Abstract
Background
Recently, procedures have been developed to model specific facets of human alcohol abuse disorders, including those that model excessive binge-like drinking (i.e., “drinking in the dark”, or DID procedures) and excessive dependence-like drinking (i.e., intermittent ethanol vapor exposure). Similar neuropeptide systems modulate excessive ethanol drinking stemming from both procedures, raising the possibility that both paradigms are actually modeling the same phenotypes and triggering the same central neuroplasticity. Therefore, the goal of the present project was to study the effects of a history of binge-like ethanol drinking, using DID procedures, on phenotypes that have previously been described with procedures to model dependence-like drinking.
Methods
Male C57BL/6J mice first experienced 0 to 10 4-day binge-like drinking episodes (3 days of rest between episodes). Beginning 24-h after the final binge-like drinking session, mice were tested for anxiety-like behaviors (with elevated plus maze (EPM) and open-field locomotor activity tests), ataxia with the rotarod test, and sensitivity to handling-induced convulsions (HICs). One week later, mice began a 40-day 2-bottle (water versus ethanol) voluntary consumption test with concentration ranging from 10 to 20% (v/v) ethanol.
Results
A prior history of binge-like ethanol drinking significantly increased subsequent voluntary ethanol consumption and preference, effects most robust in groups that initially experienced 6 or 10 binge-like drinking episodes and completely absent in mice that experienced 1 binge-like drinking episode. Conversely, a history of binge-like ethanol drinking did not influence anxiety-like behaviors, ataxia, or HICs.
Conclusions
Excessive ethanol drinking stemming from DID procedures does not initially induce phenotypes consistent with a dependence-like state. However, the subsequent increases of voluntary ethanol consumption and preference that become more robust following repeated episodes of binge-like ethanol drinking may reflect the early stages of ethanol dependence, suggesting that DID procedures may be ideal for studying the transition to ethanol dependence.
Keywords: ethanol, binge-like, dependence, C57BL/6J, anxiety-like, ataxia, seizure
INTRODUCTION
Early pre-clinical alcoholism research primarily relied on animal models that involved voluntary consumption of ethanol, in which rats or mice were given 24-h/day access to ethanol and water simultaneously in separate bottles. However, voluntary ethanol consumption may not be the most appropriate model for human alcohol abuse disorders, as rodents typically consume low amounts of ethanol that do not generate blood ethanol levels thought to be pharmacologically meaningful. Recently, procedures have been developed to model specific facets of human alcohol abuse disorders. Such procedures include those that model excessive binge-like drinking prior to the development of dependence (Boehm et al., 2008; Lowery-Gionta et al., 2012; Lowery et al., 2010; Sparrow et al., 2012; Sprow and Thiele, 2012), excessive ethanol intake stemming from ethanol dependence (Funk et al., 2007; Gilpin et al., 2011; Roberts et al., 1996), and excessive relapse-like ethanol drinking (Spanagel and Holter, 2000; Spanagel et al., 1996; Sparta et al., 2009) and ethanol seeking behaviors (Le et al., 1998; 1999; Weiss and Liu, 2002). These models have been useful for discovering the neurochemical pathways and the neurocircuitry involved in alcohol-related behaviors.
Recent evidence has been presented suggesting that neuropeptide systems, specifically neuropeptide Y (NPY) and corticotropin releasing factor (CRF), modulate excessive binge-like ethanol drinking in C57BL/6J mice that have been described as non-dependent. Using the procedure called “drinking in the dark” (DID; Rhodes et al., 2005; 2007), central administration of CRF-1 receptor (CRF1R) antagonists and NPY were found to prevent binge-like ethanol drinking in mice. Interestingly, CRF1R antagonists and NPY failed to alter low level non-binge-like ethanol drinking, suggesting that central CRF and NPY systems are recruited only after sufficient blood/brain ethanol concentrations are achieved, which may motivate continued binge-like drinking (Lowery-Gionta et al., 2012; Lowery et al., 2010; Sparrow et al., 2012). Interestingly, central administration of CRF1R antagonists or NPY have also been shown to protect against excessive dependence-like ethanol drinking in rodents that have had a prior history of repeated intermittent ethanol vapor exposure, but these compounds failed to alter low level ethanol intake in non-dependent animals (Gilpin et al., 2011; Roberto et al., 2010). The striking similarity between results obtained with models of binge-like ethanol drinking in “non-dependent” animals and in “dependent” animals has led our group to propose that overlapping systems may be involved (Lowery-Gionta et al., 2012; Sparrow et al., 2012; Thiele, 2012). Theoretically, CRF signaling is increased and NPY signaling is blunted when sufficient brain ethanol levels are achieved during binge-like drinking, stimulating continued excessive ethanol intake. Alterations in neuropeptide signaling are thought to initially be transient, but with repeated episodes of binge-like drinking these neuroplastic changes may become rigid, contributing to excessive dependence-like drinking.
