Abstract
Introduction
Micronutrient deficiencies are key concerns after bariatric surgery. We describe the prevalence of perioperative testing and diagnosis of micronutrient deficiencies among a cohort of insured bariatric surgery patients.
Methods
We used claims data from seven health insurers to identify bariatric surgery patients from 2002–2008. Our outcomes were perioperative claims for vitamin D, B12, folate, and iron testing and diagnosed deficiencies. We analyzed results by bariatric surgery type: Roux-en-Y gastric bypass (RYGB), restrictive, and malabsorptive. We calculated the prevalence of testing and deficiency diagnosis, and performed multivariate logistic regression to determine the association with surgery type.
Results
Of 21,345 eligible patients, 84% underwent RYGB. The pre-surgical testing prevalence for all micronutrients was <25%. The testing prevalence during the first 12 months after surgery varied: vitamin D (12%), vitamin B12 (60%), folate (47%) and iron (49%), and declined during 13–24 and 25–36 months. The deficiency prevalence during 0–12 months post-survey varied: vitamin D (34%), vitamin B12 (20%), folate (13%), and iron (10%). The odds of vitamin B12, folate, and iron deficiency during 0–12 months were significantly lower for restrictive as compared to RYGB, but were not different during 13–24 and 25–36 months post-surgery. The odds of vitamin D deficiency were significantly greater for malabsorptive as compared to RYGB during all post-surgical periods.
Conclusion
Many patients did not receive micronutrient testing pre- or post-surgery, yet deficiencies were relatively common among those tested. These results highlight the need for surgeons and primary care providers to test all bariatric surgery patients for micronutrient deficiencies.
Keywords: bariatric surgery, vitamin D deficiency, vitamin B12 deficiency, folate deficiency, iron deficiency
Introduction
Recent studies estimate that the current number of bariatric surgeries performed ranges between 113,000–125,000 cases per year [1–2]. These surgical procedures induce weight loss through malabsorption and/or restriction. However, malabsorptive surgeries including Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion (BPD) and biliopancreatic diversion with duodenal switch (BPD-DS) may make patients susceptible to micronutrient deficiencies. Patients who undergo restrictive surgeries such as adjustable gastric banding (AGB) and vertical banded gastroplasty (VBG) are generally considered at less risk for these deficiencies; however, micronutrient deficiencies may theoretically occur as a consequence of dietary changes that occur after the procedure.
Few studies have evaluated the use of micronutrient laboratory testing in the perioperative bariatric surgery period. In a 1999 survey, 102 surgeons self-reported ordering iron or total iron binding capacity (TIBC) in 56% of patients, vitamin B12 in 66%, and folate in 58% of RYGB patients, and 24 surgeons self-reported ordering iron or TIBC in 80%, vitamin B12 in 67%, folate in 71%, and vitamin D in 46% of BPD patients [3]. However, little is known about the actual receipt of perioperative laboratory testing at the population level. Recent guidelines have recommended routine laboratory testing to diagnose these micronutrient deficiencies as a part of the long-term medical management, although the strength of evidence for this recommendation is weak [4].
In contrast, a number of studies have documented the prevalence of post-operative diagnosis of micronutrient deficiencies. A 2005 review summarized the literature examining micronutrient deficiencies after bariatric surgery [5]. The prevalence of vitamin D, folate and iron deficiencies varied widely by surgery type and across studies, although B12 deficiency was consistently found among 33% of RYGB patients [5]. All of the studies included in this review and additional recent studies have primarily been limited to a single-site [5–9]. To date, no single site study has included more than 600 patients, and in fact, most have examined fewer than 100 patients. These small single site studies may explain the prior variable results.
Our objective was to characterize the use of micronutrient laboratory testing in both the pre- and post-bariatric surgical periods, and to test for differences in micronutrient laboratory testing according to type of bariatric surgery. We hypothesized that patients who underwent restrictive surgery would be less likely to undergo pre- and post-surgical micronutrient laboratory testing as compared to patients who underwent RYGB, while there would be no difference in pre- or post-surgical laboratory testing between patients who underwent malabsorptive surgery and RYGB. Our second objective was to determine the prevalence of micronutrient deficiencies in the post-bariatric period, and to test for differences in micronutrient deficiencies by type of bariatric surgery. We hypothesized that patients who underwent restrictive surgery would be less likely to have a diagnosed micronutrient deficiency as compared to RYGB patients. We hypothesized that patients who underwent malabsorptive surgery would be more likely to have vitamin D deficiency and be equally likely to have iron, vitamin B12 or folate deficiencies as compared to RYGB patients.
Methods
Data source
We combined 2002–2008 claims data from seven Blue Cross/Blue Shield health plans, which included enrollment, inpatient, outpatient, professional, laboratory, and pharmacy claims records. The Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB) classified this study as exempt from review.
