Abstract
Objective
Recent data reports that youth experience greater weight gain during summer than during school months. We tested the hypothesis that a difference in total energy expenditure (TEE) between school and summer months exists and may contribute to summer weight gain.
Subjects and Methods
A secondary analysis was performed on cross-sectional TEE data from school-age, sedentary African American and Caucasian youth based in or near the District of Columbia who were at-risk for adult obesity because they had BMI≥85th percentile or had overweight parents. TEE was estimated from 18-O and deuterium measurements during 1-week intervals using urine samples collected after ingestion of doubly-labeled water. Differences in summer and school time TEE were assessed using ANCOVA. The data were adjusted for fat-free mass as determined by deuterium dilution to adjust for the effect of body size on TEE.
Results
Data were collected from 162 youth (average age 10±2 years, BMI 28±8 kg/m2, and BMI z-score 1.96+0.96). Of these, 96 youth had TEE measured during the school year (September – June); 66 different youths had TEE measured during summer months (June – August). After adjustment for fat-free mass, average summertime TEE was 2450±270 kcal/day and average school-time TEE was 2510±350 kcal/day (p=0.26).
Conclusion
No difference in TEE was detected between the school year and the summer months. These data suggest that seasonal differences in youth weight gain are not necessarily due to differences in energy expenditures.
Keywords: children, adolescents, energy expenditure, overweight, physical activity, seasonal variation
Introduction
The prevalence of youth who are overweight or obese (body mass index ≥85th percentile for age and sex) has increased in the last four decades from 11% to 32% in the United States 1-3. This high prevalence is a national health concern, because high BMI in youth is predictive of adult obesity and the early onset of chronic disease 4. Failure to reverse this trend foreshadows increased prevalence of adult obesity and major health and health care consequences in the decades to come 5, 6. This increased prevalence in youth overweight and obesity has been blamed on an obesity-promoting school environment that encourages increased consumption of palatable energy dense foods, increased sedentary behaviors, and decreased physical activity 7.
Although schools provide a public health opportunity for teaching healthy lifestyles, observations suggest that the current school environment may not necessarily be a central cause of the increasing prevalence of pediatric obesity because the greatest increases in BMI and percent body fat in youth, especially overweight or obese youth, appear to occur in the summer months, when youth are not in school 8-13. Von Hippell, et al. reported that among primary school children, BMI increased 0.03 kg/m2 per month during the school year compared to 0.08 BMI units per month between the end of the school year in June and the start of the next term in September 10. A related study found that there was a significant gain in body weight during summer vacation compared to during the school year in 73 overweight youth enrolled in a weight loss program 8. Additional studies of overweight Native American children and overweight Japanese children have reported similar results of summer weight gain9, 11, 12. These data support the conclusion that overweight children experience excess weight gain over the summer months, but that there may be exceptions in certain populations where this trend is not observed13.
Little is known about the correlates of weight gain during the summer among youth; however, reports do indicate that the increase in body weight and adiposity is greatest in those already having high BMI status for age 8-12. Also, because these reports are based on studies in youth of different ages ranging from primary grade school through middle school, it would appear that the risk for summer increase in BMI is present across a broad age range. Potential contributors to summer BMI increase may include increased consumption of high energy density snacks or other foods, more time spent in sedentary activities such as screen time (TV and computer), and decreases in physical activity (PA).
The purpose of this study was to examine total energy expenditure (TEE) in youth who had these measurements during the summer or during the school year to assess the potential impact of seasonal differences in TEE on summer weight gain. Physical activity is difficult to measure accurately over long periods of time, but is related to non-basal energy expenditure (NBEE), which can be measured by subtracting resting metabolic rate (RMR) from (TEE). We examined a cross-sectional data set that included TEE and RMR measurements from youth aged 6-13 years obtained throughout the year. These data allowed us to compare TEE between summer and school months among school-aged youth. Our study design does not allow us to disentangle the separate effects of the academic year with seasonal effects on TEE.
Methods
Subjects
This is a secondary data analysis of an ongoing study to examine metabolic and behavioral factors in youth at risk for adult obesity 14. The subjects recruited for the parent study were weight stable healthy weight and overweight male and female youth ages 6-13 years who were considered at high risk for adult obesity, defined as having one of two risk factors: a BMI greater than the 85th percentile, or a BMI between the 5th and 85th percentiles and two parents who were overweight. The subject population was disproportionally overweight or obese by design. Subjects were recruited from the greater Washington DC area via posted flyers and mailings to parents, local family physicians, pediatricians, and school districts 15. Subjects provided written assent, and parents gave written consent for participation. The Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board approved the clinical protocols. Names and other potential identifiers were removed and the de-identified data set in this analysis included TEE measurement by doubly labeled water, and measurement of height, weight, total body water, age, sex, self-reported race, and resting metabolic rate (RMR).
