Abstract
Objectives
Women who experience postpartum psychosis (PP) seek guidance on further pregnancies and risk of illness; however empirical data are limited. This study describes reproductive and mental health outcomes in women diagnosed with PP and examines clinical risk factors as predictors of further illness.
Methods
A retrospective cohort design was used; 116 women who experienced episodes of mania or depression with psychotic features within six weeks of childbirth were recruited. All subjects underwent clinical diagnostic interviews and medical case notes were reviewed.
Results
Only 33% of women had an antecedent history, of which 34% had bipolar disorder and 55% unipolar depression. Only 58% of those with PP in their first pregnancy had a subsequent pregnancy, and 18% of marriages ended following the PP episode. Clinical presentation at the time of initial episode did not influence the timing of the onset of symptoms, treatment, or recovery. Although 86% of patients received treatment within 30 days of onset, 26% of women reported ongoing symptoms at a year after delivery. The recurrence rate of PP was 54.4%; a longer duration of the index episode (p < 0.05) and longer latency between the index PP and next pregnancy predicted a subsequent PP. The rate of subsequent non-puerperal episodes was 69%, and all these episodes were bipolar.
Conclusions
PP is difficult to predict in women with no antecedent history and is associated with a high rate of subsequent puerperal and non-puerperal illness. Risk of further illness needs to be conveyed in order to make fully informed decisions regarding future pregnancies.
Keywords: perinatal psychiatry, postpartum psychosis, pregnancy recurrence
Postpartum, or puerperal, psychosis (PP) is the most severe form of postpartum mood disorder. The rate of PP in the general population is approximately 1 in 1,000 (1, 2), however, the occurrence of such severe psychiatric episodes in the early postpartum is of great and disproportionate clinical importance. The tragic consequences of PP have been shown in a number of well-publicized cases in which women suffering from the illness have killed themselves or, in very rare circumstances, harmed their baby (3–5). Suicide is a leading cause of maternal death, and it is evident that a high proportion of cases represent women with acute psychosis in the early puerperium (6).
Although some have argued that PP is a condition in its own right, it is more appropriate to view childbirth as a trigger of mood episodes with psychotic features (7–10). Most cases of PP have an onset within two weeks of parturition (7–11), and a diverse range of clinical features are seen (7–11). Affective symptoms, both elation and depression, are prominent as is a disturbance of consciousness marked by an apparent confusion, bewilderment or perplexity (7–10). The nosology of PP remains controversial, which has led to problems in both research and clinical practice.
Several lines of evidence strongly support a model in which most cases of PP represent a variant of bipolar disorder. Data from epidemiological studies (12, 13) show a 22-fold increased risk of experiencing an affective psychosis in the four weeks following delivery, and a relative risk (RR) = 35 with first deliveries (12, 13). Although the rate of PP in the general population is 1 in 1,000, this rises to 260/1000 in women diagnosed with bipolar disorder and to 570/1000 in women with bipolar disorder and a family history of PP (14). In women diagnosed with bipolar disorder, between 25% and 40% of deliveries are affected by an episode of mania or depression with psychotic features (14). While a previous personal or family diagnosis of bipolar disorder is clearly a risk factor for PP, it is unclear whether a family history of mood disorders in general confers risk. Primiparity (15–19) and experiencing obstetric complications (15, 17, 19, 20) have also been established as risk factors for PP.
Long-term follow-up studies show that women who experience PP remain at high risk of experiencing future episodes that are bipolar in nature (21), related (puerperal) (40–57%) (22, 23) and unrelated (non-puerperal) (60%) to childbirth (18, 22–25). A number of women will make the decision not to extend their family. Although data are limited, they suggest that between 40% and 70% of women who experience PP do not have further children (25–27), but the rationale behind this decision-making process is not well understood. Our previous work found that many women with PP felt that they had made ill-informed decisions about their reproductive health based on a lack of information from health care professionals. Specifically, many reported avoiding pregnancy in order to remain well, only to experience further non-puerperal episodes of illness (28).
Given that women with a history of PP will seek advice on subsequent pregnancies and risk of future illness from psychiatric professionals, it is imperative that we obtain empirically derived data to aid in this decision-making process. There has been little research that examines the future reproductive and mental health of women diagnosed with PP and whether clinical factors may modify the high risk they face. Research on PP, in general and specifically on predictors of subsequent risk, is limited by the disorder’s relatively rare nature, small sample sizes, cross-sectional designs, and a broad range of phenotypes being included. We address some of these limitations in this study by using a large, well-characterized sample of women purposely ascertained on the basis of having narrowly defined PP.
The aims of this study were to:
Describe the reproductive course in women who experienced an index episode of PP and to compare those women who do and do not go on to have subsequent pregnancies.
Quantify the risk of experiencing PP in a(ny) subsequent delivery following the index episode.
Examine whether clinical factors predict: (i) a further PP in the next/subsequent delivery and (ii) non-puerperal relapse.
We proposed the following hypotheses: Women with an antecedent history of psychiatric illness would be more likely to have: (i) a subsequent pregnancy and (ii) a subsequent episode of PP. We further hypothesized that having a family history of psychiatric illness would be associated with experiencing subsequent episodes of illness (i) in the postpartum and (ii) unrelated (non-puerperal) to childbirth.
Materials and methods
Subject selection
One hundred and seventy-nine women were recruited as part of an ongoing national clinical and genetic study of PP conducted in the United Kingdom. Recruitment methods included support groups for PP and bipolar disorder, admissions to general and specialist perinatal psychiatry units and clinics, and national publicity.