Selection of study sample
We included all patients who had undergone bariatric surgery, as determined from paid claims data using either common procedural terminology codes (CPT) for those surgeries or by international classification of disease codes (ICD-9) for surgical procedures that were associated with an ICD-9 code for morbid obesity (278.01). We used this method to identify restrictive (43770, 43842–3, S2082, 44.68, 44.95, 43.89, 44.38), RYGB (43644–5, 43844, 43846–7, 43659, S2085, 44.31, 44.39), and malabsorptive procedures (43845, 45.50–1, 45.90–1). We excluded surgical procedures associated with gastrointestinal malignancy (ICD-9: 150–152; 157, 199) or gastrointestinal ulcer (ICD-9: 531–533). In addition, we required that patients be enrolled in the health plan for 6 months prior to their bariatric surgery, and to have both medical and prescription benefits coverage during this period.
Measures
Our first outcome was the receipt of micronutrient laboratory testing. We focused on four common micronutrients: vitamin D, vitamin B12, folate (vitamin B9), and iron. We used CPT codes to identify the occurrence of laboratory testing (Table 1). We determined whether or not each patient underwent micronutrient laboratory testing at any point during specific time periods both pre- and post-bariatric surgery. We defined the pre-bariatric period as the 6 months prior to the date of bariatric surgery. During the post-bariatric period, we examined several time intervals including 0–12 months, 13–24 months, and 25–36 months after the date of bariatric surgery. We also evaluated the outcome of receipt of “any micronutrient testing” and “all micronutrient testing.” We defined “any” testing by identifying patients who had received at least one laboratory test for vitamin D, vitamin B12, folate, or iron. We defined “all” testing when patients received laboratory testing for all of the micronutrients defined above.
Table 1.
Micronutrient laboratory testing and deficiency outcome variables
| Micronutrient Laboratory Testing | Micronutrient Deficiency | |||||
|---|---|---|---|---|---|---|
| Vitamin D |
25-OH Vitamin D OR 1,25-OH Vitamin D |
Vitamin D Deficiency OR | ||||
| Medication Prescription OR | ||||||
| Medication Injection | ||||||
| CPT | 82306 82652 |
ICD-9 | 268 268.0 |
268.1 268.2 |
268.9 | |
| Multum | 1418 | 1419 | 1420 | |||
| CPT | J1270 | |||||
|
| ||||||
| Vitamin B12 | Vitamin B12 | Vitamin B12 Deficiency OR | ||||
| Medication Prescription OR | ||||||
| Medication Injection | ||||||
| CPT | 82607 | ICD-9 | 281.1 | 281.3 | 266.2 | |
| Multum | 4686 3610 |
3609 | 6858 | |||
| CPT | J3420 | |||||
|
| ||||||
| Folate | Folate OR | Folic Acid Deficiency OR | ||||
| RBC Folate | Medication Prescription | |||||
| CPT | 82746 82747 |
ICD-9 | 281.2 | 281.3 | 266.2 | |
| Multum | 4686 3610 |
1197 3609 |
6858 | |||
|
| ||||||
| Iron | Iron | Iron Deficiency OR | ||||
| Medication Prescription OR | ||||||
| Medication Injection/Infusion | ||||||
| CPT | 83540 | ICD-9 | 280 | 280.1 | 280.9 | |
| Multum | 1582 | 6911 | ||||
| CPT | J1750 J1751 |
J1752 Q0138 |
Q0139 Q4098 |
|||
Abbreviations: CPT, Common Procedural Terminology Code; ICD-9, International Classification of Diseases, Ninth Revision; RBC, red blood cell.
Our second outcome was occurrence of micronutrient deficiencies during several post-bariatric surgical periods. We evaluated for vitamin D, vitamin B12, folate, and iron deficiencies at any point within the period (0–12 months, 13–24 months, 25–36 months). For each micronutrient deficiency, we only examined the outcome among patients who received laboratory testing for that micronutrient in an effort to increase our accuracy to identify a true micronutrient deficiency diagnosis. Patients were identified as having a micronutrient deficiency if they received a micronutrient deficiency diagnosis, were prescribed a micronutrient medication, or received an injection or infusion of a micronutrient (Table 1). We determined diagnosis of micronutrient deficiencies through ICD-9 codes assigned on claims for outpatient visits or inpatient admissions. We identified micronutrient prescription medications dispensed by a pharmacist though pharmacy claims data using Multum codes. We determined injection or infusion of micronutrient medications through CPT codes. We also evaluated the outcome of “any micronutrient deficiency,” which we created by identifying patients who had at least one of the micronutrient deficiencies as defined above.
