Abstract
Adiponectin and its receptors are inversely related to the degree of obesity and have been identified as potential therapeutic targets for the treatment of obesity. In this study, we evaluated the effect of hydrodynamic delivery of adiponectin and/or its receptor 2 (adipoR2) genes on controlling the development of obesity and insulin resistance in AKR/J mice fed a high-fat diet. An increase in adiponectin and adipoR2 gene expression by hydrodynamic gene delivery prevented diet-induced weight gain, reduced fat accumulation in liver and adipose tissue, and improved insulin sensitivity. Beneficial effects were seen with reduced gluconeogenesis in the liver and lipogenesis in the liver, white adipose tissue and skeletal muscle. Real-time PCR analysis demonstrated overexpression of adiponectin and adipoR2 significantly suppressed transcription of phosphoenolpyruvate carboxykinase (pepck), glucose-6-phosphatase (g6pase), stearoyl CoA desaturase 1 (scd-1), and fatty acid synthase (fas) gene. Inhibition effects were mediated by activating the AMP-activated protein kinase (AMPK). These results prove that elevation of adiponectin and/or adipoR2 expression via gene transfer is an effective approach in managing obesity epidemics.
Keywords: Obesity, insulin resistance, adiponectin, adiponectin receptor, gene therapy
Introduction
Adiponection (also known as Acrp30, AdipoQ, and GBP28) is an adipocytokine secreted from adipocytes1–4 and is present abundantly in plasma.5 Through interacting with its receptors, AdipoR1 and AdipoR2, adiponectin regulates energy homeostasis and glucose metabolism by increasing insulin sensitivity, suppressing inflammation, and inhibiting atherogenicity.6–8 Previous studies show that adiponectin expression and its plasma concentration were significantly reduced in obese/diabetic mice, monkeys and humans.9–13 Decreased serum level of adiponectin also correlates with lowered insulin sensitivity.14 Similarly, the expression of the adiponectin receptor was also significantly decreased in ob/ob mice and C57BL/6 mice fed a high-fat diet (HFD).15,16 In addition, simultaneous disruption of both AdipoR1 and AdipoR2 abolished adiponectin binding and actions, leading to insulin resistance and glucose intolerance.17 These studies suggest that obesity decreases the expression of adiponectin receptors, and consequently, reduces adiponectin sensitivity and leads to insulin resistance. Therefore, a strategy to enhance expression of