Abstract
Paraoxonase 3 (PON3) is a member of the PON family, which includes PON1, PON2, and PON3. Recently, PON3 was shown to prevent the oxidation of low-density lipoprotein in vitro. To test the role of PON3 in atherosclerosis and related traits, 2 independent lines of human PON3 transgenic (Tg) mice on the C57BL/6J (B6) background were constructed. Human PON3 mRNA was detected in various tissues, including liver, lung, kidney, brain, adipose, and aorta, of both lines of Tg mice. The human PON3 mRNA levels in the livers of PON3 Tg mice were 4- to 7-fold higher as compared with the endogenous mouse Pon3 mRNA levels. Human PON3 protein and activity were detected in the livers of Tg mice as well. No significant differences in plasma total, high-density lipoprotein, and very-low-density lipoprotein/low-density lipoprotein cholesterol and triglyceride and glucose levels were observed between the PON3 Tg and non-Tg mice. Interestingly, atherosclerotic lesion areas were significantly smaller in both lines of male PON3 Tg mice as compared with the male non-Tg littermates on B6 background fed an atherogenic diet. When bred onto the low-density lipoprotein receptor knockout mouse background, the male PON3 Tg mice also exhibited decreased atherosclerotic lesion areas and decreased expression of monocyte chemoattractant protein-1 in the aorta as compared with the male non-Tg littermates. In addition, decreased adiposity and lower circulating leptin levels were observed in both lines of male PON3 Tg mice as compared with the male non-Tg mice. In an F2 cross, adipose Pon3 mRNA levels inversely correlated with adiposity and related traits. Our study demonstrates that elevated PON3 expression significantly decreases atherosclerotic lesion formation and adiposity in male mice. PON3 may play an important role in protection against obesity and atherosclerosis.
Keywords: atherosclerosis, obesity, genetics
Atherosclerosis is characterized by chronic inflammation in the artery wall.1 The chronic inflammation is thought to be caused partly by hypercholesterolemia and subsequent oxidation of low-density lipoprotein (LDL) in the artery wall.2 When studied using in vitro assays, oxidized LDL is capable of inducing endothelial expression of adhesion molecules, chemokines, and cytokines.2 As a result of the endothelial inflammatory response, monocytes transmigrate into the subendothelial space of the artery wall, where they differentiate into macrophages. Macrophages can further oxidize LDL and take up highly oxidized LDL through scavenger receptors such as scavenger receptor class A and CD36. Accumulation of lipids in the macrophages transforms them into foam cells, the constituents of early fatty streak lesion.2
Obesity is an independent risk factor for diabetes and cardiovascular disease.3 Recent studies have shown that obesity is associated with accumulation of macrophages in adipose tissue4 and increased levels of proinflammatory molecules such as tumor necrosis factor-α, 5 Interleukin-6,6 and monocyte chemoattractant protein (MCP)-1.7 The obese state also alters the expression of circulating adipokines such as leptin and adiponectin.8 Circulating leptin levels correlate positively with body fat mass.9 In addition, recent studies suggest that leptin exerts proinflammatory and immunepotentiating effects.8 Injection of recombinant leptin has been shown to promote atherosclerosis and thrombosis in apolipoprotein E knockout (apoE KO) mice.10 Circulating adiponectin level is reduced in obesity. Transgenic mice expressing the globular domain of adiponectin are more resistant to atherosclerosis.11 Therefore, the increase in circulating proinflammatory molecules as well as leptin, and decrease in adiponectin, all of which are associated with obesity, may contribute to increased risk for cardiovascular disease.
The paraoxonase (PON) gene family contains 3 members, PON1, PON2, and PON3.12 The PON1 protein is expressed primarily in the liver and is secreted into circulation where it associates with high-density lipoprotein (HDL).13 In vitro studies have demonstrated that PON1 inhibits LDL oxidation.14–16 Epidemiological studies show that both PON1 protein levels and PON1 gene polymorphisms correlate with cardiovascular disease. 17–19 Animal studies also demonstrate that PON1 protects against lipoprotein oxidation and atherosclerosis. 20–22
PON3 shares ≈60% homology in amino acid sequence with PON1. Both rabbit and human PON3 proteins are detected on HDL.23,24 Purified rabbit PON3 prevents copper-induced LDL oxidation in vitro.23 Human PON3 protects LDL against cell-mediated accumulation of lipid hydroperoxides and inactivates oxidized phospholipids capable of inducing monocyte chemotactic activity.24 Therefore, in vitro evidence suggests that PON3 may also be atheroprotective. To understand the role of PON3 in atherosclerosis and related traits, we generated and characterized transgenic mice overexpressing human PON3. Our data suggest that PON3 protects against both obesity and atherosclerosis in male mice.