Our independent variable was type of bariatric surgery. We categorized surgery type as 1) restrictive surgery, which included adjustable gastric banding, vertical banded gastroplasty, and sleeve gastrectomy, 2) RYGB, and 3) malabsorptive surgery, which included biliopancreatic diversion and biliopancreatic diversion with duodenal switch. We have used a similar classification system previously [10]. Covariates included age, sex, and health insurance plan. We also included year of surgery to account for the temporal changes in surgical practices.
Statistical analyses
All analyses were conducted with SAS. Descriptive analyses of all variables were performed. For our first objective, we calculated the unadjusted prevalence of micronutrient laboratory testing among all eligible patients for each pre- and post-surgical period. To determine if differences in micronutrient laboratory testing occurred by bariatric surgery type, we performed multivariate logistic regression analysis adjusted for age, sex, surgery year, and health plan. Patients missing data on age or sex were excluded from the logistic regression analysis. For our second objective, we calculated the unadjusted prevalence of micronutrient deficiencies among eligible patients who had received testing for that micronutrient in each post-surgical period. We also calculated the unadjusted prevalence of any micronutrient deficiency among eligible patients who had received “all” micronutrient laboratory testing in each perioperative period. To determine if differences in micronutrient deficiencies or any micronutrient deficiency occurred by type of bariatric surgery, we performed multivariate logistic regression analysis. The models were adjusted for age, sex, surgery year, and health plan. Patients missing data on age or sex were excluded from the logistic regression analysis.
Results
Overall, our sample included 21,345 patients. The mean age was 44.1 years (SD 10.4) and 81% were female. The majority of patients underwent RYGB (84%). Table 2 displays the characteristics of the overall study sample.
Table 2.
Characteristics of the study sample
| Eligible Beneficiaries (n=21,345) | |
|---|---|
| Age in years | |
| Mean (SD) | 44.1 (10.4) |
| Sex | |
| % Female | 81% |
| Bariatric surgery type | |
| % Restrictive | 15% |
| % RYGB | 84% |
| % Malabsorptive | 1% |
| Year surgical procedure performed | |
| % 2002 | 13% |
| % 2003 | 20% |
| % 2004 | 18% |
| % 2005 | 19% |
| % 2006 | 12% |
| % 2007 | 9% |
| % 2008 | 9% |
Abbreviation: RYGB, Roux-en-Y gastric bypass.
Vitamin D
For the entire sample, only 7% underwent laboratory testing for vitamin D in the pre-surgical period, which did not vary by surgery type. Figure 1 displays the prevalence of vitamin D laboratory testing and deficiency by surgery type. After surgery, few patients received laboratory testing for vitamin D irrespective of surgery type or time period (Supplementary Table 1). In adjusted multivariate logistic regression models, patients who had restrictive surgery had significantly lower odds of receiving laboratory testing in any time period as compared to RYGB (Table 3).
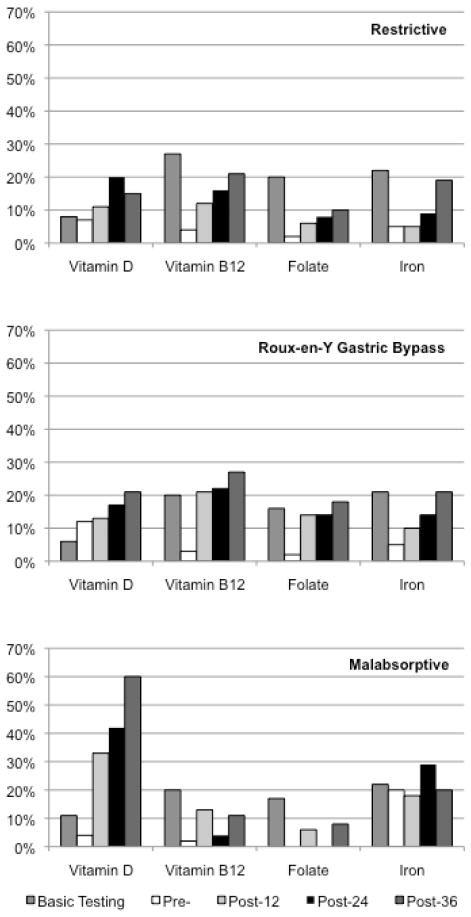
Figure 1.
Unadjusted prevalence of micronutrient laboratory testing and deficiency bariatric surgery type
Figure 1 shows the unadjusted prevalence of laboratory testing and deficiency diagnosis of vitamin D, vitamin B12, folate, and iron for each surgery type. For each type of bariatric surgery, we display the pre-surgical basic laboratory testing for each micronutrient (vitamin D, vitamin B12, folate, and iron). We also display the prevalence of diagnosed deficiency among those patients who had at least basic laboratory testing for the pre-surgical (pre-), 12 months post-surgery (post-12), 24 months post-surgery (post-24), and 36 months post-surgery (post-36).
Table 3.
