Abstract
In this minireview, we briefly survey the molecular processes that lead to reactive oxygen species (ROS) production by the respiratory complex III (CIII or cytochrome bc1). In particular, we discuss the “forward” and “reverse” electron transfer pathways that lead to superoxide generation at the quinol oxidation (Qo) site of CIII, and the components that affect these reactions. We then describe and compare the properties of a bacterial (Rhodobacter capsulatus) mutant enzyme producing ROS with its mitochondrial (human cybrids) counterpart associated with a disease. The mutation under study is located at a highly conserved tyrosine residue of cytochrome b (Y302 in R. capsulatus and Y278 in human mitochondria) that is at the heart of the quinol oxidation (Qo) site of CIII. Similarities of the major findings of bacterial and human mitochondrial cases, including decreased catalytic activity of CIII, enhanced ROS production and ensuing cellular responses and damages, are remarkable. This case illustrates the usefulness of undertaking parallel and complementary studies using biologically different yet evolutionarily related systems, such as α-proteobacteria and human mitochondria. It progresses our understanding of CIII mechanism of function and ROS production, and underlines the possible importance of supra molecular organization of bacterial and mitochondrial respiratory chains (i. e., respirasomes) and their potential disease-associated protective roles.
Keywords: Complex III, Cytochrome bc1, Mitochondria, Reactive Oxygen Species, Oxidative damages, Electron transfer, Quinone, Superoxide
1. Introduction
1.1 Reactive Oxygen Species
Reactive oxygen species (ROS) are a group of radical or non-radical oxygen containing molecules that display high reactivity with lipids, proteins and nucleic acids. Depending on the concentration, location, and molecular context, ROS can be beneficial or harmful to cells. Increasing evidence indicates that homeostatic and physiological levels of ROS are indispensable in regulating diverse cellular processes, including ion channel/transporter function [1], Ca2+ spark production [2, 3], protein kinase/phosphatase activation and gene expression [4]. A current view is that low levels of ROS production contribute to many essential intracellular signaling processes ranging from cell metabolism to ischemia preconditioning in eukaryotic cells [4–6]. Conversely, excessive ROS generation often leads to apoptotic and necrotic cell death [7, 8] as well as to a panel of clinically distinct disorders, including neurodegeneration (e.g., Alzheimer’s disease), cardiomyopathies, diabetes and cancer [9–11]. Accumulative and systemic ROS damages also underlie cell senescence and aging [6, 12].
In quiescent cells, ROS are primarily produced as byproducts of mitochondrial respiration when electrons leak from the electron transport chain (ETC) [13] (Fig. 1). Superoxide anions (O2.−) are the primary ROS species generated by the ETC, and are converted to hydrogen peroxide (H2O2) either spontaneously or by the superoxide dismutase (SOD). In the presence of transition metals, O2.− can also be transformed into hydroxyl radicals (.OH) that are considered to be more reactive and damaging. Recent studies documented that massive increases in localized ROS production could occur during metabolic stress [6, 14] and photostimulation [15]. Besides, excessive amounts of intracellular ROS ultimately contribute to necrotic or apoptotic cell death [16, 17].
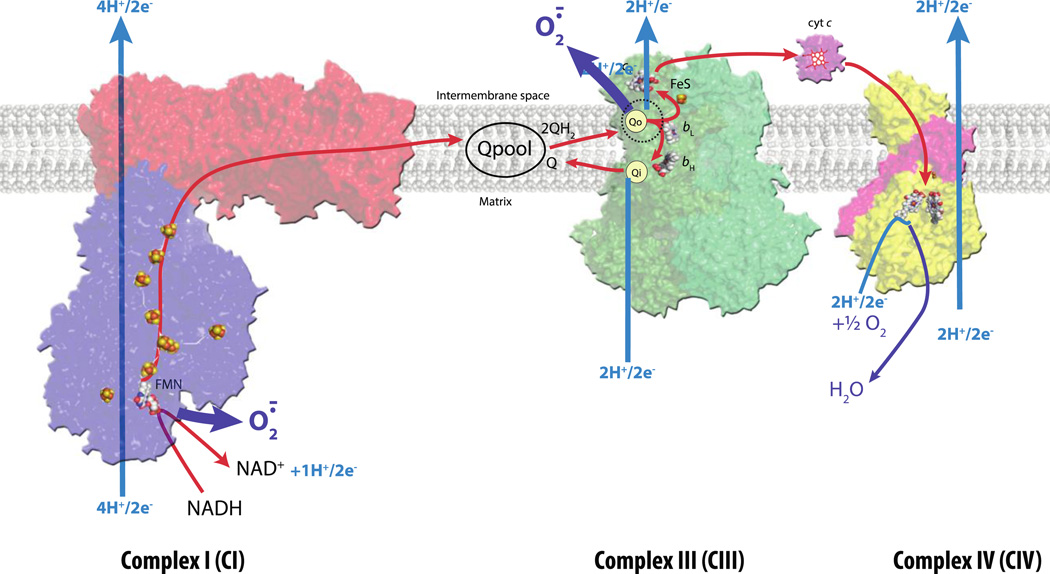
Figure 1. Structures of CI, CIII and CIV complexes of respiratory ETC and ROS production.
Overall structures of individual respiratory complexes CI (PDB 3IAM + 3M9C in red and blue for membrane integral and membrane external parts, respectively), CIII2 (PDB 1PP9 in shades of green for the two monomers) and CIV (PDB 1OCC in yellow) are shown together with the Q pool and the electron carrier cytochrome c in purple. In CIII2, the iron sulfur clusters are positioned in the stigmatellin-bound form and depicted as yellow and orange balls. All heme cofactors are shown in white, red, and blue balls, except in the case of the electron carrier cytochrome c where heme is shown in red. The Q pool (made of oxidized Q and reduced QH2) is indicated in black. The electron transfer and the proton uptake/release pathways are shown in red and blue arrows, respectively. The CI and CIII complexes of the mitochondrial respiratory ETC are generally considered to be the main sources of O2.− radicals, indicated with thick dark blue arrows. In CI, O2.− is thought to be produced primarily at the FMN (flavine mononucleotide) cofactor facing the matrix side, whereas in CIII it is generated at the ubiquinol oxidation site (Qo site) facing the inter membrane space of mitochondria.
1.2 Oxidized and Reduced Quinones
Quinone molecules (oxidized Q/reduced QH2) play a central role in O2.− generation because they act as direct electron donors/acceptors, or redox mediators, to reduce molecular oxygen (O2). They are present in energy transducing membranes in large amounts as compared with other components of the respiratory chain, and serve as intermediates during electron transfer reactions connecting together ETC complexes (Fig. 1). The redox chemistry of Q/QH2 is reversible, fast, and involves two consecutive “one-electron” reduction steps via a semiquinone radical (SQ.−) intermediate. While this property of Q/QH2 is important during energy transduction to store reducing equivalents, it also constitutes a liability for ROS generation, especially when Q/QH2 catalysis is not well confined to specific niches in related proteins. This process is often referred to as the ‘leakage of electrons’ from the ETC complexes [18]. In addition to their redox functions, QH2 molecules can also reduce O2 via a “one-electron” reduction step leading to the formation of O2.−, which is the main source of ROS in cells. However, direct oxidation of QH2 by O2 is slow and spin-forbidden, whereas the redox reactions between SQ.− and O2 ultimately yielding O2.− can be very fast [19]. These reactions strongly depend on how well SQ.− binds to its site of generation as well as the redox potential of the Q/SQ.− couple, thus on the stability constant and local factors modulating its disproportionation [20]. As O2 partitions favorably into the lipid phase, co-partitioning would allow O2 and SQ.− to react effectively unless the latter species is sequestered away from accessing O2. The local concentration and stability of SQ.− have been optimized during evolution in the case of the ETC complexes that perform Q/QH2 redox chemistry. It is also noteworthy that the reaction leading to O2.− production is reversible, which allows Q to be used as a protective agent against oxidative damages by consuming O2.− radicals [21, 22].
