Abstract
Targeting epigenetic mechanisms during initial learning or memory retrieval can lead to persistent memory. Retrieval induces plasticity that may result in reconsolidation of the original memory, in which critical molecular events are needed to stabilize the memory, or extinction, in which new learning during the retrieval trial creates an additional memory that reflects the changed environmental contingencies. A canonical feature of extinction is that the original response is temporarily suppressed, but returns under various conditions. These characteristics have defined whether a given manipulation alters extinction (when persistence does not occur) or reconsolidation (when persistence does occur). A problem arises with these behavioral definitions when considering the potential for persistent memory of extinction. Recent studies have found that epigenetic modulation of memory processes leads to surprisingly robust and persistent extinction. We discuss evidence from behavioral epigenetic approaches that forces a re-evaluation of widely used behavioral definitions of extinction and reconsolidation.
An unresolved issue in the neurobiological study of memory is the characterization of the conditions that lead to the loss of established memories. Many studies have shown that behavioral and pharmacological manipulations around the time of memory retrieval can result in a lasting loss of previously learned behaviors. These findings are consistent with the idea that memory retrieval induces a period of plasticity in which perturbations of the molecular signaling cascades involved in memory can produce enduring effects at the level of behavior. Effects on retrieval-induced plasticity have been described in terms of their effects on reconsolidation of the original memory, in which critical molecular events are needed to stabilize the memory, or extinction, in which learning during the retrieval trial creates an additional memory that reflects the changed environmental contingencies1–3. These two processes are often treated as exclusive, with manipulations being thought to affect one or the other, depending on behavioral conditions (for example, duration of retrieval) and how signaling molecules are affected by the manipulation. A key piece of behavioral evidence that has been used to distinguish these mechanisms is the finding that the loss of behavior induced by extinction is often temporary, reversing with time, changes in context or reminder treatments1.
Recent studies that manipulated epigenetic mechanisms around the time of memory retrieval and found persistent weakening of behavior challenge some widely held assumptions about the nature of reconsolidation and extinction. These studies complicate our understanding of both the behavioral conditions that engage one process or the other, as well as the behavioral, cellular and molecular evidence that can distinguish between them. Extinction and reconsolidation are complex processes that consist of interactions between molecular, cellular and systems mechanisms. What epigenetics brings to the table in attempting to distinguish these processes are distinct molecular events that lead to persistent molecular changes. Converging evidence for persistent memory resulting from epigenetic manipulations indicate that common assumptions about the nature of retrieval-induced plasticity need to be re-evaluated (Figs. 1 and 2). This is particularly exciting for extinction research because epigenetics gives us a plausible cellular and molecular mechanism for maintaining the persistent loss of behavior induced by extinction. Our focus here is on this emerging evidence and how it is becoming increasingly clear that the persistent absence of behavior is not under the exclusive domain of reconsolidation or memory erasure.
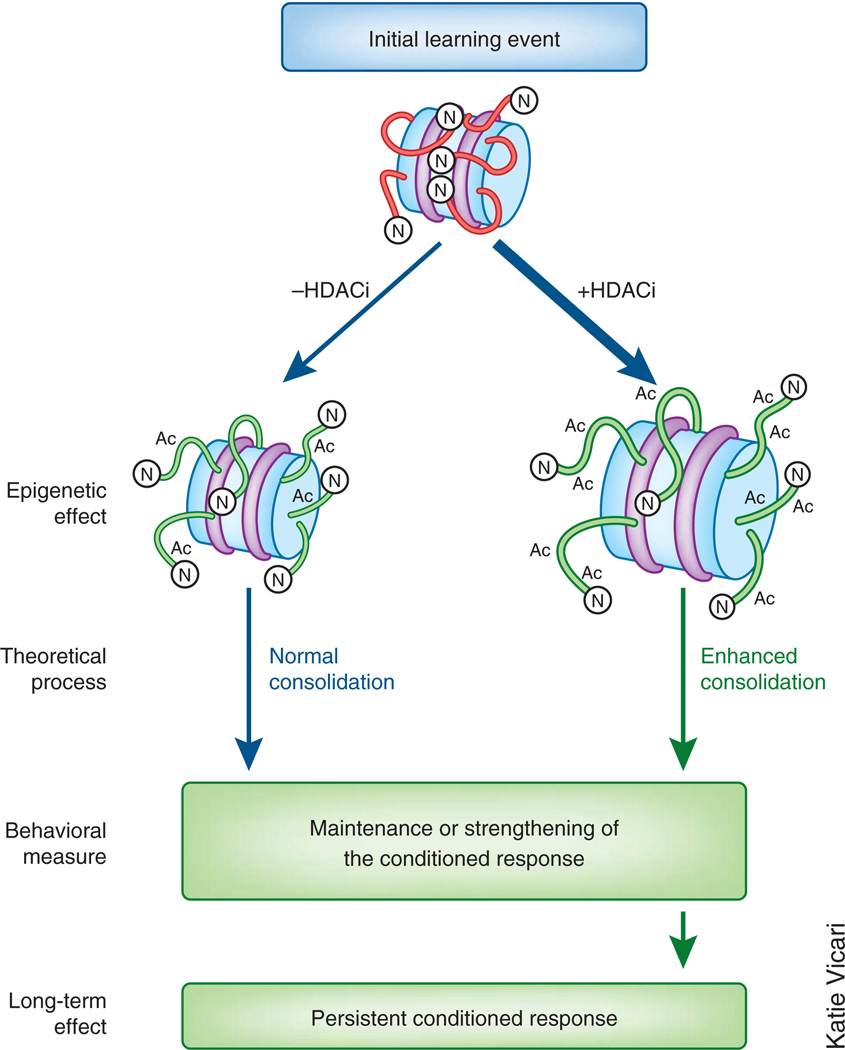
Figure 1.