Adjusted odds of receiving laboratory testing for micronutrients by type of bariatric surgery
| Pre-Surgicala | Post-Surgicala | ||||
|---|---|---|---|---|---|
| 6 months (n=21,239) | 0–12 months (n=13,267) | 12–24 months (n=8,022) | 24–36 months (n=5,390) | ||
| Vitamin D | Restrictive | 0.41b (0.35–0.48) | 0.24 b (0.20–0.28) | 0.27 b (0.20–0.36) | 0.43 b (0.29–0.61) |
| RYGB | 1.00 | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 1.88 b (1.16–3.02) | 1.26 (0.78–2.03) | 1.36 (0.72–2.59) | 2.12 (1.00–4.48) | |
|
| |||||
| Vitamin B12 | Restrictive | 0.96 (0.87–1.05) | 0.08 b (0.07–0.09) | 0.16 b (0.14–0.20) | 0.21 b (0.16–0.28) |
| RYGB | 1.00 | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 1.27 (0.88–1.82) | 0.16 b (0.11–0.24) | 0.44 b (0.27–0.72) | 1.11 (0.60–2.05) | |
|
| |||||
| Folate | Restrictive | 0.90 b (0.81–1.00) | 0.12 b (0.10–0.13) | 0.19 b (0.16–0.24) | 0.21 b (0.15–0.28) |
| RYGB | 1.00 | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 1.44 (0.98–2.12) | 0.25 b (0.16–0.37) | 0.64 (0.39–1.07) | 1.06 (0.54–2.09) | |
|
| |||||
| Iron | Restrictive | 0.83 b (0.75–0.92) | 0.14 b (0.12–0.16) | 0.22 b (0.18–0.27) | 0.32 b (0.25–0.42) |
| RYGB | 1.00 | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 1.54 b (1.08–2.18) | 0.30 b (0.20–0.44) | 0.60 (0.36–1.00) | 1.28 (0.67–2.43) | |
Abbreviation: RYGB, Roux-en-Y gastric bypass.
Multivariate logistic regression was used to calculate the displayed odds ratios and 95% confidence intervals, which were adjusted for age, sex, surgery year, and health plan. Patients missing age or sex were excluded from this analysis.
p<0.05
The prevalence of vitamin D deficiency increased after surgery for all patients; however, the prevalence was substantially higher after malabsorptive surgery (Figure 1). In adjusted models, patients who underwent malabsorptive surgery had significantly greater odds of having vitamin D deficiency in all post-surgical time periods as compared to RYGB (Table 4).
Table 4.
Adjusted odds of micronutrient deficiencies by bariatric surgery type among patients who received laboratory testing
| Post-Surgicala | ||||
|---|---|---|---|---|
| 0–12 months | 12–24 months | 24–36 months | ||
| Vitamin D | (n=1,573) | (n=810) | (n=584) | |
| Restrictive | 0.92 | 1.50 | 0.84 | |
| (0.53–1.60) | (0.73–3.04) | (0.32–2.21) | ||
| RYGB | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 2.66b | 3.77 b | 6.82 b | |
| (1.09–6.50) | (1.14–12.52) | (1.82–25.59) | ||
|
| ||||
| Vitamin B12 | (n=7,898) | (n=3,522) | (n=1,905) | |
| Restrictive | 0.53 b | 0.71 | 0.70 | |
| (0.40–0.72) | (0.46–1.09) | (0.37–1.30) | ||
| RYGB | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 0.61 | 0.20 | 0.25 | |
| (0.25–1.52) | (0.04–1.04) | (0.05–1.41) | ||
|
| ||||
| Folate | (n=6,285) | (n=2,608) | (n=1,394) | |
| Restrictive | 0.46 b | 0.54 | 0.47 | |
| (0.30–0.72) | (0.27–1.06) | (0.17–1.27) | ||
| RYGB | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 0.48 | 0.11 | 0.52 | |
| (0.13–1.77) | (0.01–1.95) | (0.09–3.09) | ||
|
| ||||
| Iron | (n=6,478) | (n=2,860) | (n=1,575) | |
| Restrictive | 0.47 b | 0.68 | 1.03 | |
| (0.30–0.73) | (0.38–1.22) | (0.55–1.92) | ||
| RYGB | 1.00 | 1.00 | 1.00 | |
| Malabsorptive | 2.03 | 2.41 | 1.16 | |
| (0.90–4.58) | (0.92–6.29) | (0.33–4.10) | ||
Abbreviation: RYGB, Roux-en-Y gastric bypass.
