Abstract
Objective
We report rates and risk factors for attrition in the first cohort of patients followed through all stages from HIV testing to ART initiation.
Design
Cohort study of all patients diagnosed with HIV between January and June, 2009.
Methods
We calculated the proportion of patients who completed CD4 cell counts and initiated ART or remained in pre-ART care during two years of follow-up, and assessed predictors of attrition.
Results
Of 1,427 patients newly diagnosed with HIV, 680 (48%) either initiated ART or were retained in pre-ART care for the subsequent two years. One thousand eighty-three patients (76%) received a CD4 cell count and 973 (90%) returned for result; 297 (31%) had CD4 cell count < 200 cells/μl and of these, 256 (86%) initiated ART. Among 429 patients with CD4 > 350 cells/μl, 215 (50%) started ART or were retained in pre-ART care. Active TB was associated with lower odds of attrition prior to CD4 cell count (OR: 0.08; 95% CI: 0.03–0.25) but also higher odds of attrition prior to ART initiation (OR: 2.46; 95% CI: 1.29–4.71). Lower annual income (≤ $US125) was associated with higher odds of attrition prior to CD4 cell count (OR 1.65; 95% CI: 1.25–2.19), and prior to ART initiation among those with CD4 cell count > 350 cells/μl (OR: 1.74; 95% CI: 1.20–2.52). After tracking patients through a national database, the retention rate increased to only 57%.
Conclusion
Fewer than half of patients newly diagnosed with HIV initiate ART or remain in pre-ART care for two years in a clinic providing comprehensive services. Additional efforts to improve retention in pre-ART are critically needed.
Keywords: HIV, HIV/AIDS, Haiti, Resource-poor setting, Attrition, Loss to follow-up, Retention in care
Introduction
The majority of patients in resource-poor settings initiate antiretroviral therapy (ART) with advanced HIV/AIDS, which is associated with higher mortality and increased HIV transmission [1–14]. Earlier ART initiation will require earlier diagnosis, effective linkage of newly diagnosed patients with HIV treatment services, and ongoing monitoring to determine when the patient qualifies for ART. Yet, several studies from resource-poor settings have reported high rates of attrition along each stage of the pathway from HIV testing to ART initiation [4, 5, 15–39].
A recently published review, which included 28 studies from Africa, found that the median (range) of patients retained at each stage was low: 59% (35%-88%) of patients were retained in care from HIV testing to completion of CD4 cell count or clinical staging, 46% (31%-95%) from staging to ART eligibility, and 68% (14%-84%) from ART eligibility to ART initiation [40]. Most studies reported on retention in only one stage in the process of ART initiation, and it is therefore not possible to determine the proportion of patients who test positive for HIV who are staged, remain in pre-ART care until they are eligible, and then initiate ART. The authors estimated a median completion of the above three stages of 17%, with an 80% confidence interval of 7 to 32%.
The Haitian Study Group on Kaposi’s Sarcoma and Opportunistic Infections (GHESKIO) in Port-au-Prince, Haiti, follows patients from HIV testing through pre-ART care, to ART initiation and follow-up. Attrition is low among patients once they start ART; only 6% of patients who receive at least one month of ART at GHESKIO are lost to follow-up within the subsequent two years [41]. We conducted a study to determine the proportion of patients newly diagnosed with HIV at GHESKIO who complete CD4 cell count testing, and either start ART or remain in pre-ART care for the subsequent two years, and we assessed risk factors for attrition prior to ART. This is the first cohort of patients followed through all stages of pre-ART care.
METHODS
Setting and Patients
This study was conducted at the GHESKIO clinic in Port-au-Prince, Haiti. GHESKIO is the oldest and largest HIV testing facility in Haiti, providing voluntary counseling and testing (VCT) for HIV since 1985. GHESKIO tests nearly 30,000 patients per year for HIV. About 10 to 14% of HIV tests at GHESKIO are positive each year, representing about 10% of new cases of HIV diagnosed nationwide. There are 176 VCT centers and 86 ART clinics in Haiti; 73 VCT and 32 ART clinics are located in the West Department, which includes Port-au-Prince.
Comprehensive HIV/AIDS treatment is provided free of charge to all who present for care, with the majority living in Port-au-Prince. Eighty-five percent of GHESKIO patients are self-referred for HIV testing; 15% are referred from other physicians or by HIV-infected partners for testing. Due to GHESKIO’s long history of providing testing and treatment services, patients come directly to GHESKIO for HIV testing, rather than being referred from mobile or community-based VCT campaigns or outside VCT clinics. All persons > 13 years of age who tested positive for HIV for the first time at GHESKIO between January 1 and June 30, 2009 were included in the analysis. Data collection continued until June 29, 2011; two years of follow-up data were collected for each patient.
Clinical Care during the Pre-ART Period at GHESKIO
At GHESKIO, pregnant women and severely ill patients receive rapid HIV testing with same-day results; pregnant women also receive same-day syphilis testing, with same-day treatment. Other patients are given an appointment with a social worker to receive HIV test results within the subsequent week. At the time of HIV testing, all patients are queried about cough, and all those with cough of at least five days duration are screened for tuberculosis (TB) by medical history and physical examination, chest radiography, and sputum smear for acid fast bacilli (AFB) as described previously [42]. TB and HIV services are co-delivered, and TB treatment is generally started on the day of TB diagnosis.
CD4 cell counts are conducted on-site at GHESKIO and results are available two days later. Patients usually receive three pre-ART visits with the physician and social worker for medical evaluations and ART readiness counseling. However, those in urgent need of therapy start ART as early as the day of HIV testing. Patients not yet eligible for ART are provided with counseling and prophylaxis against opportunistic infections; they are scheduled to return to the clinic every month for the first three months, and then evaluated quarterly by a doctor or nurse. All care is provided according to WHO guidelines [43]. Transportation subsidies and reminder phone calls for those who miss visits are provided after patients start ART, but not in the pre-ART period.
