitney U test for continuous variables and the Chi-square test for categorical variables. As better survival has been reported for non-melanoma skin cancers,23 survival and risk analyses were performed for endpoints of all de novo malignancies with and without non-melanoma skin cancers. Estimation of survival was performed using the Kaplan–Meier method and compared using the log-rank test. P value < 0.05 was considered statistically significant. Univariate and multivariate Cox regression analysis was performed to identify predictors of de novo malignancies. Factors achieving a p value of < 0.1 on univariate analysis were entered in multivariate backward logistic regression model. Descriptive analyses, Kaplan Meier and Cox regression analyses were performed with IBM SPSS 19.0 (SPSS Inc., Chicago, IL, USA.).
Results
A total of 534 Indiana residents received liver transplants at our center from 1997 to 2004. Patient demographics and clinical characteristics are summarized in table 1. With a mean follow up of 5.7 ± 3.2 years, 204 patients died, and 313 patients had complete follow up. Seventeen patients (3%) were lost to follow up (after a mean interval of 4.8 ± 3 years). During the study period 73 patients (13.7%) developed 80 de novo malignancies. These included 66 patients with a single tumor and 7 patients with two tumors. The mean interval from liver transplant to development of any de novo malignancy was 4±2.2 years. The clinical characteristics of patients developing de novo malignancy and patients not developing any cancers were compared (table 1). Patients who developed de novo malignancy were older, were more frequently non-Caucasian, tobacco users, with a personal and family history of cancer. Only 2 of 32 pediatric recipients developed de novo malignancies (PTLD in both cases), with all other tumors developing in adult recipients. Non-Caucasian patients comprised diverse races, and as a group were younger than Caucasian patients (45±19 vs. 50±13 years, p=0.02), but had similar frequency of tobacco use, personal and family history of cancer. Compared to Caucasians, non-Caucasian patients had higher incidence of non-skin cancers 17% vs. 9%, p= 0.04), but similar incidence of skin cancer (3% vs. 5%, p=0.4). Although men were more frequently transplanted for alcoholic liver disease (29% vs. 13%, p< 0.001) and had significantly higher tobacco use (42% vs. 23%, p < 0.001), there was no discernible gender-based difference in the incidence of de novo malignancy in our cohort. A personal history of cancer at the time of liver transplantation (including primary liver cancers in the explanted liver) was present in 17% of patients developing any de novo cancer. However when excluding non-melanoma skin cancers, 22.6% of patients had a previous cancer compared with 10.4% in patients without de novo cancer (p=0.009).
Table 1.
Selected Demographic and Clinical Characteristics of the Study Cohort and Subjects with and without De Novo Malignancy
| All LT Recipients N=534 |
Patients with de novo malignancy N= 73 |
Patients without de novo malignancy N=461 |
P value |
|
|---|---|---|---|---|
| Mean age at LT (years, ± sd) | 49.5 ± 14 | 53 ± 12 | 49 ± 14 | 0.01 |
| Age≥18years | 94% | 97% | 94% | 0.2 |
| Age≥60years | 19% | 25% | 18% | 0.2 |
| Male gender | 65% | 67% | 65% | 0.7 |
| Race | ||||
| Caucasian | 86% | 81% | 87% | |
| Black | 6% | 11% | 5% | 0.09 |
| Hispanic | 2% | None | 3% | |
| Other | 6% | 8% | 5% | |
| Personal history of cancer | 12% | 17% | 11% | 0.14 |
| Liver cancer | 9% | 11% | 9% | 0.5 |
| Family history of cancer | 15% | 22% | 14% | 0.07 |