Enhancing histone acetylation promotes initial memory consolidation. In normal situations, an initial learning event triggers a signaling cascade that includes activation of HATs and inactivation and/or removal of HDACs. These HATs attach acetyl groups (Ac) to histone tails, which relaxes the nucleosome, allowing transcription factors to bind to the DNA. Depending on the task and type of memory, the conditioned response may not persist under normal conditions. When an HDACi is administered, however, these tails become hyperacetylated, which promotes memory consolidation, strengthening the response. Although the schematic is simplified to focus on the main concept, there are of course other epigenetic mechanisms involved that work in concert with HATs and HDACs.
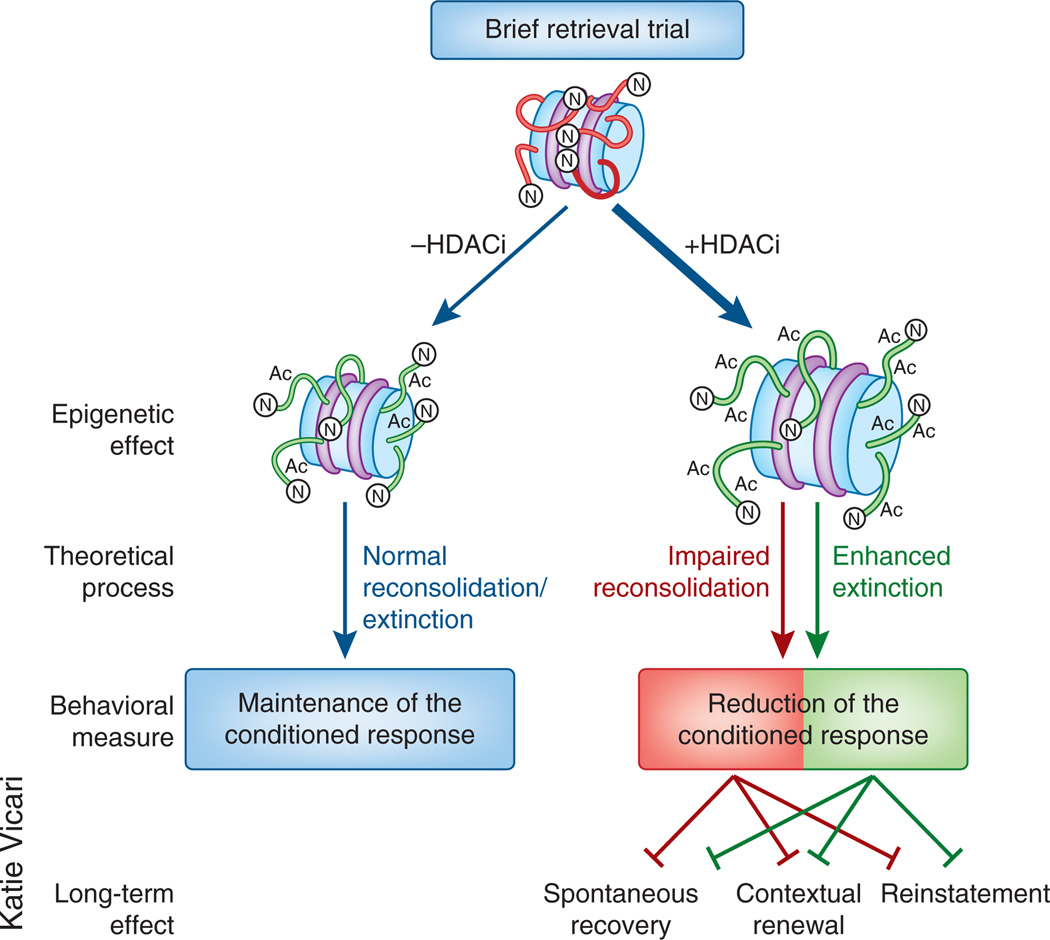
Figure 2.
