Abstract
Objective
Chronic Otitis Media (COM) develops after sustained inflammation and is characterized by secretory middle ear epithelial metaplasia and effusion, most frequently mucoid. Non-typeable Haemophilus influenzae (NTHi), the most common acute OM pathogen, is known to activate inflammation and mucin expression in vitro and in animal models of OM. The goals of this study were to examine histopathological and expression profiling epithelial effects of NTHi challenge in murine middle ears.
Study Design
In vitro and in vivo murine model of OM.
Methods
Weekly transtympanic inoculation of Balb/c mice with 300 μg/ml of NTHi lysates vs saline was performed. Histopathologic analysis was carried out at 4 weeks. Expression microarray analysis was performed at 1 and 7 days. Microarray findings were validated in independent animal samples and in a cultured murine middle ear epithelial cell (mMEEC) line.
Results
Histopathologic analyses revealed middle ear mucosal thickening after NTHi exposure. Microarray analyses of inflammatory response genes that changed significantly demonstrated that the chemokine Cxcl2 had the largest fold-change with significantly increased expression at 1 and 7 days after NTHi injection compared to either saline or no-injection (p<0.01). Validation by realtime qPCR revealed similar significantly increased relative mRNA levels for Cxcl2. NTHi lysates were also found to significantly up-regulate the transcription of Cxcl2 in mMEEC in a time and dose dependent manner (p<0.05).
Conclusions
Middle ear NTHi challenge in mice leads to chronic epithelial mucosal metaplasia and over-expression of inflammatory mediators, most notably Cxcl2. This finding is parallel to NTHi mediated pulmonary mucosal metaplasia where Cxcl2 has been identified as an important inflammatory mediator.
Keywords: NTHi, Otitis Media, Cxcl2, metaplasia
Introduction
Otitis Media (OM) is one of the most common conditions of early childhood accounting for a very high proportion of all pediatric physician office visits annually1 at a national health care cost estimated to be greater than $1 billion2. In the first years of life, a majority of children at some point will experience OM3,4. Tympanostomy tube placement to treat OM is the most common pediatric surgical procedure requiring anesthesia in the United States5. Acute Otitis Media (AOM) is defined as acute onset of middle ear effusion along with signs and symptoms of middle ear inflammation and infection. Chronic Otitis Media (COM) subsequently results as a long term sequelae of the acute middle ear infection, and is characterized by secretory epithelial metaplasia and persistence of middle ear effusion, most frequently mucoid6–8.
Since the advent of universal pneumococcal conjugate vaccination against S. pneumonia in 2000, Non-typeable Haemophilus influenzae (NTHi) has surpassed S. pneumoniae to become the most common infectious pathogen in AOM9,10. Like most bacterial infections, infectious OM is characterized by significant inflammation highlighted through the activation of a plurality of cytokines and chemokines. This inflammation is thought to induce middle ear mucosal metaplasia conducive to chronic mucin secretion11. In order to elucidate how NTHi mediates middle ear mucosal metaplasia we undertook an in vitro and in vivo approach to comprehensively analyze the effects of NTHi on middle ear epithelium. We hypothesized that conditions characteristic of AOM, comprised by an inflammatory event (NTHi), activates expression of specific inflammatory cytokines and downstream mucosal metaplasia, resulting in progression to chronic otitis media. The specific goals of this study were to: a) to examine a murine model of COM induction from recurrent inflammatory challenge with NTHi lysates as seen in recurrent AOM, and b) to determine the expression profile of middle ear epithelium over time after middle ear NTHi bacterial lysate inoculation.
Materials and Methods
Preparation of NTHi lysates
NTHi clinical strain 12 was generously provided by Dr. Xin-Xing Gu (NIDCD, Bethesda, MD). Bacteria were grown on chocolate agar at 37°C in 5% CO2 overnight and inoculated in brain heart infusion (BHI) broth supplemented with 3.5 mg of nicotinamide adenine dinucleotide per ml. After overnight incubation, bacteria were subcultured into 5 ml of fresh brain heart infusion (BHI) and upon reaching log phase growth NTHi were washed and suspended in phosphate-buffered saline (PBS) followed by sonication for lysis. After lysis, stock solutions of NTHi were in the 4mg/ml range.