One possibility is that procedures to promote binge-like ethanol drinking and procedures to induce dependence-like ethanol drinking are actually modeling the same phenotypes and trigger the same neuroplastic changes in the brain. Thus, it might be argued that the amount of ethanol exposure achieved with DID procedures is sufficient to induce a dependence-like state, and in fact that exposure to either DID procedures or ethanol vapor accomplish the same end-point. If this is true, one would predict that DID procedures should promote phenotypes consistent with ethanol dependence.
Therefore, the goal of the present project was to study the effects of a history of binge-like ethanol drinking on phenotypes that have previously been described following intermittent ethanol vapor exposure (Becker and Lopez, 2004; Crabbe et al., 1991; Kliethermes et al., 2004; Lopez et al., 2011; Philibin et al., 2012). A dependence-like state is typically associated with subsequent increases of voluntary ethanol consumption and self-administration (Becker and Lopez, 2004), and mice have been shown to exhibit elevated anxiety-like behaviors (Kliethermes et al., 2004), increased ataxia (Philibin et al., 2012), and increased sensitivity to handling-induced convulsion (HICs) following 24-h or more of withdrawal after ethanol vapor exposure (Beadles-Bohling and Wiren, 2006; Homanics et al., 1998). Here, we assessed anxiety-like behaviors, ataxia, HIC, and finally voluntary ethanol consumption in mice with varying amounts of experience with binge-like ethanol drinking using DID procedures.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were 6–8 weeks of age and weighed 20–30 g upon arrival. Mice were housed individually in standard plastic cages and allowed to habituate to the environment for at least 1 week before experimental procedures were initiated. The animal colony room was maintained at ~22°C with a 12h/12h light/dark cycle (lights on at 0700 h). Mice had ad libitum access to food throughout all experiments and ad libitum access to water except during ethanol access, as noted below. All procedures used were in accordance with the National Institute of Health guidelines and were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Drinking-in-the-dark Procedure
All experiments utilized a 4 day drinking-in-the-dark (DID) procedure that our group and others have used previously to generate high levels of ethanol intake that are associated with blood ethanol concentrations (BECs) in excess of 80 mg/dl, typically greater than 100 mg/dl (Lowery-Gionta et al., 2012; Lowery et al., 2010; Rhodes et al., 2005; 2007; Sparrow et al., 2012). On days 1–3, beginning 3 h into the dark cycle, water bottles were removed from all cages and replaced with a bottle containing 20% (v/v) ethanol solution. Mice had 2 h of access to ethanol, after which the ethanol bottles were removed from cages and water bottles were replaced. The same procedure was followed on day 4 except that ethanol access was extended to 4 h. We used this schedule of ethanol access because Rhodes et al. (2005) noted robust binge-like drinking with these procedures and because we have previously used this ethanol access schedule (Lowery-Gionta et al., 2012; Lyons et al., 2008; Sparrow et al., 2012; Sparta et al., 2008), thus allowing us to make direct comparisons of the present work with our previous research. Each 4 day DID procedure is referred to as a single binge-like ethanol drinking “cycle” or “episode” below. Between each binge-like drinking cycle, mice were given 3 days of rest with no access to ethanol. In Experiment 1 described below, mice were separated into groups balanced for body weights. Groups of mice experienced 1 (n = 13) or 6 (n = 13) binge-like drinking cycles and a third control group drank water throughout the binge-like drinking portion of the experiment (n = 13). In Experiment 2 described below, animals were divided into 5 groups balanced for body weights: The groups experienced 1, 3, 6, or 10 binge-like drinking cycles, and a fifth control group drank water only throughout the binge-like drinking portion of the experiment (n =10/group). For both experiments, initiation of binge-like drinking was staggered between groups such that all mice experienced their last binge-like drinking cycle at the same age. Though age of first ethanol exposure was different between groups (an unavoidable potential confound stemming from holding age of sacrifice constant between groups), it is important to note that all mice were of adult age at first ethanol exposure.
The EPM, open-field, rotarod, and HIC tests described below were all conducted in the animals' dark cycle to be consistent with the timing of DID procedures. The EPM and open-field tests were conducted in the dark; however, it was necessary for the rotarod and HIC tests to be conducted with light for data collection. Additionally, mice were transported to testing rooms on a cart covered with a tarp to limit light exposure.
Elevated Plus Maze (EPM): Assessment of Anxiety-Like Behavior
The EPM test is a pharmacologically validated model for the assessment of anxiety in rodents (Pellow et al., 1985). For both Experiments 1 and 2, the EPM test was initiated 24-h after the completion of the final binge-like drinking test. We have described the apparatus and the 5-min procedure previously (Fee et al., 2004). The behavior in the EPM was recorded using a digital video camera with night vision capability, and measures were scored by condition-blinded individuals trained to identify the various dependent measures. With the EPM test, anxiety-like behavior is reflected as reduced number of entries into the open arms and in the time spent in the open arms, along with an increase in the number of entries and amount of time spent in the closed arms of the EPM.