Measurements
Total Energy Expenditure
TEE was measured using the doubly labeled water (DLW) method as described previously 16, 17. Subjects provided a urine sample in the morning following a 12 hour fast. Subjects were then given a 280kcal breakfast at the National Institutes of Health Clinical Center where they remained without further energy intake for six hours and performed only sedentary activities. During this time, subjects ingested 0.25g H218O/kg total body water (estimated using height, weight, and bioelectrical impedance analysis) and 0.14g 2H20/kg total body water. Urine samples were collected serially for 4 hours after doubly-labeled water was administered. Subjects were then discharged from the clinic, but returned 7 days later to submit 2 spot urine samples to determine isotope elimination rates 15.
Resting Metabolic Rate
Resting metabolic rate (RMR) was determined using open-circuit indirect calorimetry using a metabolic cart (SensorMedics Corp, Yorba Linda, CA or ParvoMedics, Sandy, UT). The subject reclined as a plastic hood was placed over his or her head and a thin plastic apron provided a rough seal around the neck and chest. Gas analyzers measured the concentrations of O2 that entered the hood and CO2 that exited the hood at a known, controlled flow rate. Measurements were obtained from participants before breakfast, after a 12-hour fast and a 30-minute rest period as previously described 18.
Body Composition
Weight was obtained to the nearest 0.1 kg using a calibrated digital scale (Scale-Tronix, Wheaton, IL, U.S.A.) and height (measured three times) was obtained using a stadiometer (Holtain Ltd., Crymych, Wales) that was calibrated to the nearest 1 mm before each subject's height was measured. Subjects were assessed in minimal clothing (bathing suit or underwear). BMI was calculated and BMI percentile was determined using age and sex specific growth charts from National Center for Health Statistics/Centers for Disease Control and Prevention 19. Age- and sex-specific hydration factors were determined using a linear regression of hydration factor on age 20, 21. Fat-free mass (FFM) was then determined from total body water, which was measured in the DLW analysis as previously described 22. Fat mass (FM) was calculated by subtracting FFM from total body weight.
Group classification
Subjects were assigned to groups based upon whether their TEE measurement dates corresponded to the academic year or summer break in the greater Washington DC area. Subjects who had TEE measurements between June 8 and August 31 were assigned to the summer group, and those who were measured between September 1 and June 7 were the school group. A crossover group contained any subjects tested during a winter break period (December 20-January 3) and any subjects whose testing dates overlapped two groups. The subjects in the crossover group (n=26) were excluded from the analysis.
Statistical Analysis
All analyses were performed using R statistical analysis software, version 2.8.1 (R Foundation for Statistical Computing, Vienna, Austria). A Cook's distance test was performed to identify statistical outliers for TEE values. Two outliers with a Cook's distance greater than 1 were removed from the analysis. A power calculation was performed using G*Power version 3.1.223. Alpha was set at 0.05 and beta at 0.80. Given our sample size, an effect size of 0.4 would be required to detect a significant difference in TEE between the summer and school groups. This effect size corresponds to a difference of approximately 100 kcal/day between the groups. Mean TEE and standard deviations were calculated for each group. TEE was adjusted for FFM to take into account the effect of body size on TEE. TEE measurements were stratified by month. Mean TEE and standard deviations were calculated for each month. ANCOVA was used to determine whether TEE differed between specific months. To determine whether there was a difference in energy expended in physical activity between groups, non-basal energy expenditure (NBEE) was calculated (TEE-RMR). NBEE was also adjusted for FFM. Physical Activity Level (PAL) was calculated (TEE/RMR) as another indicator of physical activity. ANOVA was used to compare TEE in the summer and school groups.
Results
Subject characteristics are found in Table 1. The summer and school groups differed with respect to age, height, weight, TEE, FFM, RMR, percent fat, BMI, and BMI z-score. The school group was significantly older, taller, heavier, and had a higher percent fat, fat-free mass, and BMI z-score than the summer group. 89% of subjects in the school group and 80% of subjects in the summer group were overweight or obese by BMI percentile. These differences are reflected in a higher TEE and RMR. On average, before adjustment for subject characteristics, the summer group expended 314 kcal/day less than the school group.
