Abstract
Context
Hemorrhagic shock is the leading potentially preventable cause of death after injury. Transfusion of early and increased ratios of plasma and platelets to red blood cells (RBCs) has been associated with decreased mortality; however conflicting reports and the time-varying nature of transfusions and hemorrhagic death raise concern for the validity of the clinical conclusions drawn from the retrospective data.
Objective
To relate in-hospital mortality to: 1) early transfusion of plasma and/or platelets and 2) time-varying plasma:RBC and platelet:RBC ratios.
Design
Prospective cohort study documenting the timing of transfusions during active resuscitation and patient outcomes. Data were analyzed using time-dependent proportional hazards models.
Setting
Ten US Level 1 trauma centers.
Patients
Adult trauma patients surviving for 30 minutes after admission, transfused at least 1 unit RBC within 6 hours of admission (n=1245, the original study group) and at least 3 total units (of RBC, plasma or platelets) within 24 hours (n=905, the analysis group).
Main outcome measure
In-hospital mortality
Results
Plasma:RBC and platelet:RBC ratios were not constant over the first 24 hours (p<.001 for both). In a multivariable time-dependent Cox model, increased ratios of plasma:RBC (adjusted hazard ratio, HR=0.31, 95% CI=0.16–0.58) and platelets:RBC (adjusted HR=0.55, 95% CI=0.31–0.98) were independently associated with decreased 6-hour mortality, when hemorrhagic death predominated. In the first 6 hours, patients with ratios < 1:2 were 3–4 times more likely to die than patients with ratios ≥1:1. After 24 hours, plasma and platelet ratios were unassociated with mortality, when competing risks from non-hemorrhagic causes prevailed.
Conclusions
Higher plasma and platelet ratios early in resuscitation were associated with decreased mortality in patients transfused at least three units of blood products during the first 24 hours after admission. Among survivors at 24 hours, the subsequent risk of death by day 30 was not associated with plasma or platelet ratios.
INTRODUCTION
Injury is increasing in incidence, the second leading cause of death worldwide, and the leading cause of years of life lost in the United Sates.1,2 Uncontrolled hemorrhage after injury is the leading cause of potentially preventable death.3–9 As opposed to other major causes of traumatic death (e.g., traumatic brain injury and multiple organ failure (MOF)), hemorrhagic deaths occur quickly, and are frequently associated with massive transfusion (MT, traditionally defined as ≥ 10 units of red blood cells (RBCs) in 24 hours).10,11 Current transfusion practices consist of infusing crystalloid, RBCs, plasma, and platelets, and date back to the 1970s when separation of donated whole blood into its component parts became commonplace.12–16
A new resuscitation strategy, termed damage control resuscitation, is challenging the status quo.17 The term originated in the U.S. military and refers to the guidelines developed for combat casualties suffering substantial bleeding in Iraq and Afghanistan. Among other interventions, this approach recommends earlier and more balanced transfusion of plasma and platelets along with the first units of RBCs (i.e., maintaining plasma:platelet:RBC ratios closer to the 1:1:1 ratio of whole blood), while simultaneously minimizing crystalloid use18–27 in patients in order to avert or reverse the triad of coagulopathy, acidosis, and hypothermia25,28–30 and decrease endothelial permeability.31–33
Conflicting findings regarding the association between transfusion ratios closer to 1:1 and survival in massively transfused trauma patients have been reported29,34–36 and attributed to multiple issues, including survival bias.34,35,37,38 Survival bias, also known as reverse causation, is a prevalent, important, and often-neglected problem in clinical observational studies, systematic reviews, and comparative effectiveness research.39,40 In trauma resuscitation research, the conundrum of reverse causation is whether treatment caused patients to survive longer or patients received treatment only because they survived long enough. Without compelling evidence to guide uniform transfusion practice for trauma patients with substantial bleeding after injury, considerable variation persists across Level 1 trauma centers.14,19,41
Utilizing prospective, minute-to-minute observational data from ten Level 1 trauma centers, our objective was to accurately describe when RBCs, plasma, and platelets were infused and assess the association between inhospital mortality and the timing and amount of blood products. One purpose of observational clinical studies is to inform the design of future randomized trials, and exploratory analysis can provide critical information regarding trial feasibility, realistic estimates of expected effect size, and unique insights from real-world healthcare settings. Thus, we describe the rationale, results, and lessons learned from our exploratory analyses of observational PROMMTT data.42 We hypothesized that early transfusion of plasma and platelets in higher ratios would be associated with decreased in-hospital mortality in bleeding patients.
METHODS
Study Samples
PROMMTT was a prospective, multicenter observational cohort study conducted at ten Level 1 trauma centers in the US. At each study site and the Data Coordinating Center (DCC), the local institutional review board approved the study. The US Army Human Research Protections Office provided a second level review and approval.42
Trauma patients were enrolled in PROMMTT and data collection was begun upon ED arrival. Patients were eligible if they required the highest level of trauma activation, were age 16 or older, and were transfused at least one unit of RBCs in the first six hours after admission. Patients were excluded if: 1) transferred from other facilities; 2) declared dead within 30 minutes of admission; 3) received more than five minutes of CPR prior to or within 30 minutes of admission; 4) prisoners; 5) burn injury > 20% of total body surface area; 6) inhalation injury diagnosed by bronchoscopy; or 7) pregnant. If ineligibility was first identified sometime after enrollment, the patient was withdrawn from the study and post-enrollment data was destroyed. No changes in clinical practice were implemented in this observational study. All participating centers had massive transfusion protocols in place.42
Data Collection and Management
Standard operating procedure manuals were developed and site coordinators were trained in a series of meetings. Research assistants, available 24/7, screened and enrolled patients, recording the exact times of infused fluids and blood products, as well as patient outcomes during direct observation. Direct bedside observation began at trauma team activation and continued until active resuscitation ended (defined as the time the center transfusion protocol was discontinued, death occurred, or two hours elapsed since the last blood product transfusion, whichever came first). After direct observation ended, new interventions, complications, and outcomes were recorded daily while the patient was in the ICU and weekly thereafter during hospitalization. Cause of in-hospital death was ascribed by individual site clinicians without confirmation or central adjudication. Sites of bleeding were ascertained by data collectors. The DCC audited study data for missing values and outliers.42 Some severely injured patients did not undergo routine baseline assessments (e.g., base deficit, temperature, international normalized ratio, pH) due to the emergent nature of their injuries (Table 1).
Table 1.