Open-Field Locomotor Activity Test: Assessment of Anxiety-Like Behavior
The open-field test is commonly used to assess anxiety-like behavior in rodents, in which avoidance of the center portion of the open arena is thought to reflect heightened anxiety (Choleris et al., 2001; Fee et al., 2004). After the completion of the EPM test in Experiments 1 and 2, mice were transported in their home cages to an adjacent room and allowed to rest for 10 min. We have described the apparatus and procedure elsewhere (Fee et al., 2004). Testing sessions were 10 minutes in duration, and marginal time and distance traveled (cm), and central time and distance traveled (cm) in the open-field arena were measured over the course of the session.
Accelerating Rotarod Test: Assessment of Ataxia
Recent observations show that mice experiencing ethanol withdrawal exhibit elevated ataxia, and have difficulty maintaining balance on a rotarod apparatus (Philibin et al., 2012). After open-field testing in Experiment 2, mice were allowed to rest for 15 min in their home cage and were then placed on a rotarod apparatus (Ugo Basile, Italy) with an initial rotation speed of 4 rpm. The speed was gradually increased to 40 rpm over a period of 5 min and latency-to-fall and rpm at the time of fall were recorded. The test was repeated three more times, with 5 min between each test. Average latency-to-fall and rpm at time of fall were averaged over the 4 test trials.
Handling-Induced Convulsions (HIC): Assessment of Seizure Sensitivity
Thirty min after administration of the rotarod tests in Experiment 2, HIC tests were conducted. For HIC testing, each mouse was picked up (from its home cage) by the tail, and if this failed to elicit a convulsion, the mouse was spun gently through a 180° arc. The behavior of each mouse during the HIC test was video recorded and later scored independently by 2 condition-blinded raters (scores were averaged for analyses). The HIC rating scale ranged from 0–7, with a score of 0 given to mice showing no convulsions and a score of 7 given to mice exhibiting severe tonic-clonic convulsions. A d etailed description of HIC procedures and the scoring scale is described elsewhere (Crabbe et al., 1991).
Continuous 2-Bottle Voluntary Ethanol Consumption
Approximately one week after the completion of binge-like ethanol drinking in Experiments 1 and 2, the mice were tested for voluntary ethanol consumption using a home cage 2-bottle choice procedure. Over 8 days, mice were given 24 h access to 2-bottles in their homecage, one containing tap water and the other containing a 10% (v/v) ethanol solution. The concentration of ethanol was changed every 8 days as follows: 10, 15, 20, 15, and 10% ethanol. The positions of the bottle were alternated every day to control for position preferences. Each drinking bottle was weighed every day to calculate fluid intake and body weights were recorded every 4 days.
Data Analyses
To obtain a measure that corrected for individual differences in body weight, grams of ethanol consumed per kilogram of body weight were calculated. Ethanol preference ratios were also calculated by dividing the volume of ethanol consumed by the total fluid (ethanol + water) consumption. For all experiments, differences between groups were analyzed using analysis of variance (ANOVA). With significant interaction effects, or main effects in the absence of significant interactions, post hoc comparisons were performed using Bonferroni corrected t-tests to parse out group differences. Statistics were analyzed using SPSS (version 17.0; Armonk, NY) software. In all cases, p<0.05 (two tailed) was used to indicate statistical significance.
RESULTS
Drinking-in-the-dark
In Experiment 1, there were no significant differences between the groups that experienced 1 (5.42 ± 0.28 g/kg/4h) or 6 (6.05 ± 0.29 g/kg/4h) binge-like drinking cycles in terms of the amount of ethanol consumed during the final binge-like drinking session [F(1,25) = 2.486, p = 0.127]. Similarly, in Experiment 2 there were no significant differences between the groups that experienced 1 (4.43 ± 0.29 g/kg/4h), 3 (5.09 ± 0.41 g/kg/4h), 6 (6.16 ± 0.36 g/kg/4h), or 10 (5.36 ± 0.31 g/kg/4h) binge-like drinking cycles in the amount of ethanol consumed during the final binge-like drinking session [F(3,36) = 1.374, p = 0.226]. These data suggest that up to 10 binge-like drinking cycles did not significantly increase the level of binge-like ethanol drinking, which may reflect a ceiling effect stemming from the high levels of ethanol intake that are evident even after one DID cycle. Although we did not assess blood ethanol concentrations (BECs) in an attempt to avoid the potential confounding effects of stress on subsequent measures, the amount of ethanol consumed by mice on the final day of binge-like ethanol drinking was consistent with amounts that we have previously reported and which produced BECs of 80 mg/dL or greater (Lowery-Gionta et al., 2012; Lowery et al., 2010; Sparrow et al., 2012; Sparta et al., 2008).