Table 1.
Subject Characteristics
| Total (n=162) | Summer (n=66) | School (n=96) | |
|---|---|---|---|
| Age (y) *† | 9.7 ± 1.6 | 9.2 ± 1.5 | 10 ± 1.6 |
| Sex (% female) | 56 | 59 | 54 |
| Race | |||
| Black (%) | 34 | 35 | 34 |
| White (%) | 59 | 62 | 56 |
| Other+ (%) | 7 | 3 | 10 |
| Height (cm) *† | 144 ± 12 | 141.2 ± 11.1 | 146.5 ± 12.2 |
| Weight (kg) *† | 61 ± 26 | 51.8 ± 18.3 | 67.7 ± 27.7 |
| FFM (kg) *† | 33 ± 10 | 29.9 ± 8 | 35.1 ± 10.8 |
| Fat (%) *† | 43 ± 11 | 40.2 ± 11.2 | 45 ± 10.7 |
| BMI (kg/m2) *† | 28.4 ± 8.4 | 26.3 ± 5 | 30.4 ± 8.8 |
| BMI z-score * | 2.0 ± 1.0 | 1.77 ± 1.0 | 2.12 ± 0.91 |
| TEE (kcal/day) *†++ | 2490 ± 600 | 2303 ± 472 | 2617 ± 641 |
| TEEFFM-adjusted (kcal/day) *† | 2490 ± 360 | 2450 ± 270 | 2510 ± 350 |
| RMR (kcal/day) *†++ | 1490 ± 330 | 1391 ± 251 | 1556 ± 364 |
| NBEEFFM-adjusted (kcal/day) *† | 1000 ± 220 | 980 ± 200 | 1020 ± 280 |
| PAL (TEE/RMR) * | 1.68 ± 0.24 | 1.66 ± 0.18 | 1.70 ± 0.28 |
(mean ±SD)
Hispanic, Asian, and American Indian
These characteristics are significantly different between summer and school, p<0.05
Measured (unadjusted)
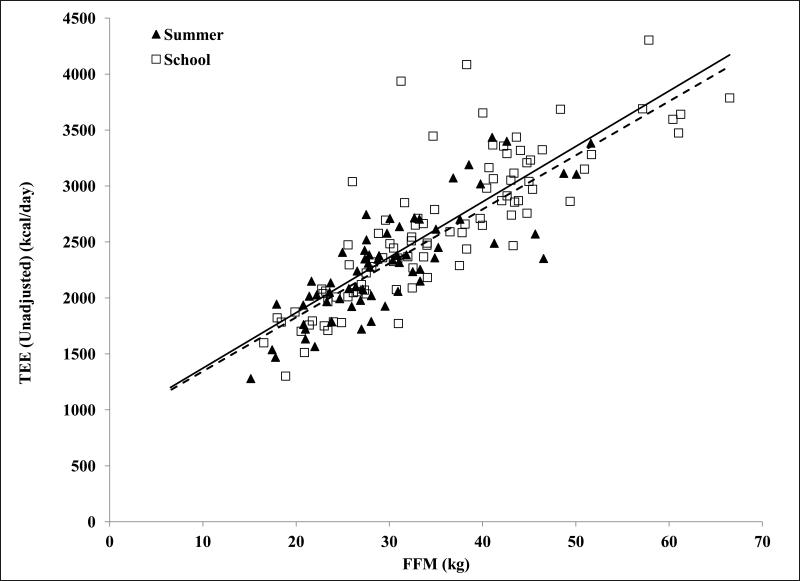
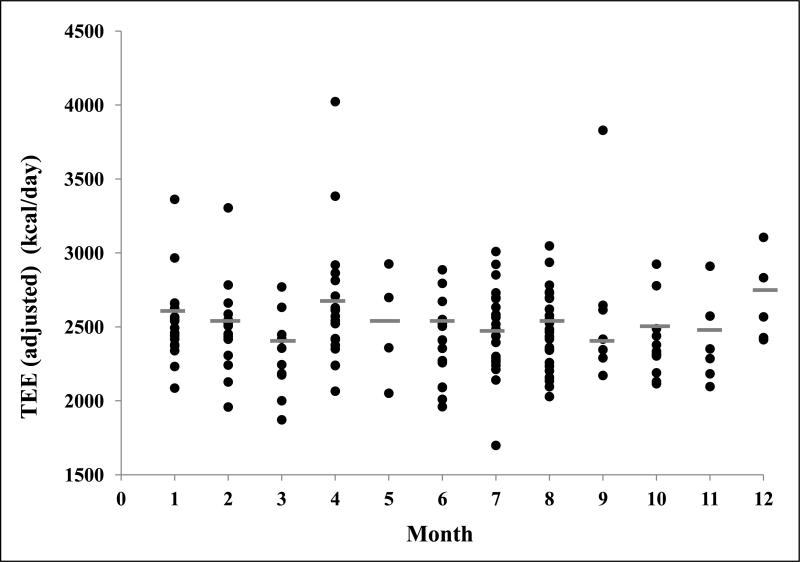
Figure 1 shows the strong relationship between TEE and FFM in both the summer (R2=0.67) and school (R2=0.70) groups, which reflects the impact of body size on TEE. After adjustment for FFM, there was no significant interaction between TEE and month (Figure 2). Further, when the months were combined to examine TEE in summer (adjusted TEE=2450±270kcal/day) versus school months (adjusted TEE=2510±350kcal/day), the non-significant difference was 58kcal/d lower TEE in summer (p=0.26, Table 1). NBEE was also not significantly different between summer (980±200kcal/day) and school (1020±280) (p=0.45, Table 1). There were no significant differences between summer and school in PAL (p=0.27, Table 1). The analysis was repeated using only the overweight and obese subjects in this cohort, and the results were not statistically different from those including all subjects.
Figure 1.
Relationship between unadjusted total energy expenditure and fat-free mass in school- (n=96) and summer-months (n=66). Energy expenditure measured by doubly labeled water 16, 17 and fat-free mass calculated from total body water 22.
Plot symbols: ▲, Summer; □, School
Trendline Styles: - - -, Summer; —, School
R2 values for total energy expenditure and fat free mass: summer (R2=0.67), school (R2=0.70).
Figure 2.
FFM-Adjusted total energy expenditure by month with averages designated by a line ( ). Months are numbered consecutively January (1) through December (12). There were no significant differences in energy expenditure between months.