Materials and Methods
An expanded Material and Methods section is available in the online data supplement at http://circres.ahajournals.org. Animals were housed in a specific pathogen-free vivarium at UCLA. All procedures were carried out in accordance with current National Institutes of Health guidelines and were approved by the Animal Research Committee at UCLA.
Statistical Analyses
Student’s t test was used for analyzing experimental data. Linear regression analysis was performed to calculate correlation coefficient (r) and significance (P) values between adipose Pon3 mRNA level and obesity-related traits in F2 mice.
Results
Human PON3 Tg Mice
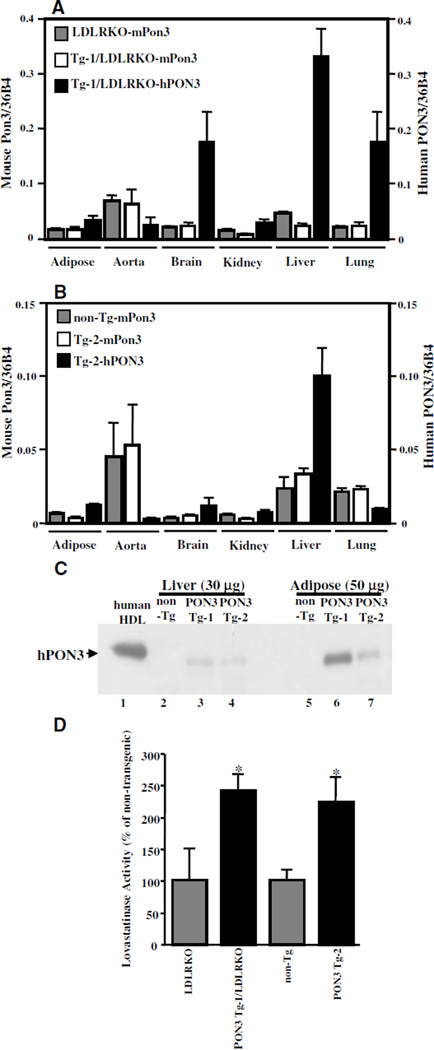
Two independent lines of human PON3 Tg mice, designated as PON3 Tg-1 and PON3 Tg-2, were generated and estimated by Southern blot analysis to carry ≈50 and 10 copies of the transgene, respectively (data not shown). From breeding records, we noticed that the female PON3 Tg-1 mice transmitted the transgene equally to male (10/19 [53%] were transgenic) and female (14/27 [52%] were transgenic) offspring. However, the male PON3 Tg-1 mice transmitted the transgene only to female (14/14 [100%] were transgenic) but not male offspring (0/21 [0%] transgenic). Therefore, we concluded that the transgene is inserted into the X chromosome in PON3 Tg-1 mice. Female and male PON3 Tg-2 mice, in contrast, transmitted the transgene equally to their male and female offspring (data not shown), suggesting an autosomal integration site of the transgene. The Tg-1 line was also bred on to the LDL receptor (LDLR)-null background. The transgene was maintained in a hemizygous state throughout the study. Human PON3 mRNA was detected in adipose tissue, aorta, brain, kidney, liver, and lung of both lines of transgenic mice according to quantitative PCR analysis (Figure 1A and 1B), with highest expression levels detected in the liver. The mRNA levels of human PON3 in the livers of male PON3 Tg-1 and PON3 Tg-2 were approximately 7-fold and 4-fold higher than the endogenous mouse Pon3 mRNA levels, respectively, as determined by quantitative RT-PCR. The human PON3 protein was detected in the livers and adipose tissues of transgenic mice by immunoblotting (Figure 1C). However, human PON3 protein was not detectable in the plasma or HDL of transgenic mice (data not shown), suggesting that the human PON3 protein remains associated with cells and is not secreted into circulation in mice. Furthermore, we could not detect mouse PON3 in HDL or plasma either (data not shown), suggesting a species difference between human and mouse in PON3 distribution.
Figure 1.