| History of tobacco use | 36% | 45% | 34% | 0.07 |
| History of alcohol use | 34% | 34% | 34% | 0.9 |
| History of diabetes mellitus | 32% | 37% | 32% | 0.4 |
| Etiology of liver disease | ||||
| Alcohol | 23% | 25% | 23% | 0.8 |
| Viral | 46% | 49% | 45% | 0.5 |
| NASH | 11% | 12% | 10% | 0.6 |
| Autoimmune | 14% | 11% | 15% | 0.4 |
| Immunosuppression | ||||
| Primary agent | ||||
| Tacrolimus | 84% | 85% | 84% | 0.6 |
| Cyclosporin | 13% | 14% | 12% | 0.6 |
| Sirolimus | 3% | 1% | 3% | 0.4 |
| Multiple agents used (Primary agent with azathioprine/ mycophenolate mofetil and/or prednisone) |
30% | 30% | 30% | 0.7 |
| Follow up (years, mean ± s.d.) | 5.7 ± 3.2 | 6.1 ± 2.6 | 5.6 ± 3.3 | 0.15 |
LT: Liver transplantation, NASH: Nonalcoholic steatohepatitis;
Patients strictly taking tacrolimus or cyclosporin for the entire post-transplant follow up period had an overall de novo cancer incidence of 15% and 19% respectively (p=0.4), but those taking tacrolimus had a lower cancer incidence when excluding non-melanoma skin cancers, 11% and 19% respectively (p= 0.09). The trend towards lower incidence of non-skin de novo malignancy with tacrolimus vs. cyclosporin use was not specific to any cancer subtype. The incidence of de novo malignancy in patients treated with sirolimus and in patients not receiving sirolimus was identical at 15%, and excluding non-melanoma skin cancer it was 12% and 7% respectively (p=0.6).
The most common malignancies were skin cancers accounting for 24 (30%) cancers, of which 21 were non-melanomas, followed by 16 (20%) hematologic cancers, and 40 (50%) solid tumors of which non-small cell lung cancer was the most common (Table 2). Twenty one patients developed aero-digestive tumors, for which tobacco use is a recognized risk factor (8 cases of squamous cell cancer of the head and neck and 13 cases of non-small cell lung cancer). These cancers developed in 7.9 % of tobacco users vs. 1.5 % of non-users (p < 0.0001). Tobacco use was also associated with increased incidence of aerodigestive cancers in patients transplanted for alcoholic liver disease (7.1% of tobacco users vs. 2.5% of non-users, P 0.3).
Table 2.
Listing of all de novo malignancies by body site and histology
| Malignancy type and site | Number of cancers |
|---|---|
| Non-melanoma skin cancers1 | 21 |
| Melanoma | 3 |
| Hematological malignancies2 | 16 |
| Solid tumors | 40 |
| Lung (all non-small cell) | 13 |
| Head and neck3 | 9 |
| Gastrointestinal tract4 | 7 |
| Genitourinary5 | 4 |
| Brain | 4 |
| Other6 | 3 |
Non-melanoma skin cancers were squamous cell (13), basal cell (6), and both squamous cell and basal cell in 2.
Hematological include lymphoma in 15 and acute myelogenous leukemia in 1 patient.
Oroharyngeal cancer (8) and parotid cancer (1)
Gastrointestinal cancers were pancreatic (2), gastric (2), 1 each of duodenum, colon and anus
Genitourinary cancers were prostate (1), female genital tract (1), and kidney (1)
Others were thymoma (1), sarcoma (1), and metastatic adenocarcinoma of unknown primary (1)
Cancer incidence
The cumulative incidence rate for all de novo malignancies was 1.3% at 1 year (number at risk n=441), 7.2% at 3 years (n=391), 11.7% at 5 years (n=346), 17.9% at 8 years (n=118) and 24.8% at 10 years (n=27) post liver transplant. The cumulative incidence rates for all de novo malignancies excluding non-melanoma skin cancers at 1, 3, 5, 8 and 10 years post transplant were 0.9%, 5.6%, 10%, 12.6% and 21.3%, respectively. The cumulative incidence of de novo malignancy, including and excluding non-melanoma skin cancers, represented by Kaplan Meier curves is described in figure 1.
Figure 1.