adiponectin or the adiponectin receptor gene or in combination should prevent obesity. The aim of this study is to assess such a possibility in AKR/J mice fed a HFD by delivering plasmids containing coding sequences of adiponectin or adipoR2 gene using hydrodynamic delivery. Our data demonstrate that an increase in adiponectin, and adipoR2 gene expression or both through gene delivery blocks HFD-induced weight gain, reduces lipids deposition and maintains glucose hemostasis.
Results
Increase of adiponectin and adipoR2 gene expression in AKR/J mice by hydrodynamic gene delivery
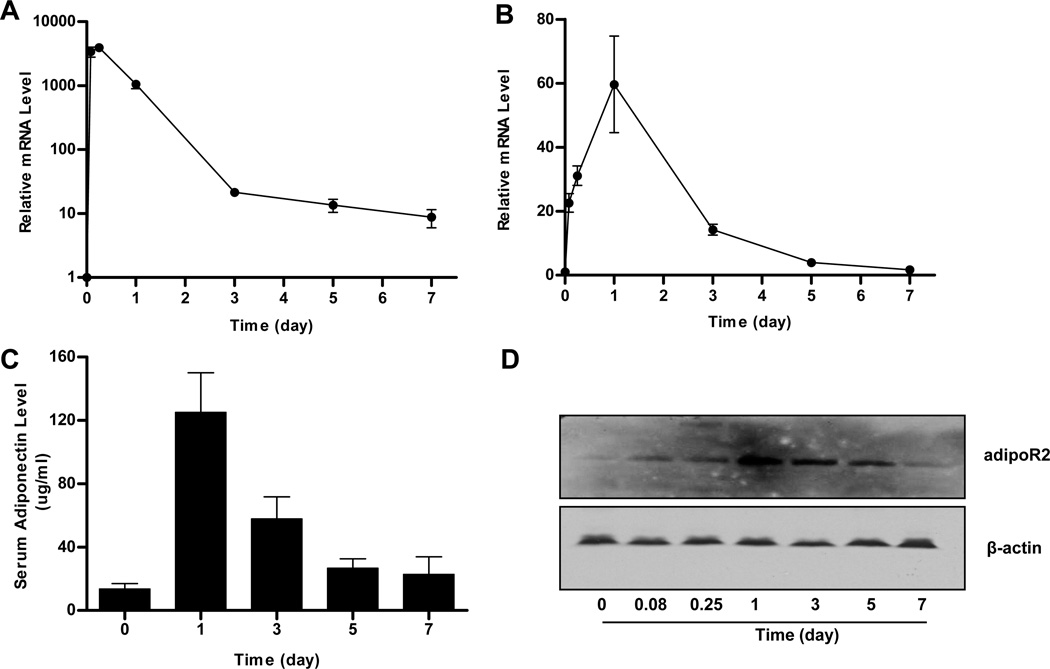
To confirm expression of adiponectin and adipoR2 in AKR/J mice, mRNA and protein levels of these gene products were determined at different times after hydrodynamic delivery of pCMV-Acrp30 or/and pCMV-adipoR2 plasmid DNA. As shown in Figure 1A, more than a 4,000-fold increase in adiponectin mRNA was detected in the livers as early as 2 hr after hydrodynamic gene delivery in AKR/J mice. Transcript levels decreased quickly over time. One week after gene delivery, transcript levels in mouse livers were 8-fold higher than that of control animals injected with an empty plasmid (pcDNA3.1). The serum concentration of adiponectin protein was 125 µg/ml 24 h after injection and remained at 22 µg/ml after 7 days, 2-fold higher than background level before gene delivery (Figure 1C, time zero). A similar gene expression pattern was also seen in animals injected with adipoR2 plasmid (Figures 1B, 1D). The mRNA levels of adipoR2 increased approximately 60-fold one day after gene delivery and returned to the background level 7 days later (Figure 1B). Western blot in mouse livers showed a peak level of adipoR2 protein on day 1 and returned to background level in day 7 (Figure 1D). These data prove that adiponectin and adipoR2 are expressed successfully in mice by hydrodynamic gene delivery.
Figure 1. Expression of adiponectin and adipoR2 in AKR/J mice by hydrodynamic gene delivery.
AKR/J mice were hydrodynamically injected via tail vein of 1 µg/g of body weight (or lean mass) of pCMV-Acrp30, pCMV-adipoR2, or both and sacrificed at desirable time. The mRNA and protein levels of adiponectin and adipoR2 were measured. The mRNA level of adiponectin (A) and adipoR2 (B) in mouse liver; adiponectin level in mouse serum (C); and protein level of adipoR2 in mouse liver (D). Data represent mean ± SD from 3 independent experiments.
