Abstract
Purpose
Exercise activity is common in female adolescents, however, excessive exercise can have detrimental effects on bone mineral density (BMD). Mechanisms underlying this decrease in bone mass are not well understood. We investigated the effects of sclerostin, a potent inhibitor of bone formation via WNT signaling inhibition, and pre-adipocyte factor (Pref)-1, a suppressor of osteoblast differentiation, on BMD, bone turnover markers and bone strength in adolescent athletes.
Methods
We studied 50 adolescents between 15-21 years of age: 17 amenorrheic athletes (AA), 17 eumenorrheic athletes (EA) and 16 nonathletic controls (NA). We measured spine and hip BMD by dual energy x-ray absorptiometry and estimated failure load and stiffness at the distal radius and tibia using micro-finite element analysis. We also measured fasting sclerostin, Pref-1, N-terminal propeptide of type 1 procollagen (P1NP) and C-terminal collagen crosslinks (CTX) levels.
Results
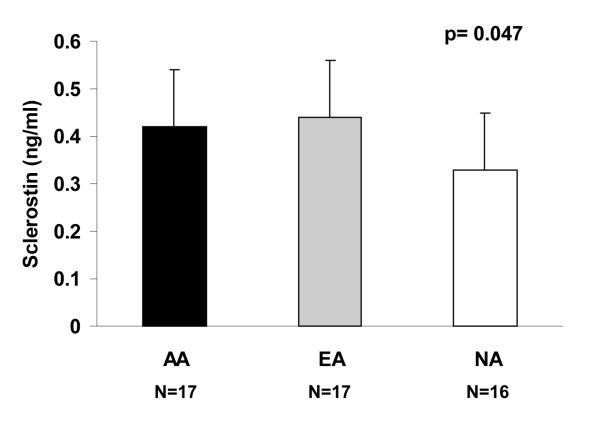
Sclerostin levels were higher in AA and EA compared with NA (AA: 0.42 ± 0.15 ng/mL, EA: 0.44 ± 0.09 ng/mL, NA: 0.33 ± 0.14 ng/mL; p=0.047). In EA, sclerostin was positively associated with lumbar spine (LS) BMD and its Z-score (R=0.52, p=0.03 and R=0.55, p=0.02, respectively) whereas in NA, sclerostin was inversely associated with LS BMD (R=−0.61, p=0.01). Pref-1 levels were similar in all three groups and there were significant inverse associations between Pref-1, BMD and estimated bone strength in NA.
Conclusions
Sclerostin and Pref-1 may have differential effects on bone in adolescent athletes compared to non-athletes.
Keywords: Sclerostin, Pref-1, Bone strength, Adolescent athletes
Introduction
Sports activities are common in female adolescents and weight-bearing activities are known to have beneficial effects on bone [1]. However, excessive athletic activity, which results in amenorrhea, has negative effects on bone mineral density (BMD). Amenorrheic athletes (AA) have lower BMD compared to eumenorrheic athletes (EA) [2] and we have previously shown that excessive athletic activity in adolescent AA is associated with lower BMD and impaired parameters of bone microarchitecture compared with EA and non-athletes (NA) [3]. Hormonal alterations such as hypogonadism contribute partially to these changes; however other determinants of impaired bone health in excessive exercisers are not well understood. Two hormones, sclerostin and preadipocyte factor (Pref-1), have negative effects on bone formation and therefore may be potential mediators of the bone phenotype of adolescent athletes.
Sclerostin is a glycoprotein, secreted primarily by the osteocyte and a potent inhibitor of bone formation through inhibition of the WNT signaling pathway. Effects of sclerostin on bone accrual are best exemplified in human disease models of sclerostin deficiency, sclerosteosis and van Buchem’s disease, both of which are characterized by increased bone mass [4-6]. Individuals with sclerosteosis have loss-of-function mutations in the sclerostin-encoding SOST gene and undetectable levels of sclerostin [7]; N-terminal propeptide of type 1 procollagen (P1NP), a marker of bone formation, and BMD are increased in individuals with sclerosteosis as compared to healthy controls [4, 7].