To be included in the study, women must have experienced at least one episode of PP, defined as an episode of mania or depression with psychotic features according to DSM-IV and ICD-10 criteria. We included episodes with onset within six weeks of childbirth to include both the postpartum onset definitions of four and six weeks employed in DSM and ICD criteria, respectively. To ensure that participants met the inclusion criteria and were willing and able to take part, participants were screened over the telephone by a study team member using a brief diagnostic interview. Fifty-six women were excluded after completing the telephone screen; some women did not meet diagnostic criteria (i.e., they experienced non-psychotic depression or had symptom onset greater than six weeks postpartum). Other women had ongoing mental health issues and did not feel well enough at that time to participate in the study.
Multicenter ethics approval was obtained, and after a complete description of the study all women provided written informed consent.
The sample was therefore comprised of 123 women who had consented and underwent a full interview. After the full interview was completed, a further seven women were excluded as they had an onset during pregnancy (n = 5) or later than six weeks postpartum (n = 2). This resulted in a final sample of 116 women.
Assessments
Each subject was interviewed by a trained psychiatrist (IJ) or psychologist (ERB) using a modified version of the Schedule for Assessment in Neuropsychiatry (SCAN) (29), a detailed semi-structured lifetime psychiatric interview used to elicit psychopathology and to generate DSM-IV diagnoses. The modified SCAN assessed subjects’ experience of manic, depressive, and psychotic symptoms both following childbirth and for their lifetime.
In addition, each woman was asked in detail about menstrual, obstetric, and gynecological events. Additional data were collected wherever possible from key informants who witnessed the PP episode(s): these included spouses, parents, siblings, and mental health professionals. In 96% of cases we were able to obtain psychiatric and obstetric case note information to supplement proband and informant data. Determination of antecedent history, defined as an episode of psychiatric illness meeting DSM-IV criteria, irrespective of whether it received medical attention, was based on all available proband, informant, and medical case note information. Postpartum episode and lifetime diagnoses were made according to DSM-IV based on all available subject and case note information. Consensus diagnoses were made by the two primary interviewers (IJ, ERB) and an independent clinician-researcher, with 98% agreement reached. In two cases where agreement could not be reached the opinion of a fourth clinician was sought and consensus reached.
Variable definitions
Data were obtained from both interview and case note review. Where case notes were unavailable we relied upon subject and key informant data.
The predominant clinical presentation of the index PP episode was categorized as mania, depression, or mixed. The mania category included, in addition to episodes of mania with no concomitant depressive features, episodes of mania that were followed by an episode of depression, and, in the case of four women, episodes of depression that were followed by a mania. In the latter group, the depressive symptoms began within four days of parturition and lasted from 8–21 days before manic symptoms appeared. Therefore, in each case the onset of postpartum mania was within four weeks of delivery. The depression category comprised episodes of major depression with psychotic features but no evidence of manic symptomatology. The core feature of the mixed category was simultaneous, rather than subsequent, symptoms of mania and depression. The DSM-IV criteria for mixed episodes states that diagnostic criteria for both mania and depression must be met for at least one week, however this is restrictive and has led to alternative definitions being proposed (30–32). For the purposes of this study we defined cases of mixed mania as DSM-IV defined mania plus at least one depressive symptom, and mixed depression, as DSM-IV defined major depressive episode (MDE) plus at least one concurrent manic symptom (e.g., 30–32).
Onset of symptoms was defined as the number of days between parturition and the onset of psychiatric symptoms. Day 1 was taken to be the day of delivery. Duration of the index episode reflected the time from illness onset until the subject was euthymic – defined by self-report and case records. Time to treatment initiation refers to the difference in days between symptom onset and the start of medication. In all but three cases, women were treated with medication.
We examined PP episodes for the presence of perplexity or confusion; the symptom was rated as present if the woman endorsed perplexity or confusion, or if she described the experience using her own terms including ‘bewilderment’, ‘having a head full of cotton wool’, ‘head fog’, and ‘being on a different planet’. Suicidal ideation and behaviors were elicited from the SCAN interview and included active and passive thoughts of death, suicidal ideation, suicidal plans, and suicide attempts. The status of the hospital admission was noted as voluntary or involuntary; involuntary means that she was detained under a section of the Mental Health Act under UK law for her own health, her safety, or for the protection of others. Postpartum episodes in female relatives were rated as postpartum depression (PPD) (non-psychotic depressive symptoms) and PP episodes (episodes of mania or depression with psychotic features). Family history of mood disorders was defined as a biological relative being diagnosed with or receiving treatment for bipolar or unipolar disorder, or another named disorder. We recorded the time from the index PP episode to when the woman completed the study interview in months (Time from index PP to interview).
Data analysis
We compared demographic and clinical factors between (i) women with and without subsequent pregnancies following the index PP episode, and (ii) between those who had and did not have subsequent PP among those women with a subsequent pregnancy.
The following factors were treated as continuous variables in analyses: age at interview, age at index PP episode, time from the index PP episode to interview (in months), time from index PP episode to subsequent pregnancy (in months), onset of symptoms (in days), onset of treatment (in days), and duration of index episode (in weeks).
The following were used as dichotomous categories (yes/no) in analyses: having an antecedent psychiatric illness, clinical presentation (categorized as mania or mixed episode versus psychotic depression), presence of suicidal behaviors, and presence of perplexity and whether the woman was involuntarily hospitalized/detained. Other variables included a family history of a mood disorder (unipolar or bipolar) and having a female relative who experienced a postpartum psychiatric illness.