Histopathological evaluation of mice middle ear upon NTHi inoculation
Three transtympanic inoculation of 6 Balb/c mice middle ears (3 animals, 6 ears) with 50 uL of 300 ug/ml of NTHi bacterial lysate and 6 Balb/c mice middle ears (3 animals, 6 ears) with 50 uL of 1X phosphate buffered saline (PBS) were carried out weekly over 4 weeks (injection on days 7, 14, and 21). On day 28, the mice were euthanized and their bullae harvested. All studies were reviewed and approved by the Institutional Animal Care and Use Committee at Children’s National Medical Center. Specimens were fixed in 10% formalin and then decalcified in Cal-Ex I for 48 hours, before being place in 70% ethanol overnight and then embedded in paraffin. For histology, sections were deparaffinized in xylene, dehydrated in graded alcohol and then water, and stained with (hematoxylin and eosin) H&E for histology, and Periodic Acid Schiff (PAS) staining for mucin glycoproteins. The thicknesses was measured over the promontory directly opposite the tympanic membrane in the mesotympanum using light microscopy, with the aid of a square grid mounted on the eyepiece as described by Tsuboi et al12. The number of PAS positive cells per high powered field, 20X (HPF) were counted and compared between saline and NTHi injected bullae.
Expression array profiling upon acute middle ear NTHI inoculation
In separate experiments with independent mice, total RNA was extracted at 0, 1, and 7 days from the middle ear after 50 μL 1X PBS or 300 μg/ml NTHi lysate transtympanic inoculation on day 0 only. Total RNA was extracted from the middle ear with three separate 50uL transtympanic bullar instillations (2 minutes each) with TRIzol reagent (Life Technologies Corp, Grand Island, NY) per ear. TRIzol from both ears was pooled together for each animal. Three animals were used for each time point (biologic triplicates). Microarray analyses were performed in the Microarray Core at Children’s National according to established protocols13. Briefly, extracted RNA was purified, quanitified, and purity/integrity control was performed. 100 ng of total RNA was used to initiate the complementary DNA–complementary RNA (cDNA-cRNA) cycle. Biotin-labeled cRNA from each sample was fragmented and hybridized to Affymetrix Mouse 430_2 Arrays (Affymetrix, Santa Clara, CA) for 16 h, followed by standard washing-scanning protocols on the Affymetrix Fluidics Station 400 (Affymetrix Inc) and incubation with phycoerythrin-streptavidin to detect bound cRNA. The signal intensity was amplified by means of biotin-labeled antistreptavidin antibody. Fluorescent images were captured by means of a gene array scanner (Hewlett-Packard G2500A; Palo Alto, CA). All data were analyzed by means of Affymetrix Microarray Analysis Software version 5.0 Plier algorithms. Only probe sets that were statistically significant with 1-way analysis of variance (ANOVA) and a p< 0.01 were used.
Real-time RT-PCR
For in vivo validation of expression array profiling, total RNA was extracted from 2 separate mice (2 mice, 4 ears) as described above prior to quantitative real-time RT-PCR (qRT-PCR). For in vitro validation, time course experiments to determine the effects of NTHi lysate exposure on Cxcl2 gene expression in an immortalized mouse middle epithelial cell line (mMEEC) were performed. This cell line was immortalized with a temperature sensitive SV40 virus allowing for cell differentiation at 37°C, and was provided to us by Dr. Jizhen Lin (University of Minnesota, Minneapolis, MN)14. mMEEC were maintained and passaged in full growth media (FGM) as previously described 15. Prior to experimentation, cells were transferred to a 37°C, 5% CO2 humidified atmosphere to inactivate the SV-40 virus. RNA was extracted from mMEEC cells exposed to NTHi lysates (300 μg/ml—1000fold NTHi lysate stock dilution) or vehicle (cell culture media) for 1 and 7 days using TRIzol reagent (Life Technologies Corp, Grand Island, NY). Reverse transcription reaction was performed using 1 μg of total RNA from each sample and the SuperScript III reverse transcriptase enzyme (Invitrogen, Carlsbad, CA), as previously described15. Generated cDNA was used for PCR using specific pairs of primers as follows: Cxcl2 forward primer, 5′-CCCAGACAGAAGTCATAGCCAC-3′; Cxcl2 reverse primer, 5′-GCCTTGCCTTTGTTCAGTATC-3′; Muc5b forward primer, 5 ′-ACTTGAGGAGGGTTCCAGGT-3′; Muc5b reverse primer, 5 ′-ACAGTGCCAGGGTTTATGC-3′. β-actin was used as an internal control; primers for mouse β-actin were obtained from GeneLink (Hawthorne, NY). Real-time RT-PCR was performed on the generated cDNA products in the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA) as described previously15. Relative quantification of Cxcl2 mRNA in control and experimental samples was obtained using the ΔΔCt or the standard method.
