Abstract
Objectives
To evaluate the effects of endurance exercise training (ET) on endothelial dependent flow-mediated arterial dilation (FMD) and carotid artery stiffness and their potential contributions to the training-related increase in peak exercise oxygen consumption (VO2) in older patients with heart failure with preserved ejection fraction (HFPEF).
Background
Elderly HFFEF patients have severely reduced peak VO2 which improves with ET, however the mechanisms of this improvement are unclear. FMD and arterial distensibility are critical components of the exercise response and are reduced with aging. However, it's unknown whether these improve with ET in elderly HFPEF or contribute to the training-related improvement in peak VO2.
Methods
63 HFPEF patients (70±7 years) were randomized to 16 weeks of ET (walking, arm and leg ergometry, n=32) or attention control (CT; n=31). Peak VO2, brachial artery FMD in response to cuff ischemia, carotid artery distensibility by high-resolution ultrasound, LV function, and QOL were measured at baseline and follow-up.
Results
ET increased peak VO2 (ET: 15.8±3.3 vs. CT: 13.8±3.1 ml/kg/min, p=0.0001) and QOL. However, brachial artery FMD (ET: 3.8±3.0% vs. CT: 4.3±3.5%, p=0.88), and carotid arterial distensibility (ET: 0.97±0.56 vs. CT: 1.07±0.34 × 10-3mm × mmHg-1 p=0.65) were unchanged. Resting LV systolic and diastolic function were unchanged by ET.
Conclusions
In elderly HFPEF patients, 16 weeks of ET improved peak VO2 without altering endothelial function or arterial stiffness. This suggests that other mechanisms, such as enhanced skeletal muscle perfusion and / or oxygen utilization, may be responsible for the ET-mediated increase in peak VO2 in older HFPEF patients.
Keywords: Heart Failure, Preserved ejection fraction, exercise, aging
INTRODUCTION
Approximately 50% of older heart failure (HF) patients have preserved left ventricular ejection fraction (HFPEF)1,2 and their primary symptom is severe exercise intolerance, measured objectively as reduced peak exercise oxygen uptake (VO2peak)3-9. Reduced peak and reserve arteriovenous oxygen difference (A-VO2Diff) are important contributors to the reduced VO2peak in HFPEF5,8. Furthermore, improved A-VO2Diff accounts for most of the increase in VO2peak following endurance exercise training (ET)10. This suggests roles for impaired arterial and/or skeletal muscle function in the severe exercise intolerance in HFPEF and its improvement with ET11.
In HFPEF, arterial stiffness is increased and endothelial function may be decreased, and both may contribute to exercise intolerance6,7,12-15. In HF with reduced ejection fraction (HFREF), ET improves endothelial function and arterial compliance16-22 and the former is correlated with the ET-related improvement in VO2peak18,23. Therefore, we performed a randomized, controlled, single-blind trial to test the hypothesis that ET would enhance endothelial function and arterial distensibility in HFPEF and that these would be important contributors to the improvement in VO2peak.
METHODS
Study Protocol
The study was approved by the Wake Forest School of Medicine Institutional Review Board for Protection of Human Subjects and registered (NCT01113840). During the screening visit, informed consent was obtained and participants were familiarized with the testing environment and procedures. During a single subsequent visit, all outcome measures were obtained. Subjects were then randomized to 16 weeks of ET or attention control (CT). Exercise performance, echocardiography, and quality-of-life (QOL) were assessed at baseline and following the 16-week intervention. Brachial artery flow mediated arterial dilation (FMD) and carotid arterial distensibility were measured at baseline, 8 and 16 weeks. Testing was performed and results were analyzed by individuals blinded to patient group. Baseline characteristics have been reported15,24.
Subjects
HFPEF patients were identified from fortified search lists4,5,10,15,25,26 and had symptoms and signs of HF defined by NHANES HF score ≥3 and the criteria of Rich et al27, LV ejection fraction ≥50%, no segmental wall motion abnormalities, and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ symptoms4,5,26. Because of the profound impact of atherosclerosis on arterial function28, patients with hyperlipidemia, cigarette smoking, coronary, cerebrovascular, or peripheral arterial disease were excluded by history, examination, exercise echocardiography, and vascular ultrasound15,24.
Echocardiography
Doppler-echocardiograms were performed and analyzed as previously described4,5,10,24,26. Doppler LV filling patterns were categorized using E/A ratio, early deceleration time, and normal reference ranges for age29,30.
Exercise testing
Exercise testing with expired gas analysis was performed as previously described in the upright position on an electronically braked bicycle using a staged protocol to exhaustion4,5,10,15,24-26,31. Peak values were averaged from the last 2 15-second intervals during peak exercise4,5,10,24-26,31. Ventilatory anaerobic threshold (VAT) and ventilation per carbon dioxide (Ve/VCO2) slope were assessed as previously described25,26,32. A six-minute walk test was performed by the method of Guyatt25,26,32,33.
Brachial artery FMD
Brachial FMD was measured as previously described in our laboratory and in accordance with international standards24,35-36. Participants were examined in the post-prandial state after 15 minutes of quiet, supine rest using a Biosound Phase II ultrasound system with a 9 mHz transducer to record images of the right brachial artery. A forearm cuff was then inflated 50 mm Hg above systolic pressure for 4 minutes. Images were recorded during the final 30 seconds prior to and 3 minutes following rapid cuff deflation32,35.
Video frames were automatically digitized and analyzed using previously described techniques 24,35. The maximum diameter from the dilation phase was automatically determined, and the change in diameter and percent change were calculated as follows: absolute change = maximum minus baseline; percent change = 100 × absolute change/baseline. Brachial FMD by these techniques is highly reproducible in our laboratory35.