Treating the two groups under comparison as independent, we used chi-square analysis for categorical variables and t-tests were conducted for continuous variables. Using group membership as a binary response multivariate logistic regression was used to estimate the effects of these variables on predicting group membership. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. However, it should be noted that predicting group membership is not totally independent, as it is based on given events occurring. For example, having a subsequent postpartum episode is predicated on having a subsequent pregnancy and delivery. Further, these events are subject to change over time, and group membership may change if the subjects were followed for a longer time period. For instance, older women are less likely to have more children. To account for the effects of time, we used survival analysis to study the relation between the covariates and occurrences of the events. These data are ‘censored’ to indicate that the period of observation was ended before the event of interest occurred. For those women who had a subsequent pregnancy, the survival analysis for another PP is based on the time from childbirth to the event of subsequent PP.
We applied Cox proportional hazards models to analyze the effect of clinical variables on the time to recurrence of non-puerperal psychosis episodes following the index PP. Hazard ratios and 95% CIs were calculated. The threshold for statistical significance was a two-tailed alpha of 0.05, and all the analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Table 1 shows the demographic and clinical characteristics of the sample at the time of interview. The majority of participants were Caucasian, married, and employed at interview. The mean time since the index PP episode was 11.9 years [standard deviation (SD) = 8.9, range: six months to 39 years].
Table 1.
Demographic and clinical characteristics of the sample at interview
| Characteristics (n = 116) | |
| Age, years, mean (SD) [range] | 40.3 (8.9) [22–69] |
| Ethnicity, n (%) | |
| Caucasian | 114 (98.3) |
| Black/Afro Caribbean | 2 (1.7) |
| Marital status (n = 113), n (%) | |
| Married/cohabiting | 96 (85.0) |
| Single/widowed | 2 (1.7) |
| Divorced/separated | 15 (13.3) |
| Employment status (n = 113), n (%) | |
| Employed | 61 (54.0) |
| Full time | 30 (49.2) |
| Part time | 31 (50.8) |
| Stay-at-home Mom | 29 (25.7) |
| Unemployed (including disability) | 15 (13.3) |
| Student/retired | 8 (7.0) |
| Currently taking psychotropic medication, n (%) | 60 (51.7) |
| Total no. of pregnancies (n = 259), median [range] | 2 [1–6] |
| Total no. of deliveries (n = 213), median [range] | 2 [1–6] |
| No. who became pregnant following the index PP, n (%) | 64/116 (55.2) |
| No. (%) whose index PP was: | |
| Their first delivery | 99/116 (85.3) |
| Their first delivery and first psychiatric episode* | 67/99 (67.7) |
| *Of these 67, no. who had no more children | 29 (43.3) |
| No. (%) of PP episodes | |
| One | 82 (70.7) |
| Two | 33 (28.4) |
| Three | 1 (0.9) |
| Family history of psychiatric illness, n (%) | |
| Yes | 70 (60.3) |
| No | 37 (31.9) |
| Unknown | 9 (7.8) |
| Type of family history (n = 70), n (%) | |
| Bipolar disorder | 23 (32.9) |
| Unipolar disorder | 31 (44.3) |
| Both bipolar and unipolar disorder | 12 (17.1) |
| Other (schizophrenia, PTSD) | 4 (5.7) |
| Female relative experienced postpartum illness, n (%) | |
| Total no. of relatives affected | 26 (22.4) |
| Type of postpartum illness | 29 |
| Non-psychotic depression | 19 (65.5) |
| Mania or depression with psychotic features | 10 (34.5) |
| Psychotropic medication taken following subsequent delivery (n = 57), n (%) | 7 (12.3) |
SD = standard deviation; PP = postpartum psychosis; PTSD = posttraumatic stress disorder.
Twenty-one subjects (18.1%) reported that their marriages ended within three years of the initial episode of PP. Five of these women stated that the relationship ended because their partner told her that he was unable to cope with her illness. A further three women said that they chose to leave an unhappy relationship. The remaining 13 women felt that the relationship changed following the illness, which lead to divorce. Of the 21 women whose marriages ended, eight were either married to or cohabiting with a new partner at the time of interview.
We examined whether the clinical presentation of the index PP episode (specifically mania or mixed versus psychotic depression) influenced the timing of the onset of symptoms, treatment or recovery. There were no significant differences between onset of symptoms (df = 114, t = −0.21, p > 0.83), duration of index episode (df = 114, t = 0.52, p > 0.61), or time to treatment (df = 111, t = −0.52, p > 0.61) between the two groups (mania or mixed versus psychotic depression).
Table 2 shows the clinical and demographic characteristics at the time of index episode of PP.
Table 2.