Elevated Plus Maze (EPM): Assessment of Anxiety-Like Behavior
Data from the EPM tests for Experiments 1 and 2 are presented in Table 1. Data sets from both experiments failed to reveal any significant effects of binge-like ethanol drinking on subsequent anxiety-like behavior. In Experiment 1, relative to the water control group, mice that experienced 1 or 6 cycles of binge-like ethanol drinking exhibited no significant differences in open arm time [F(2,36) = 0.146, p = 0.865] or percentage total time spent in open arms [F(2,36) = 0.145, p = 0.856]. Furthermore, there were no group differences in closed arm time [F(2,37) = 1.056, p = 0.358] or percentage of total time spent in closed arms [F(2,37) = 1.058, p = 0.357]. A lack of group differences in total arm entries [F(2,37) = 1.05, p= 0.360] suggests that the history of binge-like ethanol drinking did not impact overall activity (H2O = 18.38 ± 1.20 entries; 1-cycle = 20.46 ± 1.07 entries; 6-cycle = 18.92 ± 0.84 entries).
TABLE 1.
Average (Mean ± SEM) behavior in the elevated plus maze (EMP) tests from Experiments 1 and 2.
| Time in Open Arms (s) | Percentage of Time in Open Arms (%) | Time in Closed Arms (s) | Percentage of Time in Closed Arms (%) | |
|---|---|---|---|---|
| Experiment 1 | ||||
| H20 | 35.8 ± 5.58 | 11.9 ± 1.86 | 136.3 ± 10.67 | 45.4 ± 3.56 |
| 1-cycle | 41.3 ± 7.10 | 13.76 ± 2.36 | 150.6 ± 9.23 | 49.2 ± 3.15 |
| 6-cycle | 38.2 ± 7.95 | 12.74 ± 2.65 | 155.0 ± 8.66 | 51.7 ± 2.89 |
| Experiment 2 | ||||
| H20 | 70.9 ± 10.0 | 23.6 ± 3.35 | 164.2 ± 14.13 | 54.7 ± 4.71 |
| 1-cycle | 62.9 ± 12.86 | 21.0 ± 4.28 | 187.9 ± 16.13 | 62.7 ± 5.38 |
| 3-cycle | 41.3 ± 5.78 | 13.8 ± 1.93 | 214.3 ± 10.0 | 71.4 ± 3.33 |
| 6-cycle | 80.8 ± 9.79 | 26.9 ± 3.26 | 164.9± 11.12 | 55.0 ± 3.71 |
| 10-cycle | 54.3 ± 12.67 | 18.1 ± 4.22 | 189.6 ± 19.58 | 63.2 ± 6.53 |
In Experiment 2, relative to the water control group there was no evidence of alterations of anxiety-like behavior in mice that experienced 1 to 10 cycles of binge-like ethanol drinking. Thus, there were no group differences in open arm time [F(4,44)= 2.012, p = 0.109] or percentage total time spent in open arms [F(4,44)= 2.007, p = 0.110]. Furthermore, there were no group differences in closed arm time [F(4,45) = 2.016, p = 0.108] or percentage of total time spent in closed arms [F(4,45) = 2.013, p = 0.109]. Finally, there were no significant differences between groups on the number of total arm entries [F(4,45) = 0.448, p= 0.774], suggesting that the history of binge-like ethanol drinking did not impact overall activity (H2O = 22.2 ± 1.10 entries; 1-cycle = 21.3 ± 1.59 entries; 3-cycle = 21.5 ± 1.68 entries; 6-cycle = 22.7 ± 1.32 entries; 10-cycle = 20.3 ± 1.03 entries).
Open-Field Locomotor Activity Test: Assessment of Anxiety-Like Behavior
Data from the open-field locomotor activity tests from Experiments 1 and 2 are presented in Table 2. As with the EPM test, data sets from both experiments failed to provide evidence of elevated anxiety-like behavior stemming from a history of binge-like ethanol drinking. For data from Experiment 1, there were no group differences in average distance traveled in the margins [F(2,37) = 0.545, p = 0.584], average time spent in margins [F(2,37) = 2.505, p = 0.095], average distance traveled in the center [F(2,36) = 0.867, p = 0.429], or average time spent in the center [F(2,37) = 0.507, p = 0.095]. Additionally, analysis of total distance traveled data failed to achieve statistical significance [F(2,37) = 0.688, p = 0.509], providing further support that a history of binge-like ethanol drinking did not alter overall locomotor behavior (H2O = 2982.38 ± 226.83 cm2/10min; 1-cycle = 3278 ± 195.19 cm2/10min; 6-cycle = 2956.57 ± 218.52 cm2/10min; 10-cycle = 3146 ± 134.36 cm2/10min).
Table 2.