Data Collection and Statistical Analysis
We collected demographic information (age, gender, education, income, and residence zone), dates and results of HIV tests and CD4 cell counts, dates of TB treatment and ART initiation, and clinic visit dates from the electronic medical record (EMR) for a period of two years from the date of HIV testing for each patient. Attrition prior to CD4 cell count was defined as no CD4 cell count within 12 months after HIV testing, as has been recommended in other publications [44]. Patients with an initial CD4 cell count < 200 cells/μl met the definition of pre-ART attrition if they had not started ART within the study period, and were not known to have transferred care to another site. Patients with an initial CD4 cell count > 350 cells/μl met the definition of pre-ART attrition if they had not initiated ART, had no pre-ART visits in the four months prior to the end of the two-year study period, and were not known to have transferred to another site. Attrition was defined as occurring during the first year if there was no visit in the final four months of the first year.
Data were entered into an Excel Database (Microsoft, Redmond, Washington), and then converted to SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). We conducted bivariate and multivariate analyses of attrition prior to CD4 cell count, attrition prior to ART initiation (for those with CD4 cell count < 200 cells/μl), and attrition prior to ART initiation or end of the two-year study period (for those with CD4 cell count > 350 cells/μl) using the following variables: gender and active TB as binary variables; and age, education, annual income, residence zone, and baseline CD4 cell count as categorical variables. We did not conduct these analyses for patients with CD4 cell counts ranging from 200 to 350 cells/μl, because during the time period of the study, the WHO guidelines changed from recommending ART for all patients with a CD4 cell count < 200 cells/μl to recommending ART for all patients with a CD4 cell count < 350 cells/μl[43]. For all multivariate models, we used a stepwise logistic regression method. Variables that were significant at the 0.10 level in bivariate analysis were included in the initial multivariate model, and a P value of 0.05 was required for retention in the final model. We used the Wald confidence interval (CI) for adjusted odds ratios (ORs) and reported 95% CIs. Institutional review board approval was obtained from all participating institutions.
The Haitian Ministry of Health (MSPP) and the National Alliance of State and Territorial AIDS Directors (NASTAD) manage the national HIV/AIDS Surveillance System (HASS), a national case-based HIV surveillance database. Patients can be tracked through their HIV infection (reported diagnosis through reported death) based on their demographic and clinical characteristics at the national level through this system, but patient-level data is not shared between clinic sites, as it is not part of the MSPP’s HASS data sharing and confidentiality agreement. In October, 2012, the GHESKIO EMR was reviewed for all patients that were lost to care during the study period, to determine if they later returned to care. With the approval of the Ministry of Health, NASTAD (authors CD, MG, and GM) searched the national database for all patients that did not return to care at GHESKIO, to see if patients that were lost to follow-up (LTFU) at GHESKIO later received clinical care at an outside clinic.
RESULTS
From January 1 to June 30, 2009, 14,104 persons > 13 years of age were tested for HIV at the GHESKIO VCT clinic, and 1550 patients (11%) tested positive. Of these, 1427 patients (92%) had their first positive test; 123 patients (8%) were excluded because they had had a prior positive HIV test at GHESKIO. All patients with a first positive HIV test at GHESKIO during the study period were included.