Multivariate logistic regression was used to calculate the displayed odds ratios and 95% confidence intervals, which were adjusted for age, sex, surgery year, and health plan. Patients missing age or sex were excluded from this analysis.
p<0.05
Vitamin B12
For the entire sample, the pre-surgical basic laboratory testing for vitamin B12 was 21%. Figure 1 displays the prevalence of vitamin B12 laboratory testing and deficiency by surgery type. A majority of RYGB patients received laboratory testing for vitamin B12 during the first 12 months after surgery (65%), which then declined to 48% and 38% during the 13–24 and 25–36 month periods, respectively (Supplementary Table 2). The prevalence of vitamin B12 testing also decreased after surgery for patients who underwent restrictive surgery (0–12 month period 26%, 25–36 month period 13%), while the testing prevalence among those patients who had malabsorptive surgery increased post-surgery (0–12 month period 29, 25–36 month period 40%) (Supplementary Table 2). In adjusted models, patients who had restrictive surgery had significantly lower odds of receiving vitamin B12 testing in all post-surgical periods and patients who underwent malabsorptive surgery had significantly lower odds of receiving laboratory testing in the 0–12 and 13–24 month post-surgical periods as compared to RYGB (Table 3).
The prevalence of vitamin B12 deficiency increased after surgery for all patients; however, the prevalence was highest after RYGB (Figure 1). In adjusted multivariate logistic regression models, those patients who had restrictive surgery had significantly lower odds of vitamin B12 deficiency in the 0–12 month post-surgical time period as compared to RYGB (Table 4). There were no other significant differences in the odds of vitamin B12 deficiency between any other surgery types at all time periods.
Folate
For the entire sample, the pre-surgical laboratory testing for folate was 17%. Figure 1 displays the prevalence of folate testing and deficiency by surgery type. A majority of patients who had RYGB received laboratory testing for folate in the first 12 months after surgery (52%), which then declined to 35% and 28% during the 13–24 and 25–36 month periods, respectively (Supplementary Table 3). Few patients who had restrictive or malabsorptive surgery received laboratory testing for folate during any time period (Supplementary Table 3). In adjusted multivariate logistic regression models, patients who underwent restrictive surgery had significantly lower odds of receiving folate testing during all time periods as compared to RYGB (Table 3).
The prevalence of folate deficiency increased after surgery for all patients; however, the prevalence was higher among RYGB patients (Figure 1). In adjusted models, patients who underwent restrictive surgery had significantly lower odds of folate deficiency in the 0–12 month post-surgical time period as compared to RYGB (Table 4). There were no other significant differences in the odds of folate deficiency between any other surgery types at all time periods.
Iron
For the entire sample, the pre-surgical basic laboratory testing for iron was 21%. Figure 1 displays the prevalence of iron laboratory testing and deficiency by surgery type. A majority of patients who had RYGB received laboratory testing for iron in the first 12 months after surgery (53%), which then declined to 39% and 31% during the 13–24 and 25–36 month periods, respectively (Supplementary Table 4). Few patients who had restrictive or malabsorptive surgery received laboratory testing for iron during any time period (Supplementary Table 4). In adjusted models, patients who had restrictive surgery had significantly lower odds of receiving laboratory testing for iron during all time periods and patients who underwent malabsorptive surgery had significantly lower odds of receiving laboratory testing during the pre-surgical and 0–12 month post-surgical periods as compared to RYGB (Table 3).
The prevalence of iron deficiency was highest among patients who had malabsorptive surgery in the pre-surgical period (20%), as compared to patients who underwent restrictive surgery (5%) or RYGB (5%). The prevalence of iron deficiency increased among patients after restrictive surgery and RYGB (Figure 1). In adjusted multivariate logistic regression models, patients who had restrictive surgery had significantly lower odds of iron deficiency during the 0–12 month post-surgical time period as compared to RYGB (Table 4). There were no other significant differences in the odds of iron deficiency between any other surgery types during all time periods.
Any micronutrient deficiency
For the entire sample, the receipt of pre-surgical laboratory testing for all micronutrients was only 3%. The receipt of all laboratory testing remained low during all post-surgical periods irrespective of surgery type (Table 5). During the pre-surgical period, the prevalence of any micronutrient deficiency was highest among patients who had malabsorptive surgery (25%), as compared to patients who underwent restrictive surgery (12%) or RYGB (17%). The prevalence of any micronutrient deficiency increased for all bariatric surgery types during the 0–12 month period (Table 5). The prevalence of any micronutrient deficiency remained constant during the 0–12, 13–24, and 25–36 month periods for patients who underwent restrictive surgery, but continued to increase during 13–24 and 25–36 month periods among patients after RYGB and malabsorptive surgery (Table 5). In adjusted models, patients who had restrictive surgery had significantly lower odds of any micronutrient deficiency during the 0–12 month post-surgical period as compared to RYGB (OR 0.53, 95%CI 0.30–0.91). There were no other significant differences in the odds of any micronutrient deficiency between any other surgery types during all time periods.
Table 5.