In adults, sclerostin levels are influenced by gonadal status and age. Sclerostin increases with increasing age and is inversely associated with estradiol levels in women [8-10]. Treatment with estradiol in postmenopausal women causes a significant decrease in sclerostin [11, 12]. In adults and children, sclerostin is higher in males than females [8, 13], and in children, sclerostin declines after pubertal onset in both sexes [13].
Sclerostin levels are also affected by mechanical loading. In murine models, loading of the ulna and tibia results in a decrease in sclerostin-positive osteocytes in cortical and trabecular bone [14, 15]. In obese adults, sclerostin increases with diet-induced weight loss, yet this increase is not observed when weight loss is due to both diet and exercise [16]. In a study of healthy men and premenopausal women, individuals in the most physically active quartile had lower sclerostin compared to individuals in the least active quartile [17]. Yet in postmenopausal women, a year of weight bearing exercise did not result in changes in sclerostin [18]. Therefore, beneficial effects of exercise on BMD may partly be mediated by changes in sclerostin in some populations.
Preadipocyte factor (Pref-1) is a member of the epidermal growth factor-like family of proteins, which is expressed in progenitor cell types and is also a known suppressor of osteoblast differentiation. Pref-1 has been reported to be high in hypothalamic amenorrhea and anorexia nervosa [19, 20], and is inversely associated with BMD.
Although both sclerostin and Pref-1 have a negative effect on bone formation, they have not been investigated in adolescent athletes, and their relationship to bone parameters, amenorrhea and exercise in this group is unknown. We investigated the effects of sclerostin and Pref-1 on BMD and estimated bone strength in adolescent AA, EA and NA in order to compare a state of intense physical activity versus a sedentary state (athletes vs. non-athletes) and effects of a hypogonadal versus eugonadal state (AA vs. EA). We hypothesized that AA would have higher sclerostin and Pref-1 than EA and NA and that higher sclerostin and Pref-1 would be associated with lower BMD and bone strength.
Methods
Subjects
We studied 50 adolescent females between 15-21 years of age: 17 AA (mean age ± SD: 19.8 ± 1.7 years), 17 EA (18.7 ± 1.7 years) and 16 NA (19.4 ± 1.2 years). Subjects were recruited through medical clinics and advertising in local newspapers and colleges. A bone age of at least 15 years and a body mass index (BMI) between the 10th and 90th percentiles were required for inclusion in the study. Athletes participating in the study participated in at least 4 hours of aerobic, weight-bearing exercise of the legs or at least 20 miles of running/week for the preceding 6 months, at a minimum. These criteria were established in consultation with exercise physiologists. Non-weight bearing athletes, including cyclists and swimmers were excluded from participation. Gymnasts and rowers were also excluded due to differences in the nature of the impact and weight-bearing in these activities as compared to runners [21-23]. Nonathletic controls were included if they did not participate in more than 2 hrs of weight-bearing exercise per week and did not participate in organized team sports. Participants were excluded if they used medications known to affect bone metabolism, including oral contraceptives, in the three months prior to enrollment or if conditions other than exercise were a possible cause of amenorrhea.
Amenorrhea was defined as the absence of menses for ≥ 3 months in a period of oligomenorrhea (cycle length > 6 weeks) lasting at least 6 months or the absence of menarche by the age of ≥ 16 years. Eumenorrhea was defined as at least nine menstrual periods in the preceding year with a cycle length of 21-35 days. The Partners HealthCare institutional review board approved this study. Written informed consent was obtained from all participants ≥ 18 years of age or parents of those participants < 18 years old and assent was obtained from all participants < 18 years of age. Clinical characteristics, bone mineral density, bone microarchitecture and finite element analysis data have previously been reported for this cohort [3, 24].