Clinical characteristics at the index episode of postpartum psychosis
| Index PP episode (n = 116) | |||||
| Age, years, mean (SD) [range] | 28.0 (4.8) [17–43) | ||||
| Marital status (n = 112), n (%) | |||||
| Married/cohabiting | 111 (99.1) | ||||
| Single | 1 (0.9) | ||||
| Prior psychiatric history, n (%) | |||||
| No | 78 (67.2) | ||||
| Yes* | 38 (32.8) | ||||
| *Episode type (of those 38 with a previous history) | |||||
| Depression | 21 (55.3) | ||||
| Bipolar episode | 13 (34.2) | ||||
| Othera | 4 (10.5) | ||||
| No. of prior episodes, mean (SD) [range] | 1.8 (2.1) [1–10] | ||||
| No. of children, n (%) | |||||
| 0 | 99 (85.3) | ||||
| 1 | 13 (11.2) | ||||
| ≥2 | 4 (3.5) | ||||
| Experienced postpartum depression in previous delivery, n (%) | 7/17 (41.2) | ||||
| Delivery at which index episode of PP occurred, n (%) | |||||
| 1 | 99 (85.3) | ||||
| 2 | 13 (11.2) | ||||
| 3 | 1 (0.9) | ||||
| 4 | 3 (2.6) | ||||
| Clinical presentation, n (%) | |||||
| Mania | 80 (69.0) | ||||
| Psychotic depression | 14 (12.0) | ||||
| Mixed | 22 (19.0) | ||||
| Perplexity (n =112), n (%) | |||||
| Yes | 55 (49.1) | ||||
| No | 57 (50.9) | ||||
| Onset of symptoms, days, median [range] | |||||
| Entire sample | 3 [1–28] | ||||
| Mania presentationb | 3 [1–28] | ||||
| Psychotic depressionc | 6 [1–14] | ||||
| Mixed episodesd | 3 [1–21] | ||||
| Onset of treatment within: n (%) | 1 week | 2 weeks | 30 days | > 31 days | None |
| Entire sample | 59 (50.9) | 27 (23.3) | 14 (12.0) | 13 (11.2) | 3 (2.6) |
| Mania presentationb | 42 (52.5) | 20 (25.0) | 9 (11.3) | 6 (7.5) | 3 (3.7) |
| Psychotic depressionc | 6 (42.9) | 1 (7.1) | 4 (28.6) | 3 (21.4) | 0 (0) |
| Mixed episodesd | 11 (50.0) | 6 (27.3) | 1 (4.5) | 4 (18.2) | 0 (0) |
| Duration of index episode, weeks, median [range) | 30 [1–216] | ||||
| Time from onset of symptoms to resolution of symptoms within: n (%) | 3 months | 6 months | 1 year | > 1 year | |
| Entire sample | 21 (18.1) | 34 (29.3) | 31 (26.7) | 30 (25.9) | |
| Mania presentationb | 15 (18.8) | 24 (30.0) | 20 (25.0) | 21 (26.2) | |
| Psychotic depressionc | 1 (7.1) | 6 (42.9) | 5 (35.7) | 2 (14.3) | |
| Mixed episodesd | 5 (22.7) | 4 (18.2) | 6 (27.3) | 7 (31.8) | |
| Voluntary hospital admission (n = 112), n (%) | |||||
| Yes | 84 (75.0) | ||||
| No | 28 (25.0) | ||||
| Suicidal ideation or behavior present (n = 112), n (%) | |||||
| Yes | 38 (33.9) | ||||
| No | 74 (66.1) | ||||
PP = postpartum psychosis; SD = standard deviation.
The four episodes in the ‘Other’ category included anorexia nervosa, bulimia nervosa, severe anxiety symptoms following a traumatic episode/posttraumatic stress disorder symptoms, and paranoia and psychotic symptoms following illegal drug use.
n = 80.
n= 14.
n = 22.
Subsequent pregnancies and outcome
Following the index episode of PP, 55% (n = 64) of the sample had a subsequent pregnancy. The obstetric and mental health outcomes of these pregnancies are shown in Figure 1. Each subordinate level of the diagram represents the outcome of the node above.
Fig. 1.
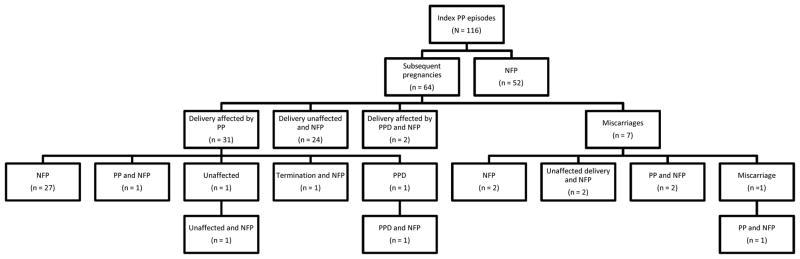
Reproductive and pregnancy outcomes following the index postpartum psychosis episode. Each subordinate level of the diagram represents the outcome of the node above. PP = postpartum psychosis; NFP = no further pregnancies; PPD = postpartum depression; unaffected = no affective disorder.
Differentiating those who became pregnant again
Logistic regression analysis was used to examine whether clinical factors differentiated those women who did or did not have a subsequent pregnancy following the index PP episode. Analysis showed that women who were older (χ2 = 4.16, df = 1, p = 0.04) and already had children (χ2 = 3.22, df = 1, p = 0.07) at the index PP episode were less likely to have a subsequent pregnancy. No significant association was found between the decision to become pregnant again and antecedent history, clinical presentation of index episode, presence of suicidal behavior or perplexity, and (in)voluntary hospital admission status. Of the 99 women who had PP with their first child, 42 women (42.4%) had no more children.
Analysis showed that the length of time from the index PP to time of interview was associated with subsequent pregnancies using both continuous (t = 4.08, p < 0.01) and dichotomous analyses (< 10 years versus ≥ 10 years) (χ2 = 9.14, p < 0.01). Those women who were diagnosed with PP > 10 years ago had a higher rate of subsequent pregnancies compared to women diagnosed within 10 years of the study.
Mental health outcomes of next delivery following the index PP
Of the 64 women who had a pregnancy subsequent to the index PP, 57 resulted in a live birth (see Fig. 1); the rate of PP in the next delivery following the index PP was 54.4% (31/57). A small number of women (n = 8) had at least one additional delivery. The rate of PP in any subsequent delivery was 52.2% (35/67).
Of the subsequent deliveries that were unaffected by PP, there were two cases of non-psychotic depression following the next delivery, and a further 2 in all subsequent deliveries (see Fig. 1). Therefore, 57.9% (33/57) of the next deliveries and 55.2% (37/67) of all subsequent deliveries were followed by an episode of mood disorder – mania or depression with psychotic features or non-psychotic depression.