Admission and treatment characteristics and unadjusted survival in 1245 PROMMTT patients
| All enrolled patients (N=1245) | Analysis cohort (N=905) | |||
|---|---|---|---|---|
| Median (IQR) | No. non-missing | Median (IQR) | No. non-missing | |
| Admission characteristics | ||||
| Age, y | 38 (24–54) | 1244 | 37 (24–53) | 904 |
| Male, No. (%) | 923 (74.2) | 1245 | 687 (75.9) | 905 |
| Blunt injury, No. (%) | 796 (64.5) | 1235 | 579 (64.4) | 899 |
| Systolic blood pressure, mm Hg | 106 (86–128) | 1213 | 102 (82–124) | 876 |
| Heart rate, bpm | 105 (86–124) | 1218 | 109 (88–128) | 887 |
| Temperature, C | 36.1 (35.6–36.6) | 630 | 36.1 (35.6–36.6) | 440 |
| Glasgow Coma Score | 14 (3–15) | 1135 | 13 (3–15) | 826 |
| Base deficit | 6 (3–10) | 960 | 7 (4–11) | 716 |
| pH | 7.3 (7.2–7.3) | 975 | 7.3 (7.2–7.3) | 730 |
| International Normalized Ratio (INR) | 1.2 (1.1–1.4) | 1081 | 1.3 (1.1–1.5) | 792 |
| Partial thromboplastin time (PTT), seconds | 27 (24–33) | 1045 | 29 (25–35) | 762 |
| Prothrombin time (PT), seconds | 15 (13–17) | 902 | 15 (14–17) | 662 |
| Hemoglobin, g/dL | 11.7 (10.1–13.3) | 1198 | 11.5 (9.9–13.1) | 869 |
| Injury Severity Score (ISS) | 25 (16–34) | 1243 | 26 (17–36) | 905 |
| Bleeding sitesa | ||||
| Head, No. (%) | 181 (14.5) | 1245 | 128 (14.1) | 905 |
| Face, No. (%) | 340 (27.3) | 1245 | 246 (27.2) | 905 |
| Neck, No. (%) | 57 (4.6) | 1245 | 41 (4.5) | 905 |
| Chest, No. (%) | 299 (24.0) | 1245 | 237 (26.2) | 905 |
| Abdomen, No. (%) | 396 (31.8) | 1245 | 320 (35.4) | 905 |
| Pelvis, No. (%) | 164 (13.2) | 1245 | 143 (15.8) | 905 |
| Limb, No. (%) | 441 (35.4) | 1245 | 334 (36.9) | 905 |
| Unknown, No. (%) | 121 (9.7) | 1245 | 79 (8.7) | 905 |
| Treatment characteristics | ||||
| Damage control surgery performed, No. (%) | 239 (19.3) | 1241 | 222 (24.6) | 904 |
| Time to first RBC transfused, min | 30 (12–99) | 1222 | 25 (11–77) | 905 |
| Time to first plasma transfused, min | 69 (35–133) | 815b | 69 (35–130) | 778b |
| Time to first platelet transfused, min | 123 (81–190) | 357b | 121 (80–187) | 343b |
| 6-hour RBC unit total | 4 (2–7) | 1224 | 5 (3–9) | 905 |
| 6-hour plasma unit total | 2 (0–5) | 1224 | 4 (2–7) | 905 |
| 6-hour platelet unit total | 0 (0–6) | 1224 | 0 (0–6) | 905 |
| 24-hour RBC unit total | 5 (2–9) | 1244 | 6 (4–11) | 905 |
| 24-hour plasma unit total | 4 (0–8) | 1245 | 5 (2–9) | 905 |
| 24-hour platelet unit total | 0 (0–6) | 1245 | 0 (0–6) | 905 |
| Unadjusted in-hospital mortality | ||||
| 30 minute – 6-hour mortality, No. (%) | 102 (8.2) | 1245 | 95 (10.5) | 905 |
| >6 hour – 24-hour mortality, No. (%) | 46 (4.0) | 1143 | 37 (4.6) | 810 |
| >24 hour – 30-day mortality, No. (%) | 112 (10.2) | 1097 | 88 (11.4) | 773 |
| Overall cumulative mortality, No. (%) | 266 (21.4) | 1245 | 226 (25.0) | 905 |
Abbreviations: IQR, Interquartile Range
Bleeding site categories are not mutually exclusive and patients were counted in multiple categories if appropriate.
Number excludes any patient who did not receive plasma or platelets during direct observation
Statistical Analysis
The primary outcome of interest was in-hospital mortality. In the original analysis plan, the primary independent variables were single plasma:RBC and platelet:RBC transfusion ratios.42 Under the assumption that each patient would receive constant ratios of plasma and platelets during the period of active resuscitation, PROMMTT was designed to enroll 1200 transfused and 300 MT patients. Previous retrospective studies suggested higher plasma and platelet ratios occurred in about 25–50% of MT patients19 and were associated with at least a 50% decrease in mortality relative to lower ratios.19,23,43 Thus, at the .05 significance level, a total of at least 300 PROMMTT MT patients was expected to provide 80% power44 to detect differences of at least 50% in mortality between two groups of patients classified by transfusion ratios (ratios closer to 1:1 vs. ratios closer to 1:2).
Previous retrospective trauma transfusion studies have focused on the subgroup of MT patients effectively excluding bleeding patients who did not survive long enough to receive 10 RBC units and heightening the concern for survival bias.19,37 Finding reliable and immediate indicators for patients’ blood loss and continuing hemorrhage rates is a challenge in trauma transfusion practice and research.45 Cumulative counts of patients’ total RBC units received within 6–24 hours (especially to identify the MT subgroup) remain a standard, though poor, surrogate. Soon after PROMMTT began, we realized the need to revise the original analysis plan to account for heterogeneity among patients (e.g., variations in the severity of blood loss and rates of continuing hemorrhage) and trauma centers (e.g., variations in blood product availability, massive transfusion protocols, and blood bank-bedside transit times).34–37 We therefore sought an exploratory approach to analysis that would incorporate the requirements for time-dependent and multi-level techniques and thereby reduce the potential for bias.
To test the hypothesis that plasma:RBC and platelet:RBC ratios closer to 1:1 were independently and jointly associated with lower in-hospital mortality than transfusion ratios closer to 1:2, we reasoned that only PROMMTT patients surviving long enough to receive at least 3 blood product units (including one RBC) should be eligible to be included in the analysis. Patients transfused less than 3 units by hour 24 (or death) had no opportunity to attain 1:1 ratios for both plasma:RBC and platelet:RBC (i.e., the same ratios as whole blood). Follow-up time at risk of death for each patient began at minute 31 or the start of the third unit transfused, whichever occurred last because eligible PROMMTT patients had to survive the first 30 minutes after admission and long enough to receive at least 3 units of blood product. Cumulative ratios of plasma:RBC and platelet:RBC and summed counts of blood products transfused were computed at baseline (entry to follow-up) and for up to 14 consecutive time intervals: 1) two 15-minute intervals between minute 31 and hour 1; 2) ten 30-minute intervals between >1 and 6 hours; 3) one 18-hour interval between >6 and 24 hours; 4) one 29-day interval between >24 hours and 30 days. The timing of transfusion was defined by the time of initiation of each transfusion. Cell-saver transfusions were not enumerated or combined with donor blood products in these analyses.
