Abstract
Background
Body mass index (BMI) is a risk factor for comorbid illnesses and cancer development. We hypothesized that obesity status affects disease outcomes and treatment-related toxicities in esophageal cancer patients treated with chemoradiotherapy (CRT).
Methods
From March 2002 to April 2010, we retrospectively analyzed 405 patients with non-metastatic esophageal carcinoma at MD Anderson Cancer Center, treated with either definitive or neoadjuvant CRT. Patients were categorized as either obese (BMI ≥ 25 kg/m2) or non-obese (BMI < 25 kg/m2). Progression-free survival (PFS) and overall survival (OS) times were examined using the Kaplan-Meier method and Cox proportional hazards regression analysis.
Results
One hundred fifteen (28.4%) patients were classified as non-obese and 290 (71.6%) as obese. Obese patients were more likely than others to have several comorbid diseases (p < 0.001), adenocarcinoma located distally (p < 0.001), and have undergone surgery (p = 0.004). Obesity was not associated with either worse operative morbidity/mortality (p > 0.05) or worse positron emission tomography (PET) tumor response (P = 0.46) on univariate analysis, nor with worse pathologic complete response (pCR) (P = 0.98) on multivariate analysis. There was also no difference in OS, locoregional control, or metastasis-free survival between obese and non-obese patients (P = 0.86). However, higher BMI was associated with reduced risk of chemoradiation-induced high-grade esophagitis (P = 0.021), esophageal stricture (P < 0.001), and high-grade hematologic toxicity (P < 0.001).
Conclusions
In esophageal cancer patients treated with CRT, obesity is not predictive of poorer disease outcomes or operative morbidities; instead, our data suggest it may be associated with decreased risk of acute chemotherapy and radiotherapy-related treatment toxicities.
Keywords: esophageal cancer, obesity, outcomes
INTRODUCTION
The prevalence of obesity in the Western world has been rising over the last quarter century (1). Up to 120 million Americans are now estimated to be overweight or obese, a figure that represents 65% of the adult population (2). Adiposity is linked to an increased risk of many cancers, including cancers of the endometrium, kidney, gallbladder, breast, and colon (3). Moreover, excess weight is an established risk factor for death from cancer (4). In breast cancer, for example, the risk of recurrent breast cancer in women who are 20% to 25% over their ideal body weight is approximately 1.3 times that in non-obese women (5); moreover, obesity has been associated with lower rates of pCR and worse OS (6).
For esophageal cancer, the relationship between obesity and oncologic outcomes is less clear. Although some studies have reported increased postoperative morbidity and duration of hospital stay in esophageal cancer patients with high BMI (7, 8), others have found no difference (9, 10). Similarly, while several studies have reported no difference in survival between obese and non-obese patients (8, 10), others (11, 12) have reported better survival outcomes in patients with high BMI.
The other challenge is that in the past 10 years, combined modality therapy (CMT) with radiotherapy, chemotherapy, and surgery have become the new standard of care (13). However, previous studies (11, 14, 15) have predominantly focused on esophageal cancer patients treated with surgery as primary therapy. Little remains known how BMI interacts with chemoradiation to affect acute treatment-related toxicities and long-term clinical outcomes. In this study, we hypothesize that obesity status affects disease outcomes and treatment-related toxicities in esophageal cancer patients treated with chemoradiotherapy.
PATIENTS AND METHODS
Patient Selection
MD Anderson Cancer Center’s tumor registry was used to identify 405 patients between March 2002 and April 2010 with biopsy-confirmed, non-metastatic esophageal cancer who received radiotherapy, chemotherapy, +/− surgery. Disease stage was determined based on the American Joint Committee on Cancer system (16). Patients were categorized into two groups, obese (BMI ≥ 25 kg/m2) and non-obese (BMI < 25 kg/m2). The study was approved by the MD Anderson institutional review board.