). Months are numbered consecutively January (1) through December (12). There were no significant differences in energy expenditure between months.
Discussion
The trend of accelerated summer weight gain in overweight youth has been reported by several groups8-13. However, there are no data comparing TEE between school and summer months in youth in the United States to determine whether energy expenditure differences could help explain a summer weight gain.
Other seasonal relationships with TEE have been investigated. Goran and colleagues examined TEE differences between spring and fall in 104 white children (ages 4-10 years) and reported that TEE was greater during the spring than it was during the fall, which was explained by physical activity differences 24. Further, Baranowski et al. reported that children's physical activity levels correlated with the amount of time spent outdoors, which was different between boys and girls and differed from month to month 25.
As a component of TEE, PA is important to consider in this discussion. Seasonality in PA has been assessed using accelerometers in several studies. Results for the UK have been very consistent, finding higher PA levels in summer compared to winter26. Unfortunately, similar studies in the United States have not been as consistent. One study in children from South Dakota found no differences in total day counts or in time spent in vigorous activity between summer and autumn27. Another study comparing parental recall to accelerometer data found no differences in PA across all four seasons in children28. A study looking at children from New York City did find a significant difference in PA, with higher accelerometer counts during the summer, compared to winter29.
We do not have a direct measure of PA in this study, so we examined summer and school-year TEE. After adjustment for body size, TEE was not different between summer and school months in this sample. Further, NBEE, which includes energy expended from physical activity as its major (75%) component (TEE–RMR=NBEE), and PAL did not differ between summer and school months, suggesting no change in physical activity from school to summer unless such changes were countered by changes in non-exercise activity thermogenesis. Our data show a non-significant TEE difference of 58 kcal/day between summer and school months. It seems intuitive that youth would spend more time outdoors during the summer months and should therefore have an increase in physical activity and TEE; however, a significant increase was not observed in our analysis.