1.3 Production of ROS by ETC
Although still subject to controversy, it is believed that ROS are produced in the cell mainly during perturbations of respiratory chain functions [6]. Increased supply of electrons to ETC, (e. g., excess of reducing equivalents) or enhanced membrane potential generation lead to an increase of SQ.− content in membranes, and subsequently, to higher O2.− production. Moreover, kinetic constraints exerted downstream of the Q pool (e. g., blocking electron flow at the level of cytochrome c oxidase, CIV) further enhance production of SQ.−. Conversely, moderate uncoupling of the membrane potential decreases the electron flux constraints across ETC therefore descreasing O2.− production by the respiratory complexes (Fig. 1) [23]. Both of these effects can also be observed upon modification of the respiratory chain by specific chemicals [24, 25]. For example, free fatty acids exert different effects on mitochondria in respect to ROS generation: they inhibit electron flux through the complex I (CI), and probably the complex III (CIII), by inducing an increase of ROS production as seen in isolated rat heart or liver mitochondria [26]. Fatty acids also induce an uncoupling effect, which seems responsible for a large decrease in ROS formation. Thus, ROS production is fine-tuned in response to changes in electron flux through ETC.
The respiratory complexes CI and CIII were well known for some time to be involved directly in superoxide production (Fig. 1). More recently, complex II (CII) is implicated in this process as well [27, 28]. It has been suggested that about 2–5% of O2 consumed might lead to O2.− generation, of which roughly 70–80% is linked to the mechanism of function of CIII [29] based on quantitative data obtained using isolated mitochondria. However O2.− production measurements depend on the cell type, respiration steady-state, materials and techniques used to quantify the chemical free radicals. More conservative predictions estimate that O2.− production by ETC is perhaps only 0.1% of physiological respiratory rates [30, 31].
2. Complex III (CIII or cytochrome bc1)
2.1 Structure and mechanism of function of CIII
CIII is a major multi-subunit, membrane-bound enzyme that is central to respiratory energy transduction pathways in many organisms [32]. In different species, it enzymatically converts various derivatives of QH2 to Q, and reduces various mobile or membrane-anchored electron carriers, typically c-type cytochromes. CIII operates via the Q-cycle mechanism, and contributes to the formation of both the membrane potential and the proton gradient used for ATP production by ATP synthase (or complex V, CV) [33–35]. Depending on the species, mitochondrial CIII contains up to eleven subunits, of which eight are not essential for enzymatic activity (referred to as the “supernumerary subunits”). These subunits are absent in most bacterial CIII, which usually contain only three subunits as the essential catalytic core of the enzyme (Fig. 2a). Due to their structural simplicity and evolutionarily conserved sequence and structure, the facultative phototrophic bacteria (Rhodobacter capsulatus and Rhodobacter sphaeroides) and Paracoccus denitrificans are widely used organisms as CIII models for their mitochondrial counterparts [36–38]. The three universally conserved catalytic subunits of CIII are the cytochrome b, the Fe/S (also called the Rieske) protein and cytochrome c1. Cytochrome b is an integral membrane protein, whereas the Fe/S protein and cytochrome c1 are membrane-anchored by their amino- and carboxyl-terminal helices, respectively. These three subunits carry specific cofactors that are required for the catalytic activity of the enzyme. These cofactors are two b-type hemes (axially coordinated protophorphyrin IX-iron) with one low (bL) and one high (bH) Em of cytochrome b, the [2Fe-2S] cluster with a high redox midpoint potential (Em) of the Fe/S protein, and a high Em c-type heme (covalently bound protophorphyrin IX-iron) of cytochrome c1 (Fig. 2a). Most bacterial and mitochondrial purified CIII form dimers, and their three-dimensional structures depict these proteins as symmetrical homodimers [36–42]. In the case of R. capsulatus CIII, monomeric forms of the enzyme are neither active nor stable. However, tetrameric forms of bacterial CIII have been reported in at least two instances. In R. capsulatus, upon fusion of cytochrome c1 with its physiological electron carrier cytochrome cy, formation of active CIII tetramers was observed [43]. The P. denitrificans native enzyme forms tetramers, but elimination of a naturally present amino-terminal extension of cytochrome c1 was reported to yield dimeric CIII [36].
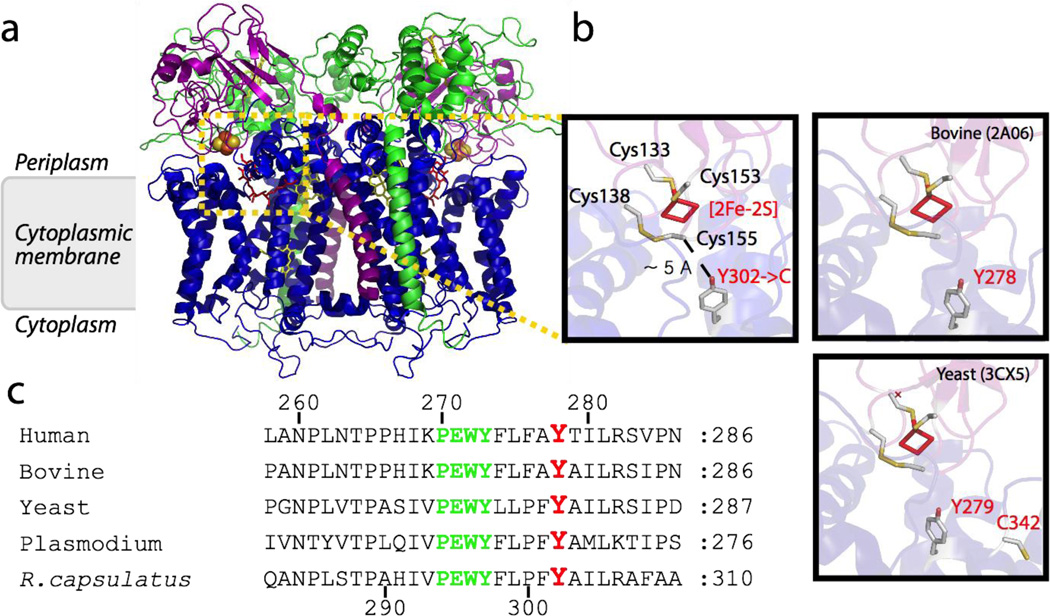
Figure 2. Structure of CIII (cytochrome bc1) and location of the conserved Tyr 302/278 of cytochrome b.