None of the women took prophylactic medication during their subsequent pregnancy but a number took medication immediately following the delivery. Two women took a mood stabilizer and remained well, and four women were participants in a double-blind placebo controlled trial of mood stabilizers so medication status is unknown. An additional five women, while not taking mood stabilizers, took progesterone for prophylaxis of whom two had PP.
Risk of subsequent PP episode following the next successful delivery
To examine clinical factors that may predict subsequent PP episodes, we selected the 62 live deliveries that followed the index PP episode and compared the 34 affected deliveries to the 28 that were unaffected. In the analysis, we controlled for age, parity, and time since the index PP episode occurred.
As seen in Table 3, a longer duration of the index PP and a longer time between the index PP episode and the subsequent pregnancy significantly predicted a subsequent PP episode. We conducted post-hoc analyses to clarify whether the time between the index PP episode and subsequent pregnancy predicting a subsequent PP (r = 0.32, p < 0.05) was an artifact of episode severity. Bivariate analyses showed that the result is partly a function of the fact that time to next pregnancy is related to the severity of the index episode, namely duration of the index episode and being involuntarily detained [after controlling for duration and being involuntarily detained (r = 0.21, p > 0.24)].
Table 3.
Estimated risk of subsequent postpartum psychosis (PP) episode for clinical factors related to the index PP based on bivariate analysis controlling for age at index PP episode, parity, and time since the index PP episode occurred
| Variable | B | SE | Odds ratio | 95% CI | p-value |
|---|---|---|---|---|---|
| Age at index PP episode (years) | −0.11 | 0.06 | 0.90 | 0.80–1.01 | 0.06 |
| Time to subsequent pregnancy (months) | 0.02 | 0.01 | 1.03 | 1.00–1.05 | 0.05 |
| Experienced a psychiatric illness prior to index PP (yes)a | 0.23 | 0.56 | 1.26 | 0.42–3.81 | 0.67 |
| Duration of index PP episode (weeks) | 0.03 | 0.01 | 1.03 | 1.01–1.05 | 0.02 |
| Had a family history of a mood disorder (yes)a | 0.27 | 0.55 | 1.31 | 0.45–3.80 | 0.62 |
| Had a female relative with postpartum illness (yes)a | 0.41 | 0.91 | 1.50 | 0.25–8.98 | 0.66 |
| Clinical presentation of PP (mania or mixed)a | 0.22 | 0.76 | 1.25 | 0.21–5.53 | 0.77 |
| Perplexity or confusion was present in index PP (yes)a | 0.05 | 0.52 | 1.05 | 0.38–2.94 | 0.92 |
| Suicidal behaviors were present in index PP (yes)a | 0.57 | 0.54 | 1.77 | 0.61–5.09 | 0.29 |
| Involuntarily detained for index PP (yes)a | 0.58 | 0.59 | 1.78 | 0.56–5.69 | 0.33 |
SE = standard error; CI = confidence interval.
Reference category is the presence of the clinical variable.
Risk of experiencing a subsequent episode unrelated to childbirth
The risk of experiencing a subsequent non-puerperal episode was 69% (80/116). Table 4 shows the results of a univariate Cox regression analysis to examine which clinical factors were associated with experiencing a non-puerperal episode of illness following the index PP controlling for time since the index PP episode to time of interview.
Table 4.
Risk of experiencing a non-puerperal episode of illness for clinical factors based on proportional hazards model controlling for time since index PP episode
| Variable | B | SE | Hazard ratio | 95% CI | p-value |
|---|---|---|---|---|---|
| Age at index PP episode (years) | 0.10 | 0.2 | 1.10 | 1.05–1.15 | 0.01 |
| Experienced psychiatric illness prior to index PP (yes) | 0.81 | 0.25 | 2.24 | 1.39–3.61 | 0.01 |
| Had a family history of a mood disorder (yes) | 0.55 | 0.27 | 1.73 | 1.03–2.91 | 0.04 |
| Had a female relative with postpartum illness (yes) | 0.68 | 0.38 | 1.97 | 0.93–4.17 | 0.07 |
| Clinical presentation of index PP (mania or mixed) | 0.22 | 0.36 | 1.25 | 0.62–2.51 | 0.54 |
| Perplexity or confusion was present in index PP (yes) | 0.08 | 0.24 | 1.08 | 0.68–1.71 | 0.75 |
| Suicidal behaviors were present in index PP (yes) | 0.18 | 0.24 | 1.20 | 0.75–1.92 | 0.45 |
| Involuntarily detained for index PP (yes) | 0.22 | 0.26 | 1.25 | 0.75–2.07 | 0.39 |
PP = postpartum psychosis; SE = standard error; CI = confidence interval.
Experiencing psychiatric illness prior to the index PP episode, being older at the index PP episode, and having a family history of a mood disorder were significantly associated with experiencing a non-puerperal affective episode.
All of the women who had a subsequent non-puerperal episode met criteria for a lifetime diagnosis of bipolar disorder. Even after excluding the postpartum episode(s), 95% (76/80) still had a diagnosis of bipolar disorder. The type of non-puerperal affective episodes varied; 16 women (20.0%) experienced only pure manic episodes (with and without psychosis), 6.3% (5) experienced only non-psychotic depressive episodes and 72.5% (59) experienced only episodes of mania followed by depression. The 80 subjects experienced a total of 374 non-puerperal episodes, (mean = 4.68, SD = 6.05, range: 1–30).
There were 28 women who, at the time of interview, had only experienced a psychotic mood episode following childbirth (mean follow-up time 8.5 years, SD = 7.8 years, range: 6 months to 8 years).