Average (Mean ± SEM) behavior in the open-field activity tests from Experiments 1 and 2.
| Marginal Distance (cm2/10min) | Time in Margin (s) | Center Distance (cm2/10min) | Time in Center (s) | |
|---|---|---|---|---|
| Experiment 1 | ||||
| H20 | 2271.4 ± 198.88 | 505.3 ± 5.58 | 711.0 ± 36.61 | 94.7 ± 5.58 |
| 1-cycle | 2501.5 ± 119.17 | 510.5 ± 7.59 | 673.8 ± 68.14 | 89.5 ± 7.59 |
| 6-cycle | 2325.9 ± 155.85 | 521.3 ± 5.53 | 609.2 ± 60.66 | 78.7 ± 5.53 |
| Experiment 2 | ||||
| H20 | 2194.7 ± 163.56 | 507.6 ± 9.60 | 747.2 ± 66.37 | 92.4 ± 9.60 |
| 1-cycle | 2351.8 ± 162.53 | 509.1 ± 8.49 | 742.6 ± 71.58 | 90.9 ± 8.49 |
| 3-cycle | 2418.1 ± 163.64 | 500.1 ± 14.81 | 655.3 ± 101.75 | 99.9 ± 14.81 |
| 6-cycle | 2311.0 ± 277.12 | 505.0 ± 7.90 | 678.4 ± 56.81 | 95.0 ± 7.90 |
| 10-cycle | 2414.6 ± 125.60 | 500.9 ± 16.04 | 732.1 ± 86.19 | 99.1 ± 16.04 |
The same pattern of results were observed from the open-field test of Experiment 2. There were no group differences in average distance traveled in the margins [F(4,45) = 0.244, p = 0.912], average time spent in margins [F(4,45) = 0.112, p = 0.978], average distance traveled in the center [F(4,45) = 0.283, p = 0.887], and average time spent in the center [F(4,45) = 0.112, p = 0.978], and analysis of total distance traveled failed to achieve statistical significance [F(4,45) = 0.133, p = 0.969] (H2O = 2942 ± 206.4 cm2/10min; 1-cycle = 3094.5 ± 212.46 cm2/10min; 3-cycle = 3037.7 ± 256.41 cm2/10min; 6-cycle = 2989.7 ± 275.36 cm2/10min; 10-cycle = 3146 ± 134.36 cm2/10min).
Accelerating Rotarod Test: Assessment of Ataxia
Data averaged over the 4 rotarod tests performed in Experiment 2 are presented in Table 3. Separate one-way ANOVAs performed on latency to fall data [F(4,45) = 0.691, p = 0.602] and average RPM at fall data [F(4,45) = 0.724, p = 0.58] both failed to achieve statistical significance, indicating that a history of binge-like ethanol drinking did not promote increased ataxia.
Table 3.
Average scores (mean ± SEM) from the rotarod and HIC test in Experiment 2.
| Rotarod: Latency to Fall (s) | Rotarod: RPM at Fall | HIC Score | |
|---|---|---|---|
| H20 | 175.4 ± 13.12 | 25.9 ± 1.82 | 0.23 ± 0.12 |
| 1-cycle | 168.0 ± 13.66 | 25.2 ± 1.96 | 0.14 ± 0.04 |
| 3-cycle | 142.9 ± 18.00 | 25.5 ± 2.46 | 0.27 ± 0.07 |
| 6-cycle | 151.8 ± 17.39 | 25.3 ± 2.54 | 0.18 ± 0.06 |
| 10-cycle | 151.7 ± 17.23 | 26.6 ± 2.50 | 0.13 ± 0.06 |
HIC = Handling-induced convulsion (scored on a scale from 0 to 7).
Handling-Induced Convulsions (HIC): Assessment of Seizure Sensitivity
Data from the HIC test performed in Experiment 2 are presented in Table 3. An ANOVA performed on these data failed to achieve statistical significance [F(4,45) = 0.651, p = 0.629], suggesting that a history of binge-like ethanol drinking did not alter seizure sensitivity.
Continuous 2-Bottle Voluntary Ethanol Consumption
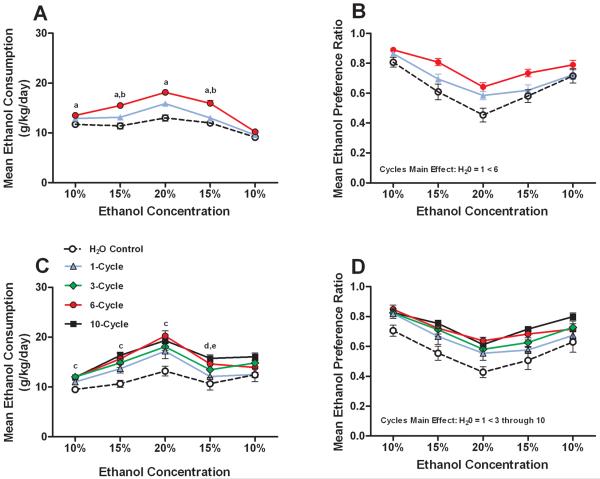
Data representing the average 8-day consumption of ethanol (g/kg) and average 8-day ethanol preference at each ethanol concentration tested in Experiment 1 (Fig. 1A and B, respectively) and Experiment 2 (Fig. 1C and D, respectively) are presented in Fig. 1. Separate two-way (ethanol concentration × number of binge-like drinking cycles) ANOVAs were performed to analyze each data set. Analysis of ethanol consumption data from Experiment 1 revealed significant main effects of ethanol concentration [F(4,148) = 49.724, p < 0.001] and binge-like drinking cycles [F(1,37) = 10.279, p < 0.001], and a significant interaction effect [F(8,148) = 2.97, p = 0.004]. Post hoc Bonferroni corrected t-tests revealed that the group with a prior history of 6 binge-like ethanol drinking cycles voluntarily drank significantly more ethanol relative to the control group during access to 4 of the 5 ethanol solutions, and more than the 1-cycle group at 2 of the 5 concentrations (Fig. 1A). Analysis of ethanol preference ratio data from Experiment 1 showed significant main effects of ethanol concentration [F(4,148) = 58.905, p < 0.001] and binge-like drinking cycles [F(1,37) = 8.357, p = 0.001], but the interaction effect was not significant [F(8,148) = 1.923, p = 0.061]. A post hoc Bonferroni corrected t-test of the binge-like drinking cycles main effect revealed that only the 6-cycle binge-like ethanol drinking group preferred ethanol significantly more than the control group (Fig. 1B).