Enhancing histone acetylation generates a persistent form of extinction. When memories are retrieved, similar signaling cascades are triggered, ultimately resulting in a loosening of chromatin structure. With a relatively brief retrieval trial, there is little to no long-term change in behavior. As a result of hyperacetylation induced by an HDACi, the behavioral effects of this brief retrieval trial are augmented. The two theoretical possibilities for these effects are an impairment of reconsolidation (shown in red) or an enhancement of extinction (shown in green). Both of these theoretical approaches predict weakened behavior and decreased persistence, as revealed through attenuated spontaneous recovery, contextual renewal and reinstatement.
Impairments in reconsolidation or enhancements in extinction?
Consider a very simple experiment in which an animal receives a mild foot shock in a previously neutral context. When tested the next day in that context, the animal will show a freezing response that occurs in anticipation of the foot shock. If the animal is then removed from that context after a relatively brief exposure, it may remember that it was not shocked during that test, but it will likely continue to freeze at high levels during subsequent tests on later days. If, however, a pharmacological perturbation of a critical signaling cascade is introduced soon after that first brief exposure, the animal will show very low levels of freezing during subsequent tests. This is a pervasive finding; many studies have documented that altering any of a number of key molecular steps along the path to transcription and translation will result in losses in performance3. At a theoretical level, these data are consistent with the idea that the act of retrieval moves memories into a labile state that is vulnerable to disruption until they are reconsolidated into a stable state. The data, however, are also consistent with the idea that the molecular manipulations promoted the new learning that occurred during retrieval, resulting in a rapidly formed memory for extinction (that is, memory that the context no longer signals shock). In both cases, the loss of behavior would be rapid and persistent (Fig. 2). How then can the field attribute effects to one of these two different processes?
There have been several answers to this question, but none are sufficient for a definitive distinction between extinction and reconsolidation. One approach has been to interpret the theoretical actions of a pharmacological or genetic manipulation on the basis of the putative effects of the manipulation at cellular and molecular levels. Because transcription and protein synthesis are critical for memory consolidation4, the effects of drugs that impair these processes have been interpreted as impairing reconsolidation. Similarly, the effects of drugs that promote these processes have been interpreted as promoting extinction5,6. At a very basic level, this type of interpretation is sensible. How could a drug that inhibits the molecular pathways that are necessary for memory formation promote extinction or a drug that promotes these pathways impair reconsolidation? But this reasoning assumes that all of the mechanisms of action of these drugs in the brain are known and that extinction and reconsolidation can be stripped down to fairly simple linear memory processes. In the case of the first point, it is quite clear that in all but a very few cases, drugs used to investigate memory have biochemical effects in addition to the intended ones7 (Box 1). In the case of the second point, extinction involves not just the formation of a single new memory (for example, context with no shock), but also changes in other memories involving previously formed associations and representations of individual stimuli in an environment8–10. Indeed, there are mounting reports of the effects of identical manipulations being interpreted as promoting extinction or impairing reconsolidation11. A consequence of these apparent contradictions in interpretation is that knowing the biochemical effects of a given compound may not necessarily reveal its effects on extinction as opposed to those on reconsolidation.
Box 1 Limitations and current directions in the epigenetics of extinction.
The studies discussed have given rise to new ideas about the mechanisms underlying the neurobiology of learning and memory. There remain numerous important questions that need to be addressed to increase our understanding and move forward.
First, what is the role of individual HDACs in extinction and reconsolidation? Much of the work discussed relates to research performed with class I–specifc HDAC inhibitors, such as sodium butyrate. Much more needs to be done with merging conditional genetic approaches and individual HDAC-specifc inhibitors (for example, see ref. 32).
Second, are HDAC4 and HDAC5 active HDACs? The class I and IIa HDACs differ by a single amino acid (tyrosine versus histidine) in the catalytic domain. Mutation of the tyrosine to a histidine in HDAC3 (class I HDAC) abolishes its deacetylase activity. HDAC4 and HDAC5 (class II HDACs) naturally have a histidine and have no detectable enzymatic activity on traditional substrates. However, mutation of the histidine to tyrosine restores the enzymatic activity50. Thus, understanding the role of HDAC4 and HDAC5 in a complex with class I HDACs is important.
Third, what is the role of protein-protein interactions in HDAC-corepressor complexes? Although most studies discuss the effects of manipulating a single HDAC either genetically or pharmacologically, there is usually little discussion of what happens to the complex to which the individual HDAC belongs. It is likely that HDAC inhibitors, either specific or nonspecific, disrupt protein-protein interactions, and the effects are therefore not limited to the simple interpretation that the enzymatic activity of that HDAC is missing. Indeed, the observed results may not have much to do with blocking enzymatic activity and more to do with disrupting the repressor complex. This is an exciting area to pursue, as it could lead to the development of small molecule drugs that disrupt specific protein-protein interactions.
Fourth, what is the actual role of the deacetylase domain? Currently no study (to the best of our knowledge) has specifically interrogated the role of the deacetylase domain when investigating the role of an HDAC in memory processes. This would require a point mutation that disrupts HDAC activity while leaving normal protein-protein interactions intact.