We first examined whether transfusion ratios among PROMMTT patients in the analysis cohort were constant across time by using mixed linear regression models46 for both continuous plasma:RBC and platelet:RBC ratios. We then performed multi-level time-dependent Cox proportional hazards regression which uses time as a continuous variable to accommodate 1) varying entry times for this dynamic analysis cohort, 2) time-varying cumulative sums of transfusion, plasma:RBC ratios and platelet:RBC ratios, 3) important patient baseline covariates, and 4) any residual variation in mortality rates due to unmeasured center influences. Center random effects were assessed using shared frailty, which assumed a single hazard factor (e.g., unmeasured clinical practices) for each trauma center shared by all of its patients. Hazard ratios (as an estimate of standard relative risk), 95% confidence intervals, and p-values were estimated.
Similar to previous retrospective studies of the association between transfusion ratios and in-hospital mortality among trauma patients,19 our initial time- dependent Cox analysis spanned the entire follow-up period of 30 days, and a separate analysis focused on the first 24 hours after ED admission. The proportional hazards assumption was tested using Schoenfeld residuals for each covariate and the global test proposed by Grambsch and Therneau.47 Results from these tests suggested significant violations of the assumptions underlying the Cox models for both the full 30 day period (global test, p=.0002) and the first 24 hours of follow-up (global test, p=.0004), so subsequent analyses are presented in three intervals (30 minutes to 6 hours, > 6 hours to 24 hours, and > 24 hours to 30 days). In the models stratified by these time intervals, the proportional hazards assumptions were not violated (global test, p=.127, .484 and .402 respectively). Because transfusions were generally completed by 6 hours, only the proportional hazards model for the first interval (30 minutes to 6 hours) included time-dependent covariates.
We applied purposeful variable selection strategies48 which retained in all models the plasma and platelet ratios as the primary independent variables of interest, and the sum of transfusions, age, time period at cohort entry and injury severity score (ISS) as the primary potential confounders of interest. The remaining covariates of head, chest and limb bleeding sites were retained in all models because they were significant at the .05 level and changed the magnitude of the plasma or platelet ratio coefficients by more than 20% when compared with models excluding them for one or more of the separate time intervals examined. The other candidate covariates listed in Table 1 did not change the magnitude of the plasma or platelet ratio coefficients by more than 20% and were not significant when compared with models excluding them; they were therefore not retained in the final models.49 No interactions (each transfusion ratio multiplied by the alternate ratio or a primary covariate) were significant at the .05 level. The transfusion ratios were also modeled categorically, using clinically relevant cut-points. The lowest ratios (< 1:2) defined the referent group; ratios > 1:2 and <1:1 defined the moderate group; and ratios > 1:1 defined the high group. Patients discharged in less than 30 days were censored alive at 30 days.
All analyses were performed using SAS/STAT50 and Stata/MP software packages.46 Manuscript preparation was guided by the STROBE statement for the reporting of cohort studies in epidemiology51 and the SQUIRE standards for the reporting of improvement studies in health care.52
RESULTS
There were 34,362 trauma admissions in the 10 centers over an average of 58 weeks. Data collection was initiated on 12,560 patients; of these, 11,315 became ineligible and were withdrawn from the study and 1,245 met all PROMMTT eligibility criteria. Of these, 905 were transfused three or more units of blood products, thus meeting the eligibility criteria for the analysis cohort. Overall in-hospital mortality was 21% for all 1245 transfused patients and 25% for patients included in the analysis cohort (Table 1).
Among cohort patients, 94% of hemorrhagic deaths occurred within 24 hours, the majority of these deaths (58%) occurred within three hours of admission (Table 2), and the median time to hemorrhagic death was 2.6 hours (interquartile range (IQR): 1.7–5.4). The principal causes of in-hospital death after 24 hours were MOF and brain injury.
Table 2.
Distribution of reported cause of death for decedent patients in the analysis cohort by the time period surviveda
| > 0.5 – ≤ 1 hour | >1 – ≤ 3 hours | > 3 – ≤ 6 hours | > 6 – ≤ 12 hours | > 12 – ≤ 24 hours | > 24 – ≤ 72 hours | > 72 hours– ≤ 30 days | 30+ days | |
|---|---|---|---|---|---|---|---|---|
| Cause of death,b No. (%) | N=8 | N=55 | N=32 | N=21 | N=16 | N=21 | N=67 | N=6 |
| Hemorrhage | 7 (88) | 46 (84) | 24 (75) | 9 (43) | 3 (19) | 3 (14) | 3 (4) | 0 |
| Brain injury | 0 | 9 (16) | 10 (31) | 10 (48) | 10 (63) | 13 (62) | 32 (48) | 1 (17) |
| Airway/respiratory | 1 (13) | 2 (4) | 3 (9) | 2 (10) | 1 (6) | 2 (10) | 15 (22) | 3 (50) |
| Sepsis | 0 | 0 | 0 | 0 | 0 | 1 (5) | 6 (9) | 2 (33) |
| Multi-organ failure | 0 | 0 | 0 | 0 | 0 | 2 (10) | 24 (36) | 5 (83) |
| Cardiovascular | 4 (50) | 16 (29) | 6 (19) | 4 (19) | 3 (19) | 3 (14) | 6 (9) | 2 (33) |
| Other | 0 | 5 (9) | 4 (13) | 2 (10) | 3 (19) | 1 (5) | 18 (27) | 1 (17) |
Column percentages sum to greater than 100% because patients may have more than one contributing cause of death
Not centrally adjudicated
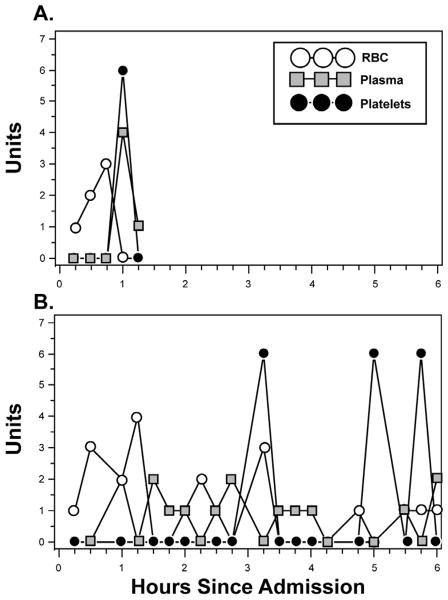
Neither plasma:RBC nor platelet:RBC ratios were constant across the first 24 hours among individual patients (Figure 1) (p<.001 for each patient in the analysis cohort). The time-varying nature of plasma and platelet transfusion practice across the analysis cohort is illustrated in Figure 2. Thirty minutes after admission, 67% of cohort patients had not received plasma, while 99% had not received platelets. Three hours after admission (the peak time of hemorrhagic death), 10% of surviving cohort patients had not received any plasma, while 28% of survivors had not received platelets. For each successive hour survived (up to hour six), patients were more likely to receive plasma and platelets and hence were more likely to approach ratios of 1:1. By 30 minutes, 1 hour, 2 hours, 3 hours and 6 hours after admission, ratios exceeded 1:2 in 29%, 47%, 69%, 78%, and 84% of surviving cohort patients for plasma and in 1%, 14%, 40%, 60%, and 80% for platelets, respectively.