Adiponectin and adipoR2 gene delivery blocks HFD-induced weight gain in AKR/J mice
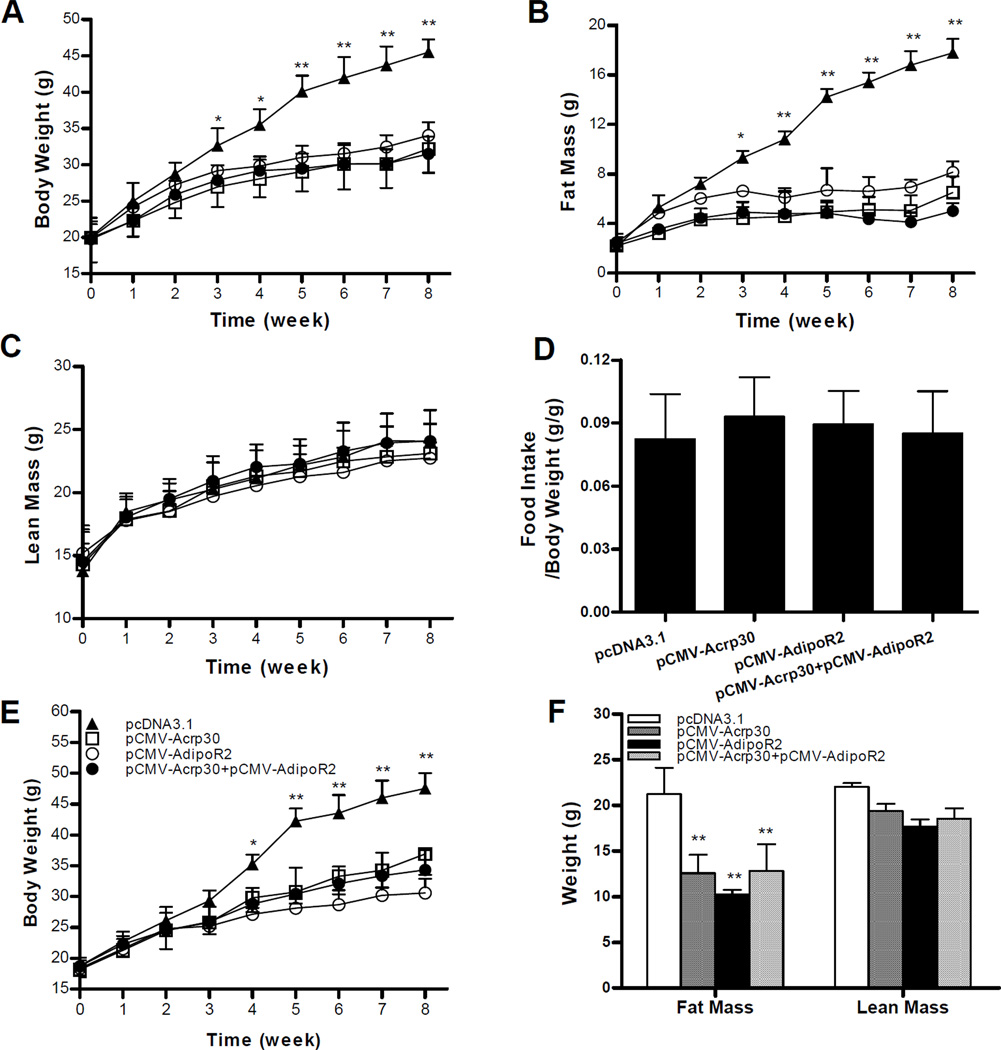
To explore the effect of adiponectin and adipoR2 gene delivery on diet-induced obesity, 4-week old male mice were injected with pCMV-Acrp30 and/or pCMV-adipoR2 once weekly and fed a HFD for 8 weeks. Figure 2A shows that delivery of adiponectin, adipoR2 gene or in combination markedly blocked body weight gain as compared to control animals. A significant difference was evidenced as early as the first three weeks of HFD feeding. After feeding with HFD for 8 weeks, control animals injected with an empty plasmid reached a body weight of 45.5±0.9g, compared to 31.2±1.6 and 30.5±1.2g for those injected with pCMV-Acrp30 or pCMV-Acrp30/pCMV-adipoR2 in combination, respectively. Animals who received pCMV-adipoR2 had an average body weight of 34.0±1.8g. Body composition analysis using the EchoMRI-100™ System revealed that an increase in adiponectin and/or adipoR2 expression in AKR/J mice significantly inhibited the fat mass gain without changing lean mass (Figures 2B, C). An eight-week treatment of pCMV-Acrp30 and/or pCMV-adipoR2 under HFD feeding slightly increased food intake when corrected for total body weight (Figure 2D). A similar pattern was seen in body weight, fat and lean mass in female mice (Figures 2E, F), suggesting the effect is not gender specific. Injection with pCMV-Acrp30 and/or pCMV-adipoR2 into animals fed a regular chow did not change body weight nor food intake (data not shown). Together, these results demonstrate that enhanced gene expression of adiponectin and its receptor by hydrodynamic gene delivery protects animals against HFD-induced obesity in AKR/J mice.
Figure 2. Delivery of adiponectin and adiponectin receptor 2 gene prevents HFD-induced weight gain.
AKR/J mice were fed a HFD for 8 weeks and received weekly injection of empty plasmid (pcDNA3.1), pCMV-Acrp30, pCMV-AdipoR2 or combination of pCMV-Acrp30/pCMV-AdipoR2 plasmids, respectively. Growth curve of male mice (A); fat (B) and lean (C) masses determined by MRI; food intake corrected for total body weight (D); growth curve in female mice (E); and fat and lean mass of female mice at the end of 8-week treatment (F). Results represent means±SD (n=4) for each group. *P<0.05; **P<0.01, comparing to control mice injected with reporter plasmid.
Hydrodynamic delivery of adiponectin and adipoR2 gene improved HFD-induced hyperinsulinemia and hyperglycemia
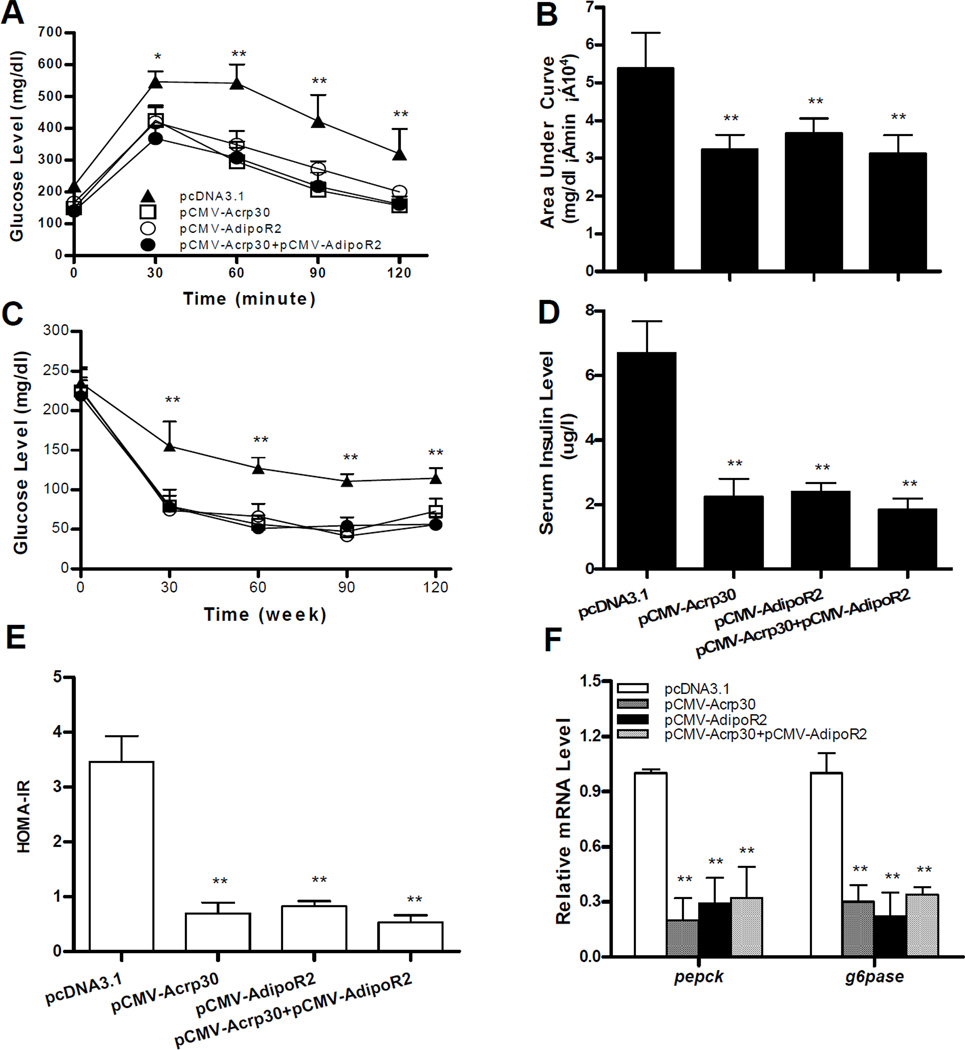
