Abstract
Prior research suggests that infantś action production affects their action understanding, but little is known about the aspects of motor experience that render these effects. In Study 1, the relative contributions of self-produced (n = 30) and observational (n = 30) action experience on 3-month-old infants’ action understanding was assessed using a visual habituation paradigm. In Study 2, generalization of training to a new context was examined (n = 30). Results revealed a unique effect of active over observational experience. Further, findings suggest that benefits of trained actions do not generalize broadly, at least following brief training.
Discerning meaningful structure in others’ actions is fundamental to human experience and survival. From early in childhood, we see others’ behaviors not just as movements of physical bodies through space but as actions structured by intentions. This basic aspect of social cognition provides a critical foundation for early social, cognitive and linguistic development. By the second year of life, children’s social referencing (Moses, Baldwin, Rosicky, & Tidball, 2001; Repacholi & Gopnik, 1997), language learning (Baldwin & Moses, 2001; Tomasello, 1999) and imitative learning (Carpenter, Akhtar, & Tomasello, 1998; Meltzoff, 1995) are guided by the analysis of others’ intentions. Foundational to this ability is the understanding that others’ actions are structured by goals and objects of attention.
Converging evidence from multiple laboratories and approaches indicates that sensitivity to others’ intentions can be traced to the first year of postnatal life (see Woodward, Sommerville, Gerson, Henderson, & Buresh, 2009 for a review). By 9 to 12 months, infants’ spontaneous social responses in naturalistic and experimental contexts strongly indicate that they understand others’ intentions in acting (Behne, Carpenter, Call, & Tomasello, 2005; Tomasello, 1999). Even before these responses are evident, by 5 to 7 months of age, infants respond selectively to the goal structure of others’ actions in looking time experiments (e.g., Biro & Leslie, 2006; Csibra, 2008; Luo & Baillargeon, 2005; Luo & Johnson, 2009; Woodward, 1998; 1999) and in their tendency to reproduce others’ action goals (e.g., Gerson & Woodward, 2012; Hamlin, Hallinan, & Woodward, 2008). To illustrate, when they are habituated to a goal-directed action, such as reaching, infants show a strong novelty response to test events in which the goal of the action has changed, but little or no novelty response to test events in which the physical movements change but the goal is preserved (e.g., Woodward, 1998). Control conditions and analyses of infants’ attention to the elements in the events have confirmed that infants’ responses reflect their representation of others’ actions as structured by the relation between the agent and his or her goal (see Woodward et al., 2009 for a review).
These findings raise the question of the factors that contribute to infants’ sensitivity to the goal structure of others’ actions. Researchers from several different theoretical and methodological perspectives have highlighted the contributions of infants’ own experience as intentional agents in structuring their understanding of others’ actions (Barresi & Moore, 1996; Daum, Prinz, & Aschersleben, 2011; Gallese, Rochat, Cossu, & Sinigaglia, 2009; Meltzoff, 2005; Tomasello, 1999; Woodward et al., 2009). These proposals rest on the idea that the cognitive representations that guide infants’ own actions provide information relevant to understanding others’ actions as structured by goals. These proposals vary along a number of dimensions (see Gerson & Woodward, 2010 for a review). For example, some hypothesize that the relevant representations involve mental state attributions (Barresi & Moore, 1996; Meltzoff, 2005) whereas others hypothesize that they reflect action-level structural descriptions (Daum, Sommerville & Prinz, 2009; Gallese et al., 2009). Despite these differences, these accounts all assume that the relevant representations involve actions as being structured with respect to goals. They hypothesize that developments in action control support the emergence of goal-based action representations, and these, in turn, support the understanding of others’ goal-directed actions. Thus, across these accounts, self-produced actions are hypothesized to provide infants with unique insight into the goals of others.
This proposal has gained further support from several bodies of work documenting that adult action production and perception recruit common cognitive representations (e.g., Hommel, Müsseler, Aschersleben, & Prinz, 2001; Prinz, 1990), and involve overlapping patterns of neural activation (e.g., Rizzolatti & Craighero, 2004; see also Di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992). Recent findings suggest similar patterns of neural activation in infants, in that neural responses associated with action production are observed when infants view others’ goal-directed actions (e.g., Marshall & Meltzoff, 2011; Saby, Marshall, & Meltzoff 2012; Shimada & Hiraki, 2006; Southgate, Johnson, Karoui, & Csibra, 2010; Southgate, Johnson, Osborne, & Csibra, 2009). As yet, however, it is not known whether these patterns of neural activation in infants reflect a functional role for the motor system in the perception of others’ goals. If this were the case, then it would be predicted that the foundational developments that occur in the motor system during infancy would influence developments in infants’ action understanding.
These theoretical perspectives and bodies of empirical work raise the question of whether, and how, infants’ experience as agents affects their sensitivity to others’ goal-directed actions. Initial evidence suggests that it does. To start, spontaneous developments in infants’ actions correlate with their understanding of those same actions as goal-directed in others. Visual habituation studies have found a relation between infants’ own action production and their recognition of the goal of the relevant action (Brune & Woodward, 2007; Daum et al., 2011; Sommerville & Woodward, 2005; Woodward & Guajardo, 2002). In addition, recent experiments have shown that infants’ anticipation of the outcomes of others’ actions (Cannon, Woodward, Gredebäck, von Hofsten, & Turek, 2011; Gredebäck & Kuchokova, 2010; Kanakogi & Itakura, 2011) and motor resonance (evidenced through mu rhythm suppression over motor areas) while observing actions (van Elk, van Schie, Hunnius, & Bekkering, 2008) correlate with infants’ own engagement in similar actions.
Further, experimental manipulations that alter infants’ own actions have been shown to influence their visual preference for those actions over others (Hauf, Aschersleben & Prinz, 2007) and to affect their perception of others’ actions as goal-directed (Sommerville, Hildebrand & Crane, 2008; Sommerville, Woodward & Needham, 2005). To illustrate, Sommerville et al. (2005) tested infants at three months, an age at which infants’ own goal-directed actions and their responses to others’ goal-directed actions are limited. Infants were given practice using Velcro-covered “sticky” mittens to apprehend objects by swiping at them (Needham, Peterman & Barrett, 2002). Infants were also tested in a habituation paradigm to assess their sensitivity to the goal structure of others’ mittened reaches. They were habituated to an event in which a mittened hand reached toward and grasped one of two toys. Following habituation, infants viewed test events in which the toys’ positions were reversed and the hand either reached toward the same toy, moving through a new path do so (old-goal events) or reached to the other toy, moving through the same path as during habituation (new-goal events).
Infants who had engaged in mittened activity prior to the habituation paradigm responded systematically to the goal structure of the events in the habituation task. That is, they looked reliably longer on new-goal trials than on old-goal trials. In contrast, infants who were not given practice acting on objects with mittens prior to the habituation paradigm did not respond systematically. Furthermore, there was a correlation between infants’ actions with the mittens and their subsequent responses in the habituation paradigm: Infants who engaged in more coordinated visual and manual contact with the toys while wearing the mittens showed a stronger novelty response to a change in the goal structure of the observed action.