Table 1 describes the baseline characteristics of the study population. The median age was 34 years (interquartile range [IQR]: 27 to 42), and 62% were women. Fifty-six percent reported no school or primary school only, and 63% reported earning ≤ $US125 per year. One thousand two hundred ninety patients (91%) lived within the greater Port-au-Prince area. The median CD4 cell count was 320 cells/μl (IQR: 163 to 495) among those who completed testing. One hundred and sixteen patients (8%) were diagnosed with TB at the time of HIV testing.
Table 1.
Baseline Characteristics of Patients Newly Diagnosed with HIV
| Characteristic | Value |
|---|---|
| Female sex – no. (%) | 889 (62) |
| Age – no. (%) | |
| ≤ 24 years | 241 (17) |
| 25 to 34 years | 477 (33) |
| 35 to 44 years | 414 (29) |
| ≥ 45 years | 295 (21) |
| Resident of Port-au-Prince – no. (%) | 1,290 (91) |
| Annual Income – no. (%) | |
| None | 640 (45) |
| $US 1 to $US125 | 260 (18) |
| > $US125 | 525 (37) |
| Education – no. (%) | |
| None | 368 (26) |
| Primary school | 430 (30) |
| Secondary school or higher | 629 (44) |
| Tuberculosis at HIV testing – no. (%) | 116 (8) |
| CD4 cell count – no. (%) | |
| < 200 cells/μl | 333 (31) |
| 200 to 349 cells/μl | 271 (25) |
| 350 to 500 cells/μl | 212 (20) |
| ≥ 500 cells/μl | 267 (25) |
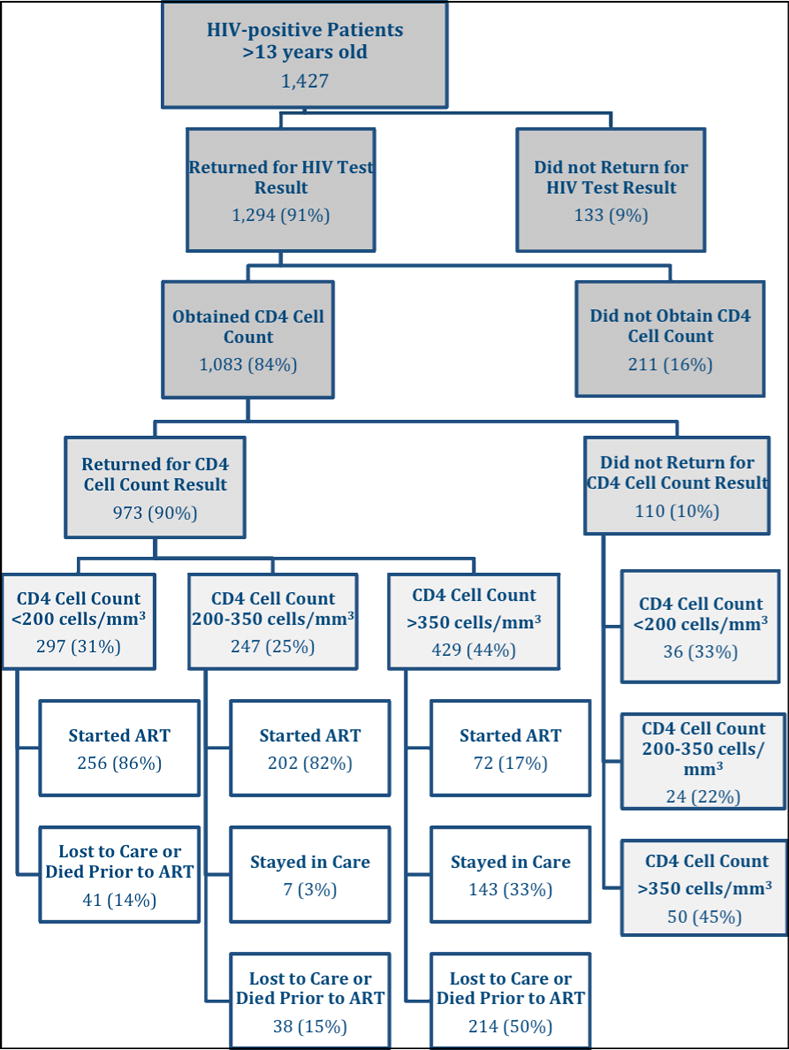
Among the 1,427 patients who tested positive for HIV, a total of 680 (48%) either initiated ART or were retained in pre-ART care for two years after HIV testing (see Figure 1). One hundred thirteen patients (8%) were pregnant women; they received same-day test results. Of these, 94 (83%) had blood drawn for CD4 cell count, and 82 (87%) returned for the result. Among these 82 patients, 64 (78%) were alive and in-care at two years, 12 (15%) were lost to follow-up during pregnancy, and 6 (7%) were lost to care after delivery. Among the other 1,314 patients, 1,181 (90%) returned for HIV test results. Of these, 989 (84%) had blood drawn for CD4 cell count and 891 (90%) returned for the test result.
Figure 1.
Patient Attrition from HIV testing to ART Initiation at GHESKIO
Among the 1,294 patients in the cohort who returned for their HIV test result, 1,083 patients (84%) had blood drawn for CD4 cell count within the subsequent 12 months. The median time from HIV testing to receipt of the CD4 cell count was nine days (IQR: 8 to 13). As illustrated in Figure 2A, 81% of patients who ever received a CD4 cell count had the test during the first two weeks after HIV testing. Among the 1,083 patients who had blood drawn for a CD4 cell count, 110 patients (10%) did not return for the test result. Of these, 36 (33%) had a CD4 cell count < 200 cells/μl, 24 (22%) had between 200 and 350 cells/μl, and 50 (45%) had > 350 cells/μl. CD4 cell counts were similar between those who returned and those who did not return for their test results. Of the 973 patients who received CD4 cell count results, 297 (31%) had CD4 cell count < 200 cells/μl, 247 (25%) had between 200 and 350 cells/μl, and 429 (44%) had a CD4 cell count > 350 cells/μl.
Figure 2A. Number of Weeks from HIV Test to CD4 Cell Count*.
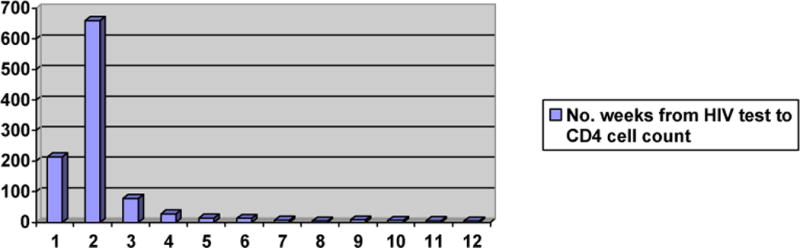
*Number of weeks from HIV test to blood draw for CD4 cell count; note that an additional 26 patients had blood drawn for CD4 cell count from weeks 13 to 52, with a maximum number of 2 tests per week.
Patients with CD4 cell counts < 200 cells/μl were eligible for ART using the WHO guidelines in place at the time of the study [43] and 256 of these patients (86%) initiated ART a median of eight days (IQR: 3 to 23) after blood was drawn for CD4 cell testing. As illustrated in Figure 2B, 82% of those who started ART did so within 6 weeks of CD4 cell testing. Among patients with CD4 cell count between 200 and 350 cells/μl, 202 (82%) initiated ART, seven (3%) were retained in pre-ART care for the subsequent two years after HIV testing, and 38 (15%) were lost to care or known to have died prior to ART initiation. Among patients with a CD4 cell count > 350 cells/μl, 72 (17%) initiated ART, 143 (33%) were retained in pre-ART care for the subsequent two years after HIV testing, and 214 (50%) were lost to care or known to have died prior to ART initiation.
Figure 2B. Number of Weeks from CD4 Cell Count to ART Initiation*.