Treatment
While surgery is the standard of care at MD Anderson for stage I esophageal cancer, CMT is used for stage II-III disease. Patients are typically treated with neoadjuvant chemoradiation to 50.4 Gy. Chemotherapy is administered in combinations of 5-flurouracil, taxanes, and platinum-based compounds. Five to 6 weeks after completion of neoadjuvant therapy, most patients are restaged using CT, PET/CT, and/or esophagogastroduodenoscopy (EGD) with biopsy of the primary disease site, and then evaluated for surgery. Surgical procedures included Ivor-Lewis, transhiatal, left thoracotomy, or minimally invasive esophagectomy using small incisions and laparoscopic instruments.
Outcome Measures
We defined pCR as the absence of disease in both the esophagus and lymph nodes in the resected specimen and pathologic near-complete response as ≤1% viable tumor cells in the resected specimen with negative lymph nodes.
Dates of death were determined by reviewing clinical follow-up information in the patients’ medical records and Social Security Death Index. OS was calculated from the date of diagnosis to the date of death or last follow-up. Data from radiographic studies, follow-up clinical examinations, surgical explorations, and endoscopy were used to assess locoregional and distant failure. PFS was calculated from the date of diagnosis to the date of documented progression. Patients who had not experienced progression or who had died by the last follow-up were censored.
Statistical Analysis
Data were collected retrospectively. BMI was examined as a binary variable, as noted above. The chi-square test or Fisher’s exact test was used to compare differences between BMI groups with respect to categorical variables. Wilcoxon rank-sum tests or Kruskal-Wallis tests were used to assess associations between BMI group and continuous variables. A multivariate logistic regression model was used to examine associations between BMI and pCR. Survival curves were constructed using the Kaplan-Meier method and compared between BMI groups with the log-rank test. Associations between BMI and patient time to event outcomes were examined by the Cox proportional hazards model. The clinical variables for the multivariable logistic regression model and the Cox proportional hazards model were selected by the backward selection procedure with an adjusted P-value ≤ 0.05.
RESULTS
BMI and Patient Characteristics
Patient characteristics are summarized in Table 1. One hundred fifteen (28.4%) patients were classified as non-obese and 290 (71.6%) as obese. Nearly all patients received radiation and concurrent chemotherapy (six patients received radiation alone). A total of 199 (49.1%) patients received definitive chemoradiation and 206 (50.9%) underwent neoadjuvant therapy followed by surgery.
Table 1. Patient Characteristics by BMI (N=405).
| Characteristic | All Patients (N = 405) |
Non-Obese (N = 115) | Obese (N = 290) | P-Value |
|---|---|---|---|---|
| Age | 0.8449 | |||
| 64.48 | ||||
| Median (range) | (22.81, 87.33) | 64.5 (22.8, 87.3) | 64.6 (30, 85.6) | |