This study provided the first direct evidence that infants’ self-produced actions support their analysis of the goal structure of others’ actions. As infants acted with the mittens, however, they also created visual examples of this novel action, and visual experience alone may have been sufficient to affect their understanding of the action in the habituation paradigm. Thus, whether active experience was important above and beyond this observational experience remained an open question. Indeed, as Biro and Leslie (2006) pointed out, infants’ mittened actions probably contained perceptual attributes that are associated with goal-directed action that may provide information about goal-directedness and that may trigger pre-existing knowledge about goal-directedness. Specifically, infants’ own actions demonstrated biological movement, self-propelled movement, and movements that resulted in displacement of the contacted object. Thus it is possible that it was the presence of this visual information, rather than the first-hand experience itself, that affected infants’ responses to others’ reaching actions.
In order to determine whether the self-produced nature of these actions was critical to action perception, self-produced experience must be compared to observational experience. A study by Sommerville, Hildebrand and Crane (2008) followed this strategy, examining active engagement in versus observation of tool use training in older infants. One group of 10-month-old infants was trained to use a cane to pull a toy into reach. A second group of infants observed an experimenter who repeatedly produced this action. Infants who had undergone active training subsequently showed evidence of representing the means-end goal structure of another person’s cane-pulling actions (as assessed in a habituation paradigm), whereas infants who observed the training did not. These findings suggest that self-produced experience provided unique information for 10-month-old infants concerning the means-end structure of a tool use event (see Hauf et al, 2007 for similar findings with regard to infants’ visual preference for actions they have performed versus observed in others).
These findings offer initial evidence that self-produced actions provide unique support for action understanding. The current studies sought to further elucidate the origins and nature of the effects of infants’ own actions on their action understanding. To start, we tested whether self-produced and observational training differ in their effects in younger infants, 3-month-olds. Accounts that posit infants’ own actions provide unique insights into others’ goals would predict greater effects of active than observational experience from the earliest points in development, but as yet this prediction has not been tested.
A second unresolved issue is whether the effects of active and observational experience are different in kind or simply different in magnitude. Observational experience may have similar, but less potent effects than active experience on action understanding. Because Sommerville and colleagues (2008) trained all infants in the observational condition to the same extent (the mean level of experience produced by infants in the active condition), any potential effects of variations in observational experience could not be assessed. In the current study, we created similar variability in infants’ active and observational training experiences by yoking each observational training protocol to an infant in the active condition. By treating yoked partners as related samples, we could examine differences between conditions while accounting for the range of variability present in mittened experience. Additionally, infants in both conditions of the current study had the opportunity to engage in barehanded activity with toys prior to training, allowing us to measure whether spontaneous activity prior to training was equivalent in each condition (as in the active condition in Sommerville et al., 2005).
In summary, in the current work we examined the effects of both active and closely matched observational experience at the origins of goal sensitivity, when infants are just beginning to control their own actions. In so doing, the current studies not only seek to clarify the factors that contribute to a foundational aspect of social cognition, namely the ability to apprehend others’ actions as goal-directed, but also inform broader consideration of the ways in which infants’ agentive experience contributes to cognitive development. Recent findings have renewed interest in this classic issue, with findings that infants own actions are linked to developments not only in social perception (Libertus & Needham, 2011; Sommerville et al., 2005), but also in knowledge about object affordances (Yang, Sidman, & Bushnell, 2010) and physical causality (Rakison & Krogh, 2011), raising the question of why and how infants’ actions support development in these domains.
Study 1
In Study 1, all infants first participated in a pre-training session in which they were given the opportunity to act on objects with their bare hands (i.e., untrained, spontaneous activity). Then, infants in the active condition underwent a training period with Velcro mittens that allowed them to produce object-directed activity, as in Sommerville et al. (2005). Infants in the observational condition underwent observational training matched to the experience of infants in the active condition. We used a yoked observational paradigm in which the self-produced activity of individual infants in the active condition was used to create scripts for infants in the observational condition. Infants in both conditions were then tested in a habituation paradigm to assess their sensitivity to the goal structure of a reaching event.
Methods
Participants
Sixty full-term (at least 37 weeks gestation) three-and-a-half-month-old infants were assigned to one of two conditions: active (n = 30; 15 males; M age = 3;16) or observational (n = 30; 12 males; M age = 3;15). Infants were recruited from the Washington, DC metropolitan area through mailings and advertisements. The sample of infants was 18% African-American, 8% Asian, 54% Caucasian, 8% Hispanic, and 12% multiracial. In order to be included in the final sample, infants had to complete the training phase and then complete at least two test trials in the looking time procedure. An additional 14 infants in the active condition and nine in the observational condition were not included in the final sample because they were unable to complete the procedure due to distress (n=11), were inattentive (n=3) or because of parental or sibling interference (n=2) or technical problems (n=1). Although observers underwent extensive training, for some infants it was very difficult to judge their looking due to movement or crying. Those infants for whom the reliability observer agreed with the original observer as to the endpoint of the trial on 50% or fewer of the test trials were not included in the sample (n=6; see Procedure section for more detail).
Procedure
Pre-training barehanded assessment
All infants were first given the opportunity to act on two toys with their bare hands while seated on a parent’s lap facing a small table. Parents were asked to support their infants but not to interfere with their actions. The experimenter sat to the side of the table and placed a toy bear (12.7 cm in length) and a toy ball (5.1 cm in diameter), both covered in Velcro, approximately eight centimeters apart in the center of the table. During the 3-minute session (and in all proceeding sessions), the experimenter ensured the infant’s hands were on the table and drew the infant’s attention to the toys by tapping or moving the toys periodically if the infant was not attending.
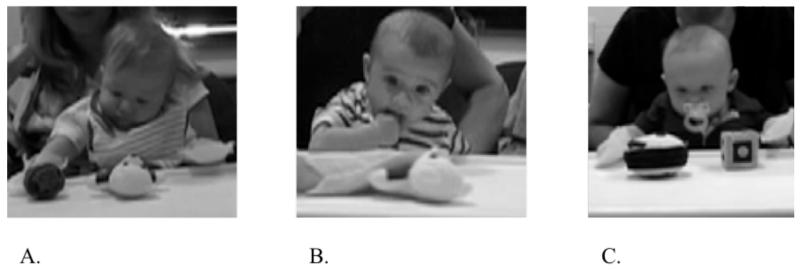
Active mittens training
In the active condition, the experimenter then fitted the infant with Velcro mittens (see Figure 1a). When the infant apprehended a toy, the experimenter allowed the infant to maintain manual contact with the toy for as long as he or she continued to look at the toy while touching it. When the infant broke visual contact, the experimenter detached the toy, placed it back on the table and drew the infant’s attention back to the toys. Infants’ coordinated visual and manual activity on the toy largely involved watching a toy while moving it back and forth across the table top with the mitten. This training lasted three minutes.
Figure 1.
Mittens training in active (A) and observational (B), and generalization conditions (C).