Expression of human PON3 mRNA and protein in transgenic mice. cDNA reverse-transcribed from total RNA isolated from various tissues of the male PON3 Tg-1/LDLRKO and LDLRKO mice (A) or from male PON3 Tg-2 and non-Tg mice (B) were analyzed by quantitative PCR using specific primers for mouse Pon3 (mPon3), human PON3 (hPON3), or mouse 36B4 cDNAs. The ratios of mouse Pon3 or human PON3 cDNAs to mouse 36B4 cDNA are calculated. The data shown are mean and SE (n=2 to 5). C, Human PON3 immunoblotting. Ten micrograms of human HDL and 30 and 50 µg of membrane protein extracts from male mouse livers and adipose tissue, respectively, were fractionated by SDS-PAGE and transferred onto a nitrocellulose membrane. Immunoblotting using an antihuman PON3 antibody was then performed. D, Lactonase assay was performed using lovastatin as the substrate, and liver membrane protein extracts were isolated from male PON3 Tg and non-Tg littermates. The relative lovastatinase activity of the PON3 Tg mice as compared with the non-Tg littermates was expressed. *P<0.05 vs non-Tg littermates.
The expression level of human PON3 mRNA in the livers of female and male PON3 Tg mice were also compared. We observed that the human PON3 mRNA levels in the livers of female PON3 Tg-1 mice were ≈50% lower as compared with those of the male PON3 Tg-1 mice (0.49±0.08 versus 1.00±0.2, P<0.05, n=3 to 4 mice for each sex). In contrast, we did not observe any significant difference in human PON3 mRNA levels between female and male PON3 Tg-2 mice (1.31±0.11 versus 1.00±0.09, n=3 to 5 mice for each sex). Because the human PON3 transgene is located on the X chromosome in PON3 Tg-1 mice, the decreased human PON3 expression in the female PON3 Tg-1 mice relative to male PON3 Tg-1 is most likely attributable to random X inactivation in the female mice.
Lovastatin Hydrolysis Activity in the Livers of Human PON3 Tg Mice
To date, the statin lactones lovastatin, mevastatin, and simvastatin are the only known exogenous substrates that are specifically hydrolyzed by PON3 but not PON1 or PON2.23,25 Membrane protein extracts isolated from the livers of human PON3 Tg-1 and Tg-2 mice exhibited 240% and 220% higher lovastatin hydrolysis activity, respectively, as compared with those of the nontransgenic littermates (Figure 1D). Because the high-performance