| Mean (SD) | 63.7 (11.13) | 63.7 (12.5) | 63.7 (10.6) | |
| Gender (%) | <0.001 | |||
| Female | 65 (16%) | 32 (27.8%) | 33 (11.4%) | |
| Male | 340 (84%) | 83 (72.2%) | 257 (88.6%) | |
| Race (%) | 0.035 | |||
| Caucasian | 353 (87.8%) | 93 (82.3%) | 260 (90%) | |
| Non-Caucasian | 49 (12.2%) | 20 (17.7%) | 29 (10%) | |
| Current Smoker (%) | 0.006 | |||
| No | 327 (80.7%) | 83 (72.2%) | 244 (84.1%) | |
| Yes | 78 (19.3%) | 32 (27.8%) | 46 (15.9%) | |
| Heavy Alcohol History (%) | 0.022 | |||
| No | 325 (80.2%) | 84 (73%) | 241 (83.1%) | |
| Yes | 80 (19.8%) | 31 (27%) | 49 (16.9%) | |
| Sum Comorbid Disease (%) | <0.001 | |||
| 0 | 139 (34.3%) | 57 (49.6%) | 82 (28.3%) | |
| 1 | 151 (37.3%) | 33 (28.7%) | 118 (40.7%) | |
| 2 or greater | 115 (28.4%) | 25 (21.7%) | 90 (31%) | |
| Histology (%) | <0.001 | |||
| Adenocarcinoma | 318 (78.5%) | 67 (58.3%) | 251 (86.6%) | |
| Squamous | 79 (19.5%) | 48 (41.7%) | 31 (10.7%) | |
| Other | 8 (2%) | 0 (0)% | 8 (2.8%) | |
| Tumor Location (%) | <0.001 | |||
| Cervical | 16 (4%) | 9 (7.8%) | 7 (2.4%) | |
| Upper Thoracic | 15 (3.7%) | 7 (6.1%) | 8 (2.8%) | |
| Mid Thoracic | 36 (8.9%) | 22 (19.1%) | 14 (4.8%) | |
| Distal | 338 (83.5%) | 77 (67%) | 261 (90%) | |
| Overall Stage | 0.301 | |||
| 1-2 | 161 (41.4%) | 41 (37.3%) | 120 (43%) | |
| 3 | 228 (58.6%) | 69 (62.7%) | 159 (57%) | |
| Type of Surgery (n = 231) | 0.28 | |||
| Transhiatal | 3 (1.7%) | 1 (2.6%) | 2 (1.4%) | |
| Ivor-Lewis | 176 (97.2%) | 36 (94.7%) | 140 (97.9%) | |
| Others | 2 (1.1%) | 1 (2.6%) | 1 (0.7%) | |
| Definitive Chemoradiation | 0.001 | |||
| No | 206 (50.9%) | 44 (38.3%) | 162 (55.9%) | |
| Yes | 199 (49.1%) | 71 (61.7%) | 128 (44.1%) | |
| Induction Chemotherapy | 0.674 | |||
| No | 247 (61%) | 72 (62.6%) | 175 (60.3%) | |
| Yes | 158 (39%) | 43 (37.4%) | 115 (39.7%) | |
| Total Radiation Dose | 0.2729 | |||
| Median (range) | 50.4 (25, 66) | 50.4 (25, 66) | 50.4 (25, 66) | |
| Mean (SD) | 50.32 (4.32) | 50.9 (5.1) | 50.1 (4) | |
| Radiation Modality | 0.779 | |||
| 3D Conformal | 76 (18.8%) | 21 (18.3%) | 55 (19%) | |
| IMRT | 280 (69.1%) | 82 (71.3%) | 198 (68.3%) | |
| Protons | 49 (12.1%) | 12 (10.4%) | 37 (12.8%) |
BMI and Surgical Complications
A total of 206 patients underwent neoadjuvant therapy followed by surgery. There was no significant difference noted in postoperative complications between obese (n = 162) and non-obese patients (n = 44), with the exception that gastrointestinal complications, such as anastomotic leaks and ileus, were significantly lower in obese patients (P = 0.011) (Table 2). In the subgroup of patients who received induction chemotherapy prior to preoperative chemoradiotherapy (n = 89), there was again no significant difference noted between obese and non-obese patients with regard to readmission within 60 days, length of hospital stay, 30-day mortality, or surgical complications (with the exception of gastrointestinal complications, which were significantly lower in obese patients, P = 0.004).
Table 2. Operative Morbidity and Mortality by BMI (N=206).