Results
Murine OM model shows chronic mucosal metaplasia
H&E and PAS staining were performed for evaluation of middle ear mucosal thickness and mucous cell metaplasia/hyperplasia after 4 weeks of weekly NTHI vs control transtympanic injections. Representative histological slides of saline-exposed mouse middle ear mucosa are shown in Figure 1A. NTHi lysate exposed mice demonstrated moderate but consistent thickening of the promontory mucosa (Figure 1A, right panel) when compared to mice exposed to PBS (Figure 1A, left panel). Quantification of the thickness by measuring matched locations in 10 sections over the promontory of the mesotympanum demonstrated a moderate, but statistically significant 2-fold increase of the epithelial thickness in NTHI stimulated ears compared to control (Figure 1B). A significant inflammatory cell infiltrate was not seen in NTHi or saline exposed ears. PAS staining (Figure 2) similarly showed a modest increase in mucin positive cells in NTHI exposed ears compared to control (2.3 cells per HPF vs. 0.2 cells per HPF, p=0.043).
Figure 1.
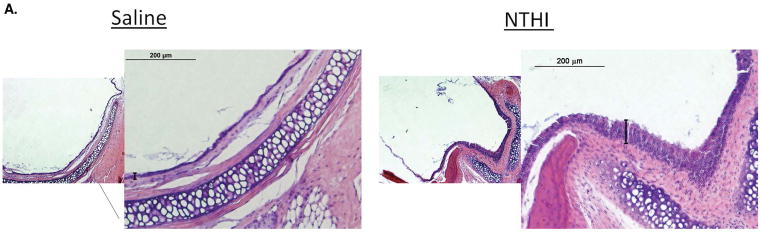
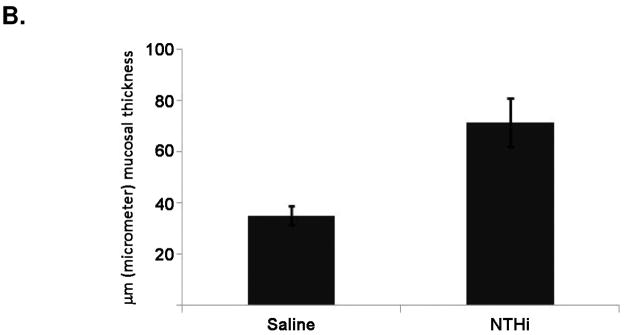
Mouse middle ear mucosa changes following NTHi lysate exposure. A. Representative H+E images of mouse middle ear mucosa after weekly exposure to 300 ug/ml NTHi lysates vs 1X PBS at 4 weeks. The left panels depict 4x magnification, and the right panels 20x magnification of the selected box area shown. The measured epithelial layer is shown by the brackets. B. Quantification of the mucosal thickness as described in the methods (p<0.05).