Fifth, how does the individual epigenome affect newly encoded information? The epigenome functions as a signal interaction platform that integrates genetic information, environmental effect and signaling cascade information with a read-out of specific neural function. There is almost nothing known about how variation in the epigenome, higher order chromatin structure or other epigenetic mechanisms (in particular nucleosome remodeling) integrate with one another to yield specific cell function.
These questions by no means encompass everything remaining to be addressed. As they imply, the idea that we can discover a single epigenetic mechanism to distinguish reconsolidation and extinction is not feasible. Hopefully answers to these questions can begin to elucidate how epigenetic mechanisms that can establish long-lasting and extremely stable cell function (as in cell fate and cellular memory) contribute to long-lasting neural plasticity that ultimately yields persistent changes in behavior.
Another way that the field has attempted to distinguish between reconsolidation and extinction is to examine the persistence of behavioral effects1,2. The logic that is often used in examining persistence is that extinguished behavior can be unmasked through spontaneous recovery with the passage of time, reinstatement after a reminder unconditioned stimulus is presented and renewal when testing occurs outside of the context of extinction1. If these tests continue to reveal low levels of performance, then the usual logic attributes this behavioral outcome to effects on reconsolidation rather than on extinction. This reasoning assumes that unmasking phenomena are defining properties of extinction and that if they do not occur, then extinction processes must not have been affected by the manipulation.
Behavioral unmasking measures may not be helpful for resolving effects on extinction or reconsolidation for several reasons. First, spontaneous recovery, reinstatement and contextual renewal are often incomplete12. Many factors determine whether behavior shows these phenomena, some of which remain to be elucidated. Second, even when behavior does not show recovery, it is often not clear how to interpret this null effect or what experimental comparisons are appropriate for making inferences about the amount of behavior that has or has not returned12. A common problem in behavioral studies is that repeated testing is often used to demonstrate persistence, but repeated testing itself is problematic because each test is an extinction session that may further weaken behavior. Subsequent tests will therefore overestimate the persistence of effects11. Third, and most important, theoretical approaches to extinction have long postulated that extinction can be enhanced even after behavior has reached asymptote.
Indeed, although Pavlov was the first to use the absence of spontaneous recovery of the behavior as a defining characteristic of extinction, he also noted that additional extinction trials after responding had ceased continued to result in learning. His evidence for this idea of “silent extinction beyond the zero” came from the observation that spontaneous recovery was weakened with additional extinction trials13. This observation was confirmed in later experiments14,15 and the idea that learning could occur beyond the point at which behavior ceases is central to the most influential associative learning theories of the last 50 years16. Many empirical studies have confirmed these ideas by demonstrating behavioral conditions that promote extinction, and weaken spontaneous recovery17, renewal18 and reinstatement after extinction19.
As “silent extinction beyond the zero” suggests, behavioral manipulations during extinction can lead to a more persistent form of extinction that resists various unmasking procedures. At the behavioral level, performance has reached a floor. But at the molecular and cellular levels, events must be continuing to allow for more stable and robust encoding of extinction. The challenge is elucidating a molecular mechanism that can underlie these persistent changes in behavior.
Epigenetics as mediator between genes and environment
Epigenetics has come to the forefront of many fields, including neuroscience20. One of the most compelling reasons to include epigenetics in an examination of key questions in neuroscience is that the epigenome reflects the interface between environmental experience and the genome. Nearly every epigenetic modification (from DNA methylation to histone acetylation) is a metabolite21, and therefore a consequence of interaction with the environment. Understanding how these environmental interactions affect the epigenome in stable, and sometimes in heritable generational and transgenerational, ways is of paramount importance for understanding basic mechanisms of experience-dependent neurobiological function that will inform applications in the clinic20,22–26.
Although the definition of epigenetics usually includes a heritable component (genomic imprinting being a classic example), neuroscientists studying learning and memory typically use the term without the heritable aspect, as neurons are post-mitotic. In neuro-science, mechanisms that directly modulate chromatin structure to regulate gene expression (DNA methylation, histone modification and nucleosome remodeling) are implicated as being important for neuronal plasticity and long-term changes in behavior.
At the molecular and cellular level, mechanisms that modulate chromatin structure can establish ‘molecular and cellular memories’27 that may maintain actively transcribed genes in a state of readiness (that is, permissive chromatin structure) for future action. At the cellular level, epigenetic mechanisms maintain inherited cell fate from mother to daughter cell. Establishment of different cell fates by cellular differentiation is carried out by defined patterns of gene expression that are put in place and stabilized by epigenetic mechanisms. This is often referred to as cellular memory27. What emerges from these seminal examples of molecular and cellular memory is that epigenetic mechanisms have the potential to establish, limit and control neuronal function in the service of synaptic plasticity and memory processes. It is important to note that there does not need to be a one-to-one correspondence between memory at the levels of cell fate and behavior. Indeed, memory at both levels is a heuristic term that reflects a combination of many different processes. Nonetheless, it will be important to determine whether neural epigenetic mechanisms can modulate learning and memory in unique and potentially persistent ways.