Mittens observational training
The amount of time each active infant engaged in object-directed activity on each toy (see coding section) was used to generate a training script for the yoked infant in the observational condition. This measure was chosen because it was the critical component relating individual differences in activity with mittens and infants’ looking times to others’ actions in Sommerville and colleagues’ (2005) study. During the training session, one experimenter wore a Velcro mitten and placed both toys just out of the infant’s reach (a few cm beyond the infants’ arm span but within the infant’s view; see Figure 1b). In order to match the type of activity produced by infants in the active condition, the experimenter reached toward, contacted, and moved each toy around on the table within the infant’s view in similar patterns to those engaged in by active infants (moving toy back and forth across the table and occasionally lifting it). The experimenter moved each of the toys (bear, ball, or both simultaneously) for approximately the amount of time the infant’s yoked partner had played with each toy. In order to match times of object-directed activity as closely as possible, the ball, the bear, and both were serially moved in pseudorandom order for each infant. The experimenter drew the infant’s attention to the toy if he or she was not attending. We ensured that infants watched the experimenter’s actions on the toys for the scripted amount of time. Thus, the scripted time was the amount of time the infant observed the experimenter’s actions, not the amount of time the experimenter acted on the toys. To ensure that infants in the observational condition received at least as much time attending to the objects being moved as did infants in the active condition (because we could not measure attention to the movements as precisely during the live training as we do for the videos offline), the scripts systematically rounded up the amount of time each infant saw the objects moved. Because we wanted to ensure extended attention to the actions on the object in the observational condition (and because of the inherent time delay caused by simultaneous script following, timing, and communication during live training), the number of contacts with the toys was not matched between the active and the observational conditions. In the active condition, the amount of object-directed activity produced was unrelated to the number of contacts made by the infant.
Habituation phase
All infants were then tested in a habituation procedure modeled after Sommerville et al.’s study (2005) and designed to assess infants’ encoding of reaching actions as goal-directed. Infants sat on a parent’s lap approximately 70 cm from a stage holding a bigger version of the bear (25.4 cm in length) and ball (10.2 cm in diameter), each on 5.1 cm high pedestals, approximately 30 cm apart. Parents were asked not to talk or gesture toward the stage, and they were asked to look down at the infant during test trials. The camera view of the infant was sent to a coder in another room who judged whether the infant was watching the event. All trials were infant-controlled and ended when infants looked away for two consecutive seconds.
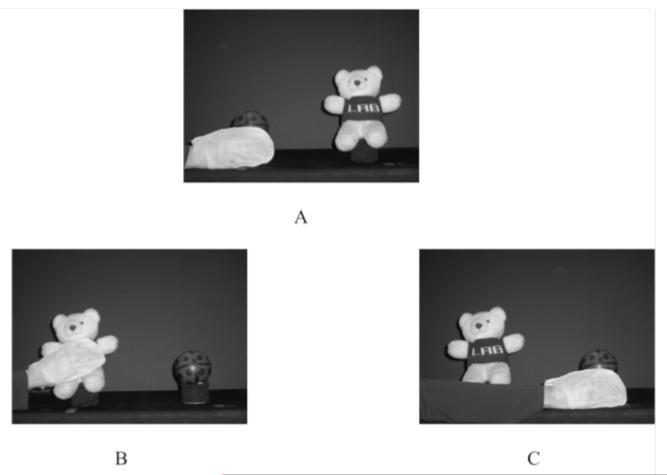
During habituation trials, the presenter sat to the side of the stage (out of view) and reached her arm through the side curtain, wearing a Velcro mitten, to grasp one of two toys (see Figure 2a). She held this position (without moving the toy) until the trial ended. This habituation procedure matched the procedure in Sommerville et al. (2005). Habituation trials were repeated until the length of the last three trials was less than half the length of the first three trials or until 14 trials had occurred.
Figure 2.
Habituation events (A), new-goal test trials (B), and old-goal test trials (C).
After habituation, the presenter switched the placement of the toys on the stage while the curtain was raised (so the infant did not see). In a familiarization trial, the infant viewed the toys in their new positions without any action. Infants were then shown six test trials alternating between new-goal and old-goal events (see Figure 2bc). On new-goal trials, the presenter reached to the same side of the stage as during habituation, this time grasping the other toy. On old-goal trials, she reached to the other side of the stage in order to grasp the same toy as during habituation. As in habituation, once she had grasped the toy, the presenter held her position until the end of the trial. The toy grasped in habituation, the side of the habituation reach, and the order of test trials were counterbalanced.
Coding of habituation paradigm responses
Infants’ looking times were measured using a coding program that calculated the habituation criterion (Casstevens, 2007; Pinto, 1994). Coders could not see the experimental event and were unaware of the order of test trials. To assess reliability, a second, independent coder coded the test trials of all of the sessions from the video record. The two coders’ judgments of trial length were strongly correlated (r ≥ .94 in both conditions). As a more stringent test, we assessed the proportion of test trials for which the online and reliability coders identified the same endpoint. Since trials ended when infants had looked away from the event for two seconds or more, observers were counted as agreeing if they identified the same shift in the infants’ gaze away from the event as ending the trial. In this way, reliability coding was unbiased by the end of the trial according to the video. Coders agreed on the end of the test trials 90%, and 89% of the time in the active and observational conditions, respectively. In both conditions, disagreements were randomly distributed with respect to the hypothesis (Fisher’s exact test, ns).
Coding of infants’ actions
Infants’ untrained and mittened actions were coded for the amount of time each infant spent looking at and touching each of the objects using a digital coding program (Mangold, 1998). Of interest was the extent to which infants engaged in coordinated object-directed actions on the toys. To operationalize this, we coded the amount of time each infant spent simultaneously looking at and touching each toy, as was done by Sommerville and colleagues (2005; henceforth referred to as object-directed activity). To obtain a parallel measure of infants’ experience in the observational condition, we coded their visual attention to the experimenter’s actions, that is, the total amount of time they watched as the experimenter’s mittened hand acted on the toys. A second independent coder coded 25% of the sessions (both barehanded and mittened training) in both the active and observational conditions. The two coders’ judgments of object-directed actions were strongly correlated (rs ≥ .97).
Results
Training experiences
We first analyzed infants’ actions during the training procedure. A paired samples t-test indicated that partners in the active and observational conditions did not differ in their untrained object-directed activity (t(29) = .15, p = .88; Cohen’s d = .028), indicating that the two groups of infants were comparable in their initial propensity to produce object-directed actions prior to mittens training. Infants produced barehanded object-directed activity for 13.26s (SE = 2.17) of the three-minute pre-training session on average, with activity ranging from 0 to 82s (two infants in the active condition and one in the observational condition did not engage in any object-directed activity with their bare hands, i.e., 0s).
We next considered the extent of infants’ experience during training. Infants in the active condition and their yoked partners in the observational condition received similar levels of exposure to object-directed activity, as indicated by a strong correlation between yoked pairs (r = .95, p < .001), though because we aimed to give infants in the observational condition at least the same amount of experience as their partner, they received more observational experience than infants in the active condition produced, t(29) = 11.37, p < .001; Cohen’s d = 2.11. Active object-directed experience ranged from 2.8s to 95.17 s (M = 50.08s [SE = 4.47]) and observational experience ranged from 18.37s to 119.97s (M = 65.8s [SE = 4.24]). Infants in both conditions gained more experience with object-directed actions during the training portion than during the untrained portion (ps < .001).
Our goal in the active manipulation was to equate for the amount of time infants spent attending to the mittened action on the toy, because this is the aspect of mittened experience that had been shown to be critical in the prior study (Sommerville et al., 2005). To achieve this, we provided uninterrupted actions for the infants to view during observation, and, as just summarized, this yielded comparable levels of sustained attention to the action in the two conditions. This strategy meant that infants in the active and observational conditions differed in the number contacts that the hand made with the toy (Median-active = 6.5; Median-observational = 3.0). The number of contacts in the active condition was unrelated to the amount of object-directed activity produced (p > .10). The lack of relation between contacts and object-directed activity was likely due to the fact that our procedure allowed infants to maintain contact with a toy as long as they were watching their actions. Therefore, infants who were more attentive to their actions were likely to make fewer total contacts during the training session. Infants who were less attentive to their actions, and thus produced less object-directed activity, however, had more opportunities to make new contacts.