a, The three dimensional structure of bacterial dimeric CIII bound with stigmatellin that mimics QH2 (PDB 1ZRT) is shown. The catalytic subunits cytochrome b, cytochrome c1 and the Fe/S protein are shown in blue, green and purple, respectively. Hemes are shown as yellow sticks, the [2Fe-2S] cluster as yellow and orange spheres, and the Qo site inhibitor stigmatellin in red sticks. b, Close-up views of bacterial (R. capsulatus, PDB 1ZRT) and bovine (PDB 2A06) and yeast (PDB3CX5) mitochondrial CIII Qo site structures. The four conserved cysteine residues of the Fe/S protein (yellow and white sticks, R. capsulatus numbering Cys 133 and Cys 153 acting as ligands of the [2Fe-2S] cluster and Cys 138 and Cys 155 forming a disulfide bond) are shown together with the conserved Tyr 302/278/279 of cytochrome b substituted with Cys (gray stick, R. capsulatus Y302->C, bovine Y278C and yeast 279). In the yeast case, the C342 is also indicated c, Sequence alignment of R. capsulatus cytochrome b residues around the conserved tyrosine 302 (278 in human and bovine, 268 in Plasmodium and 279 in yeast) and the conserved PEWY motif shown in red, and green, respectively. Human, Bovine, Yeast, Plasmodium and R. capsulatus refer to Homo sapiens (NCBI protein database accession no: P00156), Bos taurus (P00157), Saccharomyces cerevisiae (P00163), Plasmodium yoelii (AAC25924), and Rhodobacter capsulatus (P08502) cytochrome bc1, respectively.
Each monomer of the bacterial enzyme contains the three catalytic subunits in an unusual organization. Cytochrome b with its eight transmembrane helices forms the membrane-embedded core to which the other two subunits are bound. Facing the lipid layer on cytochrome b, two Q/QH2 binding (QH2 oxidation (Qo) and Q reduction (Qi)) sites are located on the positive (p) and negative (n) sides of the membrane. The carboxyl-terminal helix of cytochrome c1 interacts closely with the fifth helix of cytochrome b to form a cytochrome b-c1 core, which interacts with the mobile head domain of the Fe/S protein, leaving its amino terminal membrane helix (i. e., tail) associated with cytochrome b of the other monomer. In agreement with this organization, a stable dimeric cytochrome b-c1 subcomplex has been purified from R. capsulatus. Reconstitution of this subcomplex into an active enzyme was achieved when a full-length Fe/S protein was used, but not with a truncated Fe/S protein lacking its amino-terminal tail domain [44]. The mobility of the Fe/S protein head domain between cytochrome b and cytochrome c1 and its [2Fe-2S] cluster is essential for CIII activity [45, 46].
According to the proton-motive Q-cycle mechanism, upon the diffusion of a QH2 molecule from the Q-pool to the Qo site of CIII, the oxidized [2Fe-2S] cluster of the Fe/S protein oxidizes this QH2 and conveys a single electron via its mobile head domain to oxidized cytochrome c1. This electron is then transferred down the ETC to a terminal oxidase (e. g., CIV). The highly unstable SQ.− radical thus produced at the Qo site gives an electron to heme bL of cytochrome b, which rapidly transfers it to heme bH across the lipid bilayer, to generate membrane potential and a stable SQ.− at the Qi site. Completion of the catalytic turnover of CIII involves a second QH2 oxidation at the Qo site of the dimeric CIII, via the same sequence of events described above, converting SQ.− at the Qi site to a QH2 to be released from the enzyme. The Qo and Qi sites of CIII are not identical with respect to their ability to interact with the SQ.− species. While the SQ.− at the Qi site is well characterized by EPR spectroscopy [47–49], SQ.− at the Qo site is a subject of controversy. It is difficult to detect the latter species experimentally, and it was only seen under specific conditions at extremely low amounts [50, 51]. Moreover, no structural information is yet available about the exact position of Q/QH2 at the Qo site. Thus, detailed descriptions of the events that follow QH2 oxidation by the [2Fe-2S] cluster of the Fe/S protein until the transfer of the second electron to heme bL of cytochrome b remain unknown.
2.2 Superoxide production at the Qo site
Importance of the structural integrity of bacterial CIII for maximal rate of catalysis and minimal rate of electron leakage to O2 is known. Heat-inactivated or proteinase K digested CIII [52], and catalytically impaired mutants producing higher amounts of O2.− [53, 54] have been reported. Bifurcated electron transfer from QH2 to the [2Fe-2S] cluster of the Fe/S protein and to the heme bL of cytochrome b at the Qo site infers that either preventing the formation of a SQ.−, or entrapping it within CIII to avoid its interaction with O2, should prevent O2.− production. Conditions favoring the generation of SQ.− should enhance O2.− production as analyzed in detail by Oscyzka et al., [18, 53, 54]. Two different situations leads to SQ.− generation: a semiforward electron pathway that produces a SQ.− following oxidation of QH2 by the Fe/S protein [20, 50, 51, 53–57], and a semireverse electron transfer pathway that involves electron transfer from reduced heme bL of cytochrome b to a Q bound at the Qo site to yield a SQ.−(referred to as “forward” and “reverse” for simplicity) (Fig. 1) [53, 54, 58, 59].
2.2.1 Forward electron transfer for SQ.− generation at the Qo site of CIII
Earlier studies focused mainly on the production of O2.− at the Qo site via the forward electron transfer pathway [20, 50, 51, 55–57]. Historically, the pioneering work of Chance proposed that the residual cytochrome c reduction activity seen when CIII is inhibited with antimycin A was closely associated with O2.− production [60, 61]. Accordingly, both electrons from QH2 oxidation would be transferred to the electron carrier cytochrome c, but via two disparate pathways. One electron would be delivered to cytochrome c via the high-potential chain (i. e., the Fe/S protein and cytochrome c1), while the other electron would be conveyed to O2 to yield O2.− which would rapidly oxidize cytochrome c. Later on, occurrence of this process was supported by the fact that chemical destruction of the [2Fe-2S] cluster of the Fe/S protein [62] or maintenance of this cluster in a reduced state [20] inhibited O2.− formation. Similarly, inhibiting reduction of Q at the Qi site (e. g., using antimycin A) significantly increased Qo site mediated O2.− production. Studies of the effects of specific Qo and Qi site inhibitors on O2.− production described the complementary bypass reactions in details [20]. For instance, decreasing the rate of electron transfer between the hemes bL and bH of cytochrome b, or abolishing the subsequent oxidation of these hemes via the Qi site inhibitor antimycin A, resulted in the accumulation of electrons on cytochrome b [18]. This led to the accumulation of SQ.− at the Qo site and to the leak of electrons to O2 to generate O2.−[20]. In general, if a SQ.− is formed at the Qo site (i. e., via a non concerted electron bifurcation) during the normal turn over of a native CIII, O2.− production is expected to be quite low to minimize electron leakage and energy waste. However, O2.− production at the Qo site might become significant under compromising conditions, such as a highly reduced Q pool, presence of antimycin A-like molecules inhibiting oxidation of reduced b hemes of cytochrome b, extremely high membrane potential, or specific Qo site mutations (see section 3). Such conditions may occur in damaged CIII enzymes, or under extreme physiological situations (e. g., ischemia and reperfusion) [63, 64].