In our sample, an imbalance of 58 kcal/day, although not statistically significant, would be predicted to result in a fat gain of 0.65 kg over the summer ((58 kcal*day-1 × 85 days)/7700 kcal*kg fat-1). This is lower than the weight gain that has been reported by others. If a low TEE during summer was the driving factor in summer weight gain, a larger difference from summer to school would be necessary. It remains possible, however, that there was a statistically undetected decrease in TEE over summer months of approximately 50 kcal/day, which could explain part, but not all, of the summer weight gain reported by others. One of the limitations of our analysis is our modest sample size, which was powered to detect a difference of 100 kcal/day between school and summer months. Thus, although our power is modest, our results do not support the hypothesis that a low TEE is the primary reason for the summer weight gain that has been observed.
The major limitation of this cross-sectional designed study was that the same youth were not studied during summer and school months, thus no correlates with weight or BMI percentile change can be assessed. Also, the study was performed in a specific geographic area and with a cohort that was enriched for overweight/obesity, so this cohort may not be representative of the general population. There were significant differences between the school and summer groups in several measured variables, such as height and weight; therefore TEE needed to be adjusted to account for body size. Further, no measures of duration or intensity of physical activity were obtained, but rather we calculated non-basal energy expenditure, which is important as it can explain up to 10% of variance in body fat mass in children 30, 31.
Conclusions
These data help to inform our understanding of the increased summer weight gain that has been reported by others. Specifically, our data suggest that low energy expenditure in the summer relative to the school months is not the primary factor in promoting summer weight gain in youth (ages 6-13 years). We did not detect any difference in TEE or NBEE from school to summer months in this cohort. By deduction, this suggests that increased energy intake may be playing a greater role than decreased energy expenditure in summer weight gain. However, in using the DLW method to measure TEE, we cannot distinguish between various intensities of activity, which may have independent impacts. Future studies are needed to examine potential differences in dietary patterns and energy intake between the school year and summer months and their role in increasing adiposity in youth.
Acknowledgments
This research was supported in part by the Intramural and Extramural Research Programs of the NIH: NICHD, NIDDK, and NIMHD. Dr. J. Yanovski is a Commissioned Officer in the United States Public Health Service and is supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities of the National Institutes of Health. S. Zinkel was supported by training grant T32DK007665 from the National Institutes of Diabetes and Digestive and Kidney Diseases. The National Institute of Diabetes and Digestive and Kidney Diseases had no role in the preparation, review, or approval of the manuscript.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. Jama. 2010 Jan 20;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. Jama. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. Jama. 2002 Oct 9;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 4.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011 Nov 17;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 5.Lee JM, Pilli S, Gebremariam A, et al. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes (Lond) Apr;34(4):614–623. doi: 10.1038/ijo.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan JG. Cost and policy implications from the increasing prevalence of obesity and diabetes mellitus. Gend Med. 2009;6(Suppl 1):86–108. doi: 10.1016/j.genm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Moreno LA, Rodriguez G. Dietary risk factors for development of childhood obesity. Curr Opin Clin Nutr Metab Care. 2007 May;10(3):336–341. doi: 10.1097/MCO.0b013e3280a94f59. [DOI] [PubMed] [Google Scholar]
- 8.Gillis L, McDowell M, Bar-Or O. Relationship between summer vacation weight gain and lack of success in a pediatric weight control program. Eat Behav. 2005 Feb;6(2):137–143. doi: 10.1016/j.eatbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Kobayashi M. The relationship between obesity and seasonal variation in body weight among elementary school children in Tokyo. Econ Hum Biol. 2006 Jun;4(2):253–261. doi: 10.1016/j.ehb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.von Hippel PT, Powell B, Downey DB, Rowland NJ. The effect of school on overweight in childhood: gain in body mass index during the school year and during summer vacation. Am J Public Health. 2007 Apr;97(4):696–702. doi: 10.2105/AJPH.2005.080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato N, Sauvaget C, Kato T. Large summer weight gain in relatively overweight preschool Japanese children. Pediatr Int. 2012 Feb. doi: 10.1111/j.1442-200X.2012.03578.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith DT, Bartee RT, Dorozynski CM, Carr LJ. Prevalence of overweight and influence of out-of-school seasonal periods on body mass index among American Indian schoolchildren. Prev Chronic Dis. 2009 Jan;6(1):A20. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Himes JH, Hannan PJ, et al. Summer effects on body mass index (BMI) gain and growth patterns of American Indian children from kindergarten to first grade: a prospective study. BMC Public Health. 2011;11:951. doi: 10.1186/1471-2458-11-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anon Metabolic Differences of Overweight Children and Children of Overweight Parents. http://www.clinicaltrials.gov/ct/show/NCT00001522, http://www.clinicaltrials.gov/ct/show/NCT00001522.