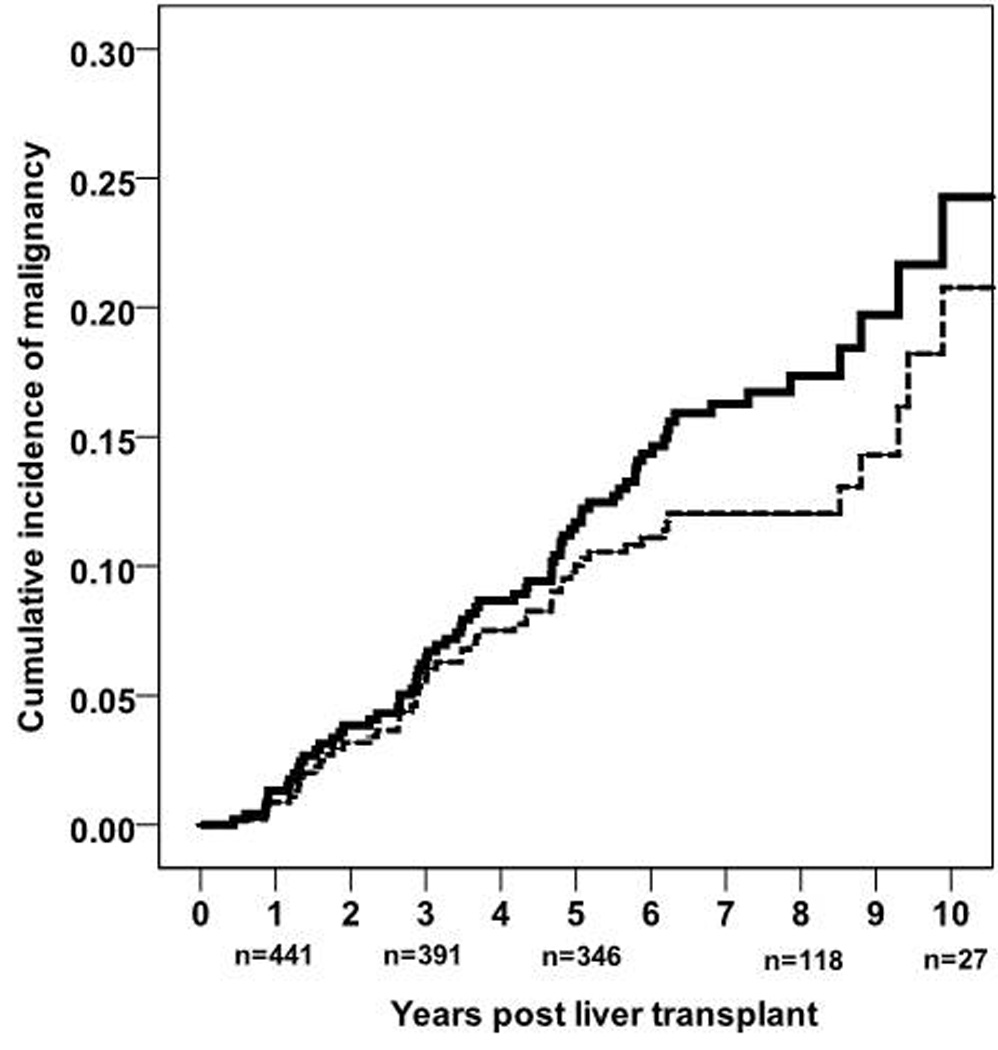
Kaplan Meier survival curves describing the cumulative incidence of de novo malignancy (solid line) b) and de novo malignancy excluding non-melanoma skin cancers (interrupted line) post liver transplantation, including number of patients at risk below the Y axis.
The clinical chart review identified all but one cancer that was reported to the ISCR based on autopsy findings not documented in our centers records. The ISCR captured the incidence of de novo cancer (excluding non-melanoma skin cancers) in 48 patients in our cohort (90% of the respective non-skin cancers in our cohort). Only the ISCR data was used to analyze the risk of cancer incidence. On comparison with the state of Indiana population controls, Indiana residents undergoing liver transplantation had significantly higher standardized incidence ratio (SIR) for all cancers, melanoma, hematological malignancies, and solid tumors (p-values were < 0.001 for all comparisons) (Table 3). While we could not control for a number of factors, according to the 2009 Census Report (http://quickfacts.census.gov/qfd/states/18000.html) and the 2009 Indiana Behavioral Risk Factor Surveillance report (http://www.in.gov/isdh/reports/brfss/2009/toc.htm), the percentage of Caucasians (84.3%) and lifetime smokers (>32.7%) in the state population were similar to those of the study cohort.