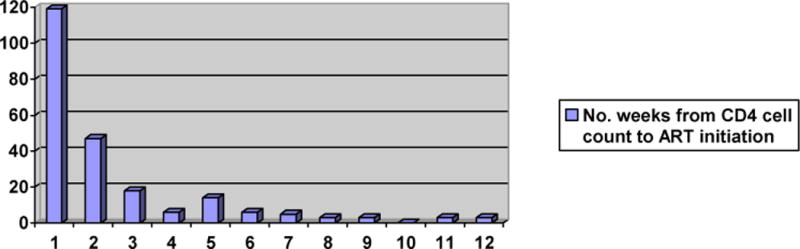
*Number of weeks from blood draw for CD4 cell count to ART initiation among patients with CD4 cell count < 200 cells/μl; note that an additional 26 patients initiated ART from weeks 13 to 73, with a maximum number of 2 patients initiating ART per week.
In patients who did not yet qualify for ART, attrition usually occurred within months after HIV testing. Among patients who were lost to care, 32 of 38 (84%) with CD4 cell count between 200 and 350 cells/μl and 141 of 220 (64%) with CD4 cell count > 350 cells/μl were lost within the first six months after HIV testing, and 35 (92%) and 178 (81%) respectively, were lost within 12 months after HIV testing. It is noteworthy that all patients in our study population had undergone HIV testing from six to 12 months prior to the devastating earthquake that struck Port-au-Prince in January 2010.
In multivariate analysis, attrition prior to CD4 cell testing was more common among patients younger than 35 years of age compared to those 35 years and older (OR: 1.84; 95% CI: 1.42–2.37), and among patients with an annual income of $US125 or less compared to those earning more than $US125 per year (OR 1.65; 95% CI: 1.25–2.19). Attrition was less common among patients with active TB at HIV testing (OR: 0.08; 95% CI: 0.03–0.25), compared to those without active TB at HIV testing (see Table 2). Among patients with CD4 cell count < 200 cells/μl, higher odds of attrition prior to ART initiation were associated with primary or lower education, compared to at least some secondary education, (OR: 2.27; 95% CI: 1.31–3.92), CD4 cell count below 150 cells/μl, compared to CD4 cell count of 150–199 cells/μl (OR: 2.36; 95% CI: 1.17–4.72), and active TB at the time of HIV testing (OR: 2.46; 95% CI: 1.29–4.71) (see Table 3).
Table 2.
Risk Factors for Attrition Prior to CD4 Cell Count
| Variable | Reference Group | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Female gender | Male | 1.13 (0.86–1.43) | 0.4088 | – | – |
| Age | |||||
| ≤ 24 years | ≥ 45 years | 2.25 (1.50–3.37) | < 0.0001 | 1.92 (1.27–2.90) | < 0.0001 |
| 25–34 years | ≥ 45 years | 1.89 (1.32–2.71) | 0.0006 | 2.04 (1.41–2.95) | 0.0021 |
| 35–44 years | ≥ 45 years | 1.06 (0.72–1.57) | 0.7763 | 1.15 (0.77–1.72) | 0.4882 |
| Residence zone | |||||
| Petionville, Port-au-Prince (PAP)* | Downtown PAP | 0.89 (0.51–1.55) | 0.6694 | – | – |
| Plaine, PAP* | Downtown PAP | 0.65 (0.41–1.02) | 0.0621 | – | – |
| Delmas, PAP* | Downtown PAP | 1.12 (0.77–1.63) | 0.5443 | – | – |
| Carrefour, PAP* | Downtown PAP | 0.89 (0.65–1.23) | 0.4866 | – | – |
| Outside Greater PAP* | Downtown PAP | 0.71 (0.44–1.14) | 0.1577 | – | – |
| Annual income | |||||
| ≤ $US125 | > $US125 | 1.73(1.32–2.26) | < 0.0001 | 1.65 (1.25–2.19) | 0.0005 |
| Educational level | |||||
| No school | Some secondary | 1.02 (0.74–1.41) | 0.8890 | – | – |
| Some primary | Some secondary | 0.89 (0.66–1.21) | 0.4614 | – | – |
| Active TB at HIV testing | None | 0.08 (0.02–0.25) | < 0.0001 | 0.08 (0.03–0.25) | < 0.0001 |
PAP is the abbreviation for Port-au-Prince
Table 3.
Risk Factors for Pre-ART Attrition with CD4 cell count < 200 cells/μl
| Variable | Reference Group | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Female gender | Male | 0.72 (0.43–1.19) | 0.2003 | – | – |
| Age | |||||
| ≤ 24 years | ≥ 45 years | 1.18 (0.41–3.39) | 0.7542 | – | – |
| 25–34 years | ≥ 45 years | 1.32 (0.64–2.74) | 0.4585 | – | – |
| 35–44 years | ≥ 45 years | 1.96 (0.99–3.90) | 0.0539 | – | – |
| Residence zone | |||||
| Petionville, Port-au-Prince (PAP)* | Downtown PAP | 0.81 (0.09–7.38) | 0.8511 | – | – |
| Plaine, PAP* | Downtown PAP | 1.06 (0.39–2.90) | 0.9125 | – | – |
| Delmas, PAP* | Downtown PAP | 2.06 (0.49–8.59) | 0.3231 | – | – |
| Carrefour, PAP* | Downtown PAP | 1.11 (0.11–11.57) | 0.9303 | – | – |
| Outside Greater PAP* | Downtown PAP | 1.28 (0.05–36.14) | 0.8833 | – | – |
| Annual income | |||||
| ≤ $US125 | > $US125 | 0.92 (0.55–1.54) | 0.7409 | – | – |
| Educational level | |||||
| No school | Some secondary | 2.27 (1.23–4.19) | 0.0086 | 2.56 (1.36–4.83) | 0.0037 |
| Some primary | Some secondary | 1.96 (1.04–3.71) | 0.0387 | 1.99 (1.03–3.83) | 0.0407 |
| Active TB at HIV testing | None | 2.37 (1.27–4.44) | 0.0070 | 2.46 (1.29–4.71) | 0.0066 |
| CD4 cell count | |||||
| < 50 cells/μl | 150–199 cells/μl | 2.03 (0.95–4.34) | 0.0693 | 2.47 (1.12–5.44) | 0.0252 |
| 50–99 cells/μl | 150–199 cells/μl | 1.88 (0.85–4.15) | 0.1188 | 2.17 (0.96–4.91) | 0.0633 |
| 100–149 cells/μl | 150–199 cells/μl | 2.42 (1.08–5.39) | 0.0314 | 2.48 (1.09–5.65) | 0.0312 |
PAP is the abbreviation for Port-au-Prince
Among patients with CD4 cell count > 350 cells/μl, in multivariate analysis, attrition was more common among those with an annual income of $US125 or less compared to those earning more than $US125 per year (OR: 1.74; 95% CI: 1.20–2.52) (see Table 4). No other factors were significantly associated with attrition in the multivariable analysis.