Arterial stiffness
As previously described, ten-second images of the left common carotid artery were recorded using a Sequoia ultrasound instrument (Accuson, Inc.) fitted with a 10 MHz linear probe15,32,34 while optimizing the four boundaries defining the media-adventitia and blood-intima interfaces on the near and far walls. Later, these boundaries were traced from the digital images on a dedicated workstation to generate the mean, maximum, and minimum values of the arterial diameter, the lumen diameter, the far wall thickness, and the near wall thickness. Carotid stiffness indexes were calculated as previously described and according to accepted formulae15,32,37,38. Overall systemic arterial stiffness was measured as pulse pressure/stroke volume.
Quality-of-life
As previously described26,32 health-related quality-of-life was assessed with the short-form healthy survey (SF-36)39 and the Minnesota Living with HF (MLHF)40 questionnaire.
B-type natriuretic peptide-32 measurement (BNP)
As previously described, a commercially available radioimmunoassay (Phoenix Pharmaceuticals Inc; Mountain View, Calif) was used for BNP4,26,32.
Endurance ET
The ET group exercised for one hour, three times per week for 16-weeks. Each session had three phases: 10-minute warm-up, stimulus, and 10-minute recovery. The stimulus phase consisted of walking on a track and cycle ergometry (Schwinn Airdyne). Isolated arm ergometry (operating the Airdyne with arms only) was performed ≥ 10 minutes each session in order to ensure upper extremity training. Patients exercised initially at 40-50% of heart rate reserve (HRR) for 5-10 minutes each of walking and ergometry. The intensity increased gradually until 70% HRR was maintained for at least 20 minutes each of walking and ergometry.
Attention Control
The CT group received telephone calls every 2 weeks for 16-weeks. These focused on retention, reminders and encouragement to keep study visits, and capture of medical events and did not address exercise behaviors.
Statistical analysis
The sample size was derived from a power analysis using data from a pilot study of 16 patients indicating that a sample size of 44 evaluable patients (22 per group) would provide 80% power to detect a 2% absolute change in brachial FMD, the primary outcome. The secondary outcome was carotid distensibility. VO2peak (ml/kg/min) was the main exercise outcome.
Baseline comparisons were made using two-sample t-tests for continuous data, Fisher's exact tests for dichotomous data, or chi-square tests for categorical data. Comparisons of exercise testing, LV function, and qualify-of-life measures were made by ANCOVA, with the baseline value of the measure as the covariate. Brachial FMD and carotid distensibility were analyzed using repeated measure ANCOVA models fitted with the week 8 and 16 values as outcomes and the baseline value as the covariate. A two-sample t-test was used to compare baseline characteristics between participants who dropped out and those who completed the 16-week study. Logarithmic transformation was performed for highly skewed data. A 5% two-sided significance level was used.
RESULTS
Participants
Fortified search lists from hospital discharges, clinic visits, and echocardiogram reports were reviewed from Wake Forest Medical Center. From 827 records reviewed, 543 patients potentially met inclusion criteria; 156 responded to an invitation, passed telephone screening, and were scheduled for a screening visit. Most common reasons for exclusion were patient unwillingness or did not meet criteria.
Sixty-three HFPEF patients (age 70±7 years) were enrolled; 32 were randomized to ET and 31 to CT (Table 1). All had NYHA class II-III symptoms. There were no significant baseline intergroup differences in key characteristics except for more beta-blockers among CT patients.
Table 1.
Baseline participant characteristics
| ET(N=32) | CT (N= 31) | p-value | |
|---|---|---|---|
| Female, % | 72 | 80 | 0.56 |
| Caucasian, % | 66 | 71 | 0.79 |
| African-American, % | 28 | 23 | 0.77 |
| Age, years | 70 ± 7 | 70 ± 7 | 0.63 |
| Body mass index, kg/m2 | 32.2 ± 6.7 | 32.0 ± 6.6 | 0.92 |
| Body surface area, m2 | 1.95 ± 0.22 | 1.94 ±0.25 | 0.84 |
| History of hypertension, % | 94 | 84 | 0.25 |
| Diabetes mellitus, % | 28 | 19 | 0.56 |
| Systolic blood pressure, mm Hg | 146 ± 17 | 147 ± 17 | 0.81 |
| Diastolic blood pressure, mm Hg | 82 ± 11 | 83 ± 10 | 0.83 |
| Left ventricular mass, g | 247 ± 95 | 266 ± 94 | 0.47 |
| Posterior wall thickness, cm | 1.3 ± 0.2 | 1.2 ± 0.2 | 0.41 |
| Left atrial diameter, cm | 3.6 ± 0.7 | 3.4 ± 0.7 | 0.27 |
| Smoking history | |||
| Never, % | 41 | 61 | |
| Former, % | 50 | 35 | |
| Current, % | 9 | 3 | 0.26 |
| New York Heart Association class | |||
| II, % | 47 | 55 | 0.62 |
| III, % | 53 | 45 | |
| Diastolic Filling | |||
| Normal, % | 0 | 0 | 1.0 |
| Impaired relaxation, % | 44 | 52 | 0.61 |
| Pseudonormal, % | 53 | 41 | 0.44 |
| Restrictive, % | 3 | 7 | 0.59 |
| B-type natriuretic peptide, pg/ml | 64.8 ± 74.5 | 72.2 ± 60.9 | 0.62 |
| Medications | |||
| Diuretics, % | 66 | 55 | 0.45 |
| Angiotensin converting enzyme inhibitors, % | 44 | 39 | 0.80 |
| Beta blockers, % | 9 | 35 | 0.02 |
| Calcium channel blockers, % | 44 | 19 | 0.06 |
| Angiotensin receptor blockers, % | 6 | 3 | 1.00 |
| Estrogen (♀), % | 34 | 36 | 0.92 |
| Nitrates, % | 13 | 10 | 1.00 |
Values are mean ± sd or %. For B-type natriutetic peptide, medians (25th percentile, 75th percentile) are 39.1 (18.8, 78.5) vs. 55.4 (22.6, 109.7) and the p-value shown is following logarithmic transformation of this highly skewed variable.