Figure 1. Blood product use in the 1st 6 hours in two PROMMTT patients.
Patient A had an ISS of 48 and died of hemorrhage at 1 hour, 7 minutes after ED admission. Patient B had an ISS of 57 and was discharged to another acute care hospital at 27 days. Note the constantly changing ratios over time. For example, patient A received cumulative plasma:platelet:RBC ratios of 0:0:1, 0:0:3, 0:0:6, 4:6:6, and 5:6:6 at 15, 30, 45, 60 and 75 minutes respectively, while patient B received 0:0:1, 0:0:4, 0:0:4, 2:0:6, and 2:0:10 at those same time points.
Figure 2.
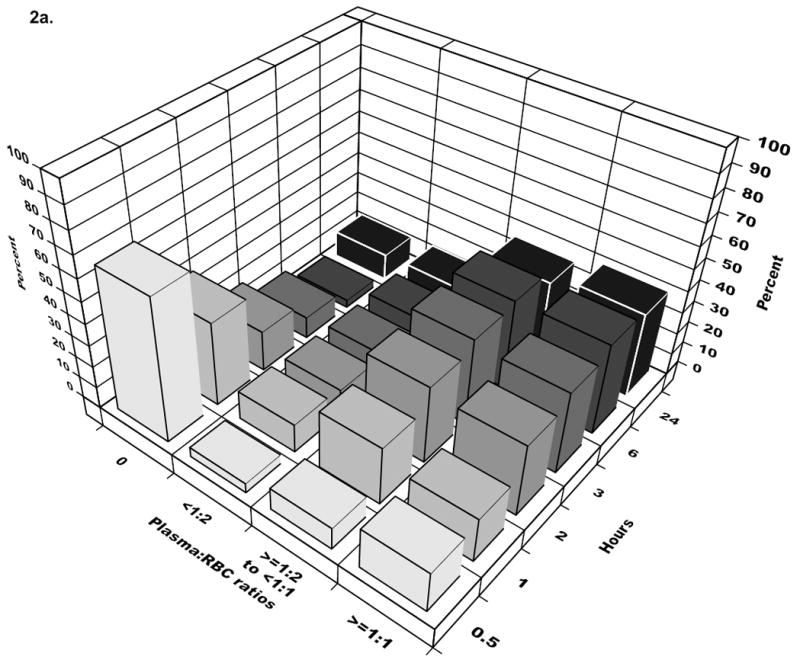
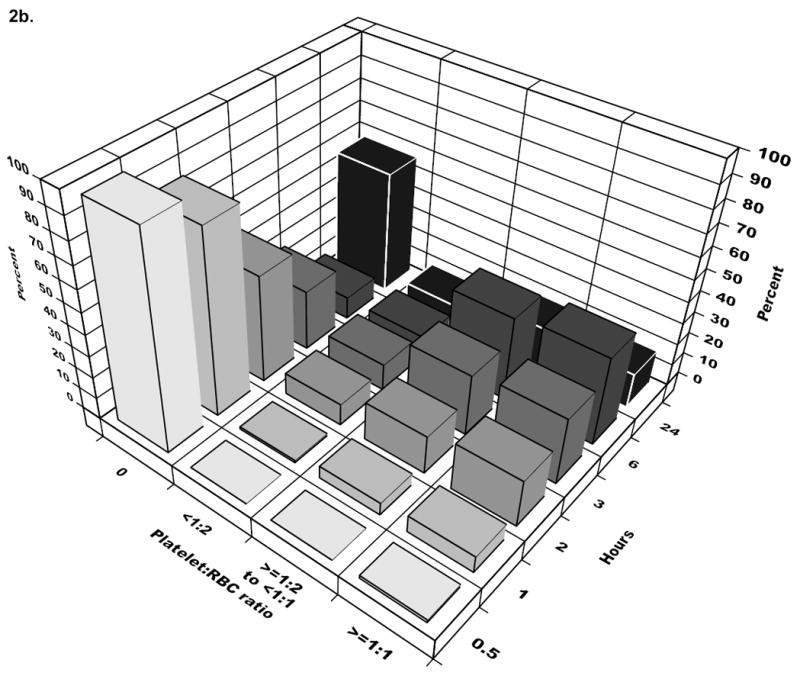
The bars represent cumulative ratios at the start of each time interval. The majority of patients received 1:2 plasma:RBC ratio or higher by three hours and for platelets:RBC, by six hours. In the last time interval (24 hours), the percentage for patients receiving 0 platelets or plasma increases, reflecting the dynamic cohort with newly eligible patients entering and others exiting due to death in the previous interval.
The protective association between higher transfusion ratios and mortality in the first time interval (minute 31 to hour 6) diminished over the next two time intervals (Table 3). The trend for plasma ratios suggested that the decreased mortality risk observed during the first 6 hours (adjusted HR=0.31, p=<.001) switched direction and became non-significant (adjusted HR=1.21, p=.20) by the final follow-up period of >24 hours- 30 days. The association between platelet:RBC ratio and mortality remained below the null but was not significant for either of the later time periods. Additionally, bleeding from the chest was associated with higher mortality during the first 6 hours; in contrast, among patients who survived longer than 6 hours, bleeding from the chest was associated with lower mortality.
Table 3.