| Variable | Total (N = 206) | Non-Obese (N = 44) | Obese (N=162) | P-Value |
|---|---|---|---|---|
| Sum Operative Complications | 0.514 | |||
| 0 | 75 (37.3%) | 13 (31%) | 62 (39%) | |
| 1 | 80 (39.8%) | 17 (40.5%) | 63 (39.6%) | |
| 2 or greater | 46 (22.9%) | 12 (28.6%) | 34 (21.4%) | |
| Pulmonary Complications | 0.736 | |||
| No | 139 (68.8%) | 28 (66.7%) | 111 (69.4%) | |
| Yes | 63 (31.2%) | 14 (33.3%) | 49 (30.6%) | |
|
Gastrointestinal
Complications |
0.011 | |||
| No | 147 (72.8%) | 24 (57.1%) | 123 (76.9%) | |
| Yes | 55 (27.2%) | 18 (42.9%) | 37 (23.1%) | |
| Other Complications | 0.574 | |||
| No | 137 (67.8%) | 30 (71.4%) | 107 (66.9%) | |
| Yes | 65 (32.2%) | 12 (28.6%) | 53 (33.1%) | |
| Re-Admission within 60 Days | 0.249 | |||
| No | 173 (89.6%) | 33 (84.6%) | 140 (90.9%) | |
| Yes | 20 (10.4%) | 6 (15.4%) | 14 (9.1%) | |
| 30 Day Mortality | 0.381 | |||
| No | 200 (99%) | 42 (97.7%) | 158 (99.4%) | |
| Yes | 2 (1%) | 1 (2.3%) | 1 (0.6%) | |
| Length of Hospital Stay | 0.2395 | |||
| Median (range) | 10 (4, 60) | 11 (7, 55) | 10 (4, 60) | |
| Mean (SD) | 13.72 (9.49) | 16 (11.6) | 13.1 (8.8) |
Treatment-Related Toxicities and BMI
The prevalence of radiation-/chemotherapy-related treatment toxicities for the overall patient cohort (n = 405) as grouped by BMI is shown in Table 3. Obese patients (n = 290) were less likely than non-obese patients (n = 115) to have high-grade esophagitis (P = 0.021), any stricture toxicity (P < 0.001), and hematologic toxicity (P < 0.05). These results were nearly identical when the analysis was restricted to the subset of patients (n = 158) who also received induction chemotherapy. Obese patients were again significantly less likely to have any stricture toxicity (P = 0.008), ≥ grade 2 hematologic toxicity (P = 0.012), ≥ grade 1 anemia (P = 0.001), or ≥ grade 1 neutropenia (P = 0.032).
Table 3. Treatment-Related Toxicities by BMI (N=405).
| Treatment Toxicity | All Patients (N=405) | Non-Obese (N = 115) | Obese (N = 290) | P-Value |
|---|---|---|---|---|
| Esophagitis | 0.021 | |||
| grade 0 | 152 (37.5%) | 33 (28.7%) | 119 (41%) | |
| grade 2 or greater | 253 (62.5%) | 82 (71.3%) | 171 (59%) | |
| Nausea | 0.177 | |||
| grade 0 | 162 (40%) | 52 (45.2%) | 110 (37.9%) | |
| grade 1 or greater | 243 (60%) | 63 (54.8%) | 180 (62.1%) | |
| Pneumonitis | 0.519 | |||
| grade 0 | 388 (95.8%) | 109 (94.8%) | 279 (96.2%) | |
| grade 2 or greater | 17 (4.2%) | 6 (5.2%) | 11 (3.8%) | |
| Fistula Toxicity | 0.284 | |||
| grade 0 | 404 (99.8%) | 114 (99.1%) | 290 (100%) | |
| grade 1 or greater | 1 (0.2%) | 1 (0.9%) | ||
| Stricture Toxicity | <0.001 | |||
| grade 0 | 347 (85.7%) | 83 (72.2%) | 264 (91%) | |
| grade 1 or greater | 58 (14.3%) | 32 (27.8%) | 26 (9%) | |
| Max Hematologic Toxicity | <0.001 | |||
| grade 0 | 263 (66.1%) | 61 (53%) | 202 (71.4%) | |
| grade 2 or greater | 135 (33.9%) | 54 (47%) | 81 (28.6%) | |
| Anemia | <0.001 | |||
| grade 0 | 217 (54.5%) | 43 (37.4%) | 174 (61.5%) | |
| grade 1 or greater | 181 (45.5%) | 72 (62.6%) | 109 (38.5%) | |
|
Anemia Treatment Break or
Dose Reduction |
0.136 | |||