ET Safety, Compliance, and Adherence
One patient developed transient hypoglycemia during an exercise session; there were no other protocol-related events. Fifty-four patients (86%) completed final testing (24 ET, 30 CT). Reasons for not completing follow-up testing were: patient unwillingness (2 ET and 1 CT), elective surgery (2 ET), non-HF illness (3 ET), and HF hospitalization (1 ET). ET patients attended 88% of ET sessions. Among participants who completed follow-up testing, there were no significant intergroup differences in key baseline variables including: VO2peak (14.2±2.2 vs.14.0±3.2 ml/kg/min, p=0.54); brachial FMD (4.0±2.2 vs. 4.7±3.5, p=0.18); and carotid distensibility (1.07±0.50 vs. 0.89±0.35, p=0.19).
Exercise testing
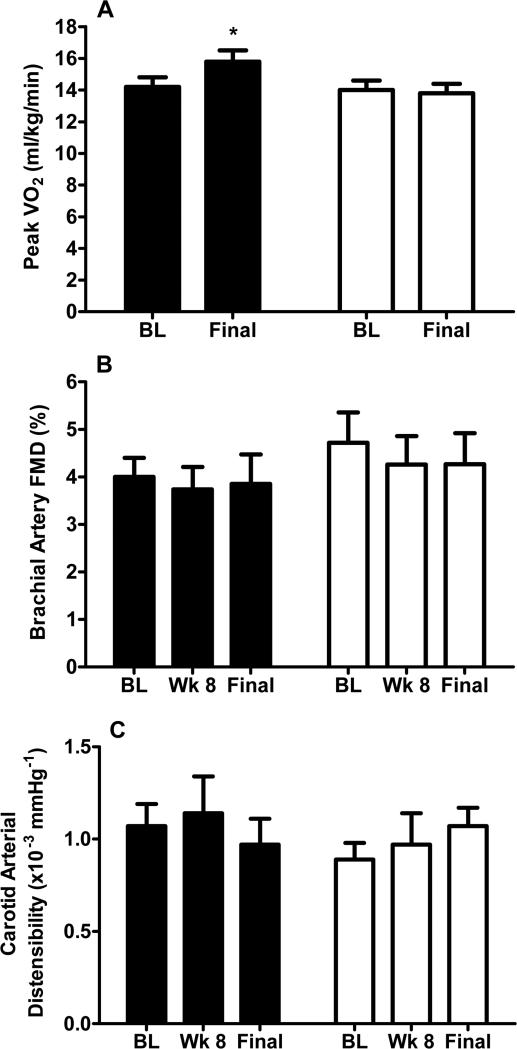
Following the 16-week intervention, VO2peak, exercise time, peak power output (all p<0.0001), and VAT (p=0.01) were significantly greater in ET than CT (Table 2, Figure 1). Although peak heart rate was mildly higher in ET than CT, there was no difference in respiratory exchange ratio (RER; p=0.68), suggesting similar, exhaustive levels of effort (Table 2). Further, 43 participants (80%) achieved RER ≥1.05 at the final visit. Of 11 participants with RER <1.05, 7 (23%) were CT and 4 (17%) were ET (p=0.74), indicating similar effort. Six-minute walk distance was greater after 16-weeks in ET than CT (p=0.009).
Table 2.
Effect of endurance exercise training on exercise performance
| ET | CT | P-Value | |||
|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | ||
| N | 24 | 24 | 30 | 30 | |
| Peak Exercise (Bike) | |||||
| Exercise time, min | 9.5 ± 3.2 | 11.4 ± 3.1 | 9.6 ± 3.1 | 9.5 ± 3.3 | <0.0001 |
| Power output, watts | 72 ± 31 | 89 ± 30 | 72 ± 26 | 67 ± 27 | <0.0001 |
| VO2, ml/kg/min | 14.2 ± 2.8 | 15.8 ± 3.3 | 14.0 ± 3.2 | 13.8 ± 3.1 | 0.0001 |
| VO2, ml/min | 1260 ± 329 | 1388 ± 378 | 1233 ± 398 | 1217 ± 370 | 0.0004 |
| Heart rate, bpm | 128 ± 18 | 132 ± 16 | 131 ± 21 | 127 ± 17 | 0.01 |
| Systolic blood pressure, mmHg | 197 ± 19 | 199 ± 21 | 192 ± 27 | 192 ± 29 | 0.49 |
| Diastolic blood pressure, mmHg | 91 ± 14 | 91 ± 11 | 91 ± 12 | 91 ± 14 | 0.99 |
| Pulse pressure, mmHg | 106 ± 21 | 107 ± 21 | 102 ± 22 | 100 ± 27 | 0.47 |
| Respiratory exchange ratio | 1.13 ± 0.07 | 1.11 ± 0.08 | 1.11 ± 0.10 | 1.09 ± 0.08 | 0.68 |
| 6-minute walk distance, m | 447 ± 107 | 486 ± 89 | 438 ± 79 | 448 ± 70 | 0.009 |
| Ventilatory anaerobic threshold, ml/min | 699 ± 178 | 796 ± 163 | 734 ± 189 | 702 ± 186 | 0.01 |
| Ve/VCO2 slope | 31.5 ± 4.4 | 32.2 ± 4.5 | 30.6 ± 3.6 | 30.2 ± 3.3 | 0.07 |
Data represent means ± SD; p-value represents comparison of least square means at final visit following adjustment for baseline values. VO2: oxygen consumption; Ve: minute ventilation; VCO2: carbon dioxide production.