Visual habituation responses
Next, we considered infants’ responses to the habituation and test events. Because of skew in habituation looking times, looking time measures during this portion of the session were log-transformed before being entered into analyses. A repeated measures analysis of variance (ANOVA) with habituation trial (sum of the log of the first three and sum of the log of the last three habituation trials) and condition (active versus observational; pairing yoked partners) as the repeated factors indicated that infants’ looking times declined across habituation trials for both groups (F(1, 29) = 81.26, p < .001; η2 = .74) and revealed no interaction between trial and condition (F(1, 29) = .19, p = .67, η2 = .007). A main effect of condition was also present (F(1,29) = 9.20, p = .005, η2 = .24), indicating that infants in the observational condition looked for longer across habituation trials than infants in the active condition.
We hypothesized that one potential source of the difference in attention to habituation trials was the amount of time infants spent in training prior to beginning the habituation session. Although infants in the observational condition spent more time attending to mittened actions during training than did infants in the active condition, the length of their training session was, on average, shorter than their partner’s (t(29) = 9.96, p < .001, Cohen’s d = 1.83; active: M = 6.32 minutes [SE = .50], observation: M = 4.87 minutes [SE = .59])). Infants in the active condition were always given three minutes to engage with the toys using mittens, whereas training ended as soon as the infant viewed the scripted amount of mittened activity in the observational condition. Across conditions, length of looking times across habituation trials was negatively correlated with the length of infants’ training sessions (r = −.36, p = .004). When the length of the training session was added into the repeated measures ANOVA described above as a covariate, the main effect of trial remained (F(1,27) = 4.95, p = .035, η2 = .16), but the main effect of condition was no longer present (F(1,27) = .98, p = .33, η2 = .035). Thus, infants demonstrated similar levels of attention prior to test trials when controlling for the length of training sessions.
Our focal question was whether responses to new-goal versus old-goal test trials were differentially affected by infants’ experience acting with mittens or observing mittened actions. Preliminary analyses revealed no effects of age, sex, goal in habituation, reach during habituation, or first test trial. Therefore, subsequent analyses collapsed across these factors. In order to control for the difference in length of training sessions between the two conditions, the length of the training session was entered as a covariate. A repeated measures analysis of variance with condition (active vs. observational) as a between subjects factor, test-trial type (average seconds looking to new-goal vs. old-goal trials) as a within subjects factor, and training session length as a covariate revealed no main effect of condition (F(1, 57) = .013, p = .91, η2 < .001), no main effect of type (F(1,57) = 2.18, p = .14, η2 = .037), no main effect of training session length (F(1,57) = .70, p = .417, η2 = .012), no interaction between test-trial type and length of training session (F(1,57) = 1.95, p = .17, η2 = .033), but, importantly, a Type × Condition interaction (F(1, 57) = 4.95, p = .030; η2 = .080).
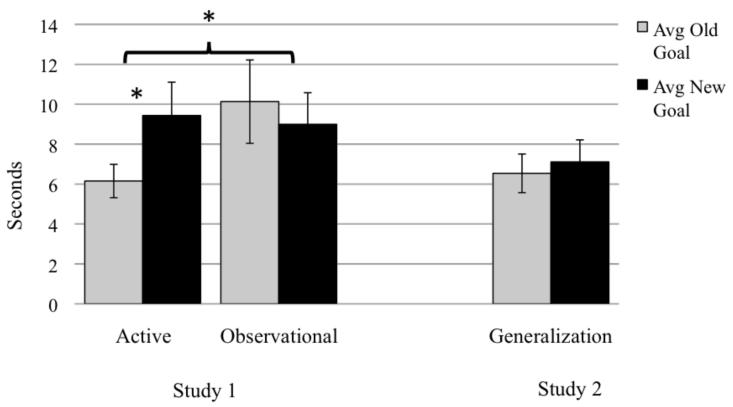
To elucidate the Type × Condition interaction, pairwise comparisons of the estimated marginal means were examined. As expected, in the active condition, infants spent significantly longer attending to new-goal test trials than old-goal test trials (md = 5.53, p = .021; see Figure 3 for raw descriptive means and standard errors of test trials). This replicates the finding from Sommerville and colleagues (2005). In contrast, infants in the observational condition did not respond systematically to new-goal versus old-goal test trials (md = 3.37, p = .15). These group level findings indicate that active training influenced infants’ responses to the goal structure of others’ actions and that observational training did not have the same effect.
Figure 3.
Average looking time to new-goal and old-goal test trials (* p < .05). Error bars represent standard errors.
In order to take advantage of our yoking procedure, we conducted an additional analysis using a generalized estimating equation (GEE; Hardin & Hilbe, 2008) with the difference between average new-goal and average old-goal trials (average new-goal preference) as the dependent variable, condition as a repeated measure (repeated within each yoked pair), and training session length as a covariate. We used a model-based estimator due to the small sample size (see Pan & Wall, 2002). This analysis revealed no effect of training session length (Wald χ2(1)= 1.89, p = .17), but critically, a significant main effect of condition (Wald χ2(1)= 4.56, p = .033). As in the ANOVA, pairwise comparisons of the estimated marginal means indicated that infants in the active condition looked longer to new-goal than old-goal trials in the active condition and not in the observational condition. Thus, when controlling for the variability in the amount of mittened experience received by yoked partners, the difference between the active and observational group remains largely the same. This implies that active experience with mittens benefited infants in the active condition in a qualitatively different way from infants in the observational condition.
Discussion
Infants who engaged in object-directed activity with Velcro mittens subsequently showed a pattern of selective attention to goal-change events that indicated sensitivity to the relational goal structure of others’ grasping actions. In contrast, infants who observed others’ actions on toys with mittens, without the opportunity to act with mittens themselves, did not recognize the goal of an experimenter’s grasping action in the habituation paradigm.
Our yoked procedure ensured that infants in the observational condition saw the same type of activity produced by infants in the active condition, namely movement of the toys on the table with the mitten. We created scripts based on the factor in active training shown to be critical to a change in action understanding in a previous study (Sommerville et al., 2005): amount of time both looking at and touching an object. Because our procedure ensured that each infant was attentive for as much or more observational experience as his or her mate in the active condition, this pattern of findings cannot be due to general inattention during training on the part of infants in the observational condition. The differing findings in the two conditions cannot be accounted for by attentional differences in the habituation paradigm because the two conditions did not differ in overall attentiveness on test trials. Further, the dependent variable was not a measure of overall attentiveness but instead a measure of infants’ sensitivity to the goal structure of the experimental events, as indexed by their relative attention to new-goal as compared to old-goal events.
Our findings argue against the suggestion that the effects in Sommerville and colleagues’ (2005) study were the product of infants’ experience viewing their own actions serving as a “trigger” for goal analysis. The contrast between the observational and active conditions provided a strong test of this hypothesis. In the observational condition, infants viewed a self-propelled entity (the experimenter’s hand) contact and move objects multiple times. Because it was important that infants did not try to act on the toys themselves (as they had just done so in the barehanded session), the toys were placed a few centimeters further from the infants in the observational training session than in the active training session. Given that we assured infants watched the entire event, that they had seen the toys at the same distance as infants in the active condition during the barehanded portion of the session, and that the toys were further away for all infants during the looking time portion of testing, it is unlikely that this had an important influence on infants’ learning from training.