liquid chromatography method that we used for determination of “lovastatinase” activity did not measure the initial reaction rate but measured the amount of hydrolysis product generated in 2 hours, this assay was only semiquantitative. This may be the reason why we observed a greater than 4-fold increase in PON3 mRNA levels, but only a 2-fold increase in PON3 activity levels in PON3 Tg livers, as compared with non-Tg livers.
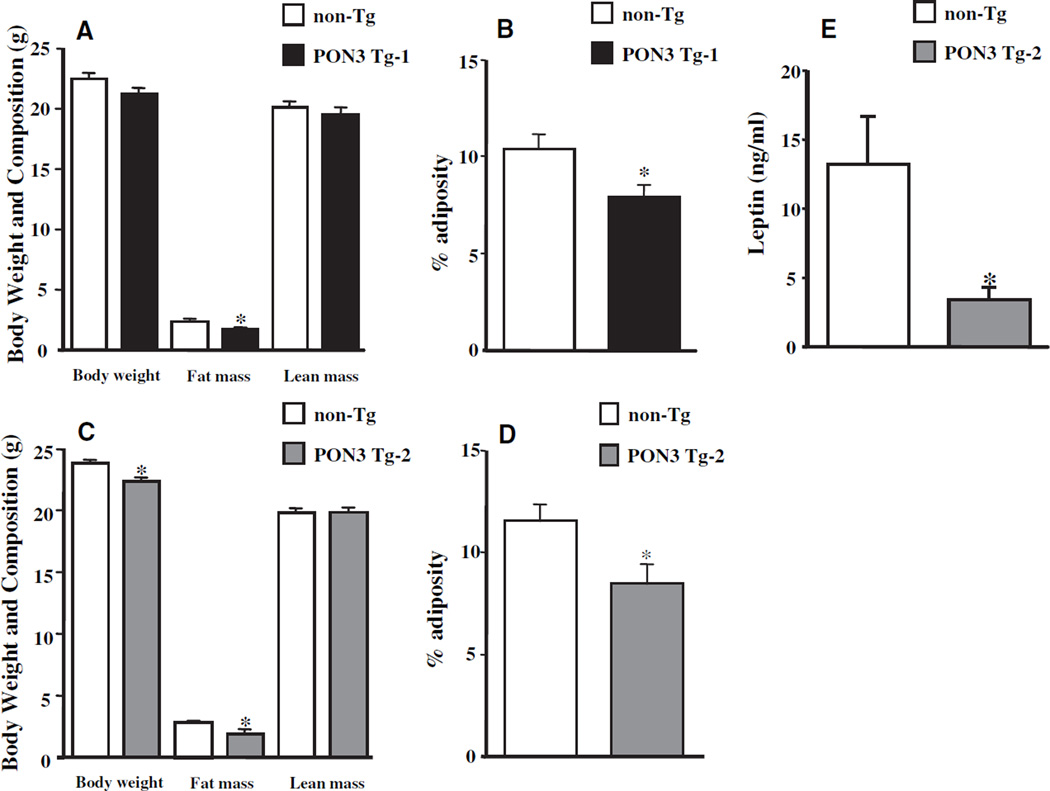
Male PON3 Transgenic Mice Exhibited Decreased Adiposity
Body weight and composition were examined in 3-month-old mice maintained on a chow diet. Body weight of the male PON3 Tg-1 mice was 6% lower as compared with those of the male non-Tg littermates, but it did not reach statistical significance (P=0.1, Figure 2A). The fat mass of PON3 Tg-1 mice was 30% lower than that of the non-Tg mice (P<0.05, Figure 2A), whereas no difference in lean mass was observed (Figure 2A). Adiposity was also decreased by 24% in the PON3 Tg-1 mice as compared with the non-Tg littermates (P<0.05, Figure 2B). We did not observe any differences in body weight, adiposity (data not shown), or circulating leptin levels (Table I in the online data supplement) between female PON3 Tg-1 and non-Tg mice. Interestingly, the body weight, fat mass, and adiposity of male PON3 Tg-2 mice were also significantly decreased by 6% (P<0.05), 33% (P<0.05), and 28% (P=0.01), respectively, as compared with those of the male non-Tg littermates (Figure 2C and 2D). Consistent with lower adiposity in the PON3 Tg-2 mice, plasma leptin levels of PON3 Tg-2 mice were 75% lower (P=0.05) as compared with those of the non-Tg littermates (Figure 2E). We did not observe significant differences in body weight, body composition (data not shown), or plasma leptin levels (supplemental Table I) between female PON3 Tg-2 and non-Tg littermates.
Figure 2.
Body weight and composition of male PON3 Tg and non-Tg mice on B6 background. Three-month-old male PON3 Tg and non-Tg littermates maintained on a low-fat chow diet were fasted for 16 hours before body weight, fat mass, and lean mass were measured. A, PON3 Tg-1 vs non-Tg. C, PON3 Tg-2 vs non-Tg. The adiposity was calculated as (fat mass/body weight)×100%. B, PON3 Tg-1 vs non-Tg. D, PON3 Tg-2 vs non-Tg. E, Plasma leptin levels of male PON3 Tg-2 and non-Tg littermates were determined by ELISA. The data shown are mean and SE. Sample sizes are 12 mice for each genotype group for A and B, 9 to 19 for C and D, and 5 to 7 for E. *P≤0.05 PON3 Tg vs non-Tg littermates.