Table 3.
Cancer Incidence Rates in Liver Transplant Recipients and Controls Based on Cancers reported to the Indiana State Cancer Registry (P value was < 0.001 for all analyses)
| Cancer incidence per 100,000 person years post liver transplantation |
Cancer incidence per 100,000 person years in the Indiana State population |
Standardized incidence ratio of cancer post liver transplant |
95% Confidence interval |
|
|---|---|---|---|---|
| All patients (n=534) | ||||
| All cancers | 1,454 | 476 | 3.1 | 2.9 – 3.2 |
| Melanoma | 95 | 16.4 | 5.8 | 4.7 – 7.0 |
| Solid tumors | 1,072 | 405 | 2.7 | 2.5 – 2.8 |
| Hematological malignancies | 286 | 40 | 7.1 | 6.3 – 8.0 |
| Male patients (n=347) | ||||
| All cancers | 1,384 | 491 | 2.8 | 2.7 – 3.0 |
| Melanoma | 73 | 19 | 3.9 | 3.0 – 4.8 |
| Solid tumors | 948 | 413 | 2.3 | 2.2 – 2.4 |
| Hematological malignancies | 364 | 44 | 8.3 | 7.5 – 9.2 |
| Female patients (n=187) | ||||
| All cancers | 1,619 | 463 | 3.5 | 3.3 – 3.7 |
| Melanoma | 141 | 14 | 10.1 | 8.4 – 11.8 |
| Solid tumors | 1,338 | 398 | 3.4 | 3.2 – 3.6 |
| Hematological malignancies | 141 | 37 | 3.8 | 3.2 – 4.5 |
Risk factors for de novo malignancy
The results of the univariate and multivariate Cox regression analysis of the predictors of de novo malignancies including and excluding non-melanoma skin cancers, and predictors of solid organ cancers are summarized in table 4. Older age was significantly associated with increased overall risk of de novo malignancy. Non-Caucasian race was associated with a significantly increased risk of de novo malignancy including and excluding non-melanoma skin cancers on univariate analysis. However given the diversity of race subsets within that group and the largely Caucasian cohort, race was not introduced in the multivariate analysis. A personal history of cancer prior to liver transplantation was associated with significant risk of de novo malignancy including (table 1) and excluding non-melanoma skin cancers. The incidence of de novo malignancy excluding non-melanoma skin cancers was increased in patients with primary liver malignancies (16.3% vs. 8.3% in those without history of any malignancy, p=0.08), and in patients with a history of non-liver malignancy at transplant ( 30.8% vs. 8.3%, p=0.007). Tobacco use was a significant predictor of non-skin de novo malignancies on univariate analysis, but approached significance on multivariate analysis. A family history was also predictive of de novo cancers on univariate analysis but not on multivariate analysis. Other factors such as gender, alcohol or primary sclerosing cholangitis as the etiology of liver disease and the type of immunosuppression were not predictive of de novo malignancy. Age and tobacco abuse were the independent predictors of solid organ cancers, and a personal history of cancer was only predictive on the univariate analysis.
Table 4.