Obesity is known to induce insulin resistance, a characteristic of type-2 diabetes. To investigate whether reduced weight gain by increasing levels of adiponectin and its receptor improves obesity-associated insulin resistance, glucose homeostasis was assessed. Glucose tolerance tests (Figure 3A) and calculated area under the curve (AUC) (Figure 3B) showed that mice injected with pCMV-Acrp30 and/or pCMV-adipoR2 exhibited a much quicker clearance rate of intra-peritoneally injected glucose comparing to that of control animals, indicating that high levels of adiponectin and its receptor maintained glucose sensitivity of mice fed a HFD. Protection against diet-induced insulin resistance was also confirmed by an insulin tolerance test (ITT) (Figure 3C). Adiponectin and adipoR2 ameliorated diet-induced hyperinsulinemia (Figure 3D) and similar patterns were observed by HOMA-IR (Figure 3E).
Figure 3. Hydrodynamic delivery of adiponectin and adipoR2 gene improves HFD-induced hyperinsulinemia and hyperglycemia.
At the end of the 8-week treatment, animals were fasted overnight to measure glucose tolerance, or 4 h for insulin sensitivity. Time-dependent blood concentration of glucose upon IP injection of glucose (2 g/kg) (A); area under the curve from glucose tolerance test (B); time dependent glucose concentration upon IP injection of insulin (C); insulin levels measured at the end of the 8-week in male mice (D); HOMA-IR value (E) calculated based on formula: [fasting insulin (mU/ml) × fasting glucose (mmol/l)/ 22.5]; and relative mRNA levels of pepck and g6pase in mouse liver (G). Data represent mean ± SD (n=4) in each group. *P<0.05; ** P<0.01, comparing to the control group.
To investigate how adiponectin and adipoR2 gene delivery maintain glucose levels in animals, expression of genes involved in hepatic gluconeogenesis were determined. Results in Figure 3F reveal that an increase in expression of adiponetin and adipoR2 gene significantly lowers the amount of transcript in phosphoenolpyruvate carboxykinase (pepck) and glucose-6-phosphatase (g6pase), concordant with a reduction in diet-induced hyperglycemia and hyperinsulinemia. At the same time, the inhibitory effect was the same between animals injected with either adiponectin or adipoR2 gene and those who received both plasmids. Overall, these results demonstrate that hydrodynamic gene delivery of adiponectin and adipoR2 blocks HFD-induced development of insulin resistance.