Clinical presentation across PP episodes
There were 34 women who experienced multiple PP episodes; analysis using McNemar’s test showed diagnostic stability between the clinical presentation of the index and subsequent PP episodes (χ2 = 14.6, df = 4, p < 0.01). All 30 women with an index mixed or manic episode had the same presentation in the subsequent episode; of the four women who had a psychotic depression for the index episode, two had another episode of psychotic depression and two had an episode of mania. Compared to the index PP episode, the subsequent episode had a significantly later onset of symptoms [eight versus four days (t = 3.18, p < 0.01)], a significantly shorter duration [19 versus 30 weeks (t = 2.33, p < 0.05)], and the time to treatment onset was significantly shorter [four versus 12 days (t = 2.08, p < 0.05)].
Discussion
This study described the reproductive outcomes and subsequent mental health of women diagnosed with PP and examined clinical factors that predicted subsequent episodes of puerperal and non-puerperal illness.
It is well established that a personal history of bipolar disorder is the strongest predictor of PP (12–14), and clinicians are acutely aware of the elevated risk of PP in these women. However, it is not uncommon for women with no antecedent history to experience PP (33, 34), a fact clearly reflected in this sample. We have argued previously that those women who experience PP and have no antecedent history may still represent an underlying bipolar disorder diathesis that is triggered by childbirth (7, 8, 10). The previous outcome data and the results of this study further support the link between PP and bipolar disorder (18, 21–24), as the majority of women who experienced PP had subsequent non-puerperal affective episodes. Almost all (95%) of those women met diagnostic criteria for bipolar disorder even when the PP episode was excluded. It is, therefore, not surprising that experiencing affective illness prior to the index PP episode and a family history of mood disorders predicted experiencing subsequent non-puerperal episodes. Moreover, the striking rarity of an antecedent history of bipolar illness in our large sample of women with PP cautions against a false sense of security on the part of health care providers in the absence of that history.
As in previous studies, mania was the most common presentation of PP, (10, 34)but in contrast to a recent study (34) we found no effect of the clinical presentation on the timing of onset of symptoms, duration of episode and time to treatment. The severity and tenacity of symptoms are suggested by the observation that despite 86% of the patients having received treatment within 30 days of symptom onset, 52% of patients required a year or more to experience symptom resolution. Despite the fact that over half of the women received medication within a week of symptom onset, a quarter of women reported ongoing symptoms at a year after delivery. Although the acute psychotic episode may have a very good prognosis (23) it is of note that many women had long periods of depression that followed the acute psychotic phase of their PP episode. The possibility of precipitating a depressive episode should therefore be taken into account when choosing medication for the acute manic and psychotic phase and mood stabilizing medications with an antidepressant action should be considered.
Subsequent PP episodes were treated significantly quicker and had a shorter duration which may reflect the fact that the woman, her family and her medical team (both psychiatric and obstetric) were more aware of the risk of illness, recognized pathological symptoms sooner and navigated the care system more quickly. The lasting impact of experiencing PP should not be underestimated, however, as approximately one in five marriages ended in the wake of the illness.
Our data strongly support the fact that experiencing PP does not discourage most women from having further children (22–27)but not surprisingly, older and multiparous women were less likely to have further pregnancies. It remains possible that having a severe postpartum episode does influence a woman’s decision about extending her family as we were not able to address this issue directly in the current analysis. It is of interest, however, that we found no evidence that the severity of the index PP episode (indexed by factors such as suicidality, perplexity, involuntary admission, previous history) influenced decision making (10) supporting similar results (35–37) in women with more broadly defined psychosis.
Nonetheless, 45% of women had no pregnancies subsequent to the index episode of PP. This is consistent with a further study from our group of women with bipolar disorder that found women experiencing severe postpartum episodes were significantly less likely to go on to have further children than women who had no postpartum mood episode (54% compared to 73%, OR = 2.3, 95% CI: 1.70–3.16, p < 0.001) (Di Florio, personal communication). Our findings in this study, however, suggest that the severity of the PP episode does not influence the decision to have further children.
Although the risk of experiencing a subsequent PP is high, with almost a third of women experiencing multiple episodes of PP, it is important to note that 45% of women who went on to have further children did not become ill again. It appears that indices of the severity of the index PP episode were associated with risk of subsequent PP: a longer duration of the index PP and a longer time between the initial episode and subsequent pregnancy predicted a subsequent PP. A longer interpregnancy interval has been associated with higher risk in second pregnancies for another pregnancy related condition, preeclampsia (38, 39). However, in contrast to our findings here for PP, there are data to suggest that in women with a previous preeclampsia, the overall risk tends to decrease with increasing time interval between deliveries (40).
A major strength of the study is that we obtained a large sample of women who were recruited specifically because they experienced PP. Limitations include the fact that it is, by design, a retrospective study and as a result, data may be subject to recall bias. To minimize bias we made diagnoses based on data from clinical interviews with several informants, and contemporaneous medical case notes. Recall surrounding perinatal events and postpartum illness, in particular, has been shown to be accurate and stable (41); this is perhaps due to the fact that it is such a major life event; this suggests that recall bias is unlikely to have had a major impact on our findings. Other limitations are that we were underpowered to detect significant differences for some risk factors, and there are potentially important clinical risk factors that were not included in this study. Despite that, this is the largest sample reported on to date. As the sample was recruited primarily for a genetic study, Caucasian women are over-represented. Whereas other studies of PP have reported on women with pre-existing bipolar spectrum disorder seeking counselling and/or treatment prior to or during pregnancy, for the majority of our sample, their PP was their first psychiatric episode. Despite this, nearly all went on to have a lifetime diagnosis of bipolar disorder consistent with the findings of other studies. We are not able to comment on the efficacy of prophylactic medications as none of the women took medications during pregnancy, and there was a low rate of prophylaxis in subsequent deliveries. This perhaps reflects the lack of evidence, and heightened concerns about teratogenicity in pregnancy and safety in breastfeeding.