We matched the observed actions to those in the active condition both in terms of duration of the critical component, object-directed activity, and in terms of the kind of actions infants produced most of the time – movement of the toys across the tabletop. Our goal in creating observational training was to maximize infants’ attention and equate infants’ experience with the factor that had been shown to be related to action understanding in the past (i.e., Sommerville et al., 2005). Thus, matching this length of time, rather than number of contacts made, gave infants in the observational condition the closest match in terms of the critical component (Note: ANCOVAs with number of contacts as a covariate revealed no main effects of number of contacts and no interactions between number of contacts and test-trial type either when conducted across conditions or within each condition).
Given this design, the sequence of actions infants observed during training differed between the conditions. Specifically, whereas infants in the observational condition saw all of the object-directed activity in a massed chunk, infants in the active condition may have had more distributed experience over the course of the three-minute training period. It is possible, therefore, that the spaced versus massed distribution of training contributed to the differences seen between the observational and active conditions. Distributed training sometimes leads to stronger retention than massed training (e.g., Cornell, 1980). Even so, massed training is sometimes more effective than distributed training in young infants’ motor learning (Enright, Rovee Collier, Fagen, & Ganiglia, 1983). Further, because the temporal gaps between mittens contact episodes in the current study were very brief (seconds in length, and all contained in one three-minute session) compared to the delays typically used in spaced training manipulations in infants (hours or days in length), it is not clear whether massing/spacing effects would be predicted in the current context. Additional research is needed to investigate this possibility.
An important remaining question concerns how and when the benefits gained from self-produced experience generalize to new events. In both Sommerville and colleagues’ (2005) study and the current study, infants played with the same toys during training that they then saw in the habituation paradigm. Although attention to objects alone cannot explain the current findings, it is possible that infants’ action representations are initially tied to the specific context in which they have gained experience. Research on reinforced motor learning in young infants has shown that newly acquired actions are (at least initially) highly context specific (e.g., DeFrancisco & Rovee-Collier, 2008). Moreover, young infants’ action learning during deferred imitation tasks is also context dependent (e.g., Hayne, MacDonald, & Barr, 1997).
On the other hand, recent findings from Yang and colleagues (Yang et al., 2010) suggest that, at least in older infants, engaging in an action facilitates generalization of action information to new instances. Yang et al. (2010) trained 15-month-old infants that a specific action (e.g. pushing a button) could lead to different effects when performed on different objects. Infants were able to transfer the means of the trained action to novel toys in test even if they had only observed the action, but they generalized to new objects more robustly if they had been allowed to push the button themselves during training.
Thus, it remains an open question whether younger infants, 3-month-olds, can generalize a goal-relation learned from active training to novel objects observed in a habituation paradigm. In Study 2, we addressed this question by assessing whether infants who received active mittens training, but with different toys than those seen in the habituation paradigm, similarly benefited from active experience producing object-directed actions with the mittens.
Study 2
Methods
Participants
Thirty full-term three-and-a-half-month-old infants (15 males; M age = 3;21) were included in the final sample of this study. The sample was 17% African-American, 3% Asian, 57% Caucasian, 10% Hispanic, 10% multiracial and 3% unreported. An additional 12 infants did not complete the procedure due to distress and five infants began testing but were excluded due to movement or crying that interfered with accurate coding. Ten additional infants were not included in the final sample due to interference, technical problems, or long breaks during the task.
Procedure
Infants in this study underwent the same habituation session as infants in Study 1 (coders agreed on the end of the trial for 91% of trials; r = .95; disagreements were randomly distributed, Fisher’s exact test, ns). The untrained and mittens training sessions were also identical except for the toys with which the infants played. Rather than playing with the ball and bear that they would later see in the habituation paradigm, infants had the opportunity to play with a block and a smiley face (see Figure 1). These toys were approximately the same size as the ball and bear and were similar in that they were different colors from one another, covered in Velcro, and one of the toys had a face. Coders once again measured infants’ object-directed activity throughout the session (reliability between coders on the 25% of sessions double-coded: r = .98).
Results
Training experiences
Preliminary analyses indicated that infants spent 15.94s (SE = 3.34; ranging from 0s to 70.57s with one infant engaging in no activity) engaged in untrained object-directed activity and 59.08s (SE = 4.76; ranging from 12.94s to 113.20s) engaged in mittened object-directed activity. They did not differ from infants in the active condition from Study 1 in their activity with or without mittens (p = .20 and p = .62, respectively). As in Study 1, infants produced more object-directed activity during the mittens training session than during the pre-training, barehanded session (p < .001).
Visual habituation responses
Next, we considered infants’ responses to the habituation and test events, comparing infants in this study to infants in the active condition in Study 1. An analysis of variance with the sum of the first three and last three habituation trials (log-transformed) as within subjects factors and condition (generalization and active from Study 1) as a between subjects factor indicated a main effect of habituation such that all infants decreased looking from the beginning to the end of habituation (F(1, 46) = 30.26, p < .001). No interaction between habituation and condition (p = .97) or main effect of condition (p = .56) emerged.
We then analyzed responses to new-goal versus old-goal test trials. Preliminary analyses revealed no effects of age, sex, goal in habituation, reach during habituation, or first test trial. Therefore, subsequent analyses collapsed across these factors. A paired samples t-test indicated that infants did not differ in their attention to the new-goal versus old-goal test trials in the generalization condition, t(29) = .65, p = .52, Cohen’s d = .10 (see Figure 3). When infants in the generalization condition from this study were compared to infants in the active condition from Study 1, a main effect of trial type emerged, (F(1, 58) = 4.54, p = .037, η2 = .073), indicating longer looking overall on new-goal than old-goal trials, but there was not a significant interaction between test-trial type and condition (F(1, 58) = 2.25, p = .14, η2 = .037).
Discussion
In this study, infants gained experience producing object-directed actions with one set of toys and then saw a different set of toys during the habituation paradigm. This training did not systematically influence infants’ subsequent looking time responses. Thus, the information that infants gained from active engagement with mittens appears to be, at least after only a brief training period, tied to the specific context in which infants gained experience.
The lack of systematicity in infants’ responses on test trials in Study 2 was not simply due to inattention to the experimental events. Infants attended to the habituation events for similar amounts of time as infants in the active condition from Study 1. Nevertheless, mittened experience seemed not to support infants’ tendency to see the experimental events as goal-directed. These findings are consistent with the general finding that young infants’ learning and memory is relatively context specific (e.g., DeFrancisco & Rovee-Collier, 2008; Greco, Hayne, & Rovee-Collier, 1990) and that this may also be the case for the effects of action on action perception (see Hauf et al., 2007).