Increase in adiponectin and adipoR2 expression suppressed HFD-induced lipid accumulation in mouse liver and adipose tissue
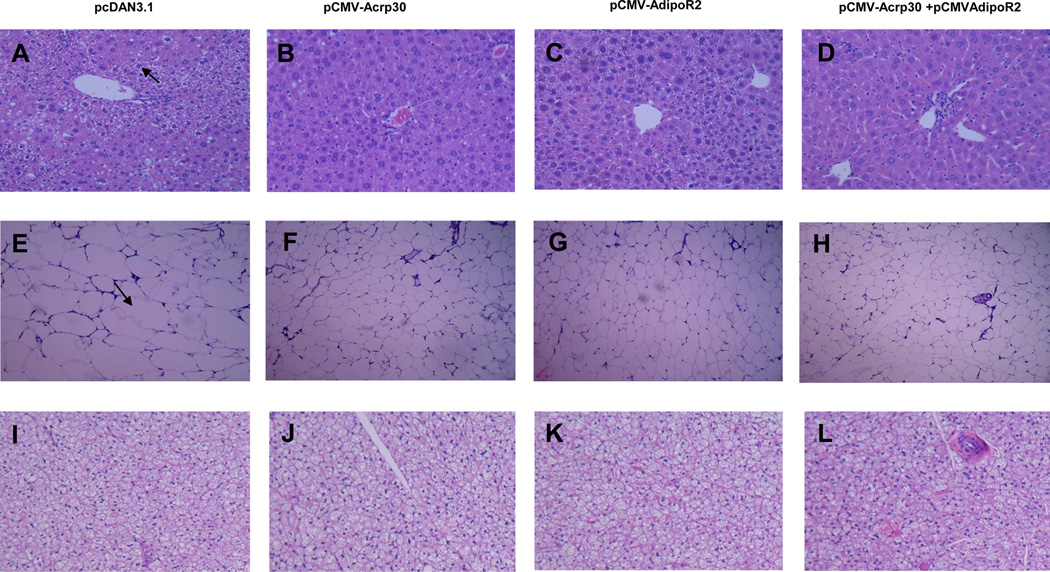
Excessive deposition of lipids in liver and adipose tissue is one of the major characteristics in obesity. Therefore, we further evaluated the effect of adiponectin and adipoR2 gene delivery on lipid accumulation in hepatic and adipose tissues. H&E-stained liver sections show extensive hepatocyte vacuolation in control mice, reflecting that severe hepatic steatosis was developed (Figure 4A). However, injection of adiponectin and adipoR2 plasmids significantly ameliorated lipid deposition in hepatocytes and resisted the development of hepatic steatosis, especially in animals injected with both types of plasmids (Figures 4B–4D). Consistent with reduction of lipids in the liver and an increased expression of adiponectin and/or adipoR2 in AKR/J mice repressed lipid accumulation in white adipose tissue (WAT) was also observed as evidenced by a unmistakable reduction in adipocyte size in animals injected with adiponectin and/or adipoR2 plasmids (Figures 4E–4H). Conversely, an analysis of a brown adipose tissue (BAT) mass revealed no significant difference in adipocyte size (Figures 4I–4L).
Figure 4. Increase of adiponectin and adipoR2 expression reduced lipid accumulation in mice fed with a HFD.
H&E staining of liver sections showing less vacuolation and lipid accumulation in livers of treated animals compared to that of control (100×) (A–D); H&E staining of WAT showing a prominent reduction of adipocyte size in treated group (100×) (E–H); and H&E staining of BAT showing no significant changes among these groups (100×) (I–L).
Increased adipnectin and adipoR2 expression inhibited lipogenesis in HFD-fed AKR/J mice
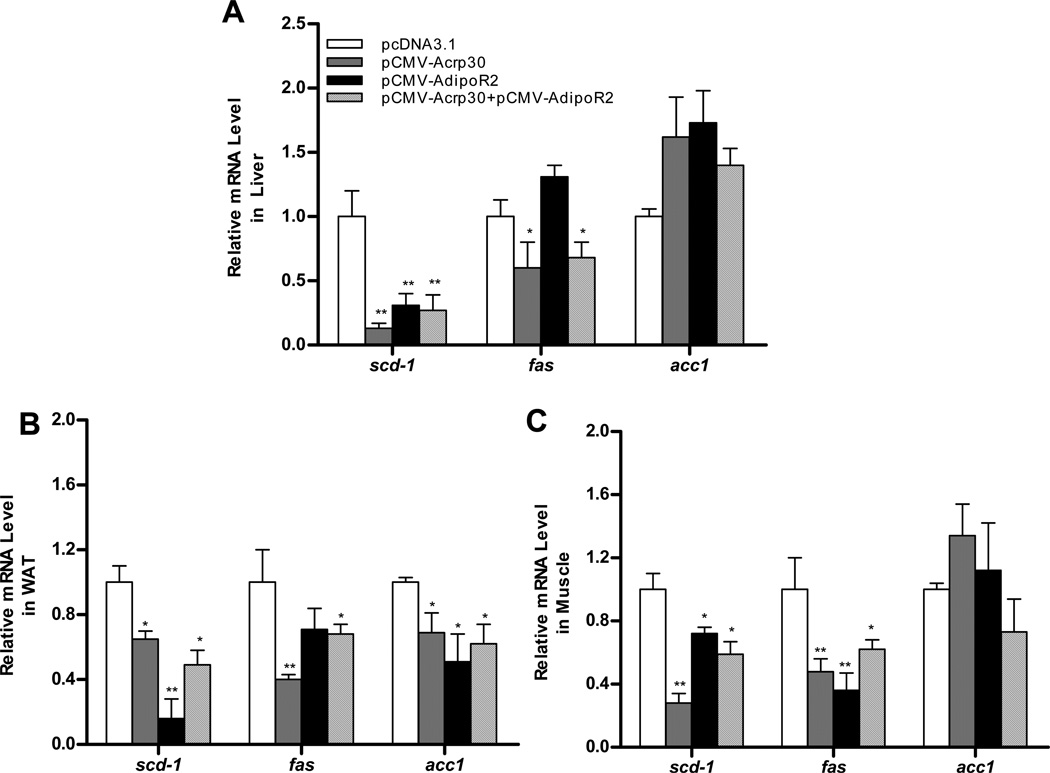
To determine how adiponectin and adipoR2 reduce excessive lipid accumulation in HFD-fed animals, we conducted a real-time PCR analysis of mRNA for genes involved in lipogenesis. As shown in Figure 5, adiponectin overexpression significantly lowered mRNA levels of stearoyl CoA desaturase 1 (scd-1) and fatty acid synthase (fas) by 87% and 40% (Figure 5A), respectively. An increase in adipoR2 gene expression did not reduce fas mRNA levels, but did decrease scd-1 transcription in the liver. Significant inhibition of fas and scd-1 gene expression was observed in WAT (Figure 5B) and skeletal muscle (Figure 5C) as compared to control animals injected with empty plasmids. Reduction of acetyl-CoA carboxylase (acc1) was only observed in WAT, no change was observed in skeletal muscle, and only a slight increase in the liver. Taken together, these data show that hydrodynamic delivery of adiponectin and adipoR2 gene prevents lipid accumulation through inhibiting lipogenesis.
Figure 5. Adipnectin- and adipoR2-overexpression inhibited lipogenesis in HFD-fed AKR/J mice.