| No | 388 (97.5%) | 110 (95.7%) | 278 (98.2%) | |
| Yes | 10 (2.5%) | 5 (4.3%) | 5 (1.8%) | |
| Leukopenia | 0.064 | |||
| grade 0 | 308 (77.4%) | 82 (71.3%) | 226 (79.9%) | |
| grade 2 or greater | 90 (22.6%) | 33 (28.7%) | 57 (20.1%) | |
|
WBC Treatment Break or
Dose Reduction |
0.039 | |||
| No | 375 (94.2%) | 104 (90.4%) | 271 (95.8%) | |
| Yes | 23 (5.8%) | 11 (9.6%) | 12 (4.2%) | |
| Neutropenia | 0.01 | |||
| grade 0 | 317 (80.3%) | 83 (72.2%) | 234 (83.6%) | |
| grade 1 or greater | 78 (19.7%) | 32 (27.8%) | 46 (16.4%) | |
|
ANC Treatment Break or
Dose Reduction |
0.005 | |||
| No | 374 (94%) | 102 (88.7%) | 272 (96.1%) | |
| Yes | 24 (6%) | 13 (11.3%) | 11 (3.9%) | |
| Thrombocytopenia | 0.02 | |||
| grade 0 | 220 (55.3%) | 74 (64.3%) | 146 (51.6%) | |
| grade 1 or greater | 178 (44.7%) | 41 (35.7%) | 137 (48.4%) | |
|
Platelet Break or Dose
Reduction |
0.035 | |||
| No | 371 (93.2%) | 112 (97.4%) | 259 (91.5%) | |
| Yes | 27 (6.8%) | 3 (2.6%) | 24 (8.5%) |
Influence of BMI on PET Tumor Response and pCR to Neoadjuvant Therapy
For patients who received definitive chemoradiation (n = 199), there was no significant difference in PET-determined tumor response between obese (n = 128) and non-obese patients (n = 71) on univariate analysis (P = 0.46). For patients who received surgery (n = 206), a 41% (n = 85) had a pCR or near-complete pathologic response to neoadjuvant therapy. In the univariate model, significant predictors of pCR included white race (P = 0.026), T1 or T2 disease (P = 0.003), and lack of residual tumor on post-treatment EGD (P < 0.001). High tumor grade (P = 0.083) trended towards a decreased likelihood of pCR. In the multivariate model, there was no significant difference in pCR or near-complete pathologic response between obese and non-obese patients (P > 0.05).
Influence of BMI on Patterns of Failure and OS
On multivariate analysis, locoregional PFS and metastasis-free survival were not associated with BMI (Table 5, Fig 1). The clinical factor most strongly associated with an increased risk of locoregional or distant progression was lack of definitive surgery. Median OS time was 42.3 months in the overall cohort, and 40.2 and 43.5 months for non-obese and obese patients, respectively. The 5-year OS rates were 41.9% for non-obese patients and 40.5% for obese patients. On both univariate and multivariate analyses, BMI was not significantly associated with OS (Table 5, Fig 1). On univariate analysis, disease stage 3 (P < 0.001), T stage 3 or 4 (P = 0.039), tumor location (P = 0.023), nodal involvement (P < 0.001), definitive chemoradiotherapy (P < 0.001), readmission within 60 days of surgery (P = 0.019), ≥ grade 2 dysphagia (P = 0.014), any anorexia (P = 0.020), ≥ grade 2 hematologic toxicity (P = 0.030), and any treatment-related anemia (P = 0.010) were significantly associated with shorter survival. Tumor location, node-status, and treatment with surgery vs. definitive CRT remained as independent prognostic factors for survival, after adjusting for covariates (Table 5).
Table 5. Multivariate Cox Proportional Hazards Model for Clinical Outcomes.