Factors associated with de novo malignancy post liver transplantation
| Hazard ratio |
95 % Confidence Interval |
P value | |
|---|---|---|---|
| Any De Novo Malignancy | |||
| Univariate analysis | |||
| Age at LT | 1.03 | 1.008 – 1.05 | 0.008 |
| Non-Caucasian race | 2 | 1.1 – 3.7 | 0.02 |
| Personal history of cancer | 2.2 | 1.2 – 4 | 0.02 |
| Multivariate analysis | |||
| Age at LT | 1.03 | 1.005–1.05 | 0.02 |
| Personal history of cancer | 1.8 | 0.98 – 3.5 | 0.06 |
|
De novo malignancy excluding non-melanoma skin cancer |
|||
| Univariate analysis | |||
| Age at LT | 1.0 | 1.001 – 1.05 | 0.04 |
| Non-Caucasian race | 2.5 | 1.3 – 4.6 | 0.005 |
| Personal history of cancer | 2.7 | 1.4 – 5.2 | 0.002 |
| Family history of cancer | 1.7 | 0.93 – 3.1 | 0.09 |
| Tobacco use | 1.7 | 1.02 – 2.9 | 0.04 |
| Multivariate analysis | |||
| Personal history of cancer | 2.5 | 1.3 – 4.9 | 0.005 |
| Tobacco use | 1.7 | 0.97 – 2.8 | 0.06 |
|
De novo Solid organ de novo malignancy |
|||
| Univariate analysis | |||
| Age at LT | 1.04 | 1.006 – 1.07 | 0.02 |
| Personal history of cancer | 2.6 | 1.2 – 5.6 | 0.02 |
| Family history of cancer | 1.8 | 0.86 – 3.6 | 0.12 |
| Tobacco use | 2.7 | 1.4 – 2.7 | 0.003 |
| Multivariate analysis | |||
| Age at LT | 1.04 | 1.003 – 1.08 | 0.03 |
| Tobacco use | 2.8 | 1.4 – 5.5 | 0.003 |
All variables achieving a p-value<0.1 on univariate analysis were included in the multivariate analysis.
Variables that were analyzed but were not predictive in the univariate analysis included; gender, a diagnosis of diabetes mellitus, alcoholic liver disease or hepatitis C, primary immunosuppressive agent used, and use of multiple immunosuppressive agents after the first year post transplant.
Patient survival
We compared patient survival with and without de novo malignancy in patients surviving more than 6 months post liver transplantation. By definition, no patients with de novo malignancy died within 6 months of liver transplantation. De novo cancer related death accounted for 29 (14.2%) of all deaths in our cohort, and 21% of all deaths in patients surviving more than 6 months post liver transplant. Categorized cause of death in patients surviving more than 6 months post liver transplant also included infection (15%), disease recurrence (13%), cardiovascular events (9%) and other causes (31%). Post transplant survival was significantly lower in patients developing de novo malignancy compared with patients without cancer (70% vs. 81% at 5 years, p= 0.006). However patient survival was diminished only in the subset of patients with de novo malignancy, excluding non-melanoma skin cancer compared with patients without cancer (62% vs. 82% at 5 years, p < 0.0001). The cause of death in this subset of patients was the de novo malignancy in 83% of cases, unrelated in 9% and unknown in 9%. One, 3 and 5 year patient survival after diagnosis of any de novo malignancy was 65%, 49% and 36% respectively. One, 3 and 5 year survival were 100%, 100% and 67% in patients developing only non-melanoma skin cancers, and 55%, 36% and 27% respectively in patients developing any other cancer (p < 0.001).
Discussion
The main findings of this study were the relatively high cumulative incidence rate of de novo malignancy in liver transplant recipients and the significantly increased risk compared to the age and gender matched control state population. A few U.S. centers have used multi-state or geographically non-restricted cancer registry data to report relative cancer risks post liver transplantation.12, 23 To our knowledge this is the first U.S. transplant center to use a state cancer registry to determine the risk of de novo malignancy for a cohort of transplant recipients relative to the state population from which it originates. It underscores the importance of de novo malignancy as the leading cause of late mortality post liver transplantation.
The overall SIR for de novo malignancy in our study (3.1) excluded non-melanoma skin cancers which were not associated with increased mortality. It was comparable to SIRs reported in European studies of similar design (2.1 to 4.3). 10, 16–17, 24–25 The overall SIR for de novo malignancy provides a simple format for conveying the meaningful cancer risk of long-term immunosuppression to patients requiring liver transplantation. The SIR for solid organ cancers in our cohort (2.7) was identical to that reported by Haagsma et al (2.7), but that was the only other study that reported this particular risk in liver transplant recipients.17 Analysis of the SIR of individual cancer types was limited by our sample size.