Unadjusted prevalence of micronutrient laboratory testing and deficiency by surgery type
| Pre-Surgical | Post-Surgical | ||||
|---|---|---|---|---|---|
| 6 months | 0–12 months | 12–24 months | 24–36 months | ||
| Restrictive | Sample size | 3,257 | 1,877 | 978 | 502 |
| Any laboratory testsa | 1,033 (32%) | 656 (35%) | 235 (24%) | 107 (21%) | |
| All laboratory testsb | 137 (4%) | 93 (5%) | 20 (2%) | 10 (2%) | |
| Any micronutrient deficiencyc | 16 (12%) | 19 (20%) | 4 (20%) | 2 (20%) | |
|
| |||||
| RYGB | Sample size | 17,880 | 11,265 | 6,966 | 4,845 |
| Any laboratory testsa | 5,106 (29%) | 7,747 (69%) | 3,646 (52%) | 2,085 (43%) | |
| All laboratory testsb | 552 (3%) | 1,006 (9%) | 482 (7%) | 306 (6%) | |
| Any micronutrient deficiencyc | 94 (17%) | 321 (32%) | 174 (36%) | 133 (43%) | |
|
| |||||
| Malabsorptive | Sample Size | 208 | 136 | 81 | 45 |
| Any laboratory testsa | 50 (24%) | 48 (35%) | 27 (33%) | 20 (44%) | |
| All laboratory testsb | 20 (10%) | 23 (17%) | 12 (15%) | 8 (18%) | |
| Any micronutrient deficiencyc | 5 (25%) | 9 (39%) | 7 (58%) | 7 (88%) | |
Abbreviation: RYGB, Roux-en-Y gastric bypass.
Any laboratory tests includes patients who underwent testing for vitamin D or vitamin B12 or folate or iron.
All laboratory tests includes patients who underwent testing for vitamin D, vitamin B12, folate and iron.
Denominator to calculate prevalence of any micronutrient deficiency is sample size of beneficiaries who received all laboratory tests for that period.
Conclusion
Despite their increased risk for micronutrient deficiencies, we found that many bariatric surgery patients were not receiving pre- or post-operative micronutrient laboratory testing between 2002–2008. We also confirmed that micronutrient deficiencies were relatively common after bariatric surgery. Recent guidelines on the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient made specific recommendations regarding routine testing for micronutrient deficiencies, although the strength of evidence for this recommendation at that time was weak [4]. Our results lend population-level evidence to support pre-surgical testing to help optimize patients prior to surgery, as well as regular postsurgical micronutrient deficiency screening for all bariatric surgery patients. Because we used data from a large, multi-site cohort over many years, we believe that our results provide better estimates of the population prevalence of testing and diagnosis of these micronutrient deficiencies than prior single site studies [5–9]. In the following paragraphs, we highlight the current screening recommendations supported by our results, as well as possible changes to current practice guidelines suggested by our findings.
For post-RYGB patients, the guidelines recommend testing for 25-OH vitamin D, vitamin B12, and iron every 3–6 months in the first year after surgery and then annually thereafter [4]. In prior studies, vitamin D deficiency has varied after RYGB ranging from 7–51% [5–6, 8–9]. We found that the prevalence of vitamin D deficiency was 21% between 25–36 months after surgery, which is consistent with these results. Across many studies, the prevalence of B12 deficiency is approximately 33% after RYGB [5–9]. We identified a slightly lower prevalence of B12 deficiency among our patients (27% between 25–36 months), which is likely an underestimation given that we were unable to identify patients who used over-the-counter supplements. Previous studies have reported an increase in iron deficiency after RYGB, although the prevalence of iron deficiency has varied between studies [5, 7–9]. We identified the prevalence of iron deficiency to be 21% within 25–36 months after surgery. We believe that our results provide additional population-level support for the routine annual screening of vitamin D, vitamin B12 and iron deficiency in RYGB patients. In contrast, the guidelines suggest RBC folate testing to be optional [4]. While folate deficiency has been identified less commonly among RYGB patients [5–9], we found that 18% of patients had folate deficiency between 25–36 months after surgery. Given that the prevalence of folate deficiency nears that of vitamin D and iron deficiency in our population, providers may need to consider routinely screening RYGB patients with RBC folate at a similar frequency to vitamin B12, vitamin D and iron rather than having it be an optional test.
For BPD or BPD-DS patients, these guidelines recommend testing for 25-OH vitamin D every 6–12 months and for vitamin B12, RBC folate, and iron every 3 months in the first year after surgery, and then every 3–6 months thereafter [4]. We found a high prevalence of vitamin D deficiency after malabsorptive surgeries, which is similar to previous studies [5]. The prevalence of B12 and folate deficiencies was low in our sample, and few studies have previously reported the prevalence of these micronutrient deficiencies after BPD or BPD-DS [11]. Results across studies for iron deficiency among patients who underwent malabsorptive surgeries have been inconsistent [5]. We found that the prevalence of iron deficiency was 20% between 25–36 months. We acknowledge that our sample size in the malabsorptive group was small (baseline n=208), which may have limited our power to detect differences. Given the higher rates of vitamin D deficiency as compared to that of vitamin B12 and folate, providers may consider screening malabsorptive patients with 25-OH vitamin D with the same frequency as vitamin B12, folate, and iron.