Recently, a variant of the forward electron transfer pathway was proposed for R. sphaeroides CIII. In contrast to the earlier studies, this model postulated that under physiological conditions, O2.− production is not the result of a bypass reaction during the Q-cycle, but is a regulatory step for enhancing Qo site catalysis [52, 65]. The authors entertained the idea that O2 might act as a redox mediator during oxidation of QH2 and reduction of heme bL of cytochrome b. Accordingly, O2.− formation and CIII activity would increase together as a function of O2 concentration available during the assay conditions. However, the relevance and validity of the relatively mild effects (< 2 fold) observed on enzyme activity require additional investigations [65].
2.2.2 Reverse electron transfer for SQ.− generation at the Qo site of CIII
In recent years, several studies focused on the bypass reactions of the Q-cycle yielding O2.− production via a reverse electron transfer pathway. It appears that partial oxidation of the Q pool in a physiologically relevant scenario significantly increases the rates of O2.− production by antimycin A inhibited CIII [58]. Using submitochondrial particles, Dröse and Brandt observed that CIII mediated ROS production was higher when CII activity was partially inhibited by malonate (or oxaloacetate), linking the Q pool redox state to O2.− production via the Qo site of CIII. They proposed that the O2.− thus generated was produced at the Qo site by reverse electron transfer from reduced heme bL of cytochrome b to O2 via a SQ.− intermediate acting as a redox mediator. Quinlan et al (2011) further supported this proposal showing that this effect might be directly driven by the redox state of hemes bL and bH of cytochrome b that are sensitive to the Q pool redox state and membrane potential [59]. Additional studies using bacterial CIII mutants indicated that O2.− production at the Qo site also involved reverse electron transfer from reduced heme bL of cytochrome b, and Osyczka’s group proposed a “kinetic” mechanism to account for its occurrence [54]. Accordingly, the movement of the Fe/S protein [2Fe-2S] cluster from the Qo site increased O2.− generation, whereas its stagnation at the Qo site decreased it. This observation correlated the production of ROS with the position of the Fe/S protein head domain on cytochrome b. Assuming that the movement of the reduced Fe/S protein is not obligatorily “concerted” with electron transfer from SQ.− to cytochrome bL heme, ROS generation could be rationalized as the result of a kinetic competition between the internal reactions involving the cofactors of CIII, the Q residing at the Qo site, and the reaction of SQ.− with O2 [54].
3. Defective CIII catalysis and enhanced ROS production due to specific mutations
3.1 Bacterial CIII mutations and ROS production
Studies using bacterial CIII also highlighted some specific amino acid residues as key contributors for affecting ROS production. For instance, the M183K or M183L substitutions in R. capsulatus cytochrome c1 drastically decreased the Em of heme c1,severely impeding electron flow kinetics through the high potential chain of CIII, and enhancing O2.− production during Q0 site catalysis [54]. In a recent study, Lee et al (2011) described a different role played by some amino acid residues of cytochrome b in controlling O2.− production via the Qo site of the bacterial CIII [66] (Fig. 2b). In R. capsulatus, substitution of the conserved Y302 of cytochrome b with any other amino acid residue decreased CIII activity. Concomitantly, it increased O2.− production independently of antimycin A inhibition or other treatments known to enhance this process [66]. These findings indicated that some cytochrome b residues are critical for suppressing ROS production at the Qo site of the enzyme. Various structures have depicted this tyrosine side chain in slightly different H-bonding patterns, depending on the position of the Fe/S protein head domain and the occupant of the Qo site [67]. Moreover, the hydroxyl group of this residue is within H-bonding distance from a cluster of H2O molecules in a high resolution structure [68]. Although it is unclear how Y302X (X being any amino acid) mutations enhance mechanistically O2.− production, the finding that even the Y302F substitution increases O2.− production suggests that it may be linked to the loss of the fixed H2O cluster coordinated in the native enzyme by the hydroxyl group of Y302 [66]. Accordingly, any mutant losing the hydroxyl group would exhibit decreased CIII activity due to the incorrect positioning of the Fe/S protein head domain. Concomitantly, it would also produce increased O2.− due to the uncoordinated mobility of the Fe/S protein head domain vis-a-vis the electron transfer from SQ.− to cytochrome bL, and the ensuing undesirable electron leakage to O2 during Qo site catalysis.
The counterparts of R. capsulatus Y302 in other species, in particular the malarial (Y268) [69, 70], yeast (Y279) [71–73] and human (Y278C) mitochondrial mutants [74, 75] were also studied with respect to decreased CIII catalysis and enhanced ROS production,. Decreased CIII activities were reported for all mutants, and enhanced O2.− production was described for several yeast and human mutants (see below). The overall findings indicate that a number of amino acid residues of cytochrome b at the Qo site affect both OH catalysis and ROS production. Whether or not all catalytically defective Qo site mutants always produce enhanced ROS, as a general property of the CIII enzyme, is unknown.
3.2 Bacterial cytochrome b Y302C mutation forms an inter subunit disulfide bond
The bacterial mutant carrying the cytochrome b Y302C mutation was studied in detail [66]. This mutant supported CHI-dependent anoxygenic photo synthetic growth of R. capsulatus. However, it progressively lost its CIII activity upon exposure to air due to slow oxidative disintegration of the [2Fe-2S] cluster in its Fe/S protein both in chromatophore membranes as well as in purified samples [66]. On the other hand, although the homologous yeast cytochrome b Y279C mutant also produced ROS [73], its Fe/S protein [2Fe-2S] cluster did not exhibit oxidative damage [71, 73]. In the case of R. capsulatus, oxidative disintegration of the [2Fe-2S] cluster required not only the presence of O2, but also the catalytic activity of the Qo site and the presence of a free thiol group at position 302. Strict anaerobiosis, highly reducing conditions, as well as use of Qo site inhibitor stigmatellin or thiol-alkylating reagents (e. g., iodoacetamide or N-ethyl-maleimide) abolished the oxidative damage in the Y302C mutant [66]. Using the bacterial mutant CIII, mass spectrometry analyses revealed for the first time that the mutant cytochrome b and the Fe/S protein subunits of CIII were covalently cross-linked to each other by an inter subunit disulfide bond formed between the thiol groups of cytochrome b Y302C and the Fe/S protein C155. It was therefore proposed that the ROS-induced cysteine redox chemistry reduced the intra molecular disulfide bridge, which is naturally present in the Fe/S protein and stabilizes its [2Fe-2S], to render this cluster oxygen labile and the mutant CIII air-sensitive (Fig. 2b) [66].