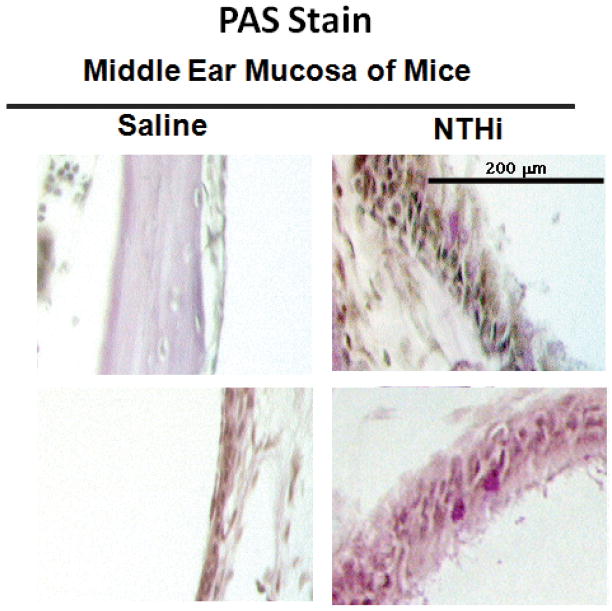
Figure 2.
PAS staining of mouse middle ear mucosa following NTHi exposure. Representative images of mouse middle ear mucosa after weekly exposure to 300 ug/ml NTHi lysates vs 1X PBS at 4 weeks. Sporadic and modest, but statistically significant increase in PAS positive cells (black arrows) was noted with NTHi.
Identification of differentially expressed gene products in mouse middle ear epithelium exposed to NTHi lysates
To identify the differential expression of genes in the middle ear mucosa of mice following NTHi lysate exposure, a genome-wide microarray analysis of mouse middle ear was performed after 0, 1, and 7 days of 50uL 1X PBS or 300ug/ml NTHi lysate trans-tympanic inoculation using Affymetrix Mouse 430_2 Arrays (Affymetrix, Santa Clara, CA). Data were analyzed separately by the Plier algorithm, and then loaded into GeneSpring G where the data were normalized and filtered. Heat map analysis of the treatment groups (Figure 3) showed that treatment with NTHi at 1 and 7 days, as well as saline treatment at 1 day, in general grouped together. We interpret this grouping as indicating that the trauma of surgery resulted in a characteristic expression profile at one day that is sustained in the presence of NTHi lysates for 7 days, but changes within 7 days with only saline exposure. ANOVA analyses of the data demonstrated that 3905 genes changed significantly with NTHi treatment at 1 and 7 days (p < 0.01). Of these, 2480 genes were up-regulated and 1425 genes were down-regulated. The up-regulated genes were analyzed with the DAVID Bioinformatics Resources 6.7 16,17. Functional annotation of this gene list demonstrated the top functional categories to include phosphoproteins, membrane associated, glycoproteins, signal peptides, acetylation, non-membrane-bounded organelles, defense response, and immune response genes. The full data set has been deposited in the NCBI GEO database (#GSE40087). Given our hypothesis that NTHi induces an inflammatory response that is critical to the progression to COM, we focused our initial analyses and validation experiments on the up-regulated inflammatory/immune response genes.
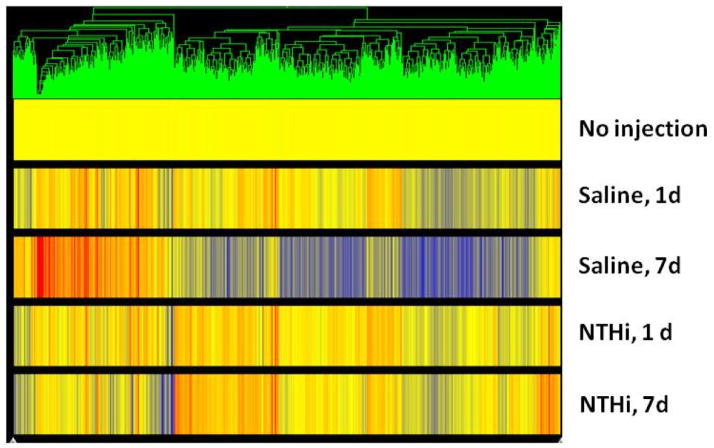
Figure 3.
Gene tree analyses (Plier) of middle ear mucosal RNA. Differential expression levels of each probe set (triplicate mice) are shown vertically for each cohort at day 1 and day 7 following surgery and exposure to saline or NTHi. Red represents increased and blue decreased mRNA expression compared with no injection control.