Epigenetic mechanisms underlying persistent memories
One way to examine the role of epigenetic mechanisms in learning and memory is to manipulate the enzymes that control the final step in modifying chromatin structure (Fig. 1). The best-studied examples thus far include histone acetyltransferases (HATs), which generally facilitate transcription, and histone deacetylases (HDACs), which generally silence transcription25,28. HATs and HDACs are found together at active genes and both are targeted to transcribed regions of active genes by phosphorylated RNA pol II29. Thus, HATs and HDACs are thought to be in a constant tug of war to acetylate or deacetylate lysine residues. Important to this discussion are the effects on synaptic plasticity and memory when the balance is offset by HDAC inhibition.
Numerous studies have found that HDAC inhibition enhances long-term potentiation (LTP) and memory, but it is important to consider how HDAC inhibition modulates memory formation and the persistence of memory. Recent experiments revealing persistent enhancements have characterized how persistent memory is mediated by the interaction between HDACs and associated epigenetic repressors. These experiments have used systemic administration of sodium butyrate, a class I HDAC inhibitor30, as well as site-specific administration of general and specific HDAC inhibitors, and even focal genetic manipulations. The key finding from these experiments is that a weak subthreshold training event (brief exposure to an object in a novel object recognition task) does not result in persistent memory unless that exposure is paired with HDAC inhibition31,32. The HDAC inhibition transformed a subthreshold learning experience into a lasting long-term memory that persisted to a test 7 d after learning, well beyond the time at which memory failed in vehicle-treated animals. This finding nicely parallels LTP experiments in which an HDAC inhibitor turned weak stimulation (which normally leads to a transcription-independent and transient form of LTP) into a robust form of transcription-dependent LTP33. Together, these results suggest that opening chromatin structure to facilitate gene expression has tremendous effects on the formation of long-lasting forms of potentiation and memory.
An open question is how long these memory effects persist. Bontempi and colleagues recently put forth a hypothesis regarding the early tagging of cortical networks as a necessary step for the formation of enduring associative memory34. They found that the early tagging of orbitofrontal cortex neurons is a prerequisite for the establishment of remote olfactory memory of the social transmission of food preference. Notably, signaling cascades shown to affect histone acetylation were found to participate in the tagging process. Furthermore, increasing histone acetylation via HDAC inhibition during the early, but not late, post-acquisition period of the task led to significantly improved remote memory retrieval probed 30 d after acquisition. This suggests that the epigenetic machinery engaged during learning may contribute to persistent long-term effects on memory processes.
It is clear from several recent studies that memory can be enhanced by the delivery of an HDAC inhibitor. The key issue now is determining the mechanism that underlies these behavioral effects. One conceptual framework, the molecular brake pad hypothesis, suggests that HDACs and associated co-repressors form complexes (molecular brake pads) that normally maintain specific genes in a silent state35. Strong activity-dependent signaling is required to temporarily remove these complexes to activate specific gene expression required for long-term memory formation. Several predictions from this hypothesis have been confirmed regarding the role of epigenetics in memory formation35,36. Briefly, epigenetic repressors (for example, HDACs) and activators (for example, HATs) are dynamically counter-opposed in the regulation of coordinate gene expression required for neuronal plasticity. They regulate gene expression in precise temporal windows, the levels of gene expression and duration of gene expression. Thus, genetic and pharmacological manipulations that affect epigenetic enzymes have critical consequences on very precise regulation of gene expression required for neuronal plasticity and memory. For example, loss of HDAC3 results in the prolonged expression of immediate early genes beyond the point at which they would normally be turned off after a learning event32. How these changes in initial expression and duration of expression lead to robust neuronal plasticity remains to be elucidated. Furthermore,understanding their contribution to other memory processes (beyond memory formation), such as extinction, is of great importance for both basic understanding and clinical relevance.
Targeting epigenetic mechanisms during extinction
The demonstration from object recognition experiments that HDAC inhibition transforms a subthreshold learning event into a persistent long-term memory suggests that this type of epigenetic manipulation can change the nature of long-term memory. Does the same hold true for extinction, in which changes in behavior are often transient? Similar to the object recognition studies, several experiments have shown that HDAC inhibition could turn a behavioral experience that does not normally result in long-term extinction into an experience that causes a lasting change in behavior29,37–42. Notably, in those studies that have examined long-term effects, the effects on extinction generally are persistent (but see ref. 40). For example, in the case of extinction of cocaine-induced conditioned place preference, HDAC inhibition generated a form of extinction that resulted in a preference that could not be reinstated by post-extinction cocaine administration39. These results have potential translational implications, as they suggest that learning and memory pathways can be modulated by epigenetic mechanisms in a way that overrides the actions of cocaine on the reward pathways (which are known to overlap with learning and memory pathways).