The most common solid organ cancers in our study were non-small cell lung cancer and squamous cell cancer of the head and neck accounting for 37% of all non-skin de novo malignancies. Increased risk of aerodigestive cancers post liver transplant is well described in patients with a history of tobacco use and alcoholic liver disease. 26–28 In our cohort tobacco use was the more important risk factor, but this may be related to a proportion of patients continuing to use tobacco post transplant, where as the majority of patients with alcoholic liver disease would have maintained abstinence post transplant. Surveillance strategies have not consistently resulted in diagnosis of aerodigestive cancers at earlier stages, however it is prudent to maintain a high level of suspicion and consider surveillance strategies that incorporate routine chest imaging and otolaryngology evaluation in patients at risk. 29 Standard cancer screening surveillance strategies used in the general population should, at a minimum, otherwise be followed.
It was not surprising that we found age and tobacco to be predictors of de novo malignancy, particularly for solid organ cancers which were predominantly aerodigestive tumors. 13, 17, 30–33 However, a personal history of cancer at liver transplantation was a novel and strong predictor of increased risk of de novo malignancy in our study, particularly for non-skin cancers, and is supported by data in kidney transplantation. 34–36 It is possible that this factor represents a composite surrogate marker of an individual's propensity for malignancy based on genetic and epigenetic factors. Non-Caucasian race was represented by a small subset of patients with diverse races and therefore could not be appropriately evaluated.
We analyzed the effect of immunosuppression on de novo malignancy according to the agent used and the use of multiple agents. Cyclosporin has been the main agent used in the majority of studies on de novo malignancy to date, 2, 12–13, 16, 33, 37. The present study, with 84% of the cohort using tacrolimus, is one of the few studies reporting on de novo cancer with tacrolimus based immunosuppression.11, 23 There was no difference in cancer incidence based on the calcineurin inhibitor used when analyzed by Cox regression which takes into account lead time bias in patients on cyclosporin who longer follow-up time to develop cancer. The use of sirolimus in our cohort included heterogeneous indications and a limited number of patients, limiting meaningful analysis. Analyzing the effect of thymoglobulin induction on cancer incidence was confounded by the concomittant protocol change in primary immunosuppressant from cyclosporin to tacrolimus. It is likely that the degree of immunosuppression itself that dictates the risk of malignancy. 38 Minimization of immunosuppression has been shown to reduce cancer risk in renal transplant recipients, and remains an important approach in mitigating the long-term risks of immunosuppression.39
The strengths of the study include; i) a large clinically well-defined cohort of Indiana State residents receiving transplantation at the sole liver transplant program in the state, ii) a long follow up period, and iii) the use of the ISCR to define the relative risk of de novo malignancies. The limitations of this study include: i) a retrospective design, based on a single center and the Indiana state population, ii) data on risk factors and cancer incidence in the state population could not be validated, and iii) some ethnic/racial groups are not represented well. Nevertheless the study highlights the increased risk and impact of de novo malignancies in liver transplant recipients, and identifies novel predictors that may aid in designing future surveillance strategies. A high index of suspicion and heightened surveillance is warranted to effectively deal with this risk. More studies are needed to obtain reliable data on cancer risk patterns, develop consensus on optimal monitoring of immunosuppression, and develop cancer surveillance programs to improve long-term outcomes.
Acknowledgments
We would like to thank Stephen Nygaard and the staff at the Indiana State Cancer Registry for their assistance in providing the data on cancer incidence in the study cohort and the state population. This work was supported in part by a National Institute of Health grant (K25 DK069290, to NC).
Abbreviations
- ISCR
Indiana State Cancer Registry
- PTLD
post transplant lymphoproliferative disorder
- SIR
standardized incidence ratio
Footnotes
Authors disclosures: None of the authors have any disclosures or conflicts of interest related to this study or manuscript.