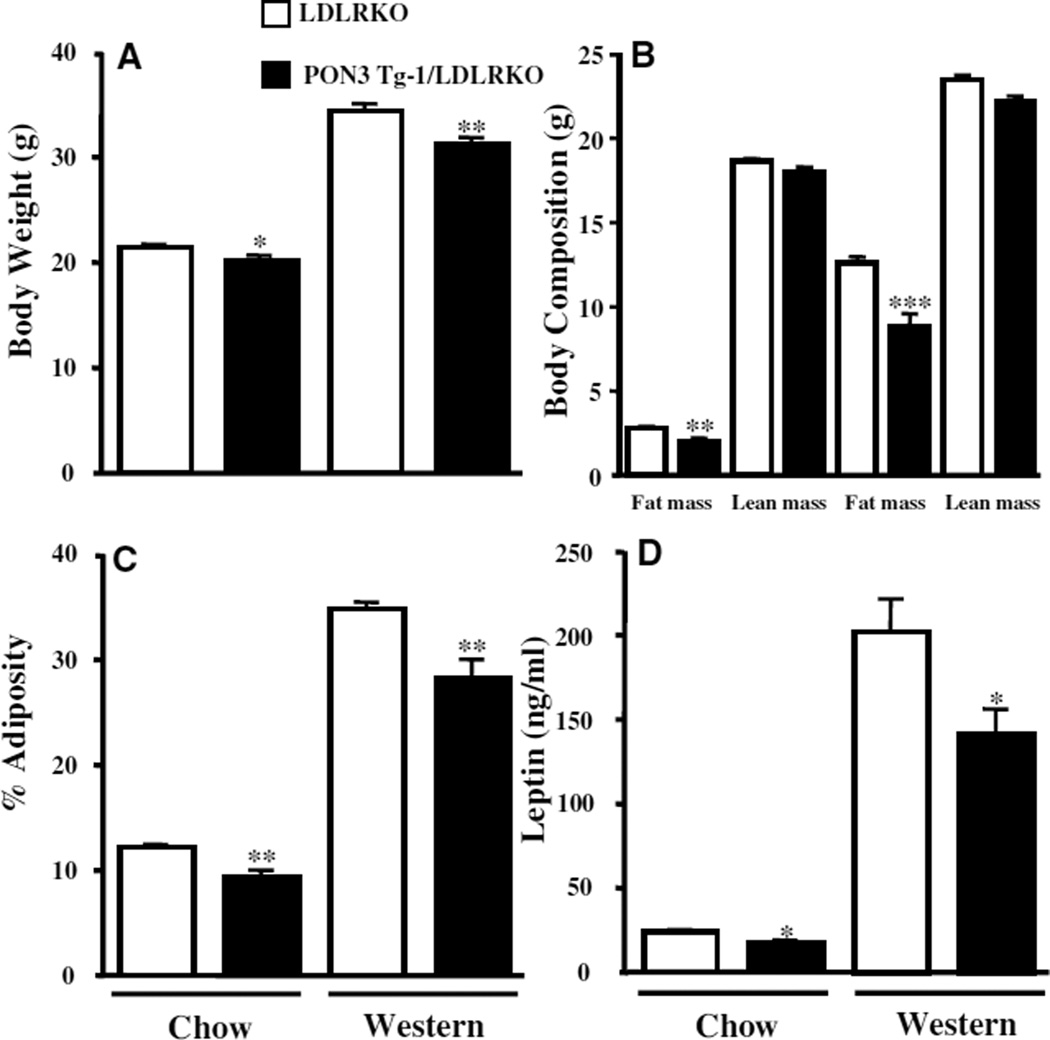
Three-month-old male PON3 Tg-1/LDLRKO mice, maintained on a chow diet, exhibited significantly decreased body weight (6%, P<0.05), fat mass (27%, P<0.01), and adiposity (23%, P<0.01), but exhibited no change in lean mass, as compared with those of the male LDLRKO littermates (Figure 3A through 3C). Daily food intake was similar between male PON3 Tg-1/LDLRKO and LDLRKO mice (4.68±0.1 g versus 4.15±0.2 g). Plasma leptin levels of the male PON3 Tg-1/LDLRKO mice were also decreased by 29% (P<0.05) as compared with those of the male LDLRKO mice (Figure 3D). After 8 weeks of feeding of a “Western diet,” the male PON3 Tg-1/LDLRKO mice exhibited significantly lower body weight (10%, P<0.01), fat mass (30%, P<0.001), and adiposity (21%, P<0.01), without a significant difference in lean mass, as compared with those of the male LDLRKO mice (Figure 3A through 3C). Plasma leptin levels were also decreased by 30% (P<0.05) in the male PON3 Tg-1/LDLRKO mice as compared with those of the male LDLRKO mice on the Western diet (Figure 3D). We did not observe any significant differences in body weight, body composition, or plasma leptin levels between female PON3 Tg-1/LDLRKO and LDLRKO mice maintained on either the chow or Western diets (data not shown and supplemental Table I). Our data suggest that PON3 overexpression attenuates the development of obesity and that this effect is specific to male but not female PON3 transgenic mice.
Figure 3.
Body weight, body composition, and plasma leptin levels of male PON3 Tg-1/LDLRKO and LDLRKO mice. Three-month-old male PON3 Tg-1/LDLRKO and LDLRKO littermates maintained on a low-fat chow diet or 5-month-old mice that had been maintained on a high-fat Western diet for 8 weeks were fasted for 16 hours before body weight (A), fat mass, and lean mass (B) were measured. C, The adiposity was calculated. D, Plasma leptin levels of these mice were determined by ELISA. The data shown are mean and SE. Sample sizes are 12 to 24 for A and B, 12 to 19 for C, and 10 to 14 for D. *P<0.05, **P<0.01, PON3 Tg-1/LDLRKO vs LDLRKO.