Although systemic approaches are more clinically relevant and necessary for preclinical evaluation, recent experiments are moving from these effects of systemic administration of general HDAC inhibitors into more regionally specific effects of intra-cranial administration of these drugs, as well as more molecularly specific effects of selective HAT and HDAC inhibitors. For example, an HDAC inhibitor delivered to the hippocampus facilitates extinction of contextual fear and increases histone acetylation and gene expression specifically in the infralimbic cortex, which is a critical region for establishing new memories during extinction. These effects were confirmed by similar behavioral extinction effects when the HDAC inhibitor was injected into the infralimbic cortex, but not into the prelimbic cortex40. The infralimbic cortex is also critical for the activity of HATs in extinction memory, in which p300 and p300/CBP-associated factor (PCAF) have been shown to regulate extinction memory41,42. In particular, PCAF activity in the infralimbic cortex is required for the extinction, but not the acquisition, of fear42. Thus, both HDACs and HATs appear to be important in the infralimbic cortex with regard to extinction learning.
Understanding the particular roles that specific HDACs have in different learning processes is critical for the ultimate goal of identifying epigenetic signatures associated with one memory or another (Box 1). Recently, HDAC1 inhibition was shown to impair extinction learning, which was associated with a decrease in c-Fos expression that was correlated with increased HDAC1 occupancy at the promoter of c-Fos, as well as decreased histone H3 lysine 9 (H3K9) acetylation43. Conversely, overexpression of wild-type HDAC1 facilitated extinction in that study. These are exciting findings that suggest a key role for HDAC1 specifically in extinction; future studies examining whether these manipulations result in a persistent effect will be critical for a comprehensive account of the role of HDAC1 in long-term mechanisms.
Together, studies examining the effects of modulating extinction learning via HDAC inhibition have shown that HDACs have a central role in regulating the gene expression required for extinction consolidation. Furthermore, similar effects are observed with extinction to those with the initial acquisition/consolidation of a memory in which HDAC inhibition (or focal deletion of an individual HDAC)32 transforms a subthreshold learning event into a robust long-term memory that is persistent. HDAC inhibition (even specific inhibition of only HDAC3)44 can enhance extinction consolidation and generate a form of extinction that is persistent (blocks reinstatement completely). These findings provide a clear example of a persistent behavioral effect that is consistent with enhanced extinction.
Epigenetics and the extinction-reconsolidation problem
It is clear that modulation of epigenetic mechanisms can lead to persistent changes at the cellular and behavioral levels. It also is clear that extinction can lead to lasting changes that result in weakened recovery, renewal and reinstatement. If impairments in reconsolidation and enhancements in extinction lead to similar behavioral predictions, how can we distinguish between the different effects? Although the absence of unmasking phenomena will not distinguish between the two accounts, there are potential behavioral solutions to this theoretical problem. These solutions, of course, depend on theoretical assumptions about mechanisms of reconsolidation, extinction under normal circumstances and enhanced extinction induced by epigenetic manipulations.
Although many different processes can explain the absence of behavior, behavioral experiments that are informed by a particular theoretical perspective are useful for distinguishing between potential effects of epigenetic manipulations on extinction or reconsolidation. As noted, many accounts of behavioral effects have relied on assumptions about what a drug is doing at the cellular and molecular level and about what sorts of theoretical processes underlie extinction and reconsolidation. A very basic assumption is that drugs, such as HDAC inhibitors, that promote transcription and translation are memory enhancing and drugs that impair these processes are memory impairing. Thus, if the hypothesis is that an HDAC inhibitor promotes extinction as a result of its general memory enhancing effects, then having that drug active during extinction should promote memory of extinction, but it also should promote memory outside of extinction while the drug is still altering histone acetylation.
A recent experiment tested this idea by asking whether an HDAC inhibitor will simultaneously enhance extinction of cocaine-induced conditioned place preference (CPP) while promoting memory in an object recognition task44. They found that a selective HDAC3 inhibitor (HDAC3i) facilitated extinction of a previously established cocaine-induced CPP, while simultaneously enhancing the long-term formation of a concurrent object location memory in the same subjects. During extinction consolidation, HDAC3i promoted a distinct pattern of histone acetylation and gene expression in the infralimbic cortex, hippocampus and nucleus accumbens44. The ability of HDAC3i to simultaneously enhance extinction of one memory while facilitating acquisition and consolidation of a different memory suggests that the effects on extinction were likely not the results of impairments in reconsolidation.
The broader challenge at a theoretical level is to determine what mechanisms underlie extinction and how epigenetic changes in these mechanisms could drive persistent extinction. Despite the simple behavioral outcomes associated with extinction, the learning process is complicated, with multiple mechanisms potentially driving the loss of behavior. Indeed, reconsolidation is often characterized as a process that antagonizes extinction, yet alterations in various aspects of the original memory are a fundamental property of extinction that theories over the years have addressed10,13,45. A noteworthy example is provided by Delamater8 who recently described that the multidimensional qualities of an unconditioned stimulus (for example, hedonic, emotional and sensory) could each become encoded as part of a memory, depending on the behavioral conditions. Extinction may alter processing of some of those aspects, but not all, which in turn will produce different outcomes on behavior. This rationale suggests that retrieval-induced plasticity involves the coordinated actions of multiple systems and that a manipulation could affect any of several aspects of motivation, emotion and memory, with any one of those aspects altering extinction10,46. Equally important to note, much of the work cited above was performed using CPP and it will be useful to examine effects of HDAC inhibition on extinction of compulsive drug-seeking and reward29. In any case, behavior alone may not be enough to disentangle extinction and reconsolidation effects.