Expression of genes involved in lipid metabolism in liver, WAT and muscle from male mice. Results are expressed as means ± SD (n=4) for each group. *P<0.05; **P<0.01, comparing to control.
Delivery of adiponectin gene activated AMP-activated protein kinase
AMP-activated protein kinase (AMPK) plays a key role in regulating energy homeostasis and mediating the activity of adiponectin and its receptors.18,19 Therefore, we assessed the effect of increased adiponectin expression on AMPK activation by measuring the amount of phosphorylated AMPK (Thr172) and total AMPK protein at different time after plasmid injection. As shown in Figure 6, phosphorylation of AMPK was increased by 1.97-fold as early as 2 h after plasmid injection and reached 2.86-fold higher than control animals 24 h later. Activated AMPK quickly reduced after one day, concordant with the expression pattern of adiponection as shown in Figure 1. These results suggest that activation of AMPK plays a critical role in adiponectin-mediated protection against HFD-induced obesity.
Figure 6. Increased expression of adiponectin activated AMPK in HFD-fed mice.
Mice were hydrodynamically injected with 20 µg/mouse of pCMV-Acrp30 plasmid DNA and sacrificed 0, 2 h, 6 h, 1 d, 3 d, 5 d, or 7d later. Phosphorylated and total AMPK were measured by Western blotting (A); and relative expression level calculated based on scanning densitometry of the phosphorylated AMPK bands (B). *P<0.05, comparing to protein at time 0.
Discussion
The overall objective of this study is to use gene transfer as a way to block HFD-induced obesity and insulin resistance. We demonstrate that increased expression of adiponectin and adipoR2 gene by hydrodynamic delivery prevented the development of HFD-induced obesity and alleviates obesity-related insulin resistance and lipid accumulation (Figures 2, 3, 4). Mechanistically, these beneficial effects are achieved by inhibition of lipogenesis and gluconeogenesis through activation of AMPK (Figures 5, 6).
Hydrodynamic gene delivery is an effective method of gene delivery20,21 and has been widely used for gene expression and functional analysis in whole animals.22 The use of the computer-controlled injection device in recent work has made the hydrodynamics-based procedure more attainable for gene delivery in both small and large animals.23–25 Results in Figure 1 show that hydrodynamic injection of pCMV-Acrp30 plasmid results in a significant production of adiponectin as early as 2 h. Secreted adiponectin levels in plasma reached 125 µg/ml in the first day, much higher than that of viral vector-based gene delivery and nonviral approach using polyethylenimine as a gene carrier.26,27 AdipoR2 also showed a similar expression pattern in the liver, as demonstrated by real-time PCR (Figure 1B) and Western blot (Figure 1D).