- 15.Savastano DM, Tanofsky-Kraff M, Han JC, et al. Energy intake and energy expenditure among children with polymorphisms of the melanocortin-3 receptor. Am J Clin Nutr. 2009 Oct;90(4):912–920. doi: 10.3945/ajcn.2009.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoeller DA, Hnilicka JM. Reliability of the doubly labeled water method for the measurement of total daily energy expenditure in free-living subjects. J Nutr. 1996 Jan;126(1):348S–354S. [PubMed] [Google Scholar]
- 17.Schoeller DA, Taylor PB, Shay K. Analytic requirements for the doubly labeled water method. Obes Res. 1995 Mar;3(Suppl 1):15–20. doi: 10.1002/j.1550-8528.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 18.McDuffie JR, Adler-Wailes DC, Elberg J, et al. Prediction equations for resting energy expenditure in overweight and normal-weight black and white children. Am J Clin Nutr. 2004 Aug;80(2):365–373. doi: 10.1093/ajcn/80.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000 Jun 8;(314):1–27. [PubMed] [Google Scholar]
- 20.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982 May;35(5 Suppl):1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 21.Hewitt MJ, Going SB, Williams DP, Lohman TG. Hydration of the fat-free body mass in children and adults: implications for body composition assessment. Am J Physiol. 1993 Jul;265(1 Pt 1):E88–95. doi: 10.1152/ajpendo.1993.265.1.E88. [DOI] [PubMed] [Google Scholar]
- 22.Robotham DR, Schoeller DA, Mercado AB, et al. Estimates of body fat in children by Hologic QDR-2000 and QDR-4500A dual-energy X-ray absorptiometers compared with deuterium dilution. J Pediatr Gastroenterol Nutr. 2006 Mar;42(3):331–335. doi: 10.1097/01.mpg.0000189373.31697.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 24.Goran MI, Nagy TR, Gower BA, et al. Influence of sex, seasonality, ethnicity, and geographic location on the components of total energy expenditure in young children: implications for energy requirements. Am J Clin Nutr. 1998 Sep;68(3):675–682. doi: 10.1093/ajcn/68.3.675. [DOI] [PubMed] [Google Scholar]
- 25.Baranowski T, Thompson WO, DuRant RH, Baranowski J, Puhl J. Observations on physical activity in physical locations: age, gender, ethnicity, and month effects. Res Q Exerc Sport. 1993 Jun;64(2):127–133. doi: 10.1080/02701367.1993.10608789. [DOI] [PubMed] [Google Scholar]
- 26.Rich C, Griffiths LJ, Dezateux C. Seasonal variation in accelerometer-determined sedentary behaviour and physical activity in children: a review. Int J Behav Nutr Phys Act. 2012 Apr 30;9(1):49. doi: 10.1186/1479-5868-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finn K, Johannsen N, Specker B. Factors associated with physical activity in preschool children. J Pediatr. 2002 Jan;140(1):81–85. doi: 10.1067/mpd.2002.120693. [DOI] [PubMed] [Google Scholar]
- 28.Burdette HL, Whitaker RC, Daniels SR. Parental report of outdoor playtime as a measure of physical activity in preschool-aged children. Arch Pediatr Adolesc Med. 2004 Apr;158(4):353–357. doi: 10.1001/archpedi.158.4.353. [DOI] [PubMed] [Google Scholar]
- 29.Rundle A, Goldstein IF, Mellins RB, Ashby-Thompson M, Hoepner L, Jacobson JS. Physical activity and asthma symptoms among New York City Head Start Children. J Asthma. 2009 Oct;46(8):803–809. [PMC free article] [PubMed] [Google Scholar]
- 30.Goran MI, Hunter G, Nagy TR, Johnson R. Physical activity related energy expenditure and fat mass in young children. Int J Obes Relat Metab Disord. 1997 Mar;21(3):171–178. doi: 10.1038/sj.ijo.0800383. [DOI] [PubMed] [Google Scholar]
- 31.Goran MI, Reynolds KD, Lindquist CH. Role of physical activity in the prevention of obesity in children. Int J Obes Relat Metab Disord. 1999 Apr;23(Suppl 3):S18–33. doi: 10.1038/sj.ijo.0800880. [DOI] [PubMed] [Google Scholar]