Hemant Chatrath, hchatrat@iupui.edu
Kenneth Berman, kenneth.berman@ucdenver.edu
Raj Vuppalanchi, rvuppala@iupui.edu
James Slaven, jslaven@iupui.edu
Paul Kwo, pkwo@iupui.edu
A. Joseph Tector, atector@iupui.edu
Naga Chalasani, nchalasa@iupui.edu
Marwan Ghabril, mghabril@iupui.edu
References
- 1.Kemmer N, Safdar K, Kaiser TE, Zacharias V, Neff GW. Liver transplantation trends for older recipients: regional and ethnic variations. Transplantation. 2008;86:104–107. doi: 10.1097/TP.0b013e318176b4c1. [DOI] [PubMed] [Google Scholar]
- 2.Jain A, Fiaz O, Sheikh B, et al. Recurrent nonhepatic and de novo malignancies after liver transplantation. Transplantation. 2009;88:706–710. doi: 10.1097/TP.0b013e3181b3918e. [DOI] [PubMed] [Google Scholar]
- 3.Pruthi J, Medkiff KA, Esrason KT, et al. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl. 2001;7:811–815. doi: 10.1053/jlts.2001.27084. [DOI] [PubMed] [Google Scholar]
- 4.Gruber SA, Matas AJ. Etiology and pathogenesis of tumors occurring after organ transplantation. Transplant Sci. 1994;4:87–104. [PubMed] [Google Scholar]
- 5.Matas AJ, Simmons RL, Najarian JS. Chronic antigenic stimulation, herpesvirus infection, and cancer in transplant recipients. Lancet. 1975;1:1277–1279. doi: 10.1016/s0140-6736(75)92555-6. [DOI] [PubMed] [Google Scholar]
- 6.Penn I, Starzl TE. Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation. 1972;14:407–417. doi: 10.1097/00007890-197210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penn I. The problem of cancer in organ transplant recipients: anoverview. Transpl Sci. 1994:4. [PubMed] [Google Scholar]
- 8.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Villeneuve PJ, Fenton SS, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008;14:1588–1597. doi: 10.1002/lt.21554. [DOI] [PubMed] [Google Scholar]
- 10.Oo YH, Gunson BK, Lancashire RJ, Cheng KK, Neuberger JM. Incidence of cancers following orthotopic liver transplantation in a single center: comparison with national cancer incidence rates for England and Wales. Transplantation. 2005;80:759–764. doi: 10.1097/01.tp.0000173775.16579.18. [DOI] [PubMed] [Google Scholar]
- 11.Saigal S, Norris S, Muiesan P, Rela M, Heaton N, O'Grady J. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transpl. 2002;8:482–487. doi: 10.1053/jlts.2002.32977. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez EQ, Marubashi S, Jung G, et al. De novo tumors after liver transplantation: a single-institution experience. Liver Transpl. 2002;8:285–291. doi: 10.1053/jlts.2002.29350. [DOI] [PubMed] [Google Scholar]
- 13.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Sanchez W, Gores GJ. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galve ML, Cuervas-Mons V, Figueras J, et al. Incidence and outcome of de novo malignancies after liver transplantation. Transplant Proc. 1999;31:1275–1277. doi: 10.1016/s0041-1345(98)01994-0. [DOI] [PubMed] [Google Scholar]
- 15.Jain A, Patil VP, Fung J. Incidence of de novo cancer and lymphoproliferative disorders after liver transplantation in relation to age and duration of follow-up. Liver Transpl. 2008;14:1406–1411. doi: 10.1002/lt.21609. [DOI] [PubMed] [Google Scholar]
- 16.Aberg F, Pukkala E, Hockerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14:1428–1436. doi: 10.1002/lt.21475. [DOI] [PubMed] [Google Scholar]
- 17.Haagsma EB, Hagens VE, Schaapveld M, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84–91. doi: 10.1016/s0168-8278(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 18.Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis. 1988;8:12–25. doi: 10.1055/s-2008-1040525. [DOI] [PubMed] [Google Scholar]
- 19.Jain A, Nalesnik M, Reyes J, et al. Posttransplant lymphoproliferative disorders in liver transplantation: a 20-year experience. Ann Surg. 2002;236:429–436. doi: 10.1097/00000658-200210000-00005. discussion 36–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tector AJ, Fridell JA, Mangus RS, et al. Promising early results with immunosuppression using rabbit anti-thymocyte globulin and steroids with delayed introduction of tacrolimus in adult liver transplant recipients. Liver Transpl. 2004;10:404–407. doi: 10.1002/lt.20085. [DOI] [PubMed] [Google Scholar]