An increased expression of adiponectin and adipoR2 gene blocked body weight gain in HFD-fed male (Figure 1A) and female (Figure 1E) AKR/J mice and reduced lipid accumulation in the liver and white adipose tissue (WAT) (Figure 4). No difference was observed between animals injected with either pCMV-Acrp30 or pCMV-AdipoR2 plasmids, although animals injected with both plasmids exhibited better hepatic protection as shown by H&E staining. The results from real-time PCR showed that a reduction in hepatic lipid accumulation involves inhibition of fas and scd-1 gene expression in the liver (Figure 5A), WAT (Figure 5B) and skeletal muscle (Figure 5C). However, elevated expression of adiponectin or adipoR2 gene did not suppress acc1 expression in the liver. Enhanced phosphorylation of AMPK in both pCMV-Acrp30 and pCMV-AdipoR2 injected animals (Figure 6) suggests reduced lipogenesis in the liver, which is in accordance with well-known pathways for adiponectin activity.28
The anti-hypoglycemic effect of adiponectin and adipoR2 in animals fed a HFD was mediated by inhibiting the expression of pepck and g6pase genes (Figure 3F), two key enzymes in gluconeogenesis. These results are in agreement with previous reports,26 confirming that AMPK activation represses the transcription of pepck and g6pase through phosphorylation of the CREB-regulated transcription co-activator 2 (CRTC2).28,29 A study using adiponectin transgenic mice showed that a reduced expression of enzymes involved in glucose metabolism is associated with elevated phosphorylation of hepatic AMPK, which may account for inhibition of endogenous glucose production by adiponectin.30
In addition to activation of AMPK, Iwabu M et al31 recently reported that adiponectin induces Ca2+ influx in skeletal muscle via adipoR1 as a mechanism for regulation of mitochondrial biogenesis that reduces oxidative stress and enhances endurance capacity. Whether adiponectin enhances Ca2+ influx in the liver via adipoR2 requires further investigation.
In summary, we demonstrate that increased expression of adiponectin and adipoR2 genes by hydrodynamic gene delivery blocks HFD-induced weight gain, lipid accumulation, and insulin resistance in AKR/J mice. The mechanism involves activation of AMPK to suppress expression of genes involved in lipogenesis and gluconeogenesis. Although further studies are needed to confirm that similar effect will be achieved in different animals or under different physiological conditions, these results strongly suggest that management of obesity epidemics through gene transfer could be an alternative option to commonly used methods including increased exercise, diet restriction and drug treatment.
Materials and Methods
Materials
Adiponectin and its receptor 2 (adipoR2) genes were from Open Biosystems and were cloned into a pcDNA3.1 plasmid. DNA plasmids were purified using CsCl–ethidium bromide density-gradient ultracentrifugation and kept in 0.9% sodium chloride. Purity of the plasmids was verified with absorbency at 260 and 280†nm and 1% agarose gel electrophoresis. High-fat diet was purchased from Bio-serv (Frenchtown, NJ, catalog number S3282). The RNeasy Tissue kit was from Qiagen (Valencia, CA). Reagents for real time PCR were obtained from AB Applied Biosystems (Foster City, CA). Antibodies against mouse adipoR2 were from Abacam (Cambrige, MA). Antibodies against mouse AMPK-alpha, phosphor-AMPK-alpha (Thr172) and β-actin were purchased from Cell Signaling (Danvers, MA). The mouse adiponectin ELISA kit was obtained from Assaypro (St. Charles, MO). The insulin assay kit was from Crystal Chem (Downers Grove, IL). The glucometer and test strips were purchased from LifeScan (Milpitas, CA). AKR/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Experimental Procedures
Animals and treatment
All procedures performed on mice were approved by the Institutional Animal Care and Use Committee at the University of Georgia. Four-week-old mice were randomly divided into 4 groups and were hydrodynamically injected with an empty vector (pcDNA3.1), pCMV-acrp30 plasmids containing mouse adiponectin gene, pCMV-adipoR2 plasmids with adiponectin receptor 2 gene, or the combination of pCMV-acrp30 and pCMV-adipoR2 plasmids. The treatment schedule included a weekly injection of plasmids at 1 µg/g of body weight for 8 weeks while keeping animals on HFD. Body weight was measured and body composition of live animals was measured and analyzed using EchoMRI-100™ from Echo Medical Systems (Houston, TX) once per week.
Analysis of insulin levels
After the final injection, blood samples were collected from fasted mice. Serum levels of insulin were measured using commercial assay kits according to the manufacturer’s instructions. HOMA-IR was calculated as follows: (fasting insulin [mU/ml] × fasting glucose [mmol/l]) / 22.5.
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
For GTT, mice were fasted overnight and injected intraperitoneally with glucose at 2 g/kg body weight. Blood samples were taken at different time points and glucose concentrations were measured using a glucometer. For ITT, mice fasted for 4 h and blood glucose levels were measured after an intraperitoneal injection of human insulin (Novolin) from Novo Nordisk (Princeton, NJ) at 1.2 U/kg.