Table 4.
Risk Factors for Attrition with CD4 cell count > 350 cells/μl
| Variable | Reference Group | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Female gender | Male | 1.18 (0.80, 1.75) | 0.4065 | – | – |
| Age | |||||
| ≤ 24 years | ≥ 45 years | 1.58 (0.85 – 2.92) | 0.1456 | – | – |
| 25–34 years | ≥ 45 years | 0.74 (0.44–1.24) | 0.2557 | – | – |
| 35–44 years | ≥ 45 years | 0.87 (0.51–1.50) | 0.6267 | – | – |
| Residence zone | |||||
| Petionville, Port-au- Prince (PAP)* | Downtown PAP | 0.99 (0.41, 2.35) | 0.9743 | – | – |
| Plaine, PAP* | Downtown PAP | 1.06 (0.59, 1.90) | 0.8400 | – | – |
| Delmas, PAP* | Downtown PAP | 0.82 (0.45, 1.51) | 0.5201 | – | – |
| Carrefour, PAP* | Downtown PAP | 0.73 (0.46, 1.17) | 0.1882 | – | – |
| Outside Greater PAP* | Downtown PAP | 0.95 (0.42, 2.12) | 0.8966 | – | – |
| Annual income | |||||
| ≤ $US125 | > $US125 | 1.74 (1.20–2.52) | 0.0034 | – | – |
| Educational level | – | – | |||
| No school | Some secondary | 1.48 (0.94–2.35) | 0.0938 | – | – |
| Some primary | Some secondary | 1.25 (0.82–1.90) | 0.2943 | – | – |
| Active TB at HIV testing | None | 1.57 (0.76–3.24) | 0.2270 | – | – |
| CD4 cell count ≥ 500 cells/μl | 350–499 cells/μl | 1.41 (0.98, 2.02) | 0.0661 | – | – |
PAP is the abbreviation for Port-au-Prince
We tracked the 747 patients who were lost to care prior to ART during the study period using the national HIV/AIDS Surveillance System (described above) and the GHESKIO EMR. Fifty-one patients (7%) returned to GHESKIO after the study period ended, and 696 (93%) did not return. Among these 696 patients who were lost to care at GHESKIO, 690 (99%) were identified in the national HIV/AIDS Surveillance System, meaning their HIV diagnosis and/or follow-up disease event was reported to the MSPP. Eighty-four of these patients (12%) received care in an outside clinic after being lost to care at GHESKIO; of these, 54 (64%) received care in only one other clinic after GHESKIO, and 30 (36%) received care at more than one other clinic. Patient-level detail was not available on these patients due to confidentially agreements with the HIV/AIDS Surveillance System. If these patients were classified as in care, then the proportion of the total cohort (n = 1,427) who started ART or remained in care would increase from 48% (n = 680) to 57% (n = 815).
DISCUSSION
We analyzed rates of attrition from HIV testing to ART initiation in a cohort of patients newly diagnosed with HIV, and found that fewer than 50% of patients initiated ART or were retained in pre-ART care during two years of follow-up. High rates of attrition were observed at each stage from HIV testing to ART initiation, regardless of baseline CD4 cell count. Nearly one-quarter of patients newly diagnosed with HIV did not remain in care long enough to complete CD4 cell testing. Among those with a CD4 cell count < 200 cells/μl, nearly one-quarter of patients were not known to have initiated ART – in nearly half of these cases, the patient had not received their CD4 cell count result to confirm they qualified for ART. Among patients with CD4 cell count > 350 cells/μl, only half initiated ART or were retained in pre-ART care in our clinic for the subsequent two years after HIV testing. We tracked patients who were lost to care using the GHESKIO EMR and Haiti’s national HIV/AIDS Surveillance System database, and found that some patients either returned to GHESKIO after the study period ended, or received care at another clinic; even with the inclusion of these patients, the retention rate increased to only 57%.
Though we report a high rate of pre-ART attrition, it is lower than those described in most published studies from other resource-poor settings. As described above, Rosen et al. estimated that 17% of patients newly diagnosed with HIV remain in care to start ART. In their review of 28 African studies, 59% of patients were retained in care from HIV testing to completion of CD4 cell count or clinical staging, 46% from staging to ART eligibility, and 68% from ART eligibility to ART initiation [40]. Studies from South Africa have reported that 39% to 86% of eligible patients initiated ART [23, 24, 26, 27, 30, 37]. This proportion ranged from 31% to 86% in reports from Mozambique, Uganda, Ethiopia, Kenya, and Malawi [16, 19, 33, 35, 39, 45]. The median time from staging to ART initiation among ART-eligible patients in these studies ranged from 16 days to 3.6 months, and all four sites with at least 30% attrition had median delays of at least two months [19, 23, 26, 37] Among patients with CD4 cell counts > 350 cells/μl, rates of retention in care (with variable definitions) range from 4% to 60% in reports from Malawi, South Africa, and Cambodia [20, 29, 31, 36]. Reasons for this variation in retention rate have not been evaluated, and comparisons are limited by differing definitions, but it is likely that both structural and patient-level factors play a role.