Figure 1. Effects of endurance training on peak VO2 (A), brachial artery flow mediated dilation (B) and carotid arterial distensibility (C) in older HFPEF patients.
Data are shown as raw mean ± SE at each time point. *=p<0.05 between groups.
Following ET, there were no intergroup differences in resting or exercise systolic, diastolic, and pulse pressure (Table 2). At baseline, 17 (27%) patients met criteria for chronotropic incompetence. Despite the ET-related improvement in peak heart rate, ET did not reduce CI significantly at follow-up (p=0.22)
Brachial FMD
Brachial FMD (the primary study outcome) was not different between groups after 8 or 16 weeks (Table 3, Figure 1). Within the ET group, FMD actually decreased slightly (4.0 ± 2.0% to 3.8 ± 3.0%). The estimated treatment effect size on absolute brachial FMD was only 0.2%; thus we can be 94% confident that the true effect is <1.0%, indicating that it is highly unlikely that the trial missed a clinically meaningful improvement in FMD.
Table 3.
Effect of endurance exercise training on brachial artery diameter in response to cuff ischemia (FMD)
| ET | CT | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8 Week | Final | Baseline | 8 Week | Final | ||
| N | 25 | 23 | 24 | 30 | 22 | 29 | |
| Baseline Diameter, mm | 4.17 ± 0.91 | 4.41 ± 0.89 | 4.30 ± 0.83 | 4.13 ± 0.87 | 4.11 ± 1.02 | 4.23 ± 0.98 | 0.78 |
| Maximum Diameter, mm | 4.33 ± 0.89 | 4.56 ± 0.88 | 4.45 ± 0.79 | 4.31 ± 0.85 | 4.28 ± 1.01 | 4.40 ± 0.95 | 0.41 |
| Absolute Change, mm | 0.16 ± 0.07 | 0.16 ± 0.08 | 0.15 ± 0.09 | 0.18 ± 0.12 | 0.16 ± 0.09 | 0.16 ± 0.10 | 0.94* |
| Brachial Artery FMD, % | 4.0 ± 2.0 | 3.7 ± 2.2 | 3.8 ± 3.0 | 4.7 ± 3.5 | 4.3 ± 2.8 | 4.3 ± 3.5 | 0.88* |
Data represent means ± SD; p-value represents comparison of least square means of the main effect across the follow-up visits following adjustment for baseline values. Abbreviations: FMD: flow-mediated dilation (the primary study outcome).
p-value is shown following logarithmic transformation of this highly skewed variable.
Arterial stiffness
Carotid distensibility, the main arterial stiffness outcome, was not different after 8 or 16 weeks (Table 4). There was also no intergroup difference in any other measure of arterial stiffness (Table 4).
Table 4.
Carotid Arterial Stiffness Measurements
| ET | CT | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8 Week | Final | Baseline | 8 Week | Final | ||
| N | 24 | 20 | 23 | 24 | 21 | 20 | |
| Main measure | |||||||
| AD (×10-3 mm*mmHg-1) | 1.07 ± 0.50 | 1.14 ± 0.70 | 0.97 ± 0.56 | 0.89 ± 0.35 | 0.97 ± 0.51 | 1.07 ± 0.34 | 0.65 |
| Other measures | |||||||
| AC (×10-3 mm*mmHg-1) | 65.1 ± 20.9 | 76.8 ± 64.7 | 58.8 ± 32.8 | 51.6 ± 23.4 | 51.3 ± 17.4 | 61.7 ± 21.1 | 0.64* |
| PEM (kPa) | 232 ± 150 | 223 ± 181 | 224 ± 198 | 242 ± 109 | 235 ± 147 | 242 ± 111 | 0.41* |
| YEM (kPa) | 1504 ± 1160 | 1302 ± 1089 | 1427 ± 1385 | 1209 ± 642 | 1195 ± 864 | 1128 ± 648 | 0.82* |
| Beta Index | 16.11 ± 9.36 | 16.45 ± 12.46 | 15.77 ± 12.66 | 16.91 ± 7.61 | 16.82 ± 10.46 | 16.58 ± 7.16 | 0.50* |
| Pulse pressure | |||||||
| PP (mmHg) | 70 ± 16 | 65 ± 12 | 62 ± 13 | 73 ± 17 | 67 ± 12 | 68 ± 12 | 0.24 |
| Arterial dimensions | |||||||
| IMT (mm) | 0.74 ± 0.08 | 0.75 ± 0.09 | 0.76 ± 0.11 | 0.84 ± 0.14 | 0.82 ± 0.10 | 0.84 ± 0.10 | 0.30 |
| SAD (mm) | 8.33 ± 1.12 | 8.31 ± 1.18 | 8.45 ± 1.20 | 8.26 ± 0.92 | 8.11 ± 0.89 | 8.35 ± 0.97 | 0.47 |
| DAD (mm) | 7.93 ± 1.15 | 7.90 ± 1.17 | 8.01 ± 1.19 | 7.89 ± 0.90 | 7.72 ± 0.89 | 8.00 ± 0.96 | 0.52 |
| SLD (mm) | 6.98 ± 1.19 | 7.02 ± 1.29 | 6.83 ± 1.02 | 6.63 ± 1.02 | 6.46 ± 0.88 | 6.53 ± 0.87 | 0.90 |
| DLD (mm) | 6.53 ± 1.22 | 6.57 ± 1.17 | 6.43 ± 1.02 | 6.21 ± 0.94 | 6.08 ± 0.91 | 6.09 ± 0.80 | 0.72 |
| ADC (mm) | 0.40 ± 0.17 | 0.41 ± 0.20 | 0.44 ± 0.24 | 0.38 ± 0.16 | 0.39 ± 0.27 | 0.35 ± 0.14 | 0.49* |
Data represent means ± SD; p-value represents comparison of least square means of the main effect across the follow-up visits following adjustment for baseline values. Abbreviations: AD: arterial distensibility; AC: arterial compliance; PEM: Peterson's elastic modulus; YEM: Young's elastic modulus; kPa: kilopascals; PP: pulse pressure; IMT: mean wall intimal medial thickness; SAD: systolic arterial diameter; DAD: diastolic arterial diameter; SLD: systolic lumen diameter; DLD: diastolic lumen diameter; ADC: arterial diameter change during cardiac cycle.
p-value is shown following logarithmic transformation of this highly skewed variable.