Plasma Lipids, Glucose, and Insulin Levels of PON3 Tg Mice
Male PON3 Tg-1 mice did not exhibit any significant differences in plasma total, HDL, or VLDL/LDL cholesterol or triglyceride or glucose levels as compared with those of the male non-Tg mice on either chow or atherogenic diets (supplemental Table II). On either chow or atherogenic diets, the female PON3 Tg-1 mice exhibited similar plasma lipids and glucose levels as compared with those of the female non-Tg littermates, except for a small increase in VLDL/LDL levels in female PON3 Tg mice when maintained on the chow diet (supplemental Table II). There were no significant differences in plasma lipids or glucose levels between sex- and diet-matched PON3 Tg-2 and non-Tg littermates (supplemental Table II).
There was no significant difference in plasma lipids or glucose or insulin levels between male PON3 Tg-1/LDLRKO and LDLRKO mice maintained on chow or Western diets (supplemental Table III). There was a small increase in total and VLDL/LDL cholesterol levels in the female PON3 Tg-1/LDLRKO mice as compared with the female LDLRKO mice on the chow diet (supplemental Table III). There was also a small increase in HDL cholesterol in the female PON3 Tg-1/LDLRKO mice as compared with the LDLRKO mice on the Western diet (supplemental Table III). Therefore, elevated PON3 expression appears to have minor effect on fasting plasma lipids levels.
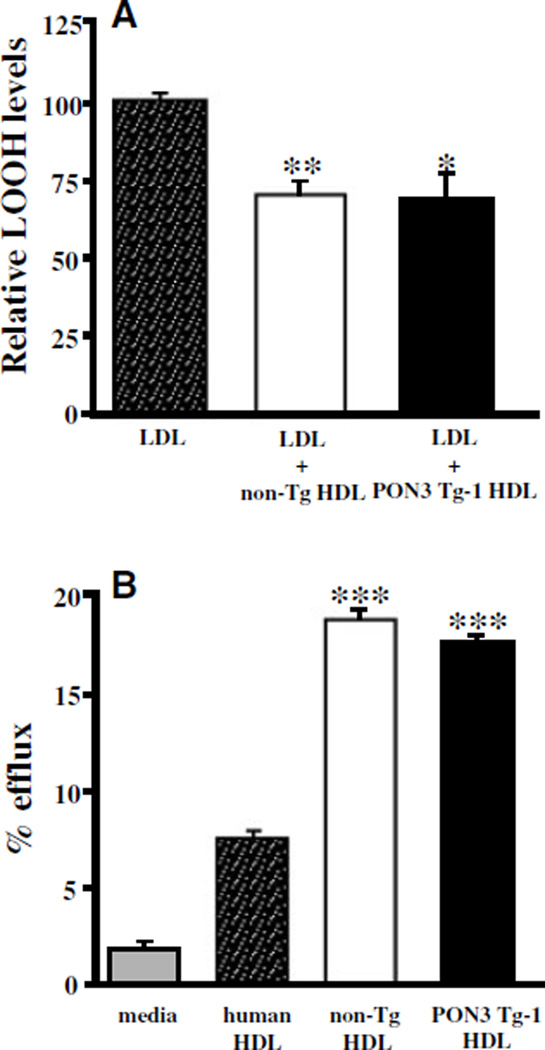
HDL Functions
We determined the ability of HDL to prevent LDL oxidation and mediate cholesterol efflux from macrophages. HDL isolated from the PON3 Tg-1 mice protected against copper-induced LDL oxidation to the same extent, as did HDL isolated from the non-Tg littermates (Figure 4A). Furthermore, there was no significant difference in the abilities of PON3 Tg-1 and non-Tg HDL to mediate cholesterol efflux from macrophages (Figure 4B).
Figure 4.
Functional evaluation of HDL from PON3 Tg and non-Tg mice. A, The ability of HDL to prevent LDL oxidation. Human LDL was incubated in the presence or absence of HDL isolated from non-Tg and PON3 Tg-1 mice in the presence of 5 µmol/L CuSO4 for 3 hours at 37°C to induce oxidation. At the end of incubation, the amount of lipid hydroperoxides (LOOH) in each sample was measured. The data shown are mean and SE. *P<0.01 vs LDL; **P<0.001 vs LDL. B, The ability of HDL to mediate cholesterol efflux from macrophage. Cholesterol-loaded macrophages were incubated with media alone or various HDL for 4 hours, as described under Materials and Methods. The data shown are mean and SE. ***P<0.0001 vs media.