Patients in the pre-ART period face many of the same barriers to attendance as patients on ART, including transportation, financial constraints, and work and child-care responsibilities [15, 16, 19, 21, 32, 33, 35, 45–55]. Yet, the attrition rates reported during the pre-ART period – both at GHESKIO and in other resource-poor settings – are much higher than those reported among patients who have already initiated ART [7, 8, 56–63]. Among patients who receive at least one month of ART at GHESKIO, only 6% are lost to follow-up within the subsequent two years [41]. Retention in care during the post-ART period is facilitated by the provision of transportation subsidies, phone calls for missed appointments, a dedicated ART clinic, and nutritional supplementation for those in need [64, 65]. In many clinics, including ours, these interventions are provided only after patients start ART, due to budget limitations. Our findings suggest that interventions to decrease barriers to attendance will be most effective in the early period after HIV testing. Most patients who had not received a CD4 cell count within two weeks after HIV testing in our cohort were already lost to follow-up. Furthermore, among those with CD4 cell count < 200 cells/μl, most patients who had not initiated ART within six weeks after CD4 cell testing were already lost to care. These findings are consistent with those of other studies finding a high rate of attrition in the early period after presentation, and preventable delays in ART initiation [45, 53, 54].
We attribute the association between annual income and retention in pre-ART care in our study to the greater ability of higher income patients to overcome financial barriers to clinic attendance. If additional funding was available, we could extend these services to patients in the pre-ART period. Such funding could have a major impact on mortality if it improved ART uptake among those who qualify for therapy, as detailed tracking studies have found that 28% to 58% of ART-eligible patients who have defaulted in the pre-ART period have died, most within 2 months of attrition [33, 35, 38, 49] We also found that higher education is associated with improved retention; we attribute this to a greater understanding of the importance of remaining in pre-ART care. Higher income and education have been associated with higher rates of ART initiation in other studies as well [18, 45, 54, 55]. It is also possible that these variables are surrogates for unstable residence. We measured residence zone (see Tables 2, 3, and 4), but change in residence was not captured. It will be important to measure change in residence in future studies.
Though pregnant patients in our cohort received same-day HIV test results, over one-fourth did not complete a CD4 cell count. Of those who did complete CD4 cell testing, an additional fifth were LTFU during pregnancy or after delivery. These findings are similar to other reports from resource-poor settings [25, 66]. Point-of-care CD4 cell testing, which is becoming more widely available, may improve test completion rates. The new WHO guidelines for mother-to-child prevention, which now recommend consideration of lifetime therapy regardless of CD4 cell count, may improve retention in care among pregnant women [67].
We found that active TB at HIV diagnosis was associated with completion of CD4 cell count; this finding has not been reported elsewhere. GHESKIO is the only site we are aware of that screens all patients for TB in the HIV VCT center, and provides same-day TB testing and treatment. We attribute the high rate of CD4 cell completion to the immediate provision of services to patients who present with TB symptoms. Interestingly, a diagnosis of active TB was associated with a lower rate of ART initiation among patients with a CD4 cell count < 200 cells/μl, which we attribute to a high rate of mortality in this group of patients, as those that do not return for follow-up in the pre-ART period are not tracked to distinguish loss to care from death.
Younger patients were more likely to drop out of care prior to receiving a CD4 cell count. Other studies have suggested that adolescents have poorer ART treatment outcomes than adult patients [68–70]. In response, GHESKIO opened an adolescent ART clinic in 2007 in a new facility with a specialized staff and the provision of patient incentives such phone cards, transportation subsidies, scholarships and prizes, free dental care, and peer counseling and other psychosocial support. Waiting times decreased from four hours to one hour, and one-year retention in care for adolescents on ART increased from 70% to 91% [71]. If further studies confirm that adolescents also have poorer pre-ART outcomes, then additional interventions will be warranted to retain them in care.
Though we were able to track patients who were lost to care at GHESKIO using the national HIV/AIDS Surveillance database to determine if they sought care at another clinic, our study was limited by our inability to determine the outcome for those who did not seek further care. We suspect that many patients with advanced AIDS who do not return for care have died. Haiti also suffered a devastating earthquake on January 12, 2010, with the displacement of large numbers of people in Port-au-Prince. However, most attrition in our cohort occurred in the first year of follow-up, prior to the earthquake. Since GHESKIO provides comprehensive testing and treatment services, our study findings may not be generalizable to settings where patients receive VCT at stand-alone or mobile VCT clinics, where patients with positive test results face the additional barrier of referral to a separate clinic for HIV treatment services.
In summary, fewer than half of patients who were newly diagnosed with HIV in our cohort either started ART or were retained in pre-ART care for the subsequent two years. There are high rates of attrition at every step from HIV testing to ART initiation, regardless of baseline CD4 cell count. In the future, it will be important to determine the complete outcome for patients that are lost to care prior to ART initiation. Additional efforts to identify barriers and improve retention in pre-ART are critically needed.
Acknowledgments
We acknowledge Jean Benisson Dumas and Clemente Jacques for their generous assistance with data collection, and Sidney Atwood and Esther Iliones for their help with database management and statistical analysis.
Funding: Pape obtained funding for this study.