An epigenetic signature of persistent extinction?
Epigenetic mechanisms can establish the stability and persistence of cell function. This has lead molecular biologists to describe the effects of epigenetic modifications in terms of creating a cellular memory (as in cell-fate decisions). Identifying the links between cellular memory and memory at the neural systems level will be essential for a complete characterization as neurobiologists continue to discover how neural epigenetic modifications result in long-term memory. Although the current body of evidence supports the idea that the epigenetic machinery has a pivotal role in establishing persistent forms of memory, including extinction memory, there is currently no demonstration of a clear epigenetic signature of persistent extinction that would help distinguish it from blocked reconsolidation. However, a very recent examination of synaptic plasticity thresholding may provide a possibility as molecular and cellular tools evolve.
Synaptic plasticity thresholding refers to the finding that adult spines have different thresholds for plasticity induction such that temporal spacing of stimulation yields increasingly higher potentiation47. Kramár et al.47 found that the increased potentiation observed with spaced simulation correlated with the additional recruitment of synapses that were missed by a single stimulation bout. This not only provides a cellular correlate of spaced training effects on memory, but also suggests that cognitive enhancers facilitate LTP and memory by engaging synapses with higher thresholds for induction (as proposed in ref. 48). This is an exciting idea that relates to other forms of enhanced and persistent memory.
It is possible that the enhanced synaptic plasticity observed in the presence of HDAC inhibition lowers these thresholds, resulting in an increased number of potentiated synapses. Thus, subthreshold learning events (as observed in behavioral experiments) or single TBS stimulation (as observed in LTP experiments) can lead to robust LTP consolidation and, ultimately, persistent long-term memory. When this occurs during extinction, the extinction memory is robust and persistent, resulting in the absence of unmasking phenomena, which at the behavioral level appears the same as if one had blocked reconsolidation. However, blocking reconsolidation would not be associated with an increased number of potentiated synapses in the brain region(s) involved in extinction, and there is very good evidence that the degree of spine remodeling (increased rate of spine formation) induced by extinction strongly correlates with the expression of extinction49. A hypothetical epigenetic signature would therefore be a combination of specific histone modifications and nucleosome remodeling (and other epigenetic machinery affecting chromatin structure) at a subset of genes necessary for the consolidation of robust potentiation, and this would correlate with an increased number of potentiated synapses in those activated neurons. Unfortunately, the cell-specific analyses do not currently exist to match epigenomic changes in a cell with synaptic analysis of the same cell, but avenues for further work are clear as these analyses become more sophisticated.
The potential of an epigenetic approach to extinction
An epigenetic approach to extinction provides a cellular mechanism for the development of Pavlov’s notion of silent extinction beyond the zero, a persistent form of extinction that challenges long-held assumptions about the fundamental behavioral properties of extinction. Behavioral evidence alone has been unconvincing when trying to disentangle theoretical accounts that appeal to extinction and reconsolidation processes. Molecular evidence offered in favor of impairments in reconsolidation or related impairments in memory erasure are almost exclusively negative, in the sense that the evidence consists of a reversal of a process to a basal state (for example, receptor internalization or depotentiation) or a loss of previously formed morphological changes (for example, dendritic spine remodeling). This molecular evidence corresponds nicely to the persistent behavioral effects because, at the molecular and behavioral levels, the memory appears to be eliminated. The challenge is that the absence of behavior or the absence of a molecular signature is open to many interpretations. The potential of an epigenetic approach to extinction is that, ultimately, positive evidence at the molecular level may be used to support negative evidence at the behavioral level. The understanding of how epigenetic machinery modulates memory formation and persistent memory processes, including extinction, is important for both basic knowledge and translating this knowledge into the completely untapped frontier of CNS epigenetic therapeutics.
ACKNOWLEDGMENTS
We thank K. Bieszczad and M. Malvaez for helpful comments. Preparation of this manuscript was supported by grants from the US National Institutes of Health (DA018165, DA025922, DA031989, MH077111 and MH081004). The order of authorship was determined by lot.
Footnotes
COMPETING FINANCIAI INTERESTS
The authors declare no competing financial interests.