Experimental Protocol
All participants had a history (including a detailed assessment of exercise activity) and physical exam performed and blood was drawn for laboratory studies. Height was measured as the average of 3 readings on a single, wall-mounted stadiometer and subjects were weighed on an electronic scale. BMI was calculated using the formula [weight (kg)/height (meter)2]. A bone age x-ray of the left hand and wrist was obtained and assessed using the standards of Greulich and Pyle [25]. Participants underwent DXA to measure BMD of the lumbar spine and hip and lumbar spine bone mineral apparent density (BMAD) was calculated using published methods [26]. All participants underwent high-resolution peripheral quantitative CT (HR-pQCT) of the non-dominant distal radius and tibia as previously described [3].
Microfinite Element Analysis
We used linear micro-finite element analysis (FEA) of HR-pQCT images to estimate biomechanical properties of the distal radius and distal tibia under uniaxial compression loading as previously described [27-29]. Outcomes from micro-FEA included stiffness (kN/m) and failure load (kN). A prior study has reported strong associations between the biomechanical properties derived from microFEA-estimated and those measured directly via ex vivo testing of elderly human cadaveric radii [30].
Biochemical Assays
Sclerostin was measured using an enzyme immunoassay (TECOmedical Group, Quidel Corporation, San Diego, CA) with a minimum detectable level of detection of 0.13 ng/mL and an intra-assay coefficient of variation (CV) of 5.48% and inter-assay CV of 5.78%. Pref-1 was measured with the Quantikine Human Pref-1 immunoassay (ELISA) (R&D Systems, Minneapolis, MN) with a mean minimum detectable level of 0.012 ng/mL, intra-assay CV of 3.7% and inter-assay CV of 6.2%. An ultrasensitive ELISA was used to measure estradiol (ALPCO Diagnostics, Salem, NH) with a minimum level of detection of 1.399 pg/mL, intra-assay CV of 6.36% and inter-assay CV of 7.60%. P1NP was measured by radioimmunoassay (Orion Diagnostics, Espoo, Finland) with a minimum reportable value of 0.7 μg/L and an intra-assay CV of 3.5-5.3% and an inter-assay CV of 3.6-5.4%. C-terminal collagen cross-links (CTX) levels were measured by immunoradiometric assay (Immunodiagnostic Systems, Fountain Hills, AZ) with a minimum reportable value of 0.02 ng/mL and intra-assay CV of 5.2-6.8% and inter-assay CV of 5.6-7.4%.
Statistical Methods
Statistical analysis was performed using JMP (version 9; SAS Institute, Inc., Cary, NC) software. Means and standard deviations are reported. The means were compared using an ANOVA and the Tukey-Kramer adjustment was used to account for multiple comparisons. A two-tailed p-value of < 0.05 was used to indicate significance. For non-normal distributions, the Wilcoxon test was used to make comparisons between the groups and the Bonferroni correction was used to account for multiple comparisons, in which case a p-value of < 0.017 was used to indicate significance. Pearson correlation coefficients, or Spearman’s coefficients, if the data were not normally distributed, were calculated to assess univariate relationships. Multivariable analyses were performed using least-squares linear regression to control for potential confounders. Outlier analysis was performed and one outlier was excluded in reporting associations between sclerostin and P1NP.
Results
Clinical characteristics
Table 1 lists the clinical characteristics of subjects in the AA, EA and NA groups. The three groups did not differ in chronologic age, bone age, BMI or height (Table 1). The AA group had a significantly older menarchal age as compared to the NA group (AA: 14.2 ± 2.4 years versus HC: 12.1 ± 1.7 years; p < 0.01) but there were no significant differences in menarchal age between AA and EA or between EA and NA. Hip BMD, Hip BMD Z-score and femoral neck (FN) BMD Z-score were significantly higher in EA as compared to both AA and NA (Table 1). Lumbar spine (LS) BMD, LS BMD Z-score and LS BMAD were all significantly lower in AA as compared to both EA and NA (Table 1). CTX levels were significantly higher in EA as compared to NA whereas P1NP levels were not significantly different in the three groups.