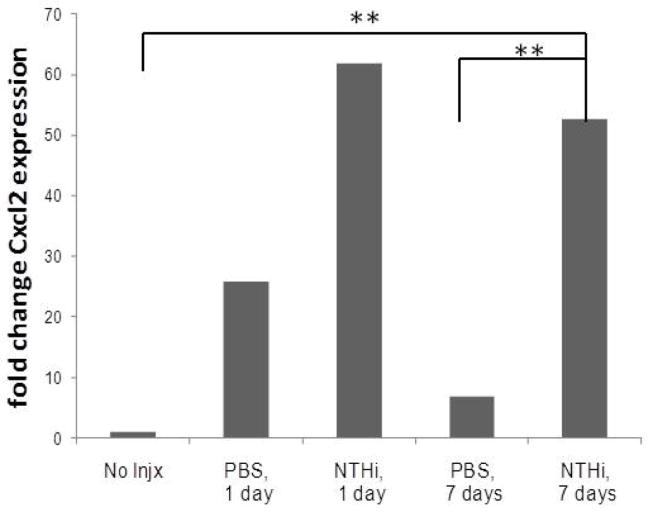
The top five genes related to inflammatory/immune response by Plier Gene Ontology that were most significantly up-regulated (p<0.01) at 1 and 7 days with with NTHi lysate exposure are depicted in Table 1. Of these genes, chemokine C-X-C motif ligand 2 (Cxcl 2) (GenBank NM_0091040) demonstrated a marked increase -- 61.9 fold at 1 day and 52.7 at 7 days with NTHi compared to no injection (Figure 4).
Table 1.
| ID No. | Gene | PBS, 1d | NTHi, 1d | PBS, 7d | NTHi, 7d | p-value | Gene Name |
|---|---|---|---|---|---|---|---|
| 1449984_at | Cxcl2 | 25.888 | 61.880 | 6.951 | 52.706 | 0.00205 | C-X-C motif ligand 2 |
|
| |||||||
| 1451713_a_at | Fcer2a | 6.699 | 5.582 | 1.676 | 8.397 | 0.00614 | Fc receptor, IgE |
| 1418747_at | Sfpi1 | 1.676 | 2.682 | 1.668 | 6.015 | 0.0095 | SFFV proviral int 1 |
| 1442233_at | Fyb | 1.841 | 4.243 | 1.978 | 5.505 | 0.0105 | FYN binding protein |
| 1422579_at | Hspe1 | 1.787 | 2.593 | 1.118 | 4.249 | 0.0102 | heat shock protein 1 |
Figure 4.
Fold change in Cxcl2 transcript expression from microarray data. Injection with either PBS or NTHi (300 ug/ml) at 1 and 7 demonstrated marked fold change increases days compared to no injection. At 7 days, this difference was significant for injection with NTHi compared to PBS (**p<0.001).
Validation of Cxcl2 mRNA expression levels by qRT-PCR
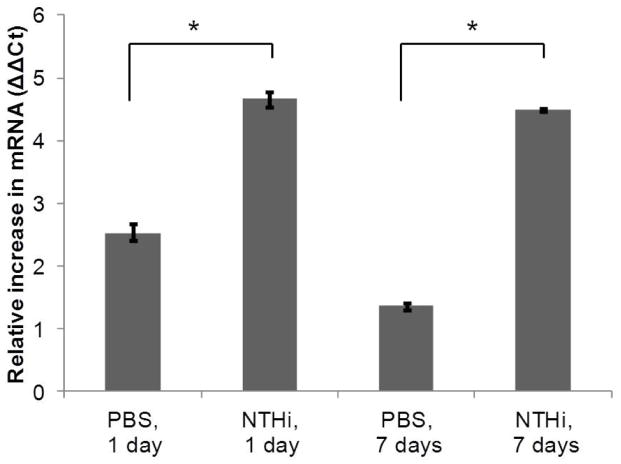
The expression level of Cxcl2 was evaluated by qRT-PCR in an independent set of mice. Middle ear mucosal RNA was isolated from 4 pooled ears after 0, 1, and 7 days of 50uL 1X PBS or 300ug/ml NTHi lysate trans-tympanic inoculation. Data showed that mRNA levels for Cxcl2 were increased in the middle ear mucosa after NTHi exposure compared to saline or no-injection in a fashion consistent with the microarray analyses (Figure 5), although the magnitude of gene expression levels was not as high with the qRT-PCR method. As in the microarray analysis, Cxcl2 levels were increased at 1 day and sustained for 7 days.