Even so, infants’ failure to generalize in the current study should not be taken as strong evidence that they are unable to generalize the information gleaned from their own actions in any circumstances. For one, although infants in Study 2 did not respond systematically on test trials, their responses did not differ reliably from infants in the active condition in Study 1, suggesting that a similar, though less robust pattern may have been present in Study 2. Further, it seems possible that longer periods of active experience would generalize more broadly. Infants in the current studies received relatively brief exposure to the mittens (three minutes) in a single training session. Extended practice using the mittens over multiple days and weeks has been shown to have wide-ranging effects on infants’ own actions (Libertus & Needham, 2010; Needham et al., 2002) and social responses (Libertus & Needham, 2011). Thus extended training with mittens might support infants’ generalization of information about the goal-structure of action to events in which the objects acted on, or even the details of the action, vary. Furthermore, the use of multiple exemplars across training might support broader generalization to new instances, just as it does in infants’ learning from contingent reinforcement (e.g., Greco et al., 1990). Further research is needed to investigate these possibilities.
General Discussion
The current studies investigated the role of self-produced experience in supporting infants’ sensitivity to others’ action goals. Prior findings have demonstrated both correlational and causal links between infants’ developing action capabilities and their responses to others’ actions (e.g., Hauf et al., 2007; Libertus & Needham, 2010, 2011; Sommerville et al., 2005, 2008). Building from these earlier findings, the current studies investigated the relative contributions of self-produced and observational action experience to young infants’ action understanding, and, within self-produced experience, evaluated whether infants could generalize gained representations to a novel context. Replicating the findings of Sommerville and colleagues (2005), we found that 3-month-old infants who had undergone active training using “sticky” mittens subsequently responded systematically to the goal structure of actions they observed in a habituation paradigm, looking longer on new-goal trials than on old-goal trials. Critically, infants who had undergone closely matched observational training did not show this group level effect. This result is consistent with the findings of Sommerville and colleagues (2008), who found that older infants’ understanding of other’s tool use actions was supported by their own engagement in tool use, but not by observational training. Together, these findings indicate that active engagement strongly affects infants’ sensitivity to action goals, and that comparable observational experience does not yield equally strong effects. These findings thus support the hypothesis that agentive experience provides infants with information that allows them to recognize the goals of other agents.
These results are consistent with the proposal that developments in action perception and action production are linked by shared neuro-cognitive representations (Decety & Sommerville, 2003; Falck-Ytter, Gredebäck, & von Hofsten, 2006; Gallese & Goldman, 1998; Lepage & Théoret, 2006; Meltzoff, 2005). As noted earlier, recent findings have revealed that infants, like adults, show activation in the motor system when viewing others’ actions (e.g., Marshall, Young, & Meltzoff, 2011; Saby et al., 2012; Shimada & Hiraki, 2006; Southgate, Johnson, Karoui, & Csibra, 2010; Southgate, Johnson, Osborne, & Csibra, 2009; van Elk et al., 2008). As yet, however, these neural responses in infants have not been shown to play a functional role in specific social cognitive abilities such as the detection of action goals. Indeed, there is currently disagreement about whether these neural responses reflect analysis of action goals (e.g., Gallese & Goldman, 1998), a mechanism of imitation (Marhsall & Meltzoff, 2011), an association between motor representations and action effects (Paulus, 2012), or the generation of predictions derived from nonmotor action representations (Southgate et al., 2009). The current findings lend support to the conclusion that motor experience shapes social perception, but the neural correlates of this effect are as yet unknown. Addressing this open question will require integrating neural measures with behavioral measures, like the ones used here, that index specific aspects of social cognition.
The current findings also highlight the need for further investigation of the learning mechanisms that support the generalization of active learning to observed actions. In Study 2 we found that the brief training used in the current studies was not sufficient to allow infants to generalize from active engagement with one set of objects to observed actions on different objects. This finding suggests that the effects of self-produced actions in young infants lead to an initially narrowly defined representation of the action goal structure. As noted earlier, young infants also demonstrate very limited abilities to generalize learning in the context of their own actions (DeFrancisco & Rovee-Collier, 2008; Hayne et al., 1997). It seems likely that the same mechanisms that support the generalization of learning in infants’ own actions could support their generalization of information to others’ actions.
Recent evidence provides support for this idea. Infants’ generalization of their own actions to novel problems is supported by conditions that allow infants to compare relational similarities across action tasks (Chen, Sanchez, & Campbell, 1997). Gerson and Woodward (2012) found that these same conditions support 7-month-old infants’ generalization of information from their own actions to the novel actions of others. Specifically, when infants’ own reaching actions were coordinated with the novel tool use actions of an adult, infants learned to see the tool use actions as goal-directed. In this case, the co-occurrence of infants’ reaching actions and the adult’s tool use actions was critical for supporting infants’ learning about the experimenter’s goal when using the tool, suggesting that the experience allowed infants to detect structural similarities between their own actions and the novel actions of the experimenter. These findings raise the question of whether similar processes would support 3-month-old infants’ generalization of active experience to observed actions.
Although our findings revealed clear effects of active experience, and no effects of observational experience on infants’ goal sensitivity, it remains possible that observational experience contributes to infants’ action knowledge in ways that were undetected in the current studies. As one possibility, observational experience could provide infants with the opportunity to relate novel actions to actions they already understand and thereby make sense of the novel action. For example, in a study by Hofer and colleagues (Hofer, Hauf, & Aschersleben, 2005), 9-month-old infants who did not initially recognize tool use actions as goal-directed did so after observing that an experimenter was responsible for the actions produced by the tool, perhaps because they could then relate the novel tool use action to actions they already understood, like grasping (see also Gerson & Woodward, under review for related evidence in 10-month-old infants). It is not currently known whether this kind of reasoning occurs in younger infants. It is possible that early in development, self-produced actions are critical for establishing action representations that can later be generalized, but further research is needed to investigate this issue.
These open questions aside, these findings add to a growing body of evidence linking self-produced actions to a number of cognitive outcomes during infancy. The effects of self-produced actions are evident not only in learning that is directly relevant to action production, such as acquiring new motor patterns (e.g. Yang et al., 2010), learning about object affordances (e.g., Yang et al., 2010), and improved object exploration (e.g., Libertus & Needham, 2010), but also cognitive outcomes that are distinct from action production, such as sensitivity to the causal structure of an event (Rakison & Krogh, 2011), perception of motor patterns in others (e.g., Daum et al., 2011), and face perception (Libertus & Needham, 2011). Self-produced actions provide rich and varied information for infants (see Campos, Anderson, Barbu-Roth, Hubbard, Hertenstein, & Witherington, 2000; Yang et al., 2010), and different aspects of this information are relevant in different cognitive domains. The current results indicate that one kind of information that infants derive from their own actions concerns the goal-structure of actions, and that this information can be recruited when interpreting the actions of others.
The current findings also situate the emergence of intentional action knowledge within a broader developmental context. Over the course of early ontogeny, infants become able to act in increasingly well-structured, goal-directed ways (Piaget, 1953; von Hofsten, 2004). In doing so, infant create for themselves the experiences that support further development. That is, like many other species-typical abilities, goal sensitivity seems to be an “experience-expectant” phenomenon (Greenough, Black, & Wallace, 1987). Research indicates that gaining experience acting in the world changes knowledge, further motor abilities, and neural responses (e.g., Libertus & Needham, 2011; Needham et al., 2002; van Elk et al., 2008). Further, the benefits of spontaneous activity prior to training suggest that initial action capacities and/or representations can be built upon in order to learn through observation or experience and expand these representations to a broader range of contexts. Considering the importance of experience-expectant processes allows us to move beyond the dichotomy between inborn and environmental factors in order to more closely examine the meaningful interactions between them.