The striking difference seen in respect to the stability of their Fe/S protein [2Fe-2S] clusters between the bacterial Y302C and its yeast counterpart Y279C is intriguing. Comparison of R. capsulatus and S. cerevisiae cytochrome b amino acid sequences show that while the yeast protein has several cysteine residues, the bacterial counterpart has none. In the former species, one of these cysteine residues (C342, yeast numbering) is structurally located nearby the Y279 (Fig. 2b). Whether the presence of additional cysteine residues counteracts the effect of Y279C mutation (for example by promoting an intra molecular disulfide bond within cytochrome b) is unknown. Mass spectrometry analyses of purified native, Y279F and Y279C yeast CIII enzymes were conducted in our group. In the case of Y279C mutant, the data indicated that the trypsin-gluC fragment encompassing Y279 (W273YLLPFX279AILR283, where X279 is Y, F or C in native, Y279F and Y279C mutants, respectively) is only detectable after dithiothreitol (DTT) reduction and iodoacetamide alkylation (unpublished data). This finding suggests that in the yeast mutant the cysteine residue at position 279 might also be modified by a DTT-cleavable chemical group of unknown identity. Inspection of the bovine (Fig. 2b) (and also human) cytochrome b sequence indicates that, although it also contains several cysteine residues, none of them is structurally located in the vicinity of Y278 (homologue of R. capsulatus Y302). Whether the oxidative disintegration of the Fe/S protein [2Fe-2S] observed with the bacterial CIII also occurs in mammalian CIII remains to be seen.
3.3 Human mitochondrial cytochrome b Y278C mutation and ROS production
Very recently, a human mitochondrial CIII produced by a homoplasmic cybrid line generated using fibroblasts of a patient bearing the m.l5579A>G (p.Y278C, i.e., the human homologue of R. capsulatus cytochrome b Y302C mutation) (Fig. 2c) heteroplasmic mutation became available [74, 75]. This mutation was identified in a patient with severe exercise intolerance and multisystem disorders [76], and provided a unique opportunity to extend the significance of the findings emanating from the bacterial case. Comparison of appropriate homoplasmic cybrids carrying either the wild type or the cytochrome b Y278C mutation showed increased intolerance to galactose (hallmark of defective oxidative phosphorylation), drastic loss of CIII activity (over ~ 90%) and highly decreased CHI-dependent oxidative phosphorylation in the mutant [75]. However, the enzymatic activities of the individual CI and CIII complexes, as well as the coupled activities of the CI+III and CII+III supercomplexes were little affected in the mutant. Moreover, no complete loss of the ETC driven membrane potential, or ATP synthesis was observed, suggesting that CI and CII were able to sustain some ATP production despite the low CIII activity. Excitingly, as observed with the bacterial CIII, enhanced O2.− production was seen in mitochondria isolated from mutant cybrids, compared to wild type cells. Moreover, an imbalance in homeostasis of the major intracellular antioxidant homeostasis, i. e., an increase of the ratio of oxidized (GSSG) versus reduced (GSH) glutathione ratio was observed, in agreement with increased oxidative stress in mutant cybrids [75]. Indeed, the CI, CIII and CI+III activities increased significantly when mitochondrial preparations were carried out in the presence of DTT. Due to material limitations, reliable detection of the Fe/S protein [2Fe-2S] cluster by EPR spectroscopy has not yet been achieved even with mitochondria from wild type human cybrids, leaving open the question of oxidative disintegration of the Fe/S protein [2Fe-2S] cluster via ROS production.
Similar to the bacterial case, no subunit assembly defect of CIII was seen with the human cytochrome b Y278C mutation as compared to wild type cybrids, based on SDS-PAGE/immunoblots. Wild type and mutant mitochondria contained similar amounts of CIII as revealed by BN-PAGE of dodecylmaltoside dispersed mitoplasts. However, BN-PAGE analyses of digitonin dispersed mitochondrial respirasomes showed decreased amounts of CIII dimers and CIII+IV supercomplexes, but slightly compensatory increased levels of CI1III2IVn (n = number of monomers) supercomplexes [75]. The overall data suggest that supra molecular interactions between the respiratory complexes are important for maintaining basal respiratory ETC function in the Y278C mutant. An emerging hypothesis from this ongoing work is that CIII activity might be better protected against oxidative damages when the mutant CIII is part of the CI1III2IVn supercomplexes (Fig. 3) [77]. Thus, comparative studies conducted for the first time with bacterial and human mitochondrial CIII bearing the same homologous mutation (Y302C and Y278C, respectively) would suggest a new protective role for supra molecular organization of respiratory complexes in membranes.
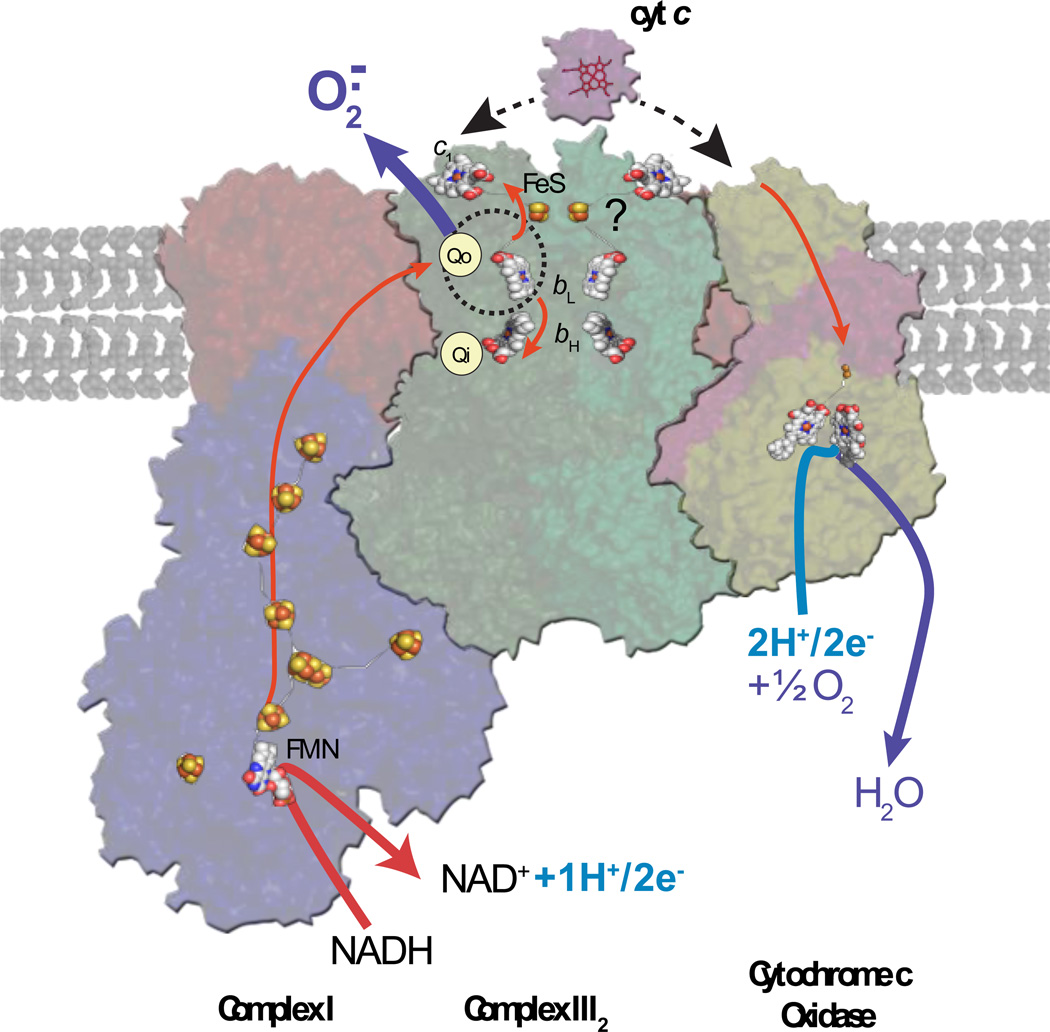
Figure 3. Organization of ETC complexes into supercomplexes.