LV function
There were no significant differences after 16 weeks for resting LV volumes, ejection fraction, Doppler LV filling, or pulse pressure/stroke volume ratio (Table 5).
Table 5.
Effect of endurance exercise training on resting left ventricular volumes and Doppler filling
| ET | CT | P-Value | |||
|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | ||
| N | 24 | 24 | 28 | 28 | |
| End-diastolic volume, ml | 103 ± 32 | 101 ± 34 | 88 ± 26 | 85 ± 22 | 0.41 |
| End-systolic volume, ml | 43 ± 13 | 43 ± 16 | 39 ± 12 | 38 ± 11 | 0.64 |
| Stroke volume, ml | 60 ± 22 | 59 ± 21 | 50 ± 15 | 47 ± 12 | 0.29 |
| Pulse pressure/stroke volume, mmHg/ml | 1.42 ± 0.47 | 1.31 ± 0.51 | 1.52 ± 0.44 | 1.38 ± 0.31 | 0.98 |
| Ejection fraction, % | 58 ± 6 | 58 ± 6 | 56 ± 5 | 56 ± 5 | 0.58 |
| Early filling velocity, cm/s | 77 ± 21 | 75 ± 19 | 72 ± 23 | 68 ± 15 | 0.24 |
| Atrial filling velocity, cm/s | 87 ± 25 | 85 ± 24 | 78 ± 24 | 78 ± 21 | 0.95 |
| Early/Atrial filling velocity ratio | 1.03 ± 0.94 | 0.99 ± 0.59 | 1.07 ± 0.87 | 0.99 ± 0.64 | 0.81* |
| Early deceleration time, ms | 225 ± 48 | 219 ± 51 | 216 ± 63 | 233 ± 57 | 0.24 |
| Isovolumic relaxation time, ms | 80 ± 24 | 80 ± 23 | 81 ± 22 | 81 ± 24 | 0.95 |
Data represent means ± SD; p-value represents comparison of least square means at final visit following adjustment for baseline values.
p-value is shown following logarithmic transformation of this highly skewed variable.
Quality-of-Life
After the 16-week intervention, the emotional and physical SF-36 scores were higher in ET than CT, but the MLHFQ scores were not significantly different (Table 6).
Table 6.
Effect of endurance exercise training on health-related quality of life
| ET | CT | P-Value | |||
|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | ||
| N | 20 | 20 | 27 | 27 | |
| SF-36 | |||||
| Physical | 48 ± 18 | 63 ± 20 | 50 ± 25 | 53 ± 27 | 0.03 |
| Emotional | 63 ± 30 | 83 ± 31 | 66 ± 37 | 62 ± 35 | 0.04 |
| MLHFQ | |||||
| Physical | 17 ± 8 | 12 ± 8 | 13 ± 11 | 11 ± 10 | 0.50 |
| Emotional | 6 ± 5 | 5 ± 4 | 4 ± 5 | 3 ± 4 | 0.19* |
| Total | 36 ± 19 | 26 ± 19 | 28 ± 23 | 25 ± 22 | 0.50 |
Data represent means ± SD; p-value represents comparison of least square means at final visit following adjustment for baseline values. Abbreviations: MLHFQ: Minnesota living with heart failure questionnaire
p-value is shown following logarithmic transformation of this highly skewed variable
Analysis based on medications and dropouts
Although beta-blockers were somewhat more prevalent in CT, the overall results were unchanged after adjusting for this. Baseline brachial FMD was not different between those who dropped out (4.4±2.9%, N=9) and those who completed the study (3.2±2.0, N=54, p=0.3). Baseline carotid distensibility was not different between those who dropped out (0.67±0.59×10-3mm*mmHg-1, N=5) compared to those who completed (0.97±0.44×10-3mm*mmHg-1, N=48, p=0.29). Baseline VO2peak was lower in participants who dropped versus completed (11.9±2.8, N=9, and 14.1±3.2, N=54, respectively, p=0.03).
Responders analyses
A responder was defined as having an absolute increase of 1.0% for brachial FMD and a 10% relative increase in carotid distensiblity36 at follow-up. There were 5 (21%) FMD responders in ET and 6 (21%) in CT (p=1.0). There were 4 (17%) carotid distensibility responders in ET and 7 (35%) in CT (p=0.29). Within ET, an additional analysis was performed to determine whether any key baseline variable differed between responders and non-responders. For FMD, only E deceleration time was significantly different (174±28 vs. 239±43 ms, respectively, p=0.004). For carotid distensibility, no variable was significantly different.