Acknowledgements
First and foremost, we would like to thank all of the families that participated in this research. We are also grateful to Laurie Eisenband, Neha Mahajan, Erin Cannon, and the many undergraduate students and high school interns who provided support for this project at the Maryland Infant Studies Laboratory. This work was funded by two grants to the second author (R01 HD35707 and HD064653).
Contributor Information
Sarah A. Gerson, Radboud University Nijmegen, Donders Institute for Brain, Cognition, and Behaviour
Amanda L. Woodward, University of Chicago
References
- Baldwin DA, Moses LJ. Links between social understanding and early word learning: Challenges to current accounts. Social Development. 2001;10:309–329. doi:10.111/1467-9507.00168. [Google Scholar]
- Barresi J, Moore C. Intentional relations and social understanding. Behavioral and Brain Sciences. 1996;19:107–122. doi:10.1017/S0140525X00041790. [Google Scholar]
- Behne T, Carpenter M, Call J, Tomasello M. Unwilling versus unable: Infants’ understanding of intentional action. Developmental Psychology. 2005;41:328–337. doi: 10.1037/0012-1649.41.2.328. doi:10.1037/0012-1649.41.2.328. [DOI] [PubMed] [Google Scholar]
- Biro S, Leslie A. Infants’ perception of goal-directed actions: Development through cue-based bootstrapping. Developmental Science. 2006;10:379–398. doi: 10.1111/j.1467-7687.2006.00544.x. doi:10.1111/j.1467-7687.2006.00544.x. [DOI] [PubMed] [Google Scholar]
- Brune C, Woodward AL. Social cognition and social responsiveness in 10-month-old infants. Journal of Cognition and Development. 2007;8:133–158. doi:10.1080/15248370701202331. [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. Travel broadens the mind. Infancy. 2000;1:149–219. doi: 10.1207/S15327078IN0102_1. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Cannon EN, Woodward AL, Gredebäck G, von Hofsten C, Turek C. Action production influences 12-month-old infants’ attention to others’ actions. Developmental Science. 2011;15:35–42. doi: 10.1111/j.1467-7687.2011.01095.x. doi: 10.1111/j.1467-7687.2011.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M, Akhtar N, Tomasello M. Fourteen- and 18-month-old infants differentially imitate intentional and accidental actions. Infant Behavior and Development. 1998;21:315–330. doi:10.1016/S0163-6383(98)90009-1. [Google Scholar]
- Casstevens RM. jHab: Java Habituation Software. Chevy Chase, MD: 2007. (version 1.0.0). [Computer Software] [Google Scholar]
- Chen Z, Sanchez RP, Campbell T. From beyond to within their grasp: the rudiments of analogical problem solving in 10- and 13-month-olds. Developmental Psychology. 1997;33:790–801. doi: 10.1037//0012-1649.33.5.790. doi:10.1037//0012-1649.33.5.790. [DOI] [PubMed] [Google Scholar]
- Cornell EH. Distributed study facilitates infants’ delayed recognition memory. Memory & Cognition. 1980;8:539–542. doi: 10.3758/bf03213773. doi: 10.3758/BF03213773. [DOI] [PubMed] [Google Scholar]
- Csibra G. Goal attribution to inanimate agents by 6.5-month-old infants. Cognition. 2008;107:705–717. doi: 10.1016/j.cognition.2007.08.001. doi:10.1016/j.cognition.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Daum MM, Prinz W, Aschersleben G. Perception and production of object-related grasping in 6-month-olds. Journal of Experimental Child Psychology. 2011;108:810–818. doi: 10.1016/j.jecp.2010.10.003. doi:10.1016/j.jecp.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Daum MM, Sommerville J, Prinz W. Becoming a social agent: Developmental foundations of an embodied social psychology. European Journal of Social Psychology. 2009;39:1196–1206. doi:10.1002/ejsp.672. [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Science. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. doi:10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- DeFrancisco BS, Rovee-Collier C. The specificity of priming effects over the first year of life. Developmental Psychobiology. 2008;50:486–501. doi: 10.1002/dev.20313. doi:10.1002/dev.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Enright MK, Rovee-Collier CK, Fagen JW, Caniglia K. The effects of distributed training on retention of operant condition in human infants. Journal of Experimental Child Psychology. 1983;36:209–225. doi: 10.1016/0022-0965(83)90030-9. doi: 10.1016/0022-0965(83)90030-9. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T, Gredebäck G, von Hofsten C. Infants predict other people’s action goals. Nature Neuroscience. 2006;9:878–879. doi: 10.1038/nn1729. doi:10.1038/nn1729. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Science. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. doi: 10.1016/S1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gallese V, Rochat M, Cossu G, Sinigaglia C. Motor cognition and its role in the phylogeny and ontogeny of action understanding. Developmental Psychology. 2009;45:103–113. doi: 10.1037/a0014436. doi: 10.1037/a0014436. [DOI] [PubMed] [Google Scholar]
- Gerson S, Woodward AL. Building intentional action knowledge with one’s hands. In: Johnson SP, editor. Neoconstructivism: The new science of cognitive development. Oxford University Press; New York: 2010. pp. 295–313. [Google Scholar]
- Gerson S, Woodward AL. A claw is like my hand: Comparison supports goal analysis in infants. Cognition. 2012;122:181–192. doi: 10.1016/j.cognition.2011.10.014. doi: 10.1016/j.cognition.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson S, Woodward A. Labels facilitate infants’ comparison of action goals. Journal of Cognition & Development. doi: 10.1080/15248372.2013.777842. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco C, Hayne H, Rovee-Collier Roles of function, reminding, and variability in categorization by 3-month-old infants. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:617–633. doi: 10.1037//0278-7393.16.4.617. doi:10.1037//0278-7393.16.4.617. [DOI] [PubMed] [Google Scholar]
- Gredebäck G, Kochukhova O. Goal anticipation during action observation is influenced by synonymous action capabilities, a puzzling developmental study. Experimental Brain Research. 2010;202:493–497. doi: 10.1007/s00221-009-2138-1. doi:10.1007/s00221-009-2138-1. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Development. 1987;58:539–559. doi:10.2307/1130197. [PubMed] [Google Scholar]
- Hamlin JK, Hallinan EV, Woodward AL. Do as I do: 7-month-old infants selectively reproduce others’ goals. Developmental Science. 2008;11:487–494. doi: 10.1111/j.1467-7687.2008.00694.x. doi:10.1111/j.1467-7687.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized estimating equations. Wiley Encyclopedia of Clinic Trials. 2008:1–8. doi:10.1002/9780471462422.eoct485. [Google Scholar]
- Hauf P, Aschersleben G, Prinz W. Baby do-baby see! How action production influences action perception in infants. Cognitive Development. 2007;22:16–32. doi: 10.1016/j.cogdev.2006.09.002. [Google Scholar]
- Hayne H, MacDonald S, Barr R. Developmental changes in the specificity of memory during second anniversary. Behavior of the infant and Development. 1997;20:233–245. doi:10.1016/S0163-6383(97)90025-4. [Google Scholar]
- Hofer T, Hauf P, Aschersleben G. Infants’ perception of goal-directed actions performed by a mechanical device. Infant Behavior and Development. 2005;28:466–480. doi:10.1016/j.infbeh.2005.04.002. [Google Scholar]