A hypothetical higher level of organization of the respiratory complexes CI, CIII and CIV, modified from the recent singleparticle electron cryo-microscopy mediated structure (PDB 2YBB) of the supercomplex CI1CIII2CIV1 from bovine CIII and CIV and bacterial CI [77], is shown. The higher-level structural organization into larger macromolecular entities is assumed to provide structural integrity to the individual ETC complexes by maximizing inter-complex electron flux, and minimizing possible oxidative damages associated with electron leakages. A plausible protective effect exerted at the Qo site of CIII via its close association with CI is depicted for illustrative purposes. All of the other features shown are as described in the legend of Figure 1.
4. Perspectives
Based on structural, biochemical and clinical studies, an integrated model for ROS production in ETC, involving CI, CII and CIII complexes with their higher order of organizations and physiological regulations is emerging [54, 78]. In the case of the Qo site of CIII, factors such as the membrane potential, availability of oxidized and reduced equivalents (Q and cytochrome c), redox state of the enzyme, and presence of critical amino acid residues located at specific locations, together control the rate and the amount of ROS production. As described here, recently initiated comparative studies of bacterial and human mitochondrial CIII suggest a new role for supra molecular organizations of the ETC complexes in membranes. Formation of respirasomes appears to improve not only the substrate/product channeling between the related enzymes [79], but might also endow them with higher degrees of stability and protection against oxidative damages by restricting ROS generation (Fig. 3) [80].
Current challenges lay in the development of new tools to improve specific detection and differentiation of various types of ROS free radicals (e. g., O2.−, .OH, .H) at very low concentrations, especially when using integrated systems like respirasomes, mitochondria or whole cells, to better define their role(s) in both signal transduction and oxidative stress. Undoubtedly, future studies will better address whether the organization into larger macromolecular entities protects the structural integrity of native and mutant ETC complexes, and minimizes oxidative damages associated with electron leakages. Emerging investigations of novel proteins promoting formation of supercomplexes [81–83], and determination of ROS-mediated damages on surrounding phospholipids promise to be very informative. The lipochaperone cardiolipin [84] (and also ornithine lipid in some species [85]) is already implicated in affecting the catalytic activity, formation, stability and reconstitution of supra molecular organization of the ETC complexes [86]. Lipidomic analyses using mass spectrometry will allow accurate evaluation of subtle changes in lipid profiles of intact mitochondria, isolated complexes and supercomplexes [84]. Hopefully, these studies will merge together to pave the way towards the development of novel and specific therapeutic interventions for patients with mitochondrial CIII related diseases.
Highlights.
Bacterial CIII cytochrome b Y302C mutation reduces enzyme activity and induces ROS.
Homologous human mitochondrial CIII cytochrome b Y278C mutation behaves similarly.
Supercomplex formation between CI, CIII and CIV might protect against oxidative damages.
Comparison of homologous bacterial and human mitochondrial mutations are informative.
Acknowledgment
This work was supported by NIH grant GM 38237 to FD and MR.
The authors thank Dr. B. Meunier for kindly providing the S. cerevisiae cytochrome b Y279C mutant, and Drs. H. De Bari and E. Berry for providing the purified samples of S. cerevisiae native CIII and its cytochrome b Y278F and Y278C mutant derivatives.
Abbreviations
- ROS
reactive oxygen species
- ETC
electron transport chain
- O2.−
superoxide radical
- .OH
hydroxyl radical
- SQ.−
semiquinone radical
- H2O2
hydrogen peroxide
- CI, CII, CIII, CIV and CV
respiratory complex I, complex II, complex III (cytochrome bc 1), complex IV and complex V, respectively
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovascular Research. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Isaeva EV, Shkryl VM, Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. The Journal of Physiology. 2005;565:855–872. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovascular Research. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 4.Dröge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 5.Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxidants & Redox signaling. 2004;6:449–469. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- 6.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Molecular cell. 2010;40:323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S-S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. AJP - Cell Physiology. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 8.Dypbukt JM, Ankarcrona M, Burkitt M, Sjöholm A, Ström K, Orrenius S, Nicotera P. Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulinsecreting RINm5F cells. The role of intracellular polyamines. The Journal of biological chemistry. 1994;269:30553–30560. [PubMed] [Google Scholar]
- 9.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nature Medicine. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 10.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. Journal of Hypertension. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 11.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Ann Rev Pharmacology and Toxicology. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 12.Beckman KB, Ames BN. The free radical theory of aging matures. Physiological Reviews. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 13.Wallace DC. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Foundation Symposium. 2001;235:247–246. doi: 10.1002/0470868694.ch20. [DOI] [PubMed] [Google Scholar]
- 14.Romashko DN, Marban EO, Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci USA. 1998;95:1618–1623. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochimica et biophysica acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. The Journal of Physiology. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vercesi AE, Kowaltowski AJ, Grijalba MT, Meinicke AR, Castilho RF. The role of reactive oxygen species in mitochondrial permeability transition. Biosci Rep. 1997;17:43–52. doi: 10.1023/a:1027335217774. [DOI] [PubMed] [Google Scholar]
- 18.Osyczka A, Moser CC, Daldal F, Dutton PL. Reversible redox energy coupling in electron transfer chains. Nature. 2004;427:607–612. doi: 10.1038/nature02242. [DOI] [PubMed] [Google Scholar]
- 19.Samoilova RI, Crofts AR, Dikanov SA. Reaction of superoxide radical with quinone molecules. J Phys Chem. 2011;115:11589–11593. doi: 10.1021/jp204891n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller F, Crofts AR, Kramer DM. Multiple Q-cycle bypass reactions at the Qo site of the cytochrome bc 1 complex. Biochemistry. 2002;41:7866–7874. doi: 10.1021/bi025581e. [DOI] [PubMed] [Google Scholar]
- 21.Fosslien E. Fosslien, Mitochondrial medicine--molecular pathology of defective oxidative phosphorylation. Annals of clinical and laboratory science. 2001;31:25–67. [PubMed] [Google Scholar]