Figure 5.
Validation by qRT-PCR analyses of Cxcl2 mRNA levels from mouse middle ear after injection with either PBS or NTHi at 1 and 7 days compared to no injection. Data show relative increase (with standard errors) in transcript levels using the ΔΔCt method. (*p<0.05)
Cxcl2 mRNA expression is increased in vitro in mouse MEEC exposed to NTHi
As an initial strategy to determine whether the observed in vivo effects of NTHi exposure parallel those seen in middle ear in vitro models, we performed experiments to determine the effects of NTHi lysate exposure on Cxcl2 gene expression in an immortalized mouse middle epithelial cell line (mMEEC). Results demonstrate that, parallel to the animal model, NTHi directly increased the abundance of Cxcl2 mRNA in mMEE cells at 1 and 7 days post innoculation (Figure 6).
Figure 6.
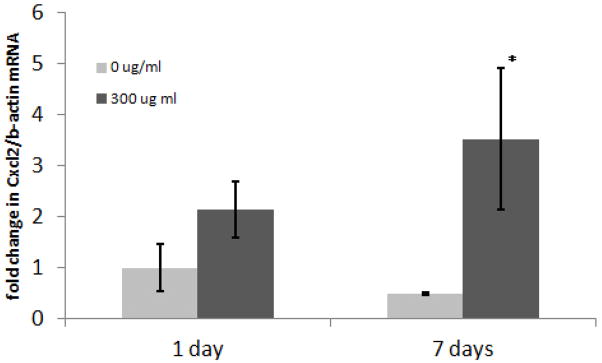
NTHi increases Cxcl2 expression in mMEEC after 1 and 7 days. Cells were stimulated with 0 or 300 μg/ml of NTHi lysates prior to RNA extraction at the indicated time points and cDNA generation. Real time RT-PCR was performed to determine the relative amounts of Cxcl2 mRNA utilizing the standard method with β-actin as an internal reference gene. Similar to in vivo results, Data show increases in Cxcl2 transcript levels at 1 and 7 days with NTHi lysate exposure in mMEEC. (*p<0.05 compared to 0 ug/ml NTHi at 1 and 7 days).
Discussion
Inflammation associated with acute bacterial infection is postulated to contribute to COM. Molecular mechanistic links of this are only recently beginning to be elucidated18,19. Work from other labs have shown that NTHi induction of the pro-inflammatory transcription factor NF-κB leads to potent induction of middle ear mucosal hyperplasia in vivo and upregulation of proinflammatory cytokines such as IL-1β and Cxcl220. In this study, in a murine model of NTHi inflammatory OM, somewhat akin to recurrent acute otitis media with repeated middle ear inoculation of NTHI lysates, we employed an unbiased gene expression microarray approach and identified Cxcl2 a markedly upregulated cytokine in the acute phase of infection, validating previous work by Lim et al20. This is also similar to findings in pulmonary disease murine models, where aerosolized NTHi challenge in control mice is marked by acute increases in pro-inflammatory cytokines, most notably Cxcl221. Of note, our microarray experiment did not identify IL-1β or TNF-α as changed with NTHi stimulation. Interestingly, our expression profiling array demonstrated a markedly different profile on day 7 after saline injection, compared to all the other conditions (Figure 3). Day 7 after saline injection demonstrates a predominant down-regulation of multiple gene products. We interpret this grouping as indicative that the injection resulted in a characteristic expression profile at one day in both the saline and NTHi groups, signifying perhaps that the surgery itself is inflammatory and produces a distinctive global gene response. After 7 days, this global profile is sustained in the presence of NTHi lysate, but changes dramatically with only saline exposure, where a lot of the upregulated inflammatory response has dissipated. Finally, a potential limitation to our mouse model is that we noted leakage of the 50 μL NTHi inoculate into the ear canal, as such some of our microarray findings may not have been specific to the middle ear mucosa.