Fig. 1.
A history of repeated cycles of binge-like ethanol (20%, v/v) drinking significantly augments subsequent 2-bottle choice voluntary ethanol consumption. Data points at each concentration of ethanol (v/v) represent an 8-day average, and data are expressed as mean g of ethanol consumed per kg of body weight per day (g/kg/day; A and C) or mean ethanol preference (ethanol consumed / total fluid intake; B and D). The top row represents data collected from Experiment 1 (A and B) and the bottom row represents data collected from Experiment 2 (C and D). Data are expressed as mean ± SEM. Significance legend (based on Bonferroni corrected t-tests): a = H20 < 6-cycles; b = 1-cycle < 6-cycles; c = H20 < 3-, 6-, and 10-cycles; d = H20 < 6- and 10-cycles; e = 1-cycle < 10-cycles. Significant differences between groups on the cycles main effect (indicated in B and D) are based on Bonferroni corrected t-tests.
Analysis of the ethanol consumption data from Experiment 2 revealed significant main effects for ethanol concentration [F(4,180) = 65.420, p < 0.001] and binge-like drinking cycles [F(4,45) = 8.173, p < 0.001], and a significant interaction effect [F(16,180) = 2.076, p = 0.011]. Post hoc Bonferroni corrected t-tests revealed that relative to the control group, mice that had a prior history for 6 or 10 binge-like drinking cycles voluntarily drank significantly more ethanol during access to 4 of the 5 ethanol solutions. Mice with a history of 3 binge-like drinking cycles drank significantly more ethanol than the control group during access to 3 of the 5 ethanol solutions. Finally, mice with a history of 10 binge-like drinking cycles drank significantly more ethanol than the 1-cycle group at one concentration (Fig. 1C). Analysis of ethanol preference data from Experiment 2 showed significant main effects of ethanol concentration [F(4,180) = 59.915, p < 0.001] and binge-like drinking cycles [F(4,45) = 5.82, p = 0.001], but the interaction effect was not significant [F(16,180) = 0.984, p = 0.476]. A post hoc Bonferroni corrected t-test of the binge-like drinking main effect showed that groups with a prior history of 3 to 10 binge-like ethanol drinking cycles showed significantly elevated ethanol preference relative to the control group (Fig. 1D).
DISCUSSION
Here we show that relative to mice with no prior history of binge-like ethanol drinking, a history of binge-like ethanol drinking using DID procedures in male C57BL/6J mice promoted subsequent increases of voluntary ethanol consumption and preference without altering anxiety-like behaviors (assessed by EPM and open-field locomotor activity tests), ataxia (assessed by the accelerating rotarod test), or sensitivity to HICs. While we cannot completely rule out the possibility that testing order could have impacted test outcomes (since test order was not counterbalanced), the lack of any trends suggests that this possibility is unlikely. Importantly, increased voluntary ethanol consumption and preference were greater in mice that experienced greater numbers of binge-like ethanol drinking cycles, with mice experiencing 6 or 10 binge-like drinking episodes showing the most robust increases of subsequent voluntary ethanol drinking and preference. Conversely, there was no instance in which mice with a prior history of just one binge-like drinking cycle exhibited a significant change from the control group in subsequent ethanol intake or preference. These observations are consistent with the hypothesis that alterations in the neurocircuitry that modulates binge-like ethanol drinking become more robust and longer-lasting with increasing numbers of binge-like drinking cycles. However, other phenotypes (i.e., anxiety-like behavior, ataxia, and HICs) that have been reported with procedures that induce a dependence-like state (i.e., ethanol vapor exposure) were not evident in mice experiencing up to 10 4-day cycles of binge-like ethanol drinking. Together, these observations make a strong case that DID and ethanol vapor procedures do not lead to the same end-point, and suggest that unlike ethanol vapor exposure procedures, excessive ethanol drinking stemming from DID procedures does not initially induce a dependence-like state. However, the subsequent increases of voluntary ethanol consumption and preference that become more robust following repeated episodes of binge-like ethanol drinking may reflect the emergence of ethanol dependence, suggesting that DID procedures may be ideal for studying the early stages of the transition to ethanol dependence. We speculate that increased anxiety-like behavior, ataxia, and sensitivity to HIC may emerge with greater experience with binge-like ethanol drinking (i.e., more than 10 cycles of DID exposure).