Multivariable Cox regression models examining the association of plasma and platelet transfusion ratios with in-hospital mortality
| A. Time Interval 1: Minute 31 to hour 6 post ED admissiona (N=876)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Continuous transfusion ratio variables | Categorical transfusion ratio variables | |||||||||
| Low <1:2 | Moderate ≥ 1:2–<1:1 | High ≥1:1 | ||||||||
| HR | 95% CI | P value | HR | P value | HR | P value | HR | P value | ||
| Early initial and time-varying plasma:RBC ratios | 0.31 | 0.16 | 0.58 | <.001 | 1.00 | Ref | 0.42 | <.001 | 0.23 | <.001 |
| Early initial and time-varying platelet:RBC ratios | 0.55 | 0.31 | 0.98 | .04 | 1.00 | Ref | 0.66 | 0.16 | 0.37 | 0.04 |
|
| ||||||||||
| Sum of blood product transfusions | 1.05 | 1.04 | 1.06 | <.001 | c | |||||
| Age | 1.01 | 1.00 | 1.02 | .03 | ||||||
| Injury Severity Score | 1.02 | 1.01 | 1.04 | .001 | ||||||
| Time interval at cohort entry | 0.73 | 0.63 | 0.86 | <.001 | ||||||
| Bleeding from the head | 3.73 | 2.15 | 6.45 | <.001 | ||||||
| Bleeding from the chest | 1.52 | 0.96 | 2.39 | .07 | ||||||
| Bleeding from a limb | 0.54 | 0.32 | 0.89 | .02 | ||||||
| B. Time Interval 2: Hour >6 to hour 24 post ED admissiond (N=809)e | Low <1:2 | Moderate ≥ 1:2–<1:1 | High ≥1:1 | |||||||
| HR | 95% CI | P value | HR | P value | HR | P value | HR | P value | ||
|
| ||||||||||
| 6-hour cumulative plasma:RBC ratio | 0.34 | 0.14 | 0.81 | .02 | 1.00 | Ref | 0.79 | 0.63 | 0.55 | 0.23 |
| 6-hour cumulative platelet:RBC ratio | 0.81 | 0.46 | 1.43 | .46 | 1.00 | Ref | 0.79 | 0.56 | 0.49 | 0.19 |
|
| ||||||||||
| Sum of blood product transfusions at hour 6 | 1.04 | 1.03 | 1.05 | <.001 | c | |||||
| Age | 1.01 | 0.99 | 1.03 | .36 | ||||||
| Injury Severity Score | 1.02 | 0.99 | 1.04 | .11 | ||||||
| Time interval at cohort entry | 0.84 | 0.72 | 0.98 | .03 | ||||||
| Bleeding from the head | 8.46 | 3.82 | 18.7 | <.001 | ||||||
| Bleeding from the chest | 0.87 | 0.39 | 1.97 | .74 | ||||||
| Bleeding from a limb | 0.96 | 0.48 | 1.92 | .90 | ||||||
| C. Time Interval 3: Hour >24 to day 30 post ED admissionf (N=773)g | Low <1:2 | Moderate ≥ 1:2–<1:1 | High ≥1:1 | |||||||
| HR | 95% CI | P value | H | P value | HR | P value | HR | P value | ||
|
| ||||||||||
| 24-hour cumulative plasma:RBC ratio | 1.21 | 0.90 | 1.61 | .20 | 1.00 | Ref | 1.41 | 0.33 | 1.47 | 0.26 |
| 24-hour cumulative platelet:RBC ratio | 0.78 | 0.57 | 1.06 | .11 | 1.00 | Ref | 1.23 | 0.46 | 0.69 | 0.19 |
|
| ||||||||||
| Sum of blood product transfusions at hour 24 | 1.02 | 1.01 | 1.03 | <.001 | c | |||||
| Age | 1.03 | 1.02 | 1.04 | <.001 | ||||||
| Injury Severity Score | 1.04 | 1.02 | 1.05 | <.001 | ||||||
| Time interval at cohort entry | 0.98 | 0.91 | 1.06 | .63 | ||||||
| Bleeding from the head | 5.96 | 3.59 | 9.90 | <.001 | ||||||
| Bleeding from the chest | 0.45 | 0.23 | 0.90 | .02 | ||||||
| Bleeding from a limb | 1.22 | 0.76 | 1.96 | .41 | ||||||
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; Ref, Referent
Time-dependent Cox model examining the association of plasma and platelet ratios with mortality within 6 hours of ED admission, adjusted for the sum of blood product transfusions (also time-varying), baseline covariates and center random effects.
Of 904 patients with complete data who entered the cohort over 24 hours; 876 entered the cohort during this initial time interval and 94 died within the 5.5 hours of follow-up.
Covariate HRs are not repeated because differences were negligible comparing the models with categorical vs. continuous transfusion ratios.
Regular Cox model examining the association of cumulative plasma and platelet ratios with mortality between >6–24 hours after ED admission, adjusted for baseline covariates and center random effects.
Of 809 patients surviving the initial 6 hours, 27 patients entered the cohort in the second time interval and 37 died within the next 18 hours of follow-up.
Regular Cox model examining the association of cumulative plasma and platelet ratios with mortality between >24 hours–30 days after ED admission, adjusted for baseline covariates and center as a fixed effect (the model did not converge with site as a random effect).
Of 773 patients surviving 24 hours, 1 patient entered the cohort in the third time interval and 88 died within the next 29 day follow-up period.
To facilitate clinical use, we repeated the same Cox models but substituted patients’ continuous transfusion ratio values with three categorical ones (Table 3). In the initial 6-hour time interval, patients in the moderate or high ratio group had lower mortality rates than the low ratio group, (p<.001 for each of the higher plasma ratio groups; p=.040 for the high platelet ratio group). In both subsequent time intervals, mortality among survivors was not associated with the categorical ratios.
DISCUSSION
In-hospital mortality among 1245 trauma patients receiving at least a single unit of RBCs within 6 hours of admission was 21% (Table 1) while cohort patients with ≥ 3 units transfused had in-hospital mortality of 25%, among the highest of any acute surgical disease process. The major findings were 1) patients did not receive a constant ratio during the period of active resuscitation, and 2) early infusion of higher plasma and platelets ratios was associated with decreased mortality within six hours of admission, during which 77% of the hemorrhagic deaths had occurred (Table 2).
The protective association between higher transfusion ratios and in-hospital mortality 1) appears strongest within 6 hours, and 2) diminishes over time as the primary causes of mortality shift from exsanguination to head injury, respiratory distress, organ failure and infection after the first 24 hours. These time trends reflect heterogeneity as the dynamic cohort of injured patients changes over the course of hospitalization in composition and risk profile due to mortality. Survivors avoiding early hemorrhage-related mortality face the longer-term competing risks of death from complications (e.g., MOF) or multiple injuries (e.g., head injury). The significant protective association between higher blood product ratios and mortality that we observed was concentrated in the first 24 hours for plasma and first 6 hours for platelets. Thereafter, during the later time periods of high competing risks for non-hemorrhagic causes of death among severely injured patients, plasma and platelet ratios were not significantly associated with mortality.
Survival bias may have threatened previous studies that used 1) the traditional definition of MT and therefore excluded patients who suffered substantial bleeding but died early;19,29,34,35,53 2) a single cumulative ratio for plasma or platelets up to the time of death or 6–24 hours after admission and therefore not accounting for time-dependent treatment;19,23,29,35,36,54–57 and 3) 30-day or overall in-hospital mortality as the primary endpoint, which conflates competing mortality risks.19,23,28,29,34–36,53–58 Our prospective study design, detailed real-time data collection methods and analysis strategies attempted to minimize the effect of survival bias.