Currently, the guidelines do not recommend routine testing for micronutrient deficiencies or routine micronutrient supplementation after restrictive surgery [4]. A few small studies have examined micronutrient deficiencies among patients after restrictive surgery, where the incidence of vitamin D, vitamin B12, folate, and iron deficiency was low [8–9, 12–13]. In contrast, our sample included a relatively large number of restrictive patients (baseline n=3,257), which enabled us to better evaluate the likelihood of post-surgical micronutrient deficiencies. Between 25–36 months, the prevalence for vitamin D deficiency was 15%, vitamin B12 was 21%, folate was 10%, and iron was 19%. While restrictive patients had a significantly lower risk of micronutrient deficiencies in the first 12 months after surgery, there was no significant difference between restrictive and RYGB patients after 12 months. Our results suggest that patients who undergo restrictive surgeries have comparable rates of micronutrient deficiencies to RYGB after one year. Therefore, providers should consider screening them for micronutrient deficiencies and providing regular supplementation similar to RYGB patients at the 1-year mark.
Our study has several limitations. We were unable to account for some potential confounding factors including race/ethnicity, BMI, or socioeconomic status, as they were not available in our data. We did not adjust for co-morbid conditions; however, bariatric surgery patients should receive micronutrient laboratory testing regardless of these conditions. Our results are likely to underestimate the true prevalence of micronutrient deficiencies for several reasons: 1) a large proportion of our population never received laboratory testing for micronutrients so were not assessed for diagnosis of micronutrient deficiency in our study, and 2) we were unable to identify patients who took over-the-counter vitamin supplements to manage their micronutrient deficiency as these purchases are not included in claims data. For example, we note that our preoperative prevalence of vitamin D deficiency is lower than compared to other reports [14–17]. Prior estimates of vitamin D deficiency among obese patients have varied widely from 21% to 84% due to the use of different thresholds to define vitamin D deficiency and location of clinics at different latitudes (southern California, Michigan, Nebraska, Wisconsin) [18–19]. We expect that our preoperative estimates of vitamin D deficiency are underestimates for several reasons: 1) we could not capture patients who took over-the-counter vitamin D supplements; 2) testing and subsequent diagnosis of vitamin D deficiency in general was lower during the years of our study as compared to more recent estimates [20]; and 3) our patients were located in a variety of latitudes (Hawaii, Tennessee, North Carolina, Pennsylvania, Iowa, Michigan, and South Dakota) where the lower rates of vitamin D deficiency in southern latitudes may balance out the more extreme rates in northern latitudes. However, underestimation of the population-level prevalence of micronutrient deficiencies would not change, and in fact, would strengthen the implications of our results.
Overall, our results support the current guidelines for routine testing and supplementation for patients before and after bariatric surgery with a few modifications. The most significant change would entail including patients who underwent restrictive surgery in routine post-surgical testing and supplementation for vitamin D, vitamin B12, folate, and iron. Our results also suggest that RYGB patients should be screened regularly for folate deficiency after surgery, and that patients who underwent malabsorptive procedures should be screened for vitamin D deficiency with the same frequency as the other micronutrients. The increasing prevalence of micronutrient deficiencies after bariatric surgery also speaks to the need for continued monitoring with laboratory testing by surgeons, or more likely, primary care providers and/or physician nutrition specialists over time. Finally, it is important to note that the recent guidelines were issued in 2009; therefore, our data cannot assess their influence on the receipt of laboratory testing. A future study should evaluate whether the receipt of micronutrient laboratory testing in bariatric surgery patients has improved since 2009, as release of guidelines alone may not be sufficient to change provider behavior [21].
Supplementary Material
Acknowledgments
Funding Sources
KAG was supported by a training grant from the Health Resources and Service Administration (T32HP10025). Creation of the database was funded by Ethicon Endo-Surgery, Inc.; GlaxoSmithKline; and Pfizer, Inc. Data and support were provided by Blue Cross Blue Shield of Michigan and Highmark. Support was provided by the Blue Cross Blue Shield Association. The supporting organizations were kept informed of the study’s progress and shared their expertise on certain aspects of the study. Also, preliminary findings were shared with them, and they were invited to review the manuscript. However, they did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation or approval of the manuscript.