| Overall Survival (N=384) | Local Failure (N=363) | Distant Failure (N=342) | ||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value |
| BMI | ||||||
| Non-Obese | 1 | 0.8591 | 1 | 0.6057 | 1 | 0.1299 |
| Obese | 1.032 (0.73 to 1.459) | 1.126 (0.717 to 1.770) |
1.366 (0.912 to 2.047) |
|||
|
Age
(increment of 1 yr) |
ns | ns | ns | ns | 0.979 (0.963 to 0.995) |
0.0113 |
| Definitive Radiation | ||||||
| No | 1 | <.0001 | 1 | <.0001 | 1 | 0.0006 |
| Yes | 2.232 (1.592 to 3.130) |
5.855 (3.457 to 9.916) |
2.001 (1.344 to 2.979) |
|||
| N-Stage | ||||||
| N0 | 1 | <.0001 | ns | ns | 1 | 0.0006 |
| N1 | 2.07 (1.458 to 2.939) | ns | 2.115 (1.382 to 3.238) |
|||
| T-Stage | ||||||
| T1/2 | ns | ns | 1 | 0.0355 | 1 | 0.0149 |
| T3/4 | ns | 2.037 (1.049 to 3.954) |
2.384 (1.185 to 4.798) |
|||
| Tumor Location | ||||||
| Distal | 1 | 0.0442 | 1 | 0.0243 | ns | ns |
| Cervical vs. Distal | 0.293 (0.117 to 0.732) |
0.623 (0.263 to 1.477) |
ns | |||
| Upper Thoracic vs. Distal |
1.087 (0.526 to 2.249) |
1.283 (0.510 to 3.224) |
ns | |||
| Mid Thoracic vs. Distal |
1.209 (0.725 to 2.016) |
2.085 (1.198 to 3.627) |
ns | |||
Abbreviation: ns = not significant.
Figure 1.
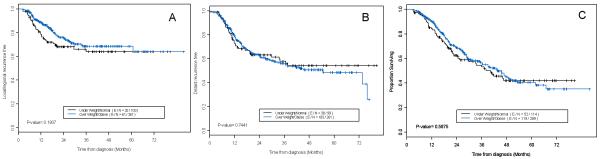
Kaplan-Meier curves of disease outcomes in the total patient cohort. Patients with BMI < 25 kg/m2 (“underweight/normal”) were compared to patients with BMI ≥ 25 kg/m2 (“overweight/obese”) in terms of (A) locoregional failure, (B) distant failure, and (C) overall survival outcomes.
DISCUSSION
As the rise in obesity rates has become a public healthcare crisis, accurate assessments of the impact of obesity on cancer-related outcomes have become critical. The current analysis represents an initial large-scale attempt to examine the effect of BMI on postoperative complications, CRT-related treatment toxicities, and long-term survival outcomes in esophageal cancer patients.
In previous studies, high BMI was found to be a risk factor for increased surgical complications (7, 8). Theoretically, although one may expect higher postoperative complication rates with higher BMI due to associations of obesity with existing medical complications and the complexity/duration of anesthesia, our results support the bulk of more recent evidence (9-12, 14) demonstrating that obesity does not increase the risk of surgical complications despite obese patients having significantly more co-morbid illnesses (p < 0.001). Obese patients were actually less likely to have GI complications (p = 0.011), perhaps as a paradoxical result of more careful dissection by the surgeons because of the patients’ obesity status. Another hypothesis may be that obese patients in our study were significantly more likely to have distal GEJ cancers (as related to reflux), whereas non-obese patients were more likely to have mid/upper thoracic tumors. Because the anastomosis is in the radiation treatment field for mid/upper thoracic tumors, leak rates may be higher than for GEJ tumors where the anastomosis is above the radiation field. This hypothesis has not been tested in previous literature and warrants further investigation. Nevertheless, our results suggest that patients who are otherwise oncologically eligible for esophagectomy should not be denied surgery based on BMI alone. A caveat to this conclusion is that the risk of complications may be evident only in extreme obese patients (BMI > 40 kg/m2) and our study included only 10 (2.5%) patients in that category. This study is also based on the experiences of high-volume esophageal surgeons, whose overall rates of post-operative complications may be too low to discern differences between the two BMI groups.
Our data also differ from that previously published by our institution in terms of the patient population analyzed. While previous studies (11, 15) have focused on patients treated with surgery as primary therapy, our study focused on patients primarily treated with chemoradiotherapy with or without surgery. Despite comparable doses of radiation delivered and no differences in radiation modality used, high-BMI patients were significantly less likely to have high-grade esophagitis, stricture, and hematologic toxicities such as anemia, leukopenia, and neutropenia. To our knowledge, no other study has examined the influence of BMI on treatment toxicities in esophageal cancer patients treated with CRT. However, our findings validate studies in other disease sites which report lower rates of grade 3-4 leukopenia and any grade ≥ 3 toxicity in obese vs. non-obese patients with colon cancer (17). Similarly, in breast cancer patients, obesity has been independently associated with lower likelihood of hospitalization for febrile neutropenia and less tendency to experience cycle delays due to prolonged myelosuppression (18). There are no previous reports on the incidence of radiation-induced esophagitis or stricture in relation to BMI; however, our findings suggest that fat may act like natural tissue separation from visceral organs, protecting normal tissue (including the normal esophagus) from radiation effects. Because low BMI may be prognostic of higher treatment-related complication rates, pre-treatment interventions to improve patient tolerance of treatment may be needed in non-obese patients. The decreased complication rate among our obese patients also suggests that achieving a higher weight before treatment may be of benefit.