DISCUSSION
To our knowledge, this is the first report on the effect of ET on endothelial function and arterial stiffness in HFPEF. The effect size of ET on peak VO2 was 1.8 ml/kg/min (13%, 15.7±0.3 vs. 13.9±0.3 ml/kg/min; least square means from ANCOVA). This supports findings from three previous trials,26,41,42 increasing the number of HFPEF patients reported in randomized ET trials nearly 50%. Despite the significant improvement in VO2peak, and in contrast to our hypothesis, ET did not improve brachial FMD (the primary outcome) or arterial stiffness. This indicates that improvements in large arterial function were not responsible for the ET-related improvement in VO2peak. There were also no changes in resting LV function. Combined with our previously reported trial and that recently reported by Fujimoto et al10,43, this suggests that the ET-related improvement in VO2peak in HFPEF may be related to microvascular and/or skeletal muscle adaptations that increase diffusive oxygen transport and/or utilization by the active muscles19,44-47.
This study was driven by evidence suggesting that impaired arterial function (vasodilation and/or arterial stiffness) contributes to exercise intolerance in HFPEF. Borlaug et al showed that in HFPEF vasodilator reserve was reduced and correlated with reduced exercise capacity.6 Puntawangkoon et al48 found that submaximal exercise leg blood flow was reduced. Borlaug et al also reported reduced digital arterial function in HFPEF7. However, the finding of abnormal vasodilation in HFPEF has not been universal, since Haykowsky et al, using high resolution brachial ultrasound, and Hundley et al, using phase contrast femoral MRI, found FMD was not reduced in older HFPEF patients screened for atherosclerosis13,24.
Arterial stiffness has been uniformly reported to be increased in HFPEF. We and others have shown that aortic and carotid distensibility are significantly reduced in elderly HFPEF and are independent predictors of reduced VO2peak 13,15,49,50. Borlaug et al reported that arterial stiffness increases during exercise and is inversely related to exercise performance7,14.
This study was also driven by several reports that ET in HFREF patients enhances arterial function16,17,19-21,23,51. Hornig et al16 and others17,21 showed that ET-mediated improvements in endothelial arterial function may be region-specific (to the locally active muscles), whereas Hambrecht et al found that effects of ET extended beyond regional effects21,23 since lower-limb ET increased upper limb FMD.21,23 Moreover, Parnell et al demonstrated that ET improves arterial stiffness in HFREF22.
In our study, 16-weeks of ET did not change endothelial function or arterial stiffness. This suggests that the vascular adaptations to ET may differ between HFREF and HFPEF patients52. If so, that may be partly explained by the fact that the effects of ET on vascular function appear to be sex and age-dependent. This is relevant since the majority of the HFPEF patients in our study were older women, as in the general population1, whereas most HFREF patients are men. Specifically, Pierce et al recently found that brachial FMD was not different between older women undergoing high-frequency, long-term ET (≥5 years) versus age-matched healthy sedentary controls, whereas it was 49% greater in older men with long-term ET compared to controls53. Also, two-months of ET improved brachial FMD in previously sedentary healthy middle-aged men but not in older post-menopausal women53. Of note, the HFREF patients in prior studies of the effect of ET on endothelial function were predominantly men1. Furthermore among master ET athletes, carotid artery compliance is lower in older women compared to men54. Thus, sex and age-dependent effects may explain, at least partly, differences in the effect of ET on arterial function in HFPEF patients in the present study compared to previous studies of HFREF.
Another potential explanation for differing results of ET on arterial function in HFPEF compared with HFREF is the profound effect of atherosclerosis on vascular function28. A strength of the present study is that patients were screened to exclude those with coronary, cerebrovascular, and peripheral vascular disease to avoid the strong, confounding influence of atherosclerosis28,55. However, this has not been accounted for in most prior studies of FMD and arterial stiffness, and atherosclerosis is more common in HFREF than HFPEF1. However, studies using animal models of HFREF and carefully selected HFREF patient groups indicate that abnormal endothelial function is present in HFREF even in the absence of atherosclerosis56,57. Our finding that ET did not improve brachial FMD and that FMD did not contribute to the ET-related improvement in VO2peak, taken together with our two prior reports that found no significant difference in brachial or femoral FMD at baseline in older patients with HFPEF versus controls13,24, suggests that in contrast to HFREF, abnormal FMD may not be a fundamental component of the pathophysiology of exercise intolerance in older HFPEF patients.
If neither increased exercise cardiac output10 or improved large arterial function primarily account for the improved VO2peak following ET in older HFPEF, this suggests a potential role for skeletal muscle adaptations, such as either increased diffusive oxygen transport of O2 from red blood cell to muscle mitochondria and / or improved O2 utilization by the active muscles, primarily via mitochondrial function. Some independent data support this possibility. Bhella et al recently showed that skeletal muscle oxidative metabolism was abnormal in a small number of older patients with HFPEF and appeared related to their severely reduced VO2peak8. Furthermore, several studies have shown that abnormal skeletal muscle perfusion and metabolism contribute significantly to exercise intolerance in HFREF and to improvements in VO2peak following ET44,58-61.
Our study also confirmed prior reports that ET improves peak heart rate response, quality-of-life, and 6-minute walk distance in HFPEF10,26,41.
Limitations
Although our study duration was long enough to produce a significant improvement in VO2peak and included upper as well as lower extremity training, we cannot exclude that training that was longer and more intense could produce detectable improvements in vascular or ventricular function. Molmen-Hansen et al compared high-intensity aerobic interval exercise with moderate intensity continuous ET and usual care in patients with essential hypertension62. They reported that change in VO2peak after high-intensity interval training was 3 and 5-fold greater than moderate continuous training or usual care groups, respectively. LV systolic and diastolic function and brachial FMD improved significantly after high-intensity training but were unchanged with moderate continuous training or usual care62. Wisloff et al showed that high-intensity aerobic interval training was superior to continuous moderate intensity ET for improving VO2peak, resting ejection fraction, and brachial FMD in older (75 years) men with HFREF20.