Atherosclerotic Lesion Formation in PON3 Tg Mice
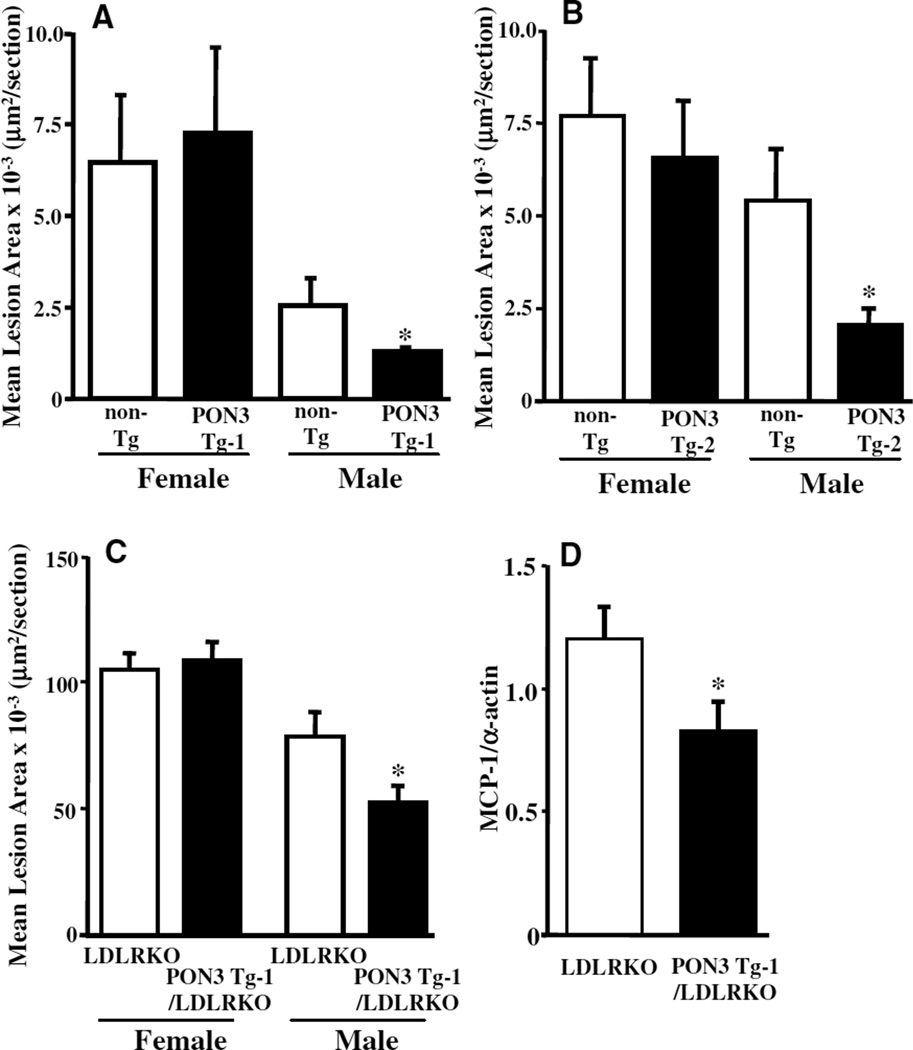
Atherosclerotic lesion areas at the aortic root of male PON3 Tg-1 mice fed an atherogenic diet for 15 weeks were decreased by 47% as compared with those of the non-Tg littermates (P<0.05), whereas there was no significant difference in lesion size between female PON3 Tg-1 and non-Tg mice (Figure 5A). Similarly, atherosclerotic lesion areas of male PON3 Tg-2 mice were decreased by 63% as compared with those of non-Tg littermates (P<0.05), whereas no difference in atherosclerotic lesion size was observed between female PON3 Tg-2 and non-Tg mice (Figure 5B).
Figure 5.
Decreased atherosclerosis in male human PON3 Tg mice. Three-month-old PON3 Tg-1 (females, n=4; males, n = 17) and non-Tg littermates (females, n=12; males, n=19) (A) or PON3 Tg-2 (females, n=12; males, n=9) and non-Tg littermates (females, n = 13; males, n=12) (B) were fed an atherogenic diet for 15 weeks before atherosclerotic lesion areas in the aortic root region were measured. C, Three-month-old PON3 Tg-1/LDLRKO (females, n=13; males, n = 18) and LDLRKO mice (females, n=11; males, n=18) were fed a Western diet for 8 weeks before atherosclerotic lesion areas in the aortic root region were measured. D, Aortic MCP-1 expression in the thoracic aorta of male PON3 Tg-1/LDLRKO (n = 15) and male LDLRKO mice (n=13) fed a Western diet for 8 weeks were measured by quantitative PCR. Relative levels of MCP-1 mRNA to α-actin mRNA were calculated. The data shown are mean and SE. *P<0.05, PON3 Tg vs non-Tg littermates.