- Hommel B, Müsseler J, Aschersleben G, Prinz W. Theory of event coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences. 2001;24:849–937. doi: 10.1017/s0140525x01000103. doi: 10.1017%2FS0140525X01000103. [DOI] [PubMed] [Google Scholar]
- Kanakogi Y, Itakura S. Developmental correspondence between action prediction and motor ability in early infancy. Nature Communications. 2011;2:341. doi: 10.1038/ncomms1342. doi:10.1038/ncomms1342. [DOI] [PubMed] [Google Scholar]
- Lepage JF, Théoret H. The mirror neuron system: grasping others’ actions from birth? Developmental Science. 2006;10:513–523. doi: 10.1111/j.1467-7687.2007.00631.x. doi: 10.1111/j.1467-7687.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- Libertus K, Needham A. Teach to reach: The effects of active versus passive reaching experiences on action and perception. Vision Research. 2010;50:2750–2757. doi: 10.1016/j.visres.2010.09.001. doi:10.1016/j.visres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus K, Needham A. Reaching experience increases face preference in 3-month-old infants. Developmental Science. 2011 doi: 10.1111/j.1467-7687.2011.01084.x. doi: 10.1111/j.1467-7687.2011.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Baillargeon R. Can a self-propelled box have a goal? Psychological reasoning in 5-month-old infants. Psychological Science. 2005;16:601–608. doi: 10.1111/j.1467-9280.2005.01582.x. doi:10.1111/j.1467-9280.2005.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Johnson SC. Recognizing the role of perception in action at 6 months. Developmental Science. 2009;12:142–149. doi: 10.1111/j.1467-7687.2008.00741.x. doi:10.1111/j.1467-7687.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Mangold P. Interact. Mangold International; Arnstorf, Germany: 1998. computer software. [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems: Exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1:110–123. doi: 10.1016/j.dcn.2010.09.001. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Young T, Meltzoff AN. Neural correlates of action observation and execution in 14-month-old infants: An event-related EEG desynchronization study. Developmental Science. 2011;14:474–480. doi: 10.1111/j.1467-7687.2010.00991.x. doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Developmental Psychology. 1995;31:1–16. doi: 10.1037/0012-1649.31.5.838. doi:10.1037//0012-1649.31.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Imitation and other minds: The “like me” hypothesis. In: Hurley S, Chater N, editors. Perspectives on imitation: From neuroscience to social science. MIT Press; Cambridge, MA: 2005. pp. 55–77. [Google Scholar]
- Moses LJ, Baldwin DA, Rosicky JG, Tidball G. Evidence for referential understanding in the emotions domain at twelve and eighteen months. Child Development. 2001;72:718–735. doi: 10.1111/1467-8624.00311. doi:10.1111/1467-8624.00311. [DOI] [PubMed] [Google Scholar]
- Needham A, Barrett T, Peterman K. A pick-me up for infants’ exploratory skills: Early simulated experiences reaching for objects using ‘sticky mittens’ enhances young infants’ exploration skills. Infant Behavior and Development. 2002;25:279–295. doi:10.1016/S0163-6383(02)00097-8. [Google Scholar]
- Pan W, Wall MM. Small-sample adjustments in using the sandwich variance estimator in generalized estimating equations. Statistics in Medicine. 2002;21:1429–1441. doi: 10.1002/sim.1142. doi: 0.1002/sim.1142. [DOI] [PubMed] [Google Scholar]
- Paulus M. Action mirroring and action understanding: An ideomotor and attentional account. Psychological Research. 2012;76:760–767. doi: 10.1007/s00426-011-0385-9. doi: 10.1007/s00426-011-0385-9. [DOI] [PubMed] [Google Scholar]
- Piaget J. The origins of intelligence in the child. Routledge & Kegan Paul; London: 1953. [Google Scholar]
- Pinto J. MacXhab. Stanford, CA: 1994. Version 1.3. [Google Scholar]
- Prinz W. A common coding approach to perception and action. In: Neumann O, Prinz W, editors. Relationships between perception and action. Springer-Verlag; Berlin: 1990. pp. 167–201. [Google Scholar]
- Rakison DH, Krogh L. Does causal action facilitate causal perception in infants younger than 6 months of age? Developmental Science. 15:45–53. doi: 10.1111/j.1467-7687.2011.01096.x. doi: 10.1111/j.1467-7687.2011.01096.x. [DOI] [PubMed] [Google Scholar]
- Repacholi BM, Gopnik A. Early reasoning about desires: Evidence from 14- and 18-month-olds. Developmental Psychology. 1997;33:12–21. doi: 10.1037//0012-1649.33.1.12. doi:10.1037/0012-1649.33.1.12. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. doi:10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Saby JN, Marshall PJ, Meltzoff AN. Neural correlates of being imitated: An EEG study in preverbal infants. Social Neuroscience. 2012;7:650–661. doi: 10.1080/17470919.2012.691429. doi: 10.1080/17470919.2012.691429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Hiraki K. Infant’s brain responses to live and televised action. NeuroImage. 2006;32:930–939. doi: 10.1016/j.neuroimage.2006.03.044. doi:10.1016/j.neuroimage.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Hildebrand E, Crane CC. Experience matters: The impact of doing versus watching on infants’ subsequent perception of tool use events. Developmental Psychology. 2008;44:1249–1256. doi: 10.1037/a0012296. doi:10.1037/a0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL. Pulling out the intentional structure of action: The relation between action processing and action production in infancy. Cognition. 2005;95:1–30. doi: 10.1016/j.cognition.2003.12.004. doi: 10.1016/j.cognition.2003.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science. 2010;21:355–359. doi: 10.1177/0956797610362058. doi:10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biology Letters. 2009;5:769–772. doi: 10.1098/rsbl.2009.0474. doi:10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. The cultural origins of human cognition. Harvard University Press; Cambridge, MA: 1999. [Google Scholar]
- van Elk M, van Schie HT, Hunnius S, Bekkering H. You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. Neuroimage. 2008;43:808–814. doi: 10.1016/j.neuroimage.2008.07.057. doi:10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. An action perspective on motor development. Trends in Cognitive Science. 2004;8:266–272. doi: 10.1016/j.tics.2004.04.002. doi:10.1016/j.tics.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants selectively encode the goal object of an actor’s reach. Cognition. 1998;69:1–34. doi: 10.1016/s0010-0277(98)00058-4. doi:10.1016/S0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants’ ability to distinguish between purposeful and non-purposeful behaviors. Infant Behavior and Development. 1999;22:145–160. doi:10.1016/S0163-6383(99)00007-7. [Google Scholar]
- Woodward AL, Guajardo JJ. Infants’ understanding of the point gesture as an object-directed action. Cognitive Development. 2002;17:1061–1084. doi:10.1016/S0885-2014(02)00074-6. [Google Scholar]
- Woodward AL, Sommerville JA, Gerson S, Henderson AME, Buresh JS. The emergence of intention attribution in infancy. In: Ross B, editor. The Psychology of Learning and Motivation. Vol. 51. Academic Press; 2009. pp. 187–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Sidman J, Bushnell EW. Beyond the information given: Infants’ transfer of actions learned through imitation. Journal of Experimental Child Psychology. 2010;106:62–81. doi: 10.1016/j.jecp.2009.12.005. doi:10.1016/j.jecp.2009.12.005. [DOI] [PubMed] [Google Scholar]