The most striking results from the present data set were the robust and long-lasting increases of voluntary ethanol consumption and preference stemming from a history of binge-like ethanol drinking. These observations parallel those obtained with vapor inhalation models that have been shown to induce subsequent increases of voluntary self-administration or consumption of ethanol in rats (Funk et al., 2007; Gilpin et al., 2011) and mice (Becker and Lopez, 2004; Finn et al., 2007; Lopez et al., 2011). Importantly, increases of subsequent voluntary ethanol consumption in the present report resulted from animals voluntarily drinking excessive amounts of ethanol in a pattern and level that closely matches human binge drinking, rather than from forced exposure to ethanol vapor. Thus, as a model to study the neurobiology underlying the transition to an ethanol dependence-like state (Thiele, 2012), repeated binge-like drinking episodes using procedures such as DID arguably have greater face validity than models employing repeated intermittent ethanol vapor exposure.
Previous reports have shown that up to 24-h of withdrawal from ethanol vapor exposure is associated with elevated anxiety-like behaviors (Kliethermes et al., 2004), increased ataxia (Philibin et al., 2012), and increased sensitivity to HICs (Beadles-Bohling and Wiren, 2006; Becker and Veatch, 2002; Homanics et al., 1998) in mice. There are numerous possibilities that may explain differences between the present report and previous studies, including the amount, pattern, and route of ethanol exposure, the amount of ethanol withdrawal time before tests were administered, and the strain of mice used. For example, while HICs have been observed up to 24-h after ethanol withdrawal, HICs tend to be more robust in the first several hours after ethanol withdrawal (Beadles-Bohling and Wiren, 2006; Homanics et al., 1998), and strains other than C57BL/6J mice are more sensitive to withdrawal-induced HICs (Homanics et al., 1998). However, C57BL/6J mice have been reported to show elevated HICs 8-h after ethanol removal following 16-weeks of intermittent access to 20% ethanol in an escalation paradigm (Hwa et al., 2011), which is why we include this test in the present set of studies. Furthermore, strains other than C57BL/6J mice have been used to assess ataxia (Philibin et al., 2012) and anxiety-like behavior (Kliethermes et al., 2004) following ethanol vapor exposure.
Importantly though, our goal was to address the possibility that models of binge-like ethanol drinking and dependence-like ethanol drinking trigger the same phenotypes and associated neuroplasticity, a possibility that might seem particularly relevant given evidence of similar involvement of CRF and NPY signaling in binge-like and dependence-like ethanol drinking (Gilpin et al., 2011; Lowery-Gionta et al., 2012; Roberto et al., 2010; Sparrow et al., 2012). However, because we found that binge-like ethanol drinking did not promote many phenotypes thought to be hallmarks of ethanol dependence, we argue that the DID procedure models excessive ethanol intake prior to the onset of dependence. Consistent with this argument, we have found that a history of binge-like ethanol drinking blocks the ability of CRF to enhance GABAergic transmission in the central amygdala (CeA) of mice (Lowery-Gionta et al., 2012), while others have found that intermittent ethanol vapor exposure augments the ability of CRF to enhance GABAergic transmission in the CeA (Roberto et al., 2010). We would predict that more extensive experience with binge-like drinking episodes would lead to the development of neuroplastic alterations in line with models of ethanol dependence.
The possible mechanisms by which a history of binge-like ethanol drinking promotes subsequent increases of voluntary ethanol intake deserve consideration. It might be argued that mice previously exposed to DID procedures were more familiar with ethanol than the water drinking control group, and thus more willing to consume ethanol during voluntary testing. However, this seems unlikely as the control mice would be expected to become familiar with ethanol during the early stages of voluntary consumption, yet group differences in voluntary drinking were evident throughout the 40-day test. Another possibility is that mice may have developed metabolic tolerance over the course of repeated DID episodes. While we did not measure BECs here, in two separate publications (Lowery-Gionta et al., 2012; Sparrow et al., 2012) we found that there were no significant differences between groups of mice experiencing 1 or 6 cycles of DID either in terms of the amount of ethanol that they consumed or the BECs that they achieved. Since similar BECs were achieved after similar levels of ethanol intake in mice with a history of 1 or 6 binge drinking cycles, metabolic tolerance does not appear to be a likely explanation for increased voluntary consumption of ethanol. The development of physiological tolerance is an attractive possibility, as repeated DID ethanol access was found to promote tolerance to the effects of ethanol injections on the balance beam test of ataxia (Linsenbardt et al., 2011). Additionally, a previous report identified low sensitivity to the aversive effects of ethanol (Holstein et al., 2011) as a potential mechanism that modulates binge-like ethanol drinking. Thus blunted sensitivity to the aversive effects of ethanol following binge-like drinking may promote subsequent increases of voluntary ethanol consumption.