Our study also adds more comprehensive data to existing evidence on the influence of BMI on prognostic outcomes. Studies of esophageal cancer patients have shown tumor stage and chemoradiation sequence as significant predictors of pCR (19). In our study, we not only validate advanced tumor stage as a poor prognostic factor for pCR, and therefore reinforce the need for early diagnosis, but also demonstrate white race and lack of residual tumor on post-treatment EGD as independent prognostic factors for improved odds of pCR. Furthermore, we demonstrate that BMI is neither a significant predictor of pCR, nor of patterns of failure or OS. These results validate a number of previous studies (8, 20) which similarly found no differences in OS or PFS between obese and non-obese esophageal cancer patients. While Melis et al (12) demonstrated longer OS/PFS for high-BMI patients, their study used pre-surgery BMI rather than pre-neoadjuvant treatment BMI, which may have been a confounding factor.
This lack of relationship between BMI and prognosis in esophageal cancer contrasts to findings in breast cancer and colon cancer, where high BMI is a more established negative prognostic factor (5, 6, 17). This disparity may be related to gender interactions and effects of estrogen. Most breast cancer patients are women, and several colon cancer studies have shown that while BMI had no significant influence in men, obese women experienced worse OS and increased risk of disease recurrence relative to non-obese women (17). A study by Calle et al (21) similarly demonstrated that the positive association between BMI and death from any cancer, including esophageal cancer, became stronger when the analysis was restricted to women. Thus, because most (84%) of the patients in our study were men, reflecting the general esophageal cancer population, it may be that any differential effect of BMI on outcomes in women were not detectable. Unfortunately, the low number (n = 65) of women in our study prohibits this subgroup analysis.
Further limitations of our study include weaknesses common to retrospective reviews, such as selection/sample bias and heterogeneity of treatment. Also, there were relatively small numbers of extremely obese and female patients. Nevertheless, we limited the heterogeneity of our patient population by examining only those patients with non-metastatic esophageal carcinoma treated with either definitive chemoradiotherapy or neoadjuvant CRT followed by surgery.
In conclusion, this is a large single-institution study of the effect of BMI on clinical outcomes in esophageal cancer patients treated primarily with chemoradiotherapy +/− surgery. Obesity was not predictive for poorer disease outcomes, such as pCR, OS, or PFS. Despite more comorbidities, higher BMI patients were not found to experience increased risk of surgical complications. Furthermore, obesity was associated with reduced risk of several radiation/chemotherapy-related treatment toxicities.
Table 4. Clinical Factors and Odds of pCR (N=199).
| Factor | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|
| BMI | |||
| Non-Obese | 1 | ||
| Obese | 0.994 | 0.454 to 2.175 | 0.9879 |
| Race | |||
| Non-White | 1 | ||
| White | 4.373 | 1.174 to 16.292 | 0.0279 |
| T-stage | |||
| T1/2 | 1 | ||
| T3/4 | 0.403 | 0.173 to 0.942 | 0.036 |
| EGD Biopsy Response | |||
| No Tumor | 1 | ||
| Residual Tumor | 0.109 | 0.032 to 0.376 | 0.0004 |
Acknowledgements
This study is supported by the NIH through the MDACC core grant (CA16672).
Footnotes
The authors have no personal conflicts of interest to declare.