Table 1.
Clinical Characteristics of Study Subjects
| Amenorrheic Athletes (n=17) |
Eumenorrheic Athletes (n=17) |
Non-athletes (n=16) |
p-value for overall group comparisons |
|
|---|---|---|---|---|
| Age (years) | 19.8 ± 1.7 | 18.7 ± 1.7 | 19.4 ± 1.2 | 0.11 |
| Bone Age (years)* | 17.6 ± 0.7 | 17.5 ± 0.9 | 17.7 ± 0.9 | 0.25 |
| BMI (kg/ m2) * | 20.8 ± 2.2 | 22.1 ± 2.2 | 21.5 ± 2.4 | 0.16 |
| Height (cm) | 166.3 ± 5.6 | 164.8 ± 6.7 | 163.2 ± 6.2 | 0.34 |
| Menarchal Age (years) | 14.2 ± 2.4 | 12.9 ± 1.2 | 12.1 ± 1.7 | <0.01 |
| LS Spine BMD (g/cm2)* | 0.89 ± 0.13a,c | 1.01 ± 0.08 | 1.02 ± 0.13 | 0.004 |
| LS Spine BMD Z-score* | −1.22 ± 1.25a,c | 0.02 ± 0.86 | −0.11 ± 1.02 | 0.004 |
| LS Spine BMAD(g/cm3)* | 0.13 ± 0.02a,c | 0.15 ± 0.01 | 0.16 ± 0.02 | 0.002 |
| Total Hip BMD (g/cm2) | 0.94 ± 0.10a | 1.05 ± 0.11d | 0.96 ± 0.08 | 0.003 |
| Total Hip BMD Z-score | −0.18 ± 0.91a | 0.85 ± 0.90g | −0.33 ± 0.59 | 0.0002 |
| Femoral Neck BMD (g/cm2) | 0.82 ± 0.09 | 0.90 ± 0.11 | 0.84 ± 0.10 | 0.06 |
| Femoral Neck BMD Z-score | −0.49 ± 0.83b | 0.26 ± 0.95d | −0.61 ± 0.71 | <0.01 |
| RADIUS | ||||
| Stiffness (kN/m) | 70.5 ± 14.8e | 79.5 ± 12.1 | 83.0 ± 15.6 | 0.04 |
| Failure Load (kN) | 3.6 ± 0.8f | 4.0 ± 0.6 | 4.2 ± 0.7 | 0.048 |
| TIBIA | ||||
| Stiffness (kN/m) | 223.2 ± 25.8 | 242.9 ± 30.8c | 211.5 ± 30.1 | 0.01 |
| Failure Load (kN) | 11.2 ± 1.3 | 12.1 ± 1.5c | 10.6 ± 1.4 | 0.01 |
| P1NP (μg/L)* | 88.9 ± 40.1 | 99.4 ± 59.8 | 81.1 ± 37.2 | 0.79 |
| CTX (ng/mL) | 1.10 ± 0.34 | 1.19 ± 0.39e | 0.87 ± 0.26 | <0.03 |
| Estradiol (pg/mL)* | 27.6 ± 25.3 | 20.5 ± 11.6 | 43 ± 50.1 | 0.54 |
Wilcoxon test used to analyze differences between groups and if p-value was significant, Bonferroni adjustment used to account for multiple comparisons
p≤0.01 as compared to EA
p=0.03 as compared to EA
p<0.01 as compared to NA
p<0.02 as compared to NA
p<0.04 as compared to NA
p<0.05 as compared to NA
p<0.001 as compared to NA.
Stiffness and failure load in the radius were significantly lower in AA as compared to NA (Table 1). In the tibia, stiffness and failure load were significantly higher in EA as compared to NA (Table 1).