In rapidly and substantially bleeding trauma patients, inadequate transfusion of plasma and platelets is associated with early death. However, the actual transfusion of blood products is a complicated balance between rapid recognition of need, ordering of appropriate products, product availability in the blood bank and ED, obtaining those products quickly, and appropriate infusion. Unless these steps are orchestrated in an integrated fashion, delayed infusion and sub-optimum ratios will occur (Figures 1 and 2). Clinicians must rapidly identify patients who are substantially bleeding, and several predictive algorithms have been developed to do this.59–69
Once bleeding patients have been identified, constant ratios are not infused and heterogeneous transfusion practice persists (Figure 2). Clinicians at PROMMTT Level 1 trauma centers ultimately delivered plasma ratios of 1:1 and 1:2 within 6–24 hours to surviving patients, but platelet infusion lagged behind with only 72% of patients receiving platelets by hour three, the median time to hemorrhagic death.
Stratifying by time interval and including time-dependent covariates (Table 3) revealed how early infusion and increased ratios were associated with decreased mortality (30 minutes to 6 hours). However, it is difficult to translate hazard ratios for continuous variables into a physician’s order to the blood bank for the delivery of specific blood product amounts. Therefore, we created three clinically-relevant categories and found a 1:1 ratio of plasma and platelets was associated with decreased early mortality compared with lower ratios (Table 3).
The strengths of this study are its prospective multicenter design and teaming a dedicated data coordinating center (epidemiologists, informatics experts, and biostatisticians) with a group of Level 1 trauma centers. By identifying patients who received at least 3 units of blood products instead of focusing on MT patients, we reduced one important source of survival bias. Accurate recording of the actual timing of blood product transfusions combined with appropriate data analysis strategies addressed another source of survival bias, i.e., the time-varying nature of blood transfusions and mortality. Limitations of our observational study include missing values on potentially important covariates, which are unavoidable in observational studies of severely injured trauma patients, and other unmeasured but potentially important confounders and effect modifiers (e.g., the time of and rationale for physician orders for RBC, plasma and platelets). Survival was not ascertained after discharge; however deaths within days of discharge from an acute care hospital are infrequent (< 2%).70 Finally, causes of death were assigned by individual site clinicians without confirmation or central adjudication.
In summary, these prospective data suggest the association between earlier and higher ratios of plasma and platelets and decreased in-hospital mortality is concentrated in the first 6 hours in patients with substantial bleeding. In the first 6 hours, patients with ratios < 1:2 were 3–4 times more likely to die than patients with ratios ≥1:1. Among survivors at 6 hours, the subsequent risk of death by hour 24 was higher for patients with low plasma ratios. Among survivors at 24 hours, the subsequent risk of death by day 30 was not associated with plasma or platelet ratios. Furthermore, these data highlight the serious problems of survival bias and competing risks in most previous trauma resuscitation studies37,58 and emphasize the need for definitive comparative effectiveness trauma transfusion research.
Survival bias can be eliminated only in a randomized trial with appropriate design and analysis strategies. However, it can threaten even a randomized trial if study patients are stratified by post-randomization events such as the conventional MT definition. This study supports a potential net survival benefit of early and higher plasma and platelet ratios to be assessed in a randomized trial.71 Our findings offer guidance and evidence for designing a rigorous, multicenter randomized transfusion trial by identifying: 1) transfusion ratios in common use at Level 1 trauma centers, 2) well-defined endpoints (e.g. 3, 6 and 24 hours and 30 day mortality), 3) appropriate data analysis strategies accounting for time-varying covariates, 4) effect size estimates for power and sample size considerations, 5) patients for whom interventions should be targeted and 6) procedures that promote integrated, consistent transfusion practices across individual clinicians, blood banks, research teams and trauma centers.
Supplementary Material
Acknowledgments
Funding/Support: This project was funded by the U.S. Army Medical Research and Materiel Command subcontract W81XWH-08-C-0712. Infrastructure for the Data Coordinating Center was supported by CTSA funds from NIH grant UL1 RR024148.
Role of the Sponsor: The sponsors did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit this manuscript for publication.
PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) Study Group
Data Coordinating Center, University of Texas Health Science Center at Houston: Mohammad H. Rahbar, PhD (principal investigator); John B. Holcomb, MD (co-investigator); Erin E. Fox, PhD (co-investigator and study coordinator); Deborah J. del Junco, PhD (co-investigator); Bryan A. Cotton, MD, MPH (co-investigator); Charles E. Wade, PhD (co-investigator); Jiajie Zhang, PhD (co-investigator); Nena Matijevic, PhD (co-investigator); Yu Bai, MD, PhD (co-investigator); Weiwei Wang, PhD (co-investigator); Jeanette Podbielski, RN (study coordinator); Sarah J. Duran, MSCIS (data manager); Ruby Benjamin-Garner, PhD (data manager); Robert J. Reynolds, MPH (data manager).
PROMMTT Clinical Sites:
Brooke Army Medical Center: Christopher E. White, MD (principal investigator); Kimberly L. Franzen, MD (co-investigator); Elsa C. Coates, MS, RN (study coordinator).
Medical College of Wisconsin: Karen J. Brasel, MD, MPH (principal investigator); Pamela Walsh (study coordinator).
Oregon Health and Sciences University: Martin A. Schreiber, MD (principal investigator); Samantha J. Underwood, MS (study coordinator); Jodie Curren, RN, BSN (study coordinator).
University of California, San Francisco: Mitchell J. Cohen, MD (principal investigator); M. Margaret Knudson, MD (co-investigator); Mary Nelson, RN, MPA (study coordinator); Mariah S. Call, BS (study coordinator).
University of Cincinnati: Peter Muskat, MD (principal investigator); Jay A. Johannigman, MD (co-investigator); Bryce RH Robinson, MD (co-investigator); Richard Branson (co-investigator); Dina Gomaa, BS, RRT (study coordinator); Cendi Dahl (study coordinator).
University of Pittsburgh Medical Center: Louis H. Alarcon, MD (principal investigator); Andrew B. Peitzman, MD (co-investigator); Stacy D. Stull, MS, CCRC (study coordinator); Mitch Kampmeyer, MPAS, CCRC, PA-C (study coordinator); Barbara J. Early, RN, BSN, CCRC (study coordinator); Helen L. Shnol, BS, CRC (study coordinator); Samuel J. Zolin, BS (research associate); Sarah B. Sears, BS (research associate).
University of Texas Health Science Center at Houston: John B. Holcomb, MD (co-principal investigator); Bryan A. Cotton, MD, MPH (co-principal investigator); Marily Elopre, RN (study coordinator); Quinton M. Hatch, MD (research associate); Michelle Scerbo (research associate); Zerremi Caga-Anan, MD (research associate).