REFERENCES
- 1.Blanck HM, Dietz WH, Galuska DA, et al. State-specific prevalence of obesity among adults-United States, 2005. Centers for Diseases Control and Prevention, Morbidity and Mortality Weekly Report. 2006;55:4. [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004 Jun 16;291(23):2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 3.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001 May 17;411(6835):390–5. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 4.Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979;32(8):563–76. doi: 10.1016/0021-9681(79)90119-x. [DOI] [PubMed] [Google Scholar]
- 5.Bastarrachea J, Hortobagyi GN, Smith TL, Kau SW, Buzdar AU. Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann Intern Med. 1994 Jan 1;120(1):18–25. doi: 10.7326/0003-4819-120-1-199401010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau SW, Bondy M, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008 Sep 1;26(25):4072–7. doi: 10.1200/JCO.2007.14.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujitani K, Ajani JA, Crane CH, Feig BW, Pisters PW, Janjan N, et al. Impact of induction chemotherapy and preoperative chemoradiotherapy on operative morbidity and mortality in patients with locoregional adenocarcinoma of the stomach or gastroesophageal junction. Ann Surg Oncol. 2007 Jul;14(7):2010–7. doi: 10.1245/s10434-006-9198-2. [DOI] [PubMed] [Google Scholar]
- 8.Healy LA, Ryan AM, Gopinath B, Rowley S, Byrne PJ, Reynolds JV. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg. 2007 Nov;134(5):1284–91. doi: 10.1016/j.jtcvs.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Kilic A, Schuchert MJ, Pennathur A, Yaeger K, Prasanna V, Luketich JD, et al. Impact of obesity on perioperative outcomes of minimally invasive esophagectomy. Ann Thorac Surg. 2009 Feb;87(2):412–5. doi: 10.1016/j.athoracsur.2008.10.072. [DOI] [PubMed] [Google Scholar]
- 10.Morgan MA, Lewis WG, Hopper AN, Escofet X, Harvard TJ, Brewster AE, et al. Prognostic significance of body mass indices for patients undergoing esophagectomy for cancer. Dis Esophagus. 2007;20(1):29–35. doi: 10.1111/j.1442-2050.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y, Correa AM, Hofstetter WL, Vaporciyan AA, Rice DC, Walsh GL, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer. Dec 15;116(24):5619–27. doi: 10.1002/cncr.25745. [DOI] [PubMed] [Google Scholar]
- 12.Melis M, Weber JM, McLoughlin JM, Siegel EM, Hoffe S, Shridhar R, et al. An elevated body mass index does not reduce survival after esophagectomy for cancer. Ann Surg Oncol. Mar;18(3):824–31. doi: 10.1245/s10434-010-1336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012 May 31;366(22):2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 14.Blom RL, Lagarde SM, Klinkenbijl JH, Busch OR, van Berge Henegouwen MI. A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol. Mar;19(3):766–71. doi: 10.1245/s10434-011-2103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi Y, Correa AM, Hofstetter WL, Vaporciyan AA, Mehran RJ, Rice DC, et al. Patients with high body mass index tend to have lower stage of esophageal carcinoma at diagnosis. Dis Esophagus. Sep;25(7):614–22. doi: 10.1111/j.1442-2050.2011.01290.x. [DOI] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, FLeming ID, et al. AJCC Cancer Staging Handbook. 6th edn. Springer-Verlag; New York: 2002. [Google Scholar]
- 17.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB, 3rd, Macdonald JS, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003 Aug 1;98(3):484–95. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 18.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005 Jun 13;165(11):1267–73. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 19.Heath EI, Burtness BA, Heitmiller RF, Salem R, Kleinberg L, Knisely JP, et al. Phase II evaluation of preoperative chemoradiation and postoperative adjuvant chemotherapy for squamous cell and adenocarcinoma of the esophagus. J Clin Oncol. 2000 Feb;18(4):868–76. doi: 10.1200/JCO.2000.18.4.868. [DOI] [PubMed] [Google Scholar]
- 20.Grotenhuis BA, Wijnhoven BP, Hotte GJ, van der Stok EP, Tilanus HW, van Lanschot JJ. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg. Nov;34(11):2621–7. doi: 10.1007/s00268-010-0697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]