Sclerostin and Pref-1 Levels
Sclerostin was higher in AA and EA as compared to NA (AA: 0.42 ± 0.15 ng/mL; EA: 0.44 ± 0.09 ng/mL; NA: 0.33 ± 0.14 ng/mL; p=0.047) (Figure 1). This relationship remained significant after controlling for total body bone mineral content (p < 0.04), or for menarchal age (p=0.03). Pref-1 levels were not significantly different in the three groups (AA: 0.35 ± 0.16 ng/mL; EA: 0.38 ± 0.25 ng/mL; NA: 0.38 ± 0.28 ng/mL; p=0.71).
Figure 1.
Sclerostin levels were higher in athletes as compared to non-athletes (p=0.047). AA: amenorrheic athletes; EA: eumenorrheic athletes; NA: non-athletes.
Associations of Sclerostin and Pref-1 with Bone Mineral Density, Bone Turnover Markers and Estimated Bone Strength in Non-Athletes and Athletes
a. Non-athletes
BMD
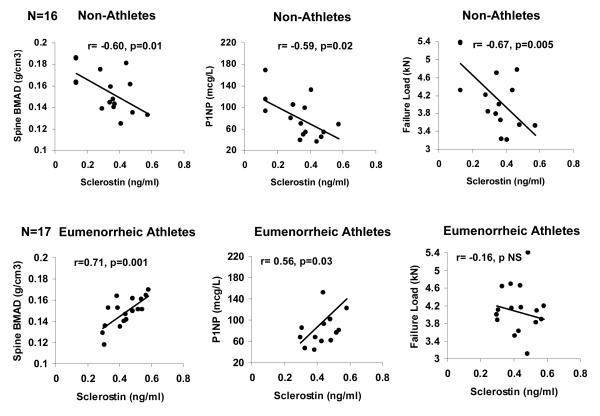
In NA, there were inverse associations between sclerostin and LS BMD (R=−0.61; p= 0.01), LS BMAD (R=−0.60; p=0.01) (Figure 2), and total hip BMD (R=−0.55; p<0.03). These relationships remained significant after controlling for estradiol levels or for menarchal age. There was also a trend towards an inverse association between sclerostin and FN BMD (R= −0.49; p=0.05) and LS BMD Z-scores (R=−0.48; p=0.06).
Figure 2.
In non-athletes, sclerostin was inversely associated with lumbar spine bone mineral apparent density (R=−0.60; p=0.01), P1NP (R=−0.59; p=0.02) and failure load at the radius (R=−0.67; p=0.005) (upper panel). In eumenorrheic athletes, sclerostin was positively associated with lumbar spine bone mineral apparent density (R=0.71; p=0.001) and P1NP (R=0.56, p=0.03) (lower panel).
There were significant inverse associations of Pref-1 with LS BMD (Spearman’s rho=−0.85; p<0.0001) and its Z-score (Spearman’s rho=−0.68; p< 0.01), LS BMAD (Spearman’s rho=−0.86; p<0.0001), hip BMD (Spearman’s rho=−0.82; p=0.0001) and its Z-score (Spearman’s rho=−0.86; p< 0.0001), and FN BMD (Spearman’s rho =−0.69; p<0.01) and its Z-score (Spearman’s rho =−0.73; p=0.002). These relationships remained significant after controlling for estradiol or for menarchal age.
Bone Turnover Markers
In NA, there were significant inverse associations between sclerostin and P1NP (R=−0.59; p<0.02) and CTX (R=−0.57; p=0.02), which remained significant after controlling for estradiol (p=0.02 and p=0.03, respectively) or for menarchal age (p=0.002 and p=0.02, respectively). There were no significant associations of Pref-1 with P1NP or CTX.
Estimated Bone Strength
In NA, sclerostin was inversely associated with stiffness and failure load at the radius (stiffness: R=−0.65; p=0.006; failure load: R=−0.67; p=0.005), even after controlling for estradiol or for menarchal age. There were trends towards an inverse association of sclerostin with estimated stiffness and failure load at the tibia (stiffness: R=−0.44; p<0.09; failure load: R=−0.47; p<0.07).