- 22.Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacology & Therapeutics. 2009;124:259–268. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Skulachev VPV. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta - Bioenergetics. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 24.Boveris AA, Boveris AA. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boveris A, Cadenas E, Stoppani AO. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schönfeld P, Wojtczak L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochimica et biophysica acta. 2007;1767:1032–1040. doi: 10.1016/j.bbabio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. The Journal of biological chemistry. 2012;287:27255–27264. doi: 10.1074/jbc.M112.374629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iverson TM, Maklashina E, Cecchini G. Structural Basis for Malfunction in Complex II. The Journal of biological chemistry. 2012;287:35430–35438. doi: 10.1074/jbc.R112.408419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chance BB, Hollunger GG. Energy-linked reduction of mitochondrial pyridine nucleotide. Nature. 1960;185:666–672. doi: 10.1038/185666a0. [DOI] [PubMed] [Google Scholar]
- 30.Loschen G, Flohé L, Chance B. Respiratory chain linked H2O2 production in pigeon heart mitochondria. FEBS Letters. 1971;18:261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 31.Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. Journal of bioenergetics and biomembranes. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- 32.Berry EA, Guergova-Kuras M, Huang LS, Crofts AR. Structure and function of cytochrome bc complexes. Annual review of biochemistry. 2000;69:1005–1075. doi: 10.1146/annurev.biochem.69.1.1005. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976;62:327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- 34.Crofts AR, Meinhardt SW, Jones KR, Snozzi M. The role of the quinone pool in the cyclic electron transfer chain of Rhodopseudomonas sphaeroides: a modified Q-cycle mechanism. Biochimica et biophysica acta. 1983;723:202–218. doi: 10.1016/0005-2728(83)90120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc 1 complex. The Journal of biological chemistry. 1990;265:11409–11412. [PubMed] [Google Scholar]
- 36.Kleinschroth T, Castellani M, Trinh CH, Morgner N, Brutschy B, Ludwig B, Hunte C. X-ray structure of the dimeric cytochrome bc 1 complex from the soil bacterium Paracoccus denitrificans at 2.7-Å resolution. Biochimica et biophysica acta. 2011;1807:1606–1615. doi: 10.1016/j.bbabio.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Berry EA, Huang L-S, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-Ray structure of Rhodobacter capsulatus cytochrome bc 1 Comparison with its mitochondrial and chloroplast counterparts. Photosynthesis research. 2004;81:251–275. doi: 10.1023/B:PRES.0000036888.18223.0e. [DOI] [PubMed] [Google Scholar]
- 38.Esser L, Gong X, Yang S, Yu L, Yu CA, Xia D. Surface-modulated motion switch: capture and release of iron-sulfur protein in the cytochrome bc 1 complex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13045–13050. doi: 10.1073/pnas.0601149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH. Electron transfer by domain movement in cytochrome bc 1 . Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 40.Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc 1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- 41.Iwata S, Lee J, Okada K, Lee J, Iwata M, Rasmussen B, Link T, Ramaswamy S, Jap B. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc 1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 42.Hunte C, Koepke J, Lange C, Rossmanith T, Michel H. Structure at 2.3 A resolution of the cytochrome bc 1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure. 2000;8:669–684. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 43.Lee DW, Ozturk Y, Osyczka A, Cooley JW, Daldal F. Cytochrome bc 1 c y fusion complexes reveal the distance constraints for functional electron transfer between photosynthesis components. The Journal of biological chemistry. 2008;283:13973–13982. doi: 10.1074/jbc.M800091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valkova-Valchanova MB, Saribas AS, Gibney BR, Dutton PL, Daldal F. Isolation and characterization of a two-subunit cytochrome b-c1 subcomplex from Rhodobacter capsulatus and reconstitution of its ubihydroquinone oxidation (Qo) site with purified Fe-S protein subunit. Biochemistry. 1998;37:16242–16251. doi: 10.1021/bi981651z. [DOI] [PubMed] [Google Scholar]
- 45.Darrouzet E, Valkova-Valchanova M, Moser CC, Dutton PL, Daldal F. Uncovering the [2Fe-2S] domain movement in cytochrome bc 1 and its implications for energy conversion. Proc Natl Acad Sci USA. 2000;97:4567–4572. doi: 10.1073/pnas.97.9.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia D, Esser L, Tang WK, Zhou F, Zhou Y, Yu L, Yu CA. Structural analysis of cytochrome bc 1 complexes: Implications to the mechanism of function. Biochimica et biophysica acta. 2012 doi: 10.1016/j.bbabio.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohnishi T, Trumpower B. Differential effects of antimycin on ubisemiquinone bound in different environments in isolated succinate : cytochrome c reductase complex. The Journal of biological chemistry. 1980;255:3278–3284. [PubMed] [Google Scholar]
- 48.de Vries S, Berden JA, Slater EC. Properties of a semiquinone anion located in the QH2:cytochrome c oxidoreductase segment of the mitochondrial respiratory chain. FEBS Letters. 1980;122:143–148. doi: 10.1016/0014-5793(80)80422-4. [DOI] [PubMed] [Google Scholar]
- 49.Robertson DE, Prince RC, Bowyer JR, Matsuura K, Dutton PL, Ohnishi T. Thermodynamic properties of the semiquinone and its binding site in the ubiquinol-cytochrome c (c2) oxidoreductase of respiratory and photosynthetic systems. The Journal of biological chemistry. 1984;259:1758–1763. [PubMed] [Google Scholar]
- 50.Zhang H, Osyczka A, Dutton PL, Moser CC. Exposing the complex III Qo semiquinone radical. Biochimica et biophysica acta. 2007;1767:883–887. doi: 10.1016/j.bbabio.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cape JL, Bowman MK, Kramer DM. A semiquinone intermediate generated at the Qo site of the cytochrome bc 1 complex: importance for the Q-cycle and superoxide production. Proc Natl Acad Sci USA. 2007;104:7887–7892. doi: 10.1073/pnas.0702621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin Y, Yang S, Yu L, Yu C-A. Reaction mechanism of superoxide generation during ubiquinol oxidation by the cytochrome bc 1 complex. The Journal of biological chemistry. 2010;285:17038–17045. doi: 10.1074/jbc.M110.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borek A, Sarewicz M, Osyczka A. Movement of the iron-sulfur head domain of cytochrome bc 1 transiently opens the catalytic Qo site for reaction with oxygen. Biochemistry. 2008;47:12365–12370. doi: 10.1021/bi801207f. [DOI] [PubMed] [Google Scholar]
- 54.Sarewicz M, Borek A, Cieluch E, Swierczek M, Osyczka A. Discrimination between two possible reaction sequences that create potential risk of generation of deleterious radicals by cytochrome bc 1. Implications for the mechanism of superoxide production. Biochim Biophys Acta - Bioenergetics. 2010;1797:1820–1827. doi: 10.1016/j.bbabio.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kramer DM, Roberts AG, Muller F, Cape J, Bowman MK. Q-cycle bypass reactions at the Qo site of the cytochrome bc 1 (and related) complexes. Methods in Enzym. 2004;382:21–45. doi: 10.1016/S0076-6879(04)82002-0. [DOI] [PubMed] [Google Scholar]
- 56.Cape JL, Strahan JR, Lenaeus MJ, Yuknis BA, Le TT, Shepherd JN, Bowman MK, Kramer DM. The respiratory substrate rhodoquinol induces Q-cycle bypass reactions in the yeast cytochrome bc 1 complex: mechanistic and physiological implications. The Journal of biological chemistry. 2005;280:34654–34660. doi: 10.1074/jbc.M507616200. [DOI] [PubMed] [Google Scholar]