CXCL2 is also called MIP2α and the murine homolog of IL-8. CXCL2 appears to be a critical mediator of bacterial induced inflammation in the airways, leading to mucin hypersecretion. In mouse inner ear, NTHi stimulation has recently also been demonstrated to significantly upregulate Cxcl2 expression22. In cystic fibrosis cells, Pseudomonal challenge potently causes epithelial release of CXCL223. The Cxcl2 receptor is critical for mucin overproduction in the lower airway epithelium of mice following viral challenge24. Transgenic mice that over-express IL1β increase production of Cxcl2 and these mice also exhibit mucoid metaplasia, potentially through Cxcl2 mediated release25. Immunomodulation of NFkB decreases Cxcl2 production and subsequent neutrophilic injury in Pseudomonal pneumonia in mice26.
It is worthy to note that in our study, however, that no mucin gene transcripts, including Muc5b, were found to significantly change in the middle ear of mice with NTHi or saline injection in the microarray data experiments. We have also not found NTHi lysates to drive up-regulation of Muc5b in vitro in independent RT-PCR experiments (data not shown). Given that COM is characterized by mucin overexpression and hypersecretion, predominantly of MUC5B mucin27 in COM fluid, this finding is difficult to reconcile. We speculate that it is not NTHi directly that leads to mucin hypersecretion in middle ear epithelium. Rather, it is likely that secondary, indirect effects of chronic bacterial stimulation such as cytokine hypersecretion, immune cell recruitment, and middle ear mucosal metaplasia, as seen in our study in vivo at 4 weeks after exposure (Figure 1 and 2), set up the conditions permissive to mucin MUC5B/Muc5b upregulation and hypersecretion as postulated by others28. Future and ongoing work in our lab aims to investigate intermediary molecular mechanisms by which NTHi affects MUC5B/Muc5b gene expression.
CONCLUSION
In conclusion, our study has demonstrated that weekly repeated NTHi lysate exposure induces middle ear mucosal thickening in mice and that NTHi lysates induce significant mouse middle ear over-expression of Cxcl2 in vivo and in vitro. Whether NTHi can directly or indirectly lead to over-expression of Muc5b mucin in the middle ear remains a subject of future investigations.
Footnotes
CONFLICT OF INTEREST: None to report.
FINANCIAL DISCLOSURE: This work was supported a K12 Genomics of Lung NHLBI/NIH HL090020-01 award.
References
- 1.Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2000 summary. Adv Data. 2002:1–32. [PubMed] [Google Scholar]
- 2.Rosenfeld RM, Casselbrant ML, Hannley MT. Implications of the AHRQ evidence report on acute otitis media. Otolaryngol Head Neck Surg. 2001;125:440–448. doi: 10.1067/mhn.2001.119326. discussion 439. [DOI] [PubMed] [Google Scholar]
- 3.Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 4.Otitis media with effusion. Pediatrics. 2004;113:1412–1429. doi: 10.1542/peds.113.5.1412. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone CD, Stool SE, Kenna MA. Pediatric otolaryngology. Philadelphia: Saunders; 1996. [Google Scholar]
- 6.Lim DJ, Birck H. Ultrastructural pathology of the middle ear mucosa in serous otitis media. Ann Otol Rhinol Laryngol. 1971;80:838–853. doi: 10.1177/000348947108000611. [DOI] [PubMed] [Google Scholar]
- 7.Tos M, Bak-Pedersen K. Goblet cell population in the pathological middle ear and eustachian tube of children and adults. Ann Otol Rhinol Laryngol. 1977;86:209–218. doi: 10.1177/000348947708600212. [DOI] [PubMed] [Google Scholar]
- 8.Ryan AF, Jung TT, Juhn SK, et al. Recent advances in otitis media. 4A. Molecular biology. Ann Otol Rhinol Laryngol Suppl. 2005;194:42–49. [PubMed] [Google Scholar]
- 9.Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004;23:824–828. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 10.Klein JO. What’s new in the diagnosis and management of otitis media? Pediatr Ann. 2002;31:777–778. doi: 10.3928/0090-4481-20021201-06. [DOI] [PubMed] [Google Scholar]