Pref-1 was also inversely associated with stiffness and failure load at the radius (stiffness: Spearman’s rho= −0.82; p<0.0001; failure load: Spearman’s rho= −0.80; p=0.0002) and tibia (stiffness: Spearman’s rho= −0.73; p=0.001; failure load: Spearman’s rho= −0.73; p=0.001). All relationships remained significant after controlling for estradiol or for menarchal age.
b. Eumenorrheic Athletes
BMD
In EA, there were significant positive correlations between sclerostin and LS BMD (R=0.52; p=0.03) and its Z-score (R=0.55; p=0.02), and LS BMAD (R=0.71; p=0.001) (Figure 2b). These relationships remained significant after controlling for estradiol, and associations with LS BMAD remained significant after controlling for menarchal age. We did not find an association between Pref-1 and BMD in EA.
Bone Turnover Markers
In EA, there was a positive association between sclerostin and P1NP (R=0.56; p=0.03). There were no significant associations between Pref-1 and P1NP or CTX in EA.
Estimated Bone Strength
In EA, there was a trend towards a positive association between Pref-1 and failure load in the radius (Spearman’s rho= 0.44; p<0.08). There were no significant associations between sclerostin and estimates of bone strength in EA.
c. Amenorrheic Athletes
BMD
We found no associations between sclerostin or Pref-1 and BMD in AA. Bone Turnover Markers: In AA, Pref-1 was positively associated with P1NP (R=0.68; p=0.004) and this relationship remained significant after controlling for estradiol. There was also a trend towards a positive association between Pref-1 and CTX (R=0.47; p=0.06). There were no associations between sclerostin and bone turnover markers in AA.
Estimated Bone Strength
We found no associations between sclerostin or Pref-1 and estimated bone strength in AA.
Discussion
We have shown that whereas sclerostin and Pref-1 have the predicted inverse association with bone parameters in non-athletes, sclerostin is positively associated with BMD in eumenorrheic adolescent athletes and Pref-1 is positively associated with bone turnover markers in amenorrheic adolescent athletes. Therefore sclerostin and Pref-1 appear to have differential effects on bone parameters in adolescent athletes as compared to non-athletes.
Sclerostin is a potent inhibitor of WNT signaling and therefore of bone formation. Both estrogen [11, 12] and physical activity [16, 17] are important potential regulators of sclerostin levels. This is the first study that uses the model of amenorrheic and eumenorrheic adolescent athletes to potentially delineate the important effects of estrogen status and physical activity on sclerostin levels. When estrogen status was held constant (by comparing menstruating athletes to menstruating non-athletes), we observed that those with higher levels of physical activity had higher sclerostin levels, in contrast to animal models showing decreased SOST expression with increased mechanical loading [14] and cross-sectional studies in adults in which increased physical was associated with lower sclerostin levels [17]. Of interest, one prior study investigating sclerostin levels in elite male and female athletes found higher sclerostin levels in males performing weight-bearing activities as compared to those performing non-weight-bearing activities [31]. All athletes in our study were weight-bearing athletes; therefore, it is possible that different types of physical activity result in differences in sclerostin secretion.
It is not clear why sclerostin levels are higher in weight-bearing adolescent athletes compared to non-athletes. One possibility is that sclerostin levels are higher in individuals with greater absolute bone mineral content, as sclerostin is secreted by osteocytes. In our study, EA had significantly higher total bone mineral content as compared to AA but similar levels compared to NA. However, even after controlling for total bone mineral content, athletes had higher sclerostin compared with NA. Of interest, in obese adults, weight loss mediated by dietary changes leads to increased sclerostin levels [16], suggesting that nutritional deficiency is associated with increased sclerostin. Therefore, it is possible that higher sclerostin in adolescent athletes is consequent to a state of relatively lower energy availability from excessive exercise compared to NA. It is also possible that effects of nutritional deprivation exceed effects of amenorrhea on sclerostin, and therefore levels do not differ between AA and EA (although we expect energy availability to be even lower in AA compared with EA). Finally, we speculate that higher sclerostin in EA (who have higher BMD at cortical weight-bearing sites than healthy NA) may act as a physiological ‘brake’ to prevent continued increases in bone formation and bone mass from repetitive mechanical loading.