The data set used in this study was originally created for a different research project on patterns of obesity care within selected BCBS plans. The previous research project (but not the current study) was funded by unrestricted research grants from Ethicon Endo-Surgery, Inc. (a Johnson & Johnson company); Pfizer, Inc and GlaxoSmithKline. The data and database development support and guidance were provided by the BCBS Association, BCBS of Tennessee, BCBS of Hawaii, BCBS of Michigan, BCBS of North Carolina, Highmark, Inc. of Pennsylvania, Independence Blue Cross of Pennsylvania, Wellmark BCBS of Iowa and Wellmark BCBS of South Dakota. We thank the Blue Cross and Blue Shield plans and their staff members.
Footnotes
Disclosure Statement
KA Gudzune: no conflicts of interest.
MM Huizinga: no conflicts of interest.
HY Chang: no conflicts of interest.
V Asamoah: no conflicts of interest.
M Gadgil: no conflicts of interest.
JM Clark: no conflicts of interest.
Description of Supplementary Information
Supplementary Tables 1–4 includes prevalence data for all individual micronutrient outcomes.
References
- 1.Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200:378–85. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003–2008. J Am Coll Surg. 2011;213:261–6. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Brolin RE, Leung M. Survey of vitamin and mineral supplementation after gastric bypass and bioliopancreatic diversion for morbid obesity. Obes Surg. 1999;9:150–4. doi: 10.1381/096089299765553395. [DOI] [PubMed] [Google Scholar]
- 4.Mechanick JI, Kushner RF, Sugerman HJ, Gonzolez-Campoy JM, Collazo-Clavell ML, Spitz AF, Apovian CM, Livingston EH, Brolin R, Sarwer DB, Anderson WA, Dixon J. AACE/TOS/ASMBS Medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring) 2009;17:S1–70. doi: 10.1038/oby.2009.28. [DOI] [PubMed] [Google Scholar]
- 5.Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nuritional deficiencies following bariatric surgery: what have we learned? Obes Surg. 2005;15:145–54. doi: 10.1381/0960892053268264. [DOI] [PubMed] [Google Scholar]
- 6.Clements RH, Katasani VG, Palepu R, Leeth RR, Leath TD, Roy BP, Vickers SM. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg. 2006;72:1196–1204. doi: 10.1177/000313480607201209. [DOI] [PubMed] [Google Scholar]
- 7.Vargas-Ruiz AG, Henandez-Rivera G, Herrera MF. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:288–93. doi: 10.1007/s11695-007-9310-0. [DOI] [PubMed] [Google Scholar]
- 8.Coupaye M, Puchaux K, Bogard C, Msika S, Jouet P, Clerici C, Larger E, Ledoux S. Nutritional consequences of adjustable gastric banding and gastric bypass: a 1-year prospective study. Obes Surg. 2009;19:56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- 9.Toh SY, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25:1150–6. doi: 10.1016/j.nut.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Segal JB, Clark JM, Shore AD, Dominici F, Magnuson T, Richards TM, Weiner JP, Bass EB, Wu AW, Makary MA. Prompt reduction in use of medications for comorbid conditions after bariatric surgery. Obes Surg. 2009;19:1646–56. doi: 10.1007/s11695-009-9960-1. [DOI] [PubMed] [Google Scholar]
- 11.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–8. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]
- 12.Kalfarentzos F, Kechagias I, Soulikia K, et al. Weight loss following vertical banded gastroplasty: intermediate results of a prospective study. Obes Surg. 2001;11:256–70. doi: 10.1381/096089201321336566. [DOI] [PubMed] [Google Scholar]
- 13.Cooper PL, Brearley LK, Jamieson AC, et al. Nutritional consequence of modified vertical gastroplasty in obese subjects. Int J Obes. 1999;23:382–8. doi: 10.1038/sj.ijo.0800830. [DOI] [PubMed] [Google Scholar]
- 14.Hamoui N, Athone G, Crookes PF. Calcium metabolism in the morbidly obese. Obes Surg. 2004;14:9–12. doi: 10.1381/096089204772787211. [DOI] [PubMed] [Google Scholar]
- 15.Carlin AM, Rao DS, Meslemani AM, et al. Prevalence of vitamin D depletion among morbidly obese patients seeking gastric bypass surgery. Surg Obes Relat Dis. 2006;2:98–103. doi: 10.1016/j.soard.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Goldner WS, Stoner JA, Thompson J, et al. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–50. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 17.Fish E, Beverstein G, Olson D, Reinhardt S, Garren M, Gould J. Vitamin D status of morbidly obese bariatric surgery patients. J Surg Res. 2010;164:198–202. doi: 10.1016/j.jss.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–5. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 20.Sattar N, Welsh P, Panarelli M, Forouhi NG. Increasing requests for vitamin D measurement: costly, confusing, and without credibility. Lancet. 2012;379:95–96. doi: 10.1016/S0140-6736(11)61816-3. [DOI] [PubMed] [Google Scholar]
- 21.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PAC, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.