In conclusion, our findings indicate that for many women developing PP, the episode would have been very difficult, if not impossible, to predict. Despite the well-known association between bipolar illness and the risk of PP, a large number of women developing PP have no antecedent history, or only a history of unipolar depression. This effectively precludes any complacent reassurance about PP only occurring in those women with a history of bipolar illness. Conversely, among the 80 women who experienced a total of 374 non-puerperal episodes, all of them were bipolar in nature. Thus although the link between bipolar illness and PP is very strong, this is of little predictive value for the women who experience PP with no antecedent history. The majority of women who experience PP will have further children and although women can be reassured that a subsequent PP is not inevitable, women, their families and all involved in their care must be aware of the high risk of recurrence. Our data demonstrate that many women with a history of PP do not go on to have a recurrence either related to childbirth or at other times. That said, it should be stressed that the risk of a subsequent non-puerperal episode is high. It is important, therefore, that women are not given the impression that avoiding future pregnancies will ensure they remain well.
Acknowledgments
This study was supported, in part, by the Wellcome Trust, the West Midlands Regional Health Authority Research & Development Directorate, and NIH grant K23MH08220290 (ERB).
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA. 2006;296:2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- 2.Terp IM, Mortensen PB. Post-partum psychoses. Clinical diagnoses and relative risk of admission after parturition. Br J Psychiatry. 1998;172:521–526. doi: 10.1192/bjp.172.6.521. [DOI] [PubMed] [Google Scholar]
- 3.Jones I, Craddock N. Bipolar disorder and childbirth: the importance of recognising risk. Br J Psychiatry. 2005;186:453–454. doi: 10.1192/bjp.186.6.453. [DOI] [PubMed] [Google Scholar]
- 4.Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166:4, 405–408. doi: 10.1176/appi.ajp.2008.08121899. [DOI] [PubMed] [Google Scholar]
- 5.McLellan F. Mental health and justice: the case of Andrea Yates. Lancet. 2006;268:1951–1954. doi: 10.1016/S0140-6736(06)69789-4. [DOI] [PubMed] [Google Scholar]
- 6.CEMACH. [Last accessed January 4, 2012];The Confidential Enquiry into Maternal and Child Health. 2007 Available at http://cemach.interface-test.com/Home.aspx.
- 7.Jones IR, Heron J, Robertson Blackmore E. Puerperal psychosis. In: Kohen D, editor. Oxford Textbook of Women and Mental Health. Oxford: Oxford University Press; 2010. pp. 179–187. [Google Scholar]
- 8.Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health. 2006;15:352–368. doi: 10.1089/jwh.2006.15.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doucet S, Dennis C-L, Letourneau N, Robertson Blackmore E. Postpartum depression and postpartum psychosis: differentiation and clinical implications. J Obstet Gynecol Neonatal Nurs. 2009;38:269–279. doi: 10.1111/j.1552-6909.2009.01019.x. [DOI] [PubMed] [Google Scholar]
- 10.Brockington IF. Puerperal Psychosis in Motherhood and Mental Health. Oxford: Oxford University Press; 1996. pp. 200–284. [Google Scholar]
- 11.Heron J, Robertson Blackmore E, McGuinness M, Craddock N, Jones I. No ‘latent period’ in the onset of bipolar affective puerperal psychosis. Arch Womens Ment Health. 2007;10:79–81. doi: 10.1007/s00737-007-0174-z. [DOI] [PubMed] [Google Scholar]
- 12.Kendell RE, Wainwright S, Hailey A, Shannon B. The influence of childbirth on psychiatric morbidity. Psychol Med. 1976;6:297–302. doi: 10.1017/s0033291700013854. [DOI] [PubMed] [Google Scholar]
- 13.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–673. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- 14.Jones I, Craddock N. Familiarity of the puerperal trigger in bipolar disorder: results of a family study. Am J Psychiatry. 2001;158:913–917. doi: 10.1176/appi.ajp.158.6.913. [DOI] [PubMed] [Google Scholar]
- 15.Robertson Blackmore E, Jones I, Doshi M, et al. Obstetric factors associated with bipolar affective puerperal psychosis. Br J Psychiatry. 2006;188:32–36. doi: 10.1192/bjp.188.1.32. [DOI] [PubMed] [Google Scholar]
- 16.Kendell RE, Rennie D, Clarke JA, Dean C. The social and obstetric correlates of psychiatric admission in the puerperium. Psychol Med. 1981;11:341–350. doi: 10.1017/s0033291700052156. [DOI] [PubMed] [Google Scholar]
- 17.Videbech P, Gouliaev G. First admission with puerperal psychosis: 7–14 years of follow-up. Acta Psychiatr Scand. 1995;91:167–173. doi: 10.1111/j.1600-0447.1995.tb09761.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirpinar I, Coskun I, Cayköylü S, Anac S, Ozer H. First-case postpartum psychosis in Eastern Turkey: a clinical case and follow-up study. Acta Psychiatr Scand. 1999;100:199–204. doi: 10.1111/j.1600-0447.1999.tb10846.x. [DOI] [PubMed] [Google Scholar]