Pref-1, a trans-membrane protein highly expressed in both pre-adipocytes and osteoblastic cell lines [32], is a member of the EGF-like family of proteins. Pref-1 is an important negative regular of both adipocyte and osteoblast differentiation. In murine models, over-expression of osteoblast-specific Pref-1 results in mice with significantly reduced BMD [33]. We have previously shown Pref-1 to be inversely associated with PA and lateral spine BMD in women with anorexia nervosa and healthy controls [20]. In this study, we also demonstrated significant inverse associations between Pref-1 and BMD in NA. However, in AA, we demonstrated significant positive associations between Pref-1 and P1NP, a marker of bone formation. We have previously shown that bone marrow fat, which is inversely associated with BMD in multiple populations including healthy Caucasian women [34], women with anorexia nervosa [35] and obese women [36], is positively associated with Pref-1 in women with anorexia nervosa, but inversely associated with Pref-1 in normal-weight controls [37]. Therefore, Pref-1 may act differentially in states of nutritional sufficiency compared with states of nutritional deficiency [37]. It is possible that Pref-1 also has differential effects on bone formation in states of intense physical activity compared to a sedentary state.
Although menarchal age was significantly greater in AA as compared to NA, there was no significant difference between menarchal age in EA as compared to NA and therefore this does not explain the contrasting relationships found in the EA and NA groups with respect to sclerostin and BMD and Pref-1 and bone strength. Additionally, even after controlling for menarchal age, reported associations of sclerostin and Pref-1 with bone density, bone turnover markers and estimated bone strength persisted.
Therefore, we have shown that both sclerostin and Pref-1 may have contrasting effects on BMD, bone microarchitecture and bone strength in athletes as compared to non-athletes. Further studies are needed to further delineate the role of sclerostin and Pref-1 in mediating BMD, bone microarchitecture and bone strength, and the association of sclerostin and Pref-1 with exercise activity, and nutritional and estrogen status.
Acknowledgements
We thank the research nurses and bionutritionists at the Clinical Research Center at Massachusetts General Hospital and our subjects, without whom this study would not have been possible. The project described was supported by NIH Grant Numbers 1 R01 HD060827-01A1, K23 DK094820-01 (Fazeli) and UL1 RR025758-03, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Non-Standard Abbreviations
- AA
Amenorrheic athletes
- EA
Eumenorrheic athletes
- NA
Non-athletes
- LS
Lumbar spine
Footnotes
The authors have no conflicts of interest to disclose
References
- 1.Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC., Jr. Role of physical activity in the development of skeletal mass in children. J Bone Miner Res. 1991;6:1227–1233. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 2.Rencken ML, Chesnut CH, 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276:238–240. [PubMed] [Google Scholar]
- 3.Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, Bouxsein ML, Misra M. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab. 2011;96:3123–3133. doi: 10.1210/jc.2011-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 5.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staehling-Hampton K, Proll S, Paeper BW, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 7.van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res. 2011;26:2804–2811. doi: 10.1002/jbmr.474. [DOI] [PubMed] [Google Scholar]
- 8.Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, Melton LJ, 3rd, Khosla S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26:373–379. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardawi MS, Al-Kadi HA, Rouzi AA, Qari MH. Determinants of serum sclerostin in healthy pre- and postmenopausal women. J Bone Miner Res. 2011;26:2812–2822. doi: 10.1002/jbmr.479. [DOI] [PubMed] [Google Scholar]