- 11.Juhn SK, Jung MK, Hoffman MD, et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008;1:117–138. doi: 10.3342/ceo.2008.1.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuboi Y, Kim Y, Giebink GS, et al. Induction of mucous cell metaplasia in the middle ear of rats using a three-step method: an improved model for otitis media with mucoid effusion. Acta Otolaryngol. 2002;122:153–160. doi: 10.1080/00016480252814153. [DOI] [PubMed] [Google Scholar]
- 13.Almon RR, DuBois DC, Yao Z, Hoffman EP, Ghimbovschi S, Jusko WJ. Microarray analysis of the temporal response of skeletal muscle to methylprednisolone: comparative analysis of two dosing regimens. Physiol Genomics. 2007;30:282–299. doi: 10.1152/physiolgenomics.00242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuchiya K, Kim Y, Ondrey FG, Lin J. Characterization of a temperature-sensitive mouse middle ear epithelial cell line. Acta Otolaryngol. 2005;125:823–829. doi: 10.1080/00016480510031533. [DOI] [PubMed] [Google Scholar]
- 15.Preciado D, Lin J, Wuertz B, Rose M. Cigarette smoke activates NF kappa B and induces Muc5b expression in mouse middle ear cells. Laryngoscope. 2008;118:464–471. doi: 10.1097/MLG.0b013e3185aedc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 17.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim DJ, Hermansson A, Hellstrom SO, et al. Recent advances in otitis media. 3. Animal models; anatomy and pathology; pathogenesis; cell biology and genetics. Ann Otol Rhinol Laryngol Suppl. 2005;194:31–41. [PubMed] [Google Scholar]
- 19.Mikami F, Lim JH, Ishinaga H, et al. The transforming growth factor-beta-Smad3/4 signaling pathway acts as a positive regulator for TLR2 induction by bacteria via a dual mechanism involving functional cooperation with NF-kappaB and MAPK phosphatase 1-dependent negative cross-talk with p38 MAPK. J Biol Chem. 2006;281:22397–22408. doi: 10.1074/jbc.M602124200. [DOI] [PubMed] [Google Scholar]
- 20.Lim JH, Jono H, Koga T, et al. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice. PLoS One. 2007;2:e1032. doi: 10.1371/journal.pone.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaschler GJ, Skrtic M, Zavitz CC, et al. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am J Respir Crit Care Med. 2009;179:666–675. doi: 10.1164/rccm.200808-1306OC. [DOI] [PubMed] [Google Scholar]
- 22.Oh S, Woo JI, Lim DJ, Moon SK. ERK2-dependent activation of c-Jun is required for nontypeable Haemophilus influenzae-induced CXCL2 upregulation in inner ear fibrocytes. J Immunol. 188:3496–3505. doi: 10.4049/jimmunol.1103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farberman MM, Ibricevic A, Joseph TD, et al. The Effect of Polarized Release of CXC-chemokines from Wild-type and Cystic Fibrosis Murine Airway Epithelial Cells. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2009-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller AL, Strieter RM, Gruber AD, Ho SB, Lukacs NW. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol. 2003;170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- 25.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 26.Sadikot RT, Zeng H, Joo M, et al. Targeted immunomodulation of the NF-kappaB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol. 2006;176:4923–4930. doi: 10.4049/jimmunol.176.8.4923. [DOI] [PubMed] [Google Scholar]
- 27.Preciado D, Goyal S, Rahimi M, et al. MUC5B Is the Predominant Mucin Glycoprotein in Chronic Otitis Media Fluid. Pediatr Res. 2010;68:231–236. doi: 10.1203/PDR.0b013e3181eb2ecc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacios SD, Pak K, Rivkin AZ, et al. Role of p38 mitogen-activated protein kinase in middle ear mucosa hyperplasia during bacterial otitis media. Infect Immun. 2004;72:4662–4667. doi: 10.1128/IAI.72.8.4662-4667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]