We did not measure FMD or arterial stiffness (other than pulse pressure which was unchanged, Table 2) during peak exercise, which would provide the most definitive evidence of relation to changes in VO2peak, but would be challenging. However, in studies of ET in HFPEF patients where hemodynamics were assessed at peak exercise, neither 16-weeks or 1 year of ET produced a change in peak exercise or reserve pulse pressure/stroke volume ratio, a measure of arterial stiffness10,43.
There were more dropouts in the ET than the CT group, however, the number of evaluable patients at the end of the study was within our sample size estimates. Furthermore, among those who completed the study, key baseline variables were similar in ET and CT participants. Also, brachial FMD and carotid distensibility were not different at baseline between those who completed compared to drop-outs.
Tissue Doppler was not measured. Thus, there may have been improvements in resting LV diastolic function following ET that were not detected.
By design, participants were stable, well compensated outpatients who were able to participate in exhaustive exercise testing and ET. As a result, the mean BNP level was less than in patients with acute, decompensated HF. BNP levels are also known to be lower in HFPEF than in HFREF, likely due to smaller LV cavity size63. The BNP levels are similar to other studies of stable HFPEF patients able to undergo maximal exercise testing7,12,32,64 and are several-fold increased compared to healthy, age-matched, normal subjects.4 However, our results may not apply to patients who are sicker, poorly compensated, or less clinically stable. Further, we enrolled HFPEF patients without known or suspected coronary, cerebrovascular, and peripheral arterial disease. Thus, we cannot exclude the possibility that ET improves arterial stiffness or endothelial function in a broader population of HFPEF patients with these comorbidities.
Conclusion
ET significantly improves VO2peak without altering brachial FMD or carotid arterial stiffness in older HFPEF patients. Combined with our previous finding that improved peripheral function (A-VO2 Diff) is an important contributor to the ET-related increase in VO2peak 10, this suggests that microvascular and/or skeletal muscle adaptations may contribute to the ET-related increase in VO2peak in older HFPEF patients.
Acknowledgements
This study was supported by the following National Institutes of Health research grants: R37AG18915, R01AG12257, and P30AG021332.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Dr. Kitzman is a consultant for Relypsa Inc., Boston Scientific Corp., Abbot, Servier, AbbVie, and GlaxoSmithKline, has received grant support from Novartis, and owns stock in Gilead Sciences. No other members of the writing group have conflicts of interest to declare.
References
- 1.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients >/= 65 Years of Age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–9. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Higginbotham MB, Cobb FR, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–72. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Brubaker PH, John JM, et al. Determinants of Exercise Intolerance in Elderly Heart Failure Patients With Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58:265–74. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Olson TP, Lam CSP, et al. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2010;56:845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeder MT, Thompson BR, Brunner-La Rocca H-P, et al. Hemodynamic Basis of Exercise Limitation in Patients With Heart Failure and Normal Ejection Fraction. J Am Coll Cardiol. 2010;56:855–63. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Haykowsky MJ, Brubaker PH, Stewart KP, et al. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60(2):120–8. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer MS, Schulze PC. Exercise intolerance in heart failure with preserved ejection fraction: shifting focus from the heart to peripheral skeletal muscle. J Am Coll Cardiol. 2012;60:129–31. doi: 10.1016/j.jacc.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2010;3:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle dependent changes in aortic area and aortic distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 14.Tartiere-Kesri L, Tartiere JM, Logeart D, et al. Increased Proximal Arterial Stiffness and Cardiac Response With Moderate Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2012;59:455–61. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 15.Kitzman DW, Herrington DM, Brubaker P, et al. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–9. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93:210–4. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 17.Katz SD, Yuen J, Bijou R, et al. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol. 1997;82:1488–92. doi: 10.1152/jappl.1997.82.5.1488. [DOI] [PubMed] [Google Scholar]
- 18.Linke A, Schoene N, Gielen S, et al. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–7. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- 19.Erbs S, Hollriegel R, Linke A, et al. Exercise Training in Patients With Advanced Chronic Heart Failure Promotes Restoration of Peripheral Vasomotor Function, Induction of Endogenous Regeneration, and Improvement of Left Ventricular Function. Circ Heart Fail. 2010;3:486–94. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 20.Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. A randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 21.Hambrecht R, Hilbrich L, Erbs S, et al. Correction of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral L-arginine supplementation. J Am Coll Cardiol. 2000;35:706–13. doi: 10.1016/s0735-1097(99)00602-6. [DOI] [PubMed] [Google Scholar]
- 22.Parnell MM, Holst DP, Kaye DM. Exercise training increases arterial compliance in patients with congestive heart failure. Clin Sci (Lond) 2002;102:1–7. [PubMed] [Google Scholar]
- 23.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 24.Haykowsky MJ, Herrington DM, Brubaker PH, et al. Relationship of flow mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. J Gerontol A Biol Sci Med Sci. 2013;68:161–7. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott JM, Haykowsky MJ, Eggebeen J, et al. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:1809–13. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitzman DW, Brubaker PH, Morgan TM, et al. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659–67. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–5. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 28.Celermajer DS, Sorensen KE, Bull C, et al. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's rosetta stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 30.Gardin JM, Arnold AM, Bild DE, et al. Left ventricular diastolic filling in the elderly: The Cardiovascular Health Study. Am J Cardiol. 1998;82:345–51. doi: 10.1016/s0002-9149(98)00339-7. [DOI] [PubMed] [Google Scholar]