Sources of Funding: The project was supported in part by the National Institutes of Health Fogarty International Center International Clinical, Operational, and Health Services Research and Training Award (ICORTHA) Grant Number 3 U2R TW006896–04S1, and the Fogarty International Center Grant Number K01 TW007142.
Footnotes
Potential conflicts of interest: All authors report no conflicts.
Description of the role of each of the authors:
Conceptualization of the study and manuscript: All authors were involved in the conceptualization of the study and the manuscript.
Patient care: Noel, Bertrand, Severe, and Pape were directly involved in patient care at GHESKIO.
Data collection and management: All authors were involved in data collection or management.
Analysis: Noel, McLaughlin, Esperance, Severe, Delcher, Pape, and Koenig
Manuscript writing and revision: Esperance and Koenig wrote the first draft and all authors reviewed and edited the manuscript.
References
- 1.Tuboi SH, Schechter M, McGowan CC, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009;51(5):615–23. doi: 10.1097/QAI.0b013e3181a44f0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keiser O, Anastos K, Schechter M, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13(7):870–9. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff MJ, Cortes CP, Shepherd BE, Beltran CJ. Long-term outcomes of a national expanded access program to antiretroviral therapy: the Chilean AIDS cohort. J Acquir Immune Defic Syndr. 2010;55(3):368–74. doi: 10.1097/QAI.0b013e3181eb4fb9. [DOI] [PubMed] [Google Scholar]
- 4.Thai S, Koole O, Un P, et al. Five-year experience with scaling-up access to antiretroviral treatment in an HIV care programme in Cambodia. Trop Med Int Health. 2009;14(9):1048–58. doi: 10.1111/j.1365-3156.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 5.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22(7):873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21(3):351–9. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 7.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24(4):563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 8.Nglazi MD, Lawn SD, Kaplan R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr. 2011;56(1):e1–8. doi: 10.1097/QAI.0b013e3181ff0bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May M, Boulle A, Phiri S, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376(9739):449–57. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 11.Palombi L, Marazzi MC, Guidotti G, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral- treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48(1):115–22. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 12.Lima VD, Johnston K, Hogg RS, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008;198(1):59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 13.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 14.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40(1):96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 15.Assefa Y, Van Damme W, Mariam DH, Kloos H. Toward universal access to HIV counseling and testing and antiretroviral treatment in Ethiopia: looking beyond HIV testing and ART initiation. AIDS Patient Care STDS. 2010;24(8):521–5. doi: 10.1089/apc.2009.0286. [DOI] [PubMed] [Google Scholar]
- 16.Mulissa Z, Jerene D, Lindtjorn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS One. 2010;5(10):e13268. doi: 10.1371/journal.pone.0013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernahu A. Confirmed referral for pre-ART and ART services: best practices from USAID/PSP-Ethiopia mobile HIV counseling and testing (abstract); Abstract 1462 HIV Implementers Conference; 10–14 June 2009; Windhoek, Namibia. 2009. [Google Scholar]
- 18.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12(5):687–94. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 19.Micek MA, Gimbel-Sherr K, Baptista AJ, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr. 2009;52(3):397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tayler-Smith K, Zachariah R, Massaquoi M, et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: can we retain such patients within the general health system? Trans R Soc Trop Med Hyg. 2010;104(5):313–9. doi: 10.1016/j.trstmh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Zachariah R, Harries AD, Manzi M, et al. Acceptance of anti-retroviral therapy among patients infected with HIV and tuberculosis in rural Malawi is low and associated with cost of transport. PLoS One. 2006;1:e121. doi: 10.1371/journal.pone.0000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.April MD, Walensky RP, Chang Y, et al. HIV testing rates and outcomes in a South African community, 2001–2006: implications for expanded screening policies. J Acquir Immune Defic Syndr. 2009;51(3):310–6. doi: 10.1097/qai.0b013e3181a248e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS. 2010;24(Suppl 1):S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51(2):135–9. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22(13):1679–81. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingle SM, May M, Uebel K, et al. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. AIDS. 2010;24(17):2717–25. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranzer K, Zeinecker J, Ginsberg P, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One. 2010;5(11):e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson BA, Brennan A, McNamara L, et al. Lost opportunities to complete CD4+ lymphocyte testing among patients who tested positive for HIV in South Africa. Bull World Health Organ. 2010;88(9):675–80. doi: 10.2471/BLT.09.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(Suppl 1):43–7. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43(6):770–6. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 31.Lessells RJ, Mutevedzi PC, Cooke GS, Newell ML. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56(3):e79–86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Losina E, Bassett IV, Giddy J, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5(3):e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amuron B, Namara G, Birungi J, et al. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19(18):2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 35.McGrath N, Glynn JR, Saul J, et al. What happens to ART-eligible patients who do not start ART? Dropout between screening and ART initiation: a cohort study in Karonga, Malawi. BMC Public Health. 2010;10:601. doi: 10.1186/1471-2458-10-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Griensven J, Sopheak T, Aseffa Y, Van Damme W, Lynen L. Attrition of HIV-infected individuals not yet eligible for antiretroviral treatment: do we care? Trans R Soc Trop Med Hyg. 2010;104(10):690–2. doi: 10.1016/j.trstmh.2010.07.006. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 37.Pepper DJ, Marais S, Wilkinson RJ, Bhaijee F, De Azevedo V, Meintjes G. Barriers to initiation of antiretrovirals during antituberculosis therapy in Africa. PLoS One. 2011;6(5):e19484. doi: 10.1371/journal.pone.0019484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuire M, Munyenyembe T, Szumilin E, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15(Suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 39.Zachariah R, Tayler-Smith K, Manzi M, et al. Retention and attrition during the preparation phase and after start of antiretroviral treatment in Thyolo, Malawi, and Kibera, Kenya: implications for programmes? Trans R Soc Trop Med Hyg. 2011;105(8):421–30. doi: 10.1016/j.trstmh.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenig SP, Rodriguez LA, Bartholomew C, et al. Long-term antiretroviral treatment outcomes in seven countries in the Caribbean. J Acquir Immune Defic Syndr. 2012;59(4):e60–71. doi: 10.1097/QAI.0b013e318245d3c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joseph P, Severe P, Ferdinand S, et al. Multidrug-resistant tuberculosis at an HIV testing center in Haiti. AIDS. 2006;20(3):415–8. doi: 10.1097/01.aids.0000206505.09159.9a. [DOI] [PubMed] [Google Scholar]
- 43.Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 44.Fox MP, Larson B, Rosen S. Defining retention and attrition in pre-antiretroviral HIV care: proposals based on experience in Africa. Trop Med Int Health. 2012 doi: 10.1111/j.1365-3156.2012.03055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon TD, Burlison JR, Blevins M, et al. Enrolment and programmatic trends and predictors of antiretroviral therapy initiation from president’s emergency plan for AIDS Relief (PEPFAR)-supported public HIV care and treatment sites in rural Mozambique. Int J STD AIDS. 2011;22(11):621–7. doi: 10.1258/ijsa.2011.010442. [DOI] [PubMed] [Google Scholar]
- 46.Orrell C. Antiretroviral adherence in a resource-poor setting. Curr HIV/AIDS Rep. 2005;2(4):171–6. doi: 10.1007/s11904-005-0012-8. [DOI] [PubMed] [Google Scholar]
- 47.Wringe A, Roura M, Urassa M, Busza J, Athanas V, Zaba B. Doubts, denial and divine intervention: understanding delayed attendance and poor retention rates at a HIV treatment programme in rural Tanzania. AIDS Care. 2009;21(5):632–7. doi: 10.1080/09540120802385629. [DOI] [PubMed] [Google Scholar]
- 48.Nsigaye R, Wringe A, Roura M, et al. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc. 2009;12(1):31. doi: 10.1186/1758-2652-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bassett IV, Giddy J, Nkera J, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46(2):181–6. doi: 10.1097/QAI.0b013e31814277c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7(4):234–44. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell SK, Kelly KJ, Potgieter FE, Moon MW. Assessing social preparedness for antiretroviral therapy in a generalized AIDS epidemic: a diffusion of innovations approach. AIDS Behav. 2009;13(1):76–84. doi: 10.1007/s10461-007-9293-9. [DOI] [PubMed] [Google Scholar]
- 52.Jarvis JN, Meintjes G, Wood R, Harrison TS. Testing but not treating: missed opportunities and lost lives in the South African antiretroviral therapy programme. AIDS. 2010;24(8):1233–5. doi: 10.1097/QAD.0b013e3283383aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcellin F, Abe C, Loubiere S, et al. Delayed first consultation after diagnosis of HIV infection in Cameroon. AIDS. 2009;23(8):1015–9. doi: 10.1097/QAD.0b013e32832a5996. [DOI] [PubMed] [Google Scholar]
- 54.Guthrie BL, Choi RY, Liu AY, et al. Barriers to antiretroviral initiation in HIV-1-discordant couples. J Acquir Immune Defic Syndr. 2011;58(3):e87–93. doi: 10.1097/QAI.0b013e31822f064e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unge C, Johansson A, Zachariah R, Some D, Van Engelgem I, Ekstrom AM. Reasons for unsatisfactory acceptance of antiretroviral treatment in the urban Kibera slum, Kenya. AIDS Care. 2008;20(2):146–9. doi: 10.1080/09540120701513677. [DOI] [PubMed] [Google Scholar]
- 56.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367(9519):1335–42. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 57.Bussmann H, Wester CW, Ndwapi N, et al. Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. AIDS. 2008;22(17):2303–11. doi: 10.1097/QAD.0b013e3283129db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auld AF, Mbofana F, Shiraishi RW, et al. Four-year treatment outcomes of adult patients enrolled in Mozambique’s rapidly expanding antiretroviral therapy program. PLoS One. 2011;6(4):e18453. doi: 10.1371/journal.pone.0018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Etard JF, Ndiaye I, Thierry-Mieg M, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20(8):1181–9. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 60.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leger P, Charles M, Severe P, Riviere C, Pape JW, Fitzgerald DW. 5-year survival of patients with AIDS receiving antiretroviral therapy in Haiti. N Engl J Med. 2009;361(8):828–9. doi: 10.1056/NEJMc0809485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ochieng-Ooko V, Ochieng D, Sidle JE, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88(9):681–8. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353(22):2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 65.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363(3):257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong’ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17(5):564–80. doi: 10.1111/j.1365-3156.2012.02958.x. [DOI] [PubMed] [Google Scholar]
- 67.Programmatic update: Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants - executive summary. Geneva: World Health Organization; 2012. [Google Scholar]
- 68.Charles M, Noel F, Leger P, et al. Survival, plasma HIV-1 RNA concentrations and drug resistance in HIV-1-infected Haitian adolescents and young adults on antiretrovirals. Bull World Health Organ. 2008;86(12):970–7. doi: 10.2471/BLT.07.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flynn PM, Rudy BJ, Douglas SD, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190(2):271–9. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 70.Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159(8):764–70. doi: 10.1001/archpedi.159.8.764. [DOI] [PubMed] [Google Scholar]
- 71.Bertrand R, Edmond C, Jean-Paul K, Verdier R, Pape JW. Adolescents, a population with special needs: the GHESKIO experience; Abstract 2911 Caribbean HIV Conference; 18–21 November, 2011; Nassau, Bahamas. 2011. [Google Scholar]