University of Texas Health Science Center at San Antonio: John G. Myers, MD (co-principal investigator); Ronald M. Stewart, MD (co-principal investigator); Rick L. Sambucini, RN, BS (study coordinator); Marianne Gildea, RN, BSN, MS (study coordinator); Mark DeRosa CRT (study coordinator); Rachelle Jonas, RN, BSN (study coordinator); Janet McCarthy, RN (study coordinator).
University of Texas Southwestern Medical Center: Herbert A. Phelan, MD, MSCS (principal investigator); Joseph P. Minei, MD (co-investigator); Elizabeth Carroll, BS, BA (study coordinator).
University of Washington: Eileen M. Bulger, MD (principal investigator); Patricia Klotz, RN (study coordinator); Keir J. Warner, BS (research coordinator).
Footnotes
Previous Presentation of the Information Reported in the Manuscript: Portions of these data were presented at the Advanced Technology Applications for Combat Causality Care (ATACCC) Annual Scientific Meeting, August 15–18, 2011, Fort Lauderdale, FL.
Disclaimer: The views and opinions expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Army Medical Department, Department of the Army, the Department of Defense, or the United States Government.
Online-only Material: eFigure 1 are available at http://www.jama.com.
Author Contributions: Drs del Junco and Fox had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Rahbar served as PI of PROMMTT and is the senior author on this manuscript.
Study concept and design: Holcomb, del Junco, Rahbar, Fox, Zhang
Acquisition of data: Alarcon, Bai, Brasel, Bulger, Cohen, Cotton, Holcomb, Matijevic, Muskat, Myers, Phelan, Schreiber, White
Analysis and interpretation of data: del Junco, Fox, Rahbar, Holcomb, Wade
Drafting of the manuscript: Holcomb, Fox, del Junco, Wade
Critical revision of the manuscript for important intellectual content: del Junco, Holcomb, Fox, Rahbar, Wade, Alarcon, Bai, Brasel, Bulger, Cohen, Cotton, Matijevic, Muskat, Myers, Phelan, Schreiber, White, Zhang
Statistical analysis: del Junco, Fox, Rahbar
Obtained funding: Rahbar
Administrative, technical, or material support: Rahbar, Holcomb, Fox, del Junco, Alarcon, Bai, Brasel, Bulger, Cohen, Cotton, Muskat, Myers, Phelan, Schreiber, Wade, White, Zhang,
Study supervision: Rahbar, Holcomb
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Holcomb reported serving on the board for Tenaxis, the Regional Advisory Council for Trauma, and the National Trauma Institute; providing expert testimony for the Department of Justice; grants funded by the Haemonetics Corporation, and KCI USA, Inc. and consultant fees from the Winkenwerder Company. Dr Wade reported serving on the Science Board for Resuscitation Products, Inc. and the Advisory Board for Astrazeneca. No other disclosures were reported.
References
- 1.Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann Trop Med Parasitol. 2006;100(5–6):481–499. doi: 10.1179/136485906X97417. [DOI] [PubMed] [Google Scholar]
- 2.Sleet DA, Moffett DB. Framing the problem: injuries and public health. Fam Community Health. 2009;32(2):88–97. doi: 10.1097/01.FCH.0000347985.67681.9d. [DOI] [PubMed] [Google Scholar]
- 3.Eastman AB. Wherever the dart lands: toward the ideal trauma system. J Am Coll Surg. 2010;211(2):153–168. doi: 10.1016/j.jamcollsurg.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Hoyt DB. Blood and war-lest we forget. J Am Coll Surg. 2009;209(6):681–686. doi: 10.1016/j.jamcollsurg.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158–163. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 6.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 7.Kelly JF, Ritenour AE, McLaughlin DF, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64(2 Suppl):S21–S26. doi: 10.1097/TA.0b013e318160b9fb. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, McMullin NR, Pearse L, et al. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245(6):986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruen RL, Jurkovich GJ, McIntyre LK, Foy HM, Maier RV. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg. 2006;244(3):371–380. doi: 10.1097/01.sla.0000234655.83517.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demetriades D, Murray J, Charalambides K, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004;198(1):20–26. doi: 10.1016/j.jamcollsurg.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Moore FA, Nelson T, McKinley BA, et al. Massive transfusion in trauma patients: tissue hemoglobin oxygen saturation predicts poor outcome. J Trauma. 2008;64(4):1010–1023. doi: 10.1097/TA.0b013e31816a2417. [DOI] [PubMed] [Google Scholar]
- 12.Counts RB, Haisch C, Simon TL, Maxwell NG, Heimbach DM, Carrico CJ. Hemostasis in massively transfused trauma patients. Ann Surg. 1979;190(1):91–99. doi: 10.1097/00000658-197907000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledgerwood AM, Lucas CE. A review of studies on the effects of hemorrhagic shock and resuscitation on the coagulation profile. J Trauma. 2003;54(5 Suppl):S68–S74. doi: 10.1097/01.TA.0000064513.59253.70. [DOI] [PubMed] [Google Scholar]
- 14.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60(6 Suppl):S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 15.Moore FA, McKinley BA, Moore EE. The next generation in shock resuscitation. Lancet. 2004;363(9425):1988–1996. doi: 10.1016/S0140-6736(04)16415-5. [DOI] [PubMed] [Google Scholar]
- 16.American College of Surgeons Committee on Trauma. Advanced Trauma Life Support for Doctors Student Manual. 8. Chicago: American College of Surgeons; 2008. [Google Scholar]
- 17.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 18.Duchesne JC, Barbeau JM, Islam TM, Wahl G, Greiffenstein P, McSwain NE., Jr Damage control resuscitation: from emergency department to the operating room. Am Surg. 2011;77(2):201–206. doi: 10.1177/000313481107700222. [DOI] [PubMed] [Google Scholar]
- 19.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 20.Johansson PI, Stensballe J. Effect of Haemostatic Control Resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96(2):111–118. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95(2):112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 22.Shaz BH, Dente CJ, Nicholas J, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50(2):493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 23.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 24.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64(5):1177–1182. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 25.Duchesne JC, Islam TM, Stuke L, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009;67(1):33–37. doi: 10.1097/TA.0b013e31819adb8e. [DOI] [PubMed] [Google Scholar]
- 26.Cotton BA, Reddy N, Hatch QM, et al. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holcomb JB, Zarzabal LA, Michalek JE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–S328. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 29.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–270. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 30.Davenport R, Curry N, Manson J, et al. Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma. 2011;70(1):90–95. doi: 10.1097/TA.0b013e318202e486. [DOI] [PubMed] [Google Scholar]