- 10.Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab. 2010;95:1991–1997. doi: 10.1210/jc.2009-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S. Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res. 2011;26:27–34. doi: 10.1002/jbmr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modder UI, Roforth MM, Hoey K, McCready LK, Peterson JM, Monroe DG, Oursler MJ, Khosla S. Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone. 2011;49:202–207. doi: 10.1016/j.bone.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirmani S, Amin S, McCready LK, Atkinson EJ, Melton LJ, 3rd, Muller R, Khosla S. Sclerostin levels during growth in children. Osteoporos Int. 2012;23:1123–1130. doi: 10.1007/s00198-011-1669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 15.Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–1234. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armamento-Villareal R, Sadler C, Napoli N, Shah K, Chode S, Sinacore DR, Qualls C, Villareal DT. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amrein K, Amrein S, Drexler C, Dimai HP, Dobnig H, Pfeifer K, Tomaschitz A, Pieber TR, Fahrleitner-Pammer A. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab. 2012;97:148–154. doi: 10.1210/jc.2011-2152. [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom I, Parini P, Gustafsson SA, Andersson G, Brinck J. Physical training increases osteoprotegerin in postmenopausal women. J Bone Miner Metab. 2012;30:202–207. doi: 10.1007/s00774-011-0304-6. [DOI] [PubMed] [Google Scholar]
- 19.Aronis KN, Kilim H, Chamberland JP, Breggia A, Rosen C, Mantzoros CS. Preadipocyte factor-1 levels are higher in women with hypothalamic amenorrhea and are associated with bone mineral content and bone mineral density through a mechanism independent of leptin. J Clin Endocrinol Metab. 2011;96:E1634–1639. doi: 10.1210/jc.2011-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95:407–413. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J Bone Miner Res. 1995;10:26–35. doi: 10.1002/jbmr.5650100107. [DOI] [PubMed] [Google Scholar]
- 22.Fehling PC, Alekel L, Clasey J, Rector A, Stillman RJ. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17:205–210. doi: 10.1016/8756-3282(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 23.Mudd LM, Fornetti W, Pivarnik JM. Bone mineral density in collegiate female athletes: comparisons among sports. J Athl Train. 2007;42:403–408. [PMC free article] [PubMed] [Google Scholar]
- 24.Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, Klibanski A, Bouxsein M, Misra M. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone. 2012 doi: 10.1016/j.bone.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greulich WPS. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford University Press; Stanford: 1959. 2nd ed. Stanford University Press, Stanford. [Google Scholar]
- 26.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 27.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25:983–993. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008;42:1203–1213. doi: 10.1016/j.bone.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Pistoia W, van Rietbergen B, Lochmuller EM, Lill CA, Eckstein F, Ruegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30:842–848. doi: 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- 30.Mueller TL, Christen D, Sandercott S, Boyd SK, van Rietbergen B, Eckstein F, Lochmuller EM, Muller R, van Lenthe GH. Computational finite element bone mechanics accurately predicts mechanical competence in the human radius of an elderly population. Bone. 2011;48:1232–1238. doi: 10.1016/j.bone.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Lombardi G, Lanteri P, Colombini A, Mariotti M, Banfi G. Sclerostin concentrations in athletes: role of load and gender. J Biol Regul Homeost Agents. 2012;26:157–163. [PubMed] [Google Scholar]
- 32.Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–852. doi: 10.1359/JBMR.040118. [DOI] [PubMed] [Google Scholar]
- 33.Abdallah BM, Ditzel N, Mahmood A, Isa A, Traustadottir GA, Schilling AF, Ruiz-Hidalgo MJ, Laborda J, Amling M, Kassem M. DLK1 is a novel regulator of bone mass that mediates estrogen deficiency-induced bone loss in mice. J Bone Miner Res. 2011;26:1457–1471. doi: 10.1002/jbmr.346. [DOI] [PubMed] [Google Scholar]
- 34.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–647. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]