Histology analysis
After mice were sacrificed, the liver, white and brown adipose tissues were removed and fixed in 10% formalin. Tissues were processed, embedded in paraffin, sectioned at a thickness of 5 µm and stained with hemotoxylin and eosin. Microscopic examination was performed and photographed under a regular light microscope
Gene expression analysis by real-time PCR
Total RNA was isolated from the mouse liver, white adipose tissue (WAT) and muscle tissue using the RNeasy kit. Two micrograms of total RNA were used for first-strand cDNA synthesis, as recommended by the manufacturers (Invitrogen, Carlsbad, CA). Real-time PCR was performed using SYBR Green as an indicator on the ABI 7300 Fast Real-Time PCR system. The final reaction mixture contained 10 ng of cDNA, 100 nM of each primer, 10 µl of 2x SYBR Green PCR Master and RNase-free water to complete the reaction mixture volume to 20 µl. All reactions were performed in triplicate. PCR was carried out for 40 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescence was read during the reaction, allowing a continuous monitoring of the amount of PCR product. Data were normalized to internal control GAPDH mRNA. The sequences of primers are as shown in Table 1.
Table 1.
Primer sets for real time PCR analysis of gene expression
| Name | Forward sequence | Reverse sequence |
|---|---|---|
| adiponectin | AGCCGCTTATATGTATCGCTCA | TGCCGTCATAATGATTCTGTTGG |
| adipoR2 | TACCAAGGAGATTTGGAGCCC | GCCCATAAACCCTTCATCTTCC |
| pepck | AAGCATTCAACGCCAGGTTC | GGGCGAGTCTGTCAGTTCAAT |
| g6pase | CGACTCGCTATCTCCAAGTGA | GTTGAACCAGTCTCCGACCA |
| acc1 | GCCTCTTCCTGACAAACGAG | TGACTGCCGAAACATCTCTG |
| fas | AGAGATCCCGAGACGCTTCT | GCCTGGTAGGCATTCTGTAGT |
| scd-1 | TTCTTACACGACCACCACCA | CCGAAGAGGCAGGTGTAGAG |
| gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Western blot analysis
The frozen samples were thawed on ice and homogenized using a Tissue Tearor in 1 ml lysis buffer with protease. The tissue homogenate was centrifuged for 10 min in a microcentrifuge and total protein was extracted from the supernatant. Fifty micrograms of total protein were separated on 7.5% SDS-PAGE gels and transferred onto a polyvinylidene difluoride membrane using a Bio-Rad Mini-Blot transfer apparatus. Membranes were blocked in tris-buffered solution (TBS) containing 5% BSA for 1 h. Immunoblotting was performed at 4°C overnight by shaking using antibodies against adipoR2, AMPKα, phosphor-AMPKα (Thr172) and β-actin, respectively. After being washed, the membrane was incubated in a 1:3,000 dilution of a secondary antibody at room temperature for 1 h in TBS washing buffer containing 0.5% of Tween-20. Protein bands were visualized using Pierce ECL Western Blotting substrate.
Statistics analysis
A statistical analysis was performed using one-way ANOVA. All data are reported as mean ± standard deviation (SD) and a P value < 0.05 was considered statistically significant.
Acknowledgements
This work was supported in part by the National Institute of Health [RO1EB007357 and RO1HL098295]. The authors would like to thank Ms. Ryan Fugett for proofreading the manuscript.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum-protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K, et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (Adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 4.Nakano Y, Tobe T, ChoiMiura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Capeau J. The story of adiponectin and its receptors AdipoR1 and R2: to follow. J Hepatol. 2007;47:736–738. doi: 10.1016/j.jhep.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershawand EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocr Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 9.Diezand JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 10.Statnick MA, Beavers LS, Conner LJ, Corominola H, Johnson D, Hammond CD, et al. Decreased expression of apM1 in omental and subcutaneous adipose tissue of humans with type 2 diabetes. Int J Exp Diabetes Res. 2000;1:81–88. doi: 10.1155/EDR.2000.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends Endocrinol Metab. 2003;14:137–145. doi: 10.1016/s1043-2760(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 12.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 14.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 16.Peng YH, Rideout D, Rakita S, Sajan M, Farese R, You M, et al. Downregulation of Adiponectin/AdipoR2 is Associated with Steatohepatitis in Obese Mice. J Gastrointest Surg. 2009;13:2043–2049. doi: 10.1007/s11605-009-1032-2. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 18.Hardie DG. The AMP-activated protein kinase pathway - new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F, Song YK, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GF, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 22.Suda T, Liu D. Hydrodynamic gene delivery: Its principles and applications. Mol Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 23.Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16:1098–1104. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura K, Suda T, Xu W, Zhang GS, Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamimura K, Zhang GS, Liu D. Image-guided, Intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol Ther. 2010;18:93–100. doi: 10.1038/mt.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA. 2003;100:14217–14222. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Lee M, Kim SW. Non-viral adiponectin gene therapy into obese 2 type diabetic mice ameliorates insulin resistance. J Control Release. 2006;114:118–125. doi: 10.1016/j.jconrel.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Dzamkoand NL, Steinberg GR. AMPK-dependent hormonal regulation of whole-body energy metabolism. Acta Physiol. 2009;196:115–127. doi: 10.1111/j.1748-1716.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- 29.Koo SH, Flechner L, Qi L, Zhang XM, Screaton RA, Jeffries S, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1114. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 30.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 31.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]