- 31.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pati S, Matijevic N, Doursout MF, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma. 2010;69 (Suppl 1):S55–S63. doi: 10.1097/TA.0b013e3181e453d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder CW, Weinberg JA, McGwin G, Jr, et al. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66(2):358–362. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 35.Magnotti LJ, Zarzaur BL, Fischer PE, et al. Improved survival after hemostatic resuscitation: does the emperor have no clothes? J Trauma. 2011;70(1):97–102. doi: 10.1097/TA.0b013e3182051691. [DOI] [PubMed] [Google Scholar]
- 36.Scalea TM, Bochicchio KM, Lumpkins K, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248(4):578–584. doi: 10.1097/SLA.0b013e31818990ed. [DOI] [PubMed] [Google Scholar]
- 37.Ho AM, Dion PW, Yeung JH, et al. Prevalence of Survivor Bias in Observational Studies on Fresh Frozen Plasma: Erythrocyte Ratios in Trauma Requiring Massive Transfusion. Anesthesiology. 2012 doi: 10.1097/ALN.0b013e318245c47b. [DOI] [PubMed] [Google Scholar]
- 38.Dimick JB, Livingston EH. Comparing treatments using observational study designs: what can we do about selection bias? Arch Surg. 2010;145(10):927. doi: 10.1001/archsurg.2010.223. [DOI] [PubMed] [Google Scholar]
- 39.van WC, Davis D, Forster AJ, Wells GA. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol. 2004;57(7):672–682. doi: 10.1016/j.jclinepi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Austin PC, Platt RW. Survivor treatment bias, treatment selection bias, and propensity scores in observational research. J Clin Epidemiol. 2010;63(2):136–138. doi: 10.1016/j.jclinepi.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Wade CE, del Junco DJ, Holcomb JB, et al. Variations between level I trauma centers in 24-hour mortality in severely injured patients requiring a massive transfusion. J Trauma. 2011;71(2 Suppl 3):S389–S393. doi: 10.1097/TA.0b013e318227f307. [DOI] [PubMed] [Google Scholar]
- 42.Rahbar MH, Fox EE, delJunco DJ, et al. Coordination and management of multicenter clinical studies in trauma: Experience from the PRospective Observational Multicenter Major Trauma (PROMMTT) Study. Resuscitation. 2011 doi: 10.1016/j.resuscitation.2011.09.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duchesne JC, Kimonis K, Marr AB, et al. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma. 2010;69(1):46–52. doi: 10.1097/TA.0b013e3181df91fa. [DOI] [PubMed] [Google Scholar]
- 44.Hintze J. Pass. Vol. 11. Kaysville, UT: NCSS, LLC; 2011. [Google Scholar]
- 45.Holcomb JB, Weiskopf R, Champion H, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35(2):107–113. doi: 10.1097/SHK.0b013e3181f7fd01. [DOI] [PubMed] [Google Scholar]
- 46.Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 47.Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51(4):1469–1482. [PubMed] [Google Scholar]
- 48.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: John Wiley & Sons; 1999. [Google Scholar]
- 50.SAS/STAT software for Windows. Version 9.2. Cary, NC: SAS Institute, Inc; 2008. [Google Scholar]
- 51.Von EE, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17 (Suppl 1):i13–i32. doi: 10.1136/qshc.2008.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riskin DJ, Tsai TC, Riskin L, et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209(2):198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 54.Cinat ME, Wallace WC, Nastanski F, et al. Improved survival following massive transfusion in patients who have undergone trauma. Arch Surg. 1999;134(9):964–968. doi: 10.1001/archsurg.134.9.964. [DOI] [PubMed] [Google Scholar]
- 55.Duchesne JC, Hunt JP, Wahl G, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65(2):272–276. doi: 10.1097/TA.0b013e31817e5166. [DOI] [PubMed] [Google Scholar]
- 56.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65(3):527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 57.Wafaisade A, Maegele M, Lefering R, et al. High plasma to red blood cell ratios are associated with lower mortality rates in patients receiving multiple transfusion (4</=red blood cell units<10) during acute trauma resuscitation. J Trauma. 2011;70(1):81–88. doi: 10.1097/TA.0b013e3182032e0b. [DOI] [PubMed] [Google Scholar]
- 58.Kent DM, Alsheikh-Ali A, Hayward RA. Competing risk and heterogeneity of treatment effect in clinical trials. Trials. 2008;9:30. doi: 10.1186/1745-6215-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60(6):1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205(4):541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Nunez TC, Dutton WD, May AK, Holcomb JB, Young PP, Cotton BA. Emergency department blood transfusion predicts early massive transfusion and early blood component requirement. Transfusion. 2010;50(9):1914–1920. doi: 10.1111/j.1537-2995.2010.02682.x. [DOI] [PubMed] [Google Scholar]
- 62.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66(2):346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 63.McLaughlin DF, Niles SE, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–S63. doi: 10.1097/TA.0b013e318160a566. [DOI] [PubMed] [Google Scholar]
- 64.Rainer TH, Ho AM, Yeung JH, et al. Early risk stratification of patients with major trauma requiring massive blood transfusion. Resuscitation. 2011;82(6):724–729. doi: 10.1016/j.resuscitation.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Borgman MA, Spinella PC, Holcomb JB, et al. The effect of FFP:RBC ratio on morbidity and mortality in trauma patients based on transfusion prediction score. Vox Sang. 2011;101(1):44–54. doi: 10.1111/j.1423-0410.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larson CR, White CE, Spinella PC, et al. Association of shock, coagulopathy, and initial vital signs with massive transfusion in combat casualties. J Trauma. 2010;69 (Suppl 1):S26–S32. doi: 10.1097/TA.0b013e3181e423f4. [DOI] [PubMed] [Google Scholar]
- 67.Cancio LC, Wade CE, West SA, Holcomb JB. Prediction of mortality and of the need for massive transfusion in casualties arriving at combat support hospitals in Iraq. J Trauma. 2008;64(2 Suppl):S51–S55. doi: 10.1097/TA.0b013e3181608c21. [DOI] [PubMed] [Google Scholar]
- 68.Maegele M, Lefering R, Wafaisade A, et al. Revalidation and update of the TASH-Score: a scoring system to predict the probability for massive transfusion as a surrogate for life-threatening haemorrhage after severe injury. Vox Sang. 2011;100(2):231–238. doi: 10.1111/j.1423-0410.2010.01387.x. [DOI] [PubMed] [Google Scholar]
- 69.Krumrei NJ, Park MS, Cotton BA, Zielinski MD. Comparison of massive blood transfusion predictive models in the rural setting. J Trauma. 2012;72(1):211–215. doi: 10.1097/TA.0b013e318240507b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mackenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354(4):366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 71. [Accessed March 8, 2012];Pragmatic, Randomized Optimal Platelets and Plasma Ratios (PROPPR) http://clinicaltrials.gov/ct2/show/NCT01545232.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.