At the level of neurocircuitry (Sprow and Thiele, 2012), CRF and NPY are two candidate pathways of particular interest. We have recently found that in mice with a 3-cycle history of binge-like ethanol drinking, CRF and NPY immunoreactivity and the effects of CRF or NPY on GABAergic transmission in the CeA were significantly altered for up to 24-h after ethanol removal (Lowery-Gionta et al., 2012; Sparrow et al., 2012). These observations suggest that binge-like ethanol drinking is associated with rigid neuroplastic alterations of CRF and NPY signaling in the CeA. We speculate that these changes contribute to the transition to ethanol dependence. Evidence of increased voluntary ethanol drinking resulting from a history of binge-like ethanol drinking in the present report may reflect, in part, such changes in CRF and NPY signaling.
In summary, here we report that while repeated cycles of binge-like ethanol drinking promoted subsequent increases of voluntary ethanol intake, an effect that becomes more robust with increasing numbers of binge-like drinking episodes, a history of binge-like ethanol drinking did not impact other phenotypes thought to characterize a dependence-like state, including anxiety-like behavior, ataxia, and HICs. Together, these observations make a strong case that DID procedures (to model binge-like ethanol drinking) and ethanol vapor procedures (to model dependence-like drinking) do not lead to the same neurobiological end-point, at least initially. Unlike ethanol vapor exposure procedures, excessive ethanol drinking stemming from DID procedures does not initially induce a dependence-like state. However, the subsequent increases of voluntary ethanol consumption and preference that become more robust following repeated episodes of binge-like ethanol drinking may reflect the early stages of ethanol dependence, suggesting that DID procedures may be ideal for studying the transition to ethanol dependence.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health grants AA015148 and AA013573, AA019454, AA017668 and AA020911. Portions of this work were support by Department of Defense grant W81XWH-09-1-0293.
REFERENCES
- Beadles-Bohling AS, Wiren KM. Anticonvulsive effects of kappa-opioid receptor modulation in an animal model of ethanol withdrawal. Genes Brain Behav. 2006;5:483–496. doi: 10.1111/j.1601-183X.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Becker HC, Veatch LM. Effects of lorazepam treatment for multiple ethanol withdrawals in mice. Alcohol Clin Exp Res. 2002;26:371–380. [PubMed] [Google Scholar]
- Boehm SL, 2nd, Moore EM, Walsh CD, Gross CD, Cavelli AM, Gigante E, Linsenbardt DN. Using drinking in the dark to model prenatal binge-like exposure to ethanol in C57BL/6J mice. Dev Psychobiol. 2008;50:566–578. doi: 10.1002/dev.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill C, Belknap JK. Acute dependence on depressant drugs is determined by common genes in mice. J Pharmacol Exp Ther. 1991;257:663–667. [PubMed] [Google Scholar]
- Fee JR, Sparta DR, Knapp DJ, Breese GR, Picker MJ, Thiele TE. Predictors of high ethanol consumption by RIIbeta knockout mice: Assessment of anxiety and ethanol-induced sedation. Alcohol Clin Exp Res. 2004;28:1459–1468. doi: 10.1097/01.ALC.0000141809.53115.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased Drinking During Withdrawal From Intermittent Ethanol Exposure Is Blocked by the CRF Receptor Antagonist d-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M. Neuropeptide y opposes alcohol effects on gamma-aminobutyric Acid release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry. 2011;69:1091–1099. doi: 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, Le NQ, Kist F, Mihalek R, Hart AR, Quinlan JJ. Ethanol tolerance and withdrawal responses in GABA(A) receptor alpha 6 subunit null allele mice and in inbred C57BL/6J and strain 129/SvJ mice. Alcohol Clin Exp Res. 1998;22:259–265. [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL., 2nd Tolerance to ethanol's ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcohol Clin Exp Res. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol Clin Exp Res. 2011;35:953–962. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AM, Lowery EG, Sparta DR, Thiele TE. Effects of food availability and administration of orexigenic and anorectic agents on elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:1962–1968. doi: 10.1111/j.1530-0277.2008.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Philibin SD, Cameron AJ, Schlumbohm JP, Metten P, Crabbe JC. Ethanol withdrawal-induced motor impairment in mice. Psychopharmacology (Berl) 2012;220:367–378. doi: 10.1007/s00213-011-2483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self- administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM. Pharmacological validation of a new animal model of alcoholism. Journal of Neural Transmission - General Section. 2000;107:669–680. doi: 10.1007/s007020070068. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, Rinker JA, Jijon AM, Pena J, Navarro M, Kash TL, Thiele TE. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology. 2012;37:1409–1421. doi: 10.1038/npp.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM, 3rd, Fee JR, Knapp DJ, Breese GR, Thiele TE. The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin Exp Res. 2009;33:31–42. doi: 10.1111/j.1530-0277.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav. 2012;106:325–331. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE. Commentary: studies on binge-like ethanol drinking may help to identify the neurobiological mechanisms underlying the transition to dependence. Alcohol Clin Exp Res. 2012;36:193–196. doi: 10.1111/j.1530-0277.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Liu X. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neuroci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]