- 21.Berman K, Tandra S, Vuppalanchi R, et al. Hepatic and extrahepatic cancer in cirrhosis: a longitudinal cohort study. Am J Gastroenterol. 2011;106:899–906. doi: 10.1038/ajg.2010.477. [DOI] [PubMed] [Google Scholar]
- 22.Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ. 1987:1–406. [PubMed] [Google Scholar]
- 23.Jain AB, Yee LD, Nalesnik MA, et al. Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation. 1998;66:1193–1200. doi: 10.1097/00007890-199811150-00014. [DOI] [PubMed] [Google Scholar]
- 24.Baccarani U, Piselli P, Serraino D, et al. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Dig Liver Dis. 2010;42:55–60. doi: 10.1016/j.dld.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–1896. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 26.Dumortier J, Guillaud O, Adham M, et al. Negative impact of de novo malignancies rather than alcohol relapse on survival after liver transplantation for alcoholic cirrhosis: a retrospective analysis of 305 patients in a single center. Am J Gastroenterol. 2007;102:1032–1041. doi: 10.1111/j.1572-0241.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez C, Marques E, Loinaz C, et al. Upper aerodigestive tract and lung tumors after liver transplantation. Transplant Proc. 2003;35:1900–1901. doi: 10.1016/s0041-1345(03)00641-9. [DOI] [PubMed] [Google Scholar]
- 28.Zanus G, Carraro A, Vitale A, et al. Alcohol abuse and de novo tumors in liver transplantation. Transplant Proc. 2009;41:1310–1312. doi: 10.1016/j.transproceed.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 29.Bellil Y, Edelman MJ. Bronchogenic carcinoma in solid organ transplant recipients. Curr Treat Options Oncol. 2006;7:77–81. doi: 10.1007/s11864-006-0034-5. [DOI] [PubMed] [Google Scholar]
- 30.Herrero JI, Lucena JF, Quiroga J, et al. Liver transplant recipients older than 60 years have lower survival and higher incidence of malignancy. Am J Transplant. 2003;3:1407–1412. doi: 10.1046/j.1600-6143.2003.00227.x. [DOI] [PubMed] [Google Scholar]
- 31.Finkenstedt A, Graziadei IW, Oberaigner W, et al. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant. 2009;9:2355–2361. doi: 10.1111/j.1600-6143.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 32.Xiol X, Guardiola J, Menendez S, et al. Risk factors for development of de novo neoplasia after liver transplantation. Liver Transpl. 2001;7:971–975. doi: 10.1053/jlts.2001.28744. [DOI] [PubMed] [Google Scholar]
- 33.Herrero JI, Lorenzo M, Quiroga J, et al. De Novo neoplasia after liver transplantation: an analysis of risk factors and influence on survival. Liver Transpl. 2005;11:89–97. doi: 10.1002/lt.20319. [DOI] [PubMed] [Google Scholar]
- 34.Danpanich E, Kasiske BL. Risk factors for cancer in renal transplant recipients. Transplantation. 1999;68:1859–1864. doi: 10.1097/00007890-199912270-00008. [DOI] [PubMed] [Google Scholar]
- 35.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 36.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am J Transplant. 2007;7:2140–2151. doi: 10.1111/j.1600-6143.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 37.Benlloch S, Berenguer M, Prieto M, et al. De novo internal neoplasms after liver transplantation: increased risk and aggressive behavior in recent years? Am J Transplant. 2004;4:596–604. doi: 10.1111/j.1600-6143.2004.00380.x. [DOI] [PubMed] [Google Scholar]
- 38.Berenguer M, Prieto M, Bustamante M, et al. [Incidence of de novo neoplasms after liver transplantation] Med Clin (Barc) 1998;111:481–484. [PubMed] [Google Scholar]
- 39.van Leeuwen MT, Webster AC, McCredie MR, et al. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ. 2010;340:c570. doi: 10.1136/bmj.c570. [DOI] [PMC free article] [PubMed] [Google Scholar]