- 57.Forquer I, Covian R, Bowman MK, Trumpower BL, Kramer DM. Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc 1 complex. The Journal of biological chemistry. 2006;281:38459–38465. doi: 10.1074/jbc.M605119200. [DOI] [PubMed] [Google Scholar]
- 58.Dröse S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc 1 complex. The Journal of biological chemistry. 2008;283:21649–21654. doi: 10.1074/jbc.M803236200. [DOI] [PubMed] [Google Scholar]
- 59.Quinlan CL, Gerencser AA, Treberg JR, Brand MD. The Mechanism of Superoxide Production by the Antimycin-inhibited Mitochondrial Q-cycle. The Journal of biological chemistry. 2011;286:31361–31372. doi: 10.1074/jbc.M111.267898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiological Reviews. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 61.Boveris A, Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Letters. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 62.Slater EC, de Vries S. Identification of the BAL-labile factor. Nature. 1980;288:717–718. doi: 10.1038/288717a0. [DOI] [PubMed] [Google Scholar]
- 63.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Ann Rev Pharmacology and Toxicology. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 64.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. AJP - Cell Physiology. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 65.Zhou F, Yin Y, Su T, Yu L, Yu C-A. Oxygen dependent electron transfer in the cytochrome bc 1 complex. Biochim Biophys Acta - Bioenergetics. 2012;1817:2103–2109. doi: 10.1016/j.bbabio.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Lee D-W, Selamoglu N, lanciano p, Cooley JW, Forquer I, Kramer DM, Daldal F. Loss of a conserved tyrosine residue of cytochrome b induces reactive oxygen species production by cytochrome bc 1 . The Journal of biological chemistry. 2011;286:18139–18148. doi: 10.1074/jbc.M110.214460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berry EA, Huang LS. Conformationally linked interaction in the cytochrome bc 1 complex between inhibitors of the Qo site and the Rieske iron-sulfur protein. Biochimica et biophysica acta. 2011;1807:1349–1363. doi: 10.1016/j.bbabio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Solmaz S, Hunte C. Structure of complex III with bound cytochrome c in reduced state and definition of a minimal core interface for electron transfer. The Journal of biological chemistry. 2008;283:17542–17549. doi: 10.1074/jbc.M710126200. [DOI] [PubMed] [Google Scholar]
- 69.Mather MW, Darrouzet E, Valkova-Valchanova M, Cooley JW, McIntosh MT, Daldal F, Vaidya AB. Uncovering the molecular mode of action of the antimalarial drug atovaquone using a bacterial system. The Journal of biological chemistry. 2005;280:27458–27465. doi: 10.1074/jbc.M502319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fisher N, Abd Majid R, Antoine T, Al-Helal M, Warman AJ, Johnson DJ, Lawrenson AS, Ranson H, O'Neill PM, Ward SA, Biagini GA. Cytochrome b mutation Y268S conferring the atovaquone resistance phenotype in the malaria parasite results in reduced parasite bc 1 catalytic turnover and protein expression. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M111.324319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher N, Castleden CK, Bourges I, Brasseur G, Dujardin G, Meunier B. Human disease-related mutations in cytochrome b studied in yeast. The Journal of biological chemistry. 2004;279:12951–12958. doi: 10.1074/jbc.M313866200. [DOI] [PubMed] [Google Scholar]
- 72.Wenz T, Hellwig P, MacMillan F, Meunier B, Hunte C. Probing the role of E272 in quinol oxidation of mitochondrial complex III. Biochemistry. 2006;45:9042–9052. doi: 10.1021/bi060280g. [DOI] [PubMed] [Google Scholar]
- 73.Wenz T, Covian R, Hellwig P, Macmillan F, Meunier B, Trumpower BL, Hunte C. Mutational analysis of cytochrome b at the ubiquinol oxidation site of yeast complex III. The Journal of biological chemistry. 2007;282:3977–3988. doi: 10.1074/jbc.M606482200. [DOI] [PubMed] [Google Scholar]
- 74.Ghelli A, Tropeano CV, Calvaruso MA, Marchesini A, Iommarini L, Porcelli AM, Zanna C, Gasparre G, Kurelac I, De Nardo V, Martinuzzi A, Vissing J, Selamoglu N, Daldal F, Rugolo M. Alterations in the supramolecular interactions of respiratory chain complexes and enhanced superoxide production by the cytochrome b Y278C mutation which causes a multisystem disorder. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1817. 2012;(Supplement):S139. [Google Scholar]
- 75.Ghelli A, Tropeano CV, Calvaruso MA, Marchesini A, Iommarini L, Porcelli AM, Zanna C, De Nardo V, Martinuzzi A, Wimbrand F, Vissing J, Kurelac I, Gasparre G, Selamoglu N, Daldal F, Rugolo M. The cytochrome b p.278Y>C mutation causative of a multisystem disorder enhances superoxide production and alters supramolecular interactions of respiratory chain complexes. Human Molecular Genetics. 2013 doi: 10.1093/hmg/ddt067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wibrand F, Ravn K, Schwartz M, Rosenberg T, Horn N, Vissing J. Multisystem disorder associated with a missense mutation in the mitochondrial cytochrome b gene. Annals of Neurology. 2001;50:540–543. doi: 10.1002/ana.1224. [DOI] [PubMed] [Google Scholar]
- 77.Althoff T, Mills DJ, Popot JL, Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. The EMBO journal. 2011;30:4652–4664. doi: 10.1038/emboj.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dröse S, Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Advances in experimental medicine and biology. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 79.Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, Del Sole M, Faccioli M, Falasca A, Fato R, Genova ML, Sgarbi G, Solaini G. Mitochondrial respiratory chain supercomplex I-III in physiology and pathology. Biochimica et biophysica acta. 2010;1797:633–640. doi: 10.1016/j.bbabio.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 80.Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- 81.Strogolova V, Furness A, Robb-McGrath M, Garlich J, Stuart RA. Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc 1-cytochrome c oxidase supercomplex. Molecular and cellular biology. 2012;32:1363–1373. doi: 10.1128/MCB.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen YC, Taylor EB, Dephoure N, Heo JM, Tonhato A, Papandreou I, Nath N, Denko NC, Gygi SP, Rutter J. Identification of a protein mediating respiratory supercomplex stability. Cell metabolism. 2012;15:348–360. doi: 10.1016/j.cmet.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vukotic M, Oeljeklaus S, Wiese S, Vogtle FN, Meisinger C, Meyer HE, Zieseniss A, Katschinski DM, Jans DC, Jakobs S, Warscheid B, Rehling P, Deckers M. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell metabolism. 2012;15:336–347. doi: 10.1016/j.cmet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Wenz T, Hielscher R, Hellwig P, Schagger H, Richers S, Hunte C. Role of phospholipids in respiratory cytochrome bc 1 complex catalysis and supercomplex formation. Biochimica et biophysica acta. 2009;1787:609–616. doi: 10.1016/j.bbabio.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 85.Aygun-Sunar S, Mandaci S, Koch HG, Murray IV, Goldfine H, Daldal F. Ornithine lipid is required for optimal steady-state amounts of c-type cytochromes in Rhodobacter capsulatus . Molecular microbiology. 2006;61:418–435. doi: 10.1111/j.1365-2958.2006.05253.x. [DOI] [PubMed] [Google Scholar]
- 86.Bazan S, Mileykovskaya E, Mallampalli VK, Heacock P, Sparagna GC, Dowhan W. Cardiolipin-dependent reconstitution of respiratory supercomplexes from purified Saccharomyces cerevisiae complexes III and IV. The Journal of biological chemistry. 2013;288:401–411. doi: 10.1074/jbc.M112.425876. [DOI] [PMC free article] [PubMed] [Google Scholar]