- 31.Marburger CT, Brubaker PH, Pollock WE, et al. Reproducibility of cardiopulmonary exercise testing in elderly heart failure patients. Am J Cardiol. 1998;82:905–9. doi: 10.1016/s0002-9149(98)00502-5. [DOI] [PubMed] [Google Scholar]
- 32.Kitzman DW, Hundley WG, Brubaker P, et al. A randomized, controlled, double-blinded trial of enalapril in older patients with heart failure and preserved ejection fraction; effects on exercise tolerance, and arterial distensibility. Circulation Heart Failure. 2010;3:477–85. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyatt GH, Sullivan M, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 35.Herrington DM, Fan L, Drum M, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8:319–28. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 36.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 37.Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34:201–6. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 38.Smilde TJ, van den Berkmortel FW, Borres GH. Carotid and femoral artery wall thickness and stiffness in patients at risk for cardiovascular disease, with special emphasis on hyperhomocysteinemia. Arterioscler Throm Vasc Biol. 1998;18:1958–63. doi: 10.1161/01.atv.18.12.1958. [DOI] [PubMed] [Google Scholar]
- 39.Ware JEJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 40.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire. Am J Cardiol. 1993;71:1106–7. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 41.Edelmann F, Gelbrich G, Dungen HD, et al. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF Pilot Study. J Am Coll Cardiol. 2011;58:1780–91. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 42.Smart NA, Haluska B, Jeffriess L, et al. Exercise training in heart failure with preserved systolic function: A randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012;18:295–301. doi: 10.1111/j.1751-7133.2012.00295.x. [DOI] [PubMed] [Google Scholar]
- 43.Fujimoto N, Prasad A, Hastings JL, et al. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–77. doi: 10.1016/j.ahj.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minotti JR, Johnson EC, Hudson TL, et al. Skeletal muscle response to exercise training in congestive heart failure. J Clin Invest. 1990;86:751–8. doi: 10.1172/JCI114771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esposito F, Reese V, Shabetai R, et al. Isolated Quadriceps Training Increases Maximal Exercise Capacity in Chronic Heart Failure: The Role of Skeletal Muscle Convective and Diffusive Oxygen Transport. J Am Coll Cardiol. 2011;58:1353–62. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hambrecht R, Fiehn E, Yu J, et al. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol. 1997;29:1067–73. doi: 10.1016/s0735-1097(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 47.Tyni-Lenne R, Gordon A, Europe E, et al. Exercise-based rehabilitation improves skeletal muscle capacity, exercise tolerance, and quality of life in both women and men with chronic heart failure. J Cardiac Failure. 1998;4:9–17. doi: 10.1016/s1071-9164(98)90503-6. [DOI] [PubMed] [Google Scholar]
- 48.Puntawangkoon C, Kitzman D, Kritchevsky S, et al. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. Journal of Cardiovascular Magnetic Resonance. 2009;11:48. doi: 10.1186/1532-429X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular Features of Heart Failure With Preserved Ejection Fraction Versus Nonfailing Hypertensive Left Ventricular Hypertrophy in the Urban Baltimore Community: The Role of Atrial Remodeling/Dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 50.Kawaguchi M, Hay I, Fetics B, et al. Combined Ventricular Systolic and Arterial Stiffening in Patients With Heart Failure and Preserved Ejection Fraction. Circulation. 2003;107:714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi N, Tsuruya Y, Iwasawa T, et al. Exercise Training in Patients With Chronic Heart Failure Improves Endothelial Function Predominantly in the Trained Extremities. Circulation Journal. 2003;67:505–10. doi: 10.1253/circj.67.505. [DOI] [PubMed] [Google Scholar]
- 52.Schwartzenberg S, Redfield MM, From AM, et al. Effects of Vasodilation in Heart Failure With Preserved or Reduced Ejection Fraction: Implications of Distinct Pathophysiologies on Response to Therapy. J Am Coll Cardiol. 2012;59:442–51. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 53.Pierce GL, Eskurza I, Walker AE, et al. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clinical Science. 2011;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeVan AE, Seals DR. Vascular health in the ageing athlete. Experimental Physiology. 2012;97:305–10. doi: 10.1113/expphysiol.2011.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrington DM, Brown WV, Mosca L, et al. Relationship Between Arterial Stiffness and Subclinical Aortic Atherosclerosis. Circulation. 2004;110:432–7. doi: 10.1161/01.CIR.0000136582.33493.CC. [DOI] [PubMed] [Google Scholar]
- 56.Kaiser L, Spickard RC, Olivier NB. Heart failure depresses endothelium-dependent responses in canine femoral artery. Am J Physiol. 1989;256:H962–H967. doi: 10.1152/ajpheart.1989.256.4.H962. [DOI] [PubMed] [Google Scholar]
- 57.Drexler H, Lu W. Endothelial dysfunction of hindquarter resistance vessels in experimental heart failure. Am J Physiol. 1992;262:H1640–H1645. doi: 10.1152/ajpheart.1992.262.6.H1640. [DOI] [PubMed] [Google Scholar]
- 58.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 59.Sullivan M, Higginbotham MB, Cobb FC. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988;78:506–515. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–27. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 61.Coats A. Exercise training for heart failure: coming of age. Circulation. 1999;99:1138–40. doi: 10.1161/01.cir.99.9.1138. [DOI] [PubMed] [Google Scholar]
- 62.Molmen-Hansen HE, Stolen T, Tjonna AE, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. European Journal of Cardiovascular Prevention & Rehabilitation. 2011;19:151–60. doi: 10.1177/1741826711400512. [DOI] [PubMed] [Google Scholar]
- 63.From AM, Borlaug BA. Heart Failure with Preserved Ejection Fraction: Pathophysiology and Emerging Therapies. Cardiovascular Therapeutics. 2011;29:e6–e21. doi: 10.1111/j.1755-5922.2010.00133.x. [DOI] [PubMed] [Google Scholar]
- 64.Mottram PM, Haluska B, Leano R, et al. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation. 2004;110:558–65. doi: 10.1161/01.CIR.0000138680.89536.A9. [DOI] [PubMed] [Google Scholar]