- 19.Paffenbarger RS. Epidemiological aspects of mental illness associated with childbearing. In: Brockington IF, Kumar R, editors. Motherhood and Mental Illness. London: Academic Press; 1982. pp. 21–36. [Google Scholar]
- 20.Nager A, Sundquist K, Ramírez-León V, Johansson LM. Obstetric complications and postpartum psychosis: a follow-up study of 1. 1 million first-time mothers between 1975–2003 in Sweden. Acta Psychiatr Scand. 2008;117:12–19. doi: 10.1111/j.1600-0447.2007.01096.x. [DOI] [PubMed] [Google Scholar]
- 21.Munk-Olsen T, Munk Laursen T, Meltzer-Brody S, Bo Mortensen P, Jones I. Psychiatric disorders with postpartum onset: possible early manifestations of bipolar affective disorders. Arch Gen Psychiatry. 2012;69:428–434. doi: 10.1001/archgenpsychiatry.2011.157. [DOI] [PubMed] [Google Scholar]
- 22.Robertson E, Jones I, Haque S, Holder R, Craddock N. Risk of puerperal and non-puerperal recurrence of illness following bipolar affective puerperal (post-partum) psychosis. Br J Psychiatry. 2005;186:258–259. doi: 10.1192/bjp.186.3.258. [DOI] [PubMed] [Google Scholar]
- 23.Robling SA, Paykel ES, Dunn VJ, Abbott R, Katona C. Long-term outcome of severe puerperal psychiatric illness: a 23 year follow-up study. Psychol Med. 2000;30:1263–1271. doi: 10.1017/s0033291799003025. [DOI] [PubMed] [Google Scholar]
- 24.Terp IM, Engholm G, Moller H, Mortensen PB. A follow-up study of postpartum psychoses: prognosis and risk factors for readmission. Acta Psychiatr Scand. 1999;100:40–46. doi: 10.1111/j.1600-0447.1999.tb10912.x. [DOI] [PubMed] [Google Scholar]
- 25.Dean C, Williams RJ, Brockington IF. Is puerperal psychosis the same as bipolar manic-depressive disorder? A family study. Psychol Med. 1989;19:637–647. doi: 10.1017/s0033291700024235. [DOI] [PubMed] [Google Scholar]
- 26.Rohde A, Marneros A. Postpartum psychoses: onset and long-term course. Psychopathology. 1993;26:203–209. doi: 10.1159/000284823. [DOI] [PubMed] [Google Scholar]
- 27.Schöpf J, Rust B. Follow-up and family study of postpartum psychoses. Part I. Overview Eur Arch Psychiatry Clin Neurosci. 1994;244:101–111. doi: 10.1007/BF02193527. [DOI] [PubMed] [Google Scholar]
- 28.Robertson E, Lyons A. Living with puerperal psychosis: A qualitative analysis. Psychol Psychother Theor Res Pract. 2003;76:411–431. doi: 10.1348/147608303770584755. [DOI] [PubMed] [Google Scholar]
- 29.Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 30.Benazzi F. Reviewing the diagnostic validity and utility of mixed depression (depressive mixed states) Eur Psychiatry. 2008;23:40–48. doi: 10.1016/j.eurpsy.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Cassidy F, Yatham LN, Berk M, Grof P. Pure and mixed manic subtypes: a review of diagnostic classification and validation. Bipolar Disord. 2008;10:131–143. doi: 10.1111/j.1399-5618.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 32.Akiskal HS, Bourgeois ML, Angst J, Post R, Moller HJ, Hirschfeld R. Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord. 2000;59:S5–S30. doi: 10.1016/s0165-0327(00)00203-2. [DOI] [PubMed] [Google Scholar]
- 33.Viguera AC, Tondo L, Koukopoulos AE, Reginaldi D, Lepri B, Baldessarini RJ. Episodes of mood disorders in 2,252 pregnancies and postpartum periods. Am J Psychiatry. 2011;168:1179–1185. doi: 10.1176/appi.ajp.2011.11010148. [DOI] [PubMed] [Google Scholar]
- 34.Bergink V, Lambregtse-van den Berg M, Koorengevel KM, Kupka R, Kushner SA. First-onset psychosis occurring in the postpartum period: a prospective cohort study. J Clin Psychiatry. 2011;72:1531–1537. doi: 10.4088/JCP.10m06648. [DOI] [PubMed] [Google Scholar]
- 35.Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry. 2001;178:427–432. doi: 10.1192/bjp.178.5.427. [DOI] [PubMed] [Google Scholar]
- 36.Laursen TM, Munk-Olsen T. Reproductive patterns in psychotic patients. Schizophr Res. 2010;121:234–240. doi: 10.1016/j.schres.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Howard LM, Kumar C, Leese M, Thornicroft G. The general fertility rate in women with psychotic disorders. Am J Psychiatry. 2002;159:991–997. doi: 10.1176/appi.ajp.159.6.991. [DOI] [PubMed] [Google Scholar]
- 38.Basso O, Christensen K, Olsen J. Higher risk of pre-eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology. 2001;12:624–629. doi: 10.1097/00001648-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346:33–38. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 40.Trogstad LI, Eskild A, Magnus P, Samuelsen SO, Nesheim BI. Changing paternity and time since last pregnancy; the impact on pre-eclampsia risk. A study of 547 238 women with and without previous pre-eclampsia. Int J Epidemiol. 2001;30:1317–1322. doi: 10.1093/ije/30.6.1317. [DOI] [PubMed] [Google Scholar]
- 41.Cox JL, Rooney A, Thomas PF, Wrate RW. How accurately do mothers recall postnatal depression? Further data from a 3-year follow up study. J Psychosom Obstet Gynec. 1984;3:185–189. [Google Scholar]