References
- 1.Maren S. Seeking a spotless mind: extinction, deconsolidation and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quirk GJ, et al. Erasing fear memories with extinction training. J. Neurosci. 2010;30:14993–14997. doi: 10.1523/JNEUROSCI.4268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 2012 May 26; doi: 10.1007/s00213-012-2750-9. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberini CM. The role of protein synthesis during the labile phases of memory: revisiting the skepticism. Neurobiol. Learn. Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl.) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- 7.Qi Z, Gold PE. Intrahippocampal infusions of anisomycin produce amnesia: contribution of increased release of norepinephrine, dopamine and acetylcholine. Learn. Mem. 2009;16:308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delamater AR. Issues in the extinction of specific stimulus-outcome associations in Pavlovian conditioning. Behav. Processes. 2012;90:9–19. doi: 10.1016/j.beproc.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lattal KM, Lattal KA. Facets of Pavlovian and operant extinction. Behav. Processes. 2012;90:1–8. doi: 10.1016/j.beproc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Rev. Neurosci. 2010;21:1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stafford JM, Lattal KM. Is an epigenetic switch the key to persistent extinction? Neurobiol. Learn. Mem. 2011;96:35–40. doi: 10.1016/j.nlm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rescorla RA. Spontaneous recovery. Learn. Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- 13.Pavlov IP. Conditioned Reflexes: an Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brogden WJ, Lipman EA, Culler E. The role of incentive in conditioning and extinction. Am. J. Psychol. 1938;51:109–117. [Google Scholar]
- 15.Reynolds GS. Operant extinction near zero. J. Exp. Anal. Behav. 1964;7:173–176. doi: 10.1901/jeab.1964.7-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current research and Theory. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- 17.Leung HT, Westbrook RF. Spontaneous recovery of extinguished fear responses deepens their extinction: a role for error-correction mechanisms. J. Exp. Psychol. Anim. Behav. Process. 2008;34:461–474. doi: 10.1037/0097-7403.34.4.461. [DOI] [PubMed] [Google Scholar]
- 18.Denniston JC, Chang RC, Miller RR. Massive extinction treatment attenuates the renewal effect. Learn. Motiv. 2003;34:68–86. [Google Scholar]
- 19.Rescorla RA. Deepened extinction from compound stimulus presentation. J. Exp. Psychol. Anim. Behav. Process. 2006;32:135–144. doi: 10.1037/0097-7403.32.2.135. [DOI] [PubMed] [Google Scholar]
- 20.Lester BM, et al. Behavioral epigenetics. Ann. NY Acad. Sci. 2011;1226:14–33. doi: 10.1111/j.1749-6632.2011.06037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katada S, Imhof A, Sassone-Corsi P. Connecting threads: epigenetics and metabolism. Cell. 2012;148:24–28. doi: 10.1016/j.cell.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci. 2012;35:145–155. doi: 10.1016/j.tins.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Zovkic IB, Sweatt JD. Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology. 2013;38:77–93. doi: 10.1038/npp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day JJ, Sweatt JD. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology. 2012;37:247–260. doi: 10.1038/npp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakovcevski M, Akbarian S. Epigenetic mechanisms in neurological disease. Nat. Med. 2012;18:1194–1204. doi: 10.1038/nm.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner BM. Cellular memory and the histone code. Cell. 2002;111:285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- 28.Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, et al. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2010;35:913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilgore M, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc. Natl. Acad. Sci. USA. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuown SC, et al. HDAC3 is a critical negative regulator of long-term memory formation. J. Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecsey CG, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J. Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesburguères E, et al. Early tagging of cortical networks is required for the formation of enduring associative memory. Science. 2011;331:924–928. doi: 10.1126/science.1196164. [DOI] [PubMed] [Google Scholar]
- 35.McQuown SC, Wood MA. HDAC3 and the molecular brake pad hypothesis. Neurobiol. Learn. Mem. 2011;96:27–34. doi: 10.1016/j.nlm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel-Ciernia A, Wood MA. Molecular brake pad hypothesis: pulling off the brakes for emotional memory. Rev. Neurosci. 2012 Aug 24; doi: 10.1515/revneuro-2012-0050. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav. Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bredy TW, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol. Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol. Psychiatry. 2012;72:25–33. doi: 10.1016/j.biopsych.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marek R, et al. Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. J. Neurosci. 2011;31:7486–7491. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei W, et al. p300/CBP-associated factor selectively regulates the extinction of conditioned fear. J. Neurosci. 2012;32:11930–11941. doi: 10.1523/JNEUROSCI.0178-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahari-Javan S, et al. HDAC1 regulates fear extinction in mice. J. Neurosci. 2012;32:5062–5073. doi: 10.1523/JNEUROSCI.0079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malvaez M, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc. Natl. Acad. Sci. USA. doi: 10.1073/pnas.1213364110. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker KD, McNally GP, Richardson R. d-cycloserine does not facilitate fear extinction by reducing conditioned stimulus processing or promoting conditioned inhibition to contextual cues. Learn. Mem. 2012;19:461–469. doi: 10.1101/lm.026674.112. [DOI] [PubMed] [Google Scholar]
- 46.Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramár EA, et al. Synaptic evidence for the efficacy of spaced learning. Proc. Natl. Acad. Sci. USA. 2012;109:5121–5126. doi: 10.1073/pnas.1120700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch G, Kramar EA, Babayan AH, Rumbaugh G, Gall CM. Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology. 2013;64:27–36. doi: 10.1016/j.neuropharm.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodeling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 50.Lahm A, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl. Acad. USA. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]