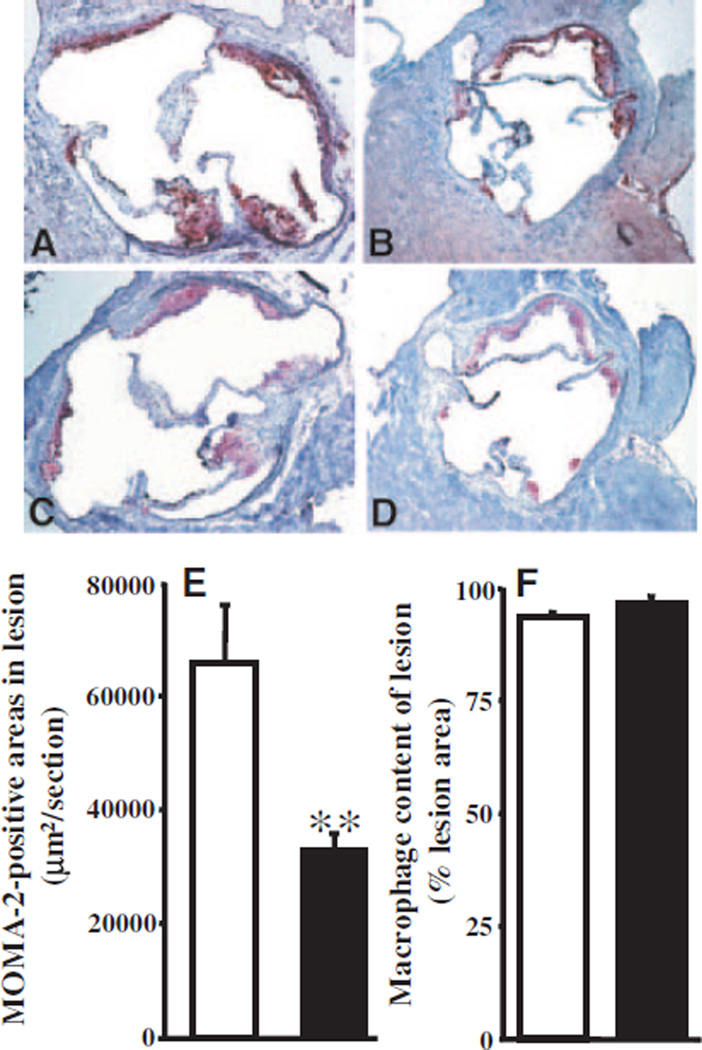
Furthermore, male PON3 Tg-1/LDLRKO mice developed significantly smaller lesion areas (34% reduction, P<0.05) in the aortic root as compared with the male LDLRKO littermates when maintained on the Western diet for 8 weeks (Figure 5C). In both groups, the lesions are mostly fatty streaks (Figure 6A and 6B). Macrophage-positive areas in the lesions, as determined by monocyte/macrophage (MOMA-2) immunostaining, were decreased significantly in the male PON3 Tg-1/LDLRKO mice as compared with the male LDLRKO mice (Figure 6C through 6E). However, macrophage contents were similar between the 2 groups (Figure 6F). We also observed significantly decreased MCP-1 mRNA levels in the thoracic aorta of male PON3 Tg-1/LDLRKO mice as compared with male LDLRKO mice (Figure 5D), suggesting decreased inflammation in the artery of male PON3 Tg mice. In contrast, there was no difference in lesion size between female PON3 Tg-1/LDLRKO and LDLRKO littermates (Figure 5C). Our data indicate that PON3 overexpression attenuates atherosclerotic lesion formation in mice and that this effect is sex-specific, observed only in male and not female mice.
Figure 6.
Atherosclerotic lesions of male LDLRKO and PON3 Tg-1/LDLRKO mice fed the Western diet for 8 weeks. Representative sections of lesion areas at the aortic root region of male LDLRKO (A) and PON3 Tg-1/LDLRKO (B) stained with oil red O (red) and counterstained with hematoxylin (blue) are shown. Representative macrophage-positive areas of the atherosclerotic lesions at the aortic root region of male LDLRKO (C) and PON3 Tg-1/LDLRKO (D) mice stained with an antibody against MOMA-2 (red) and counterstained with hematoxylin are shown. Magnification is ×50 for A through D. MOMA-2 (macrophage)-positive areas (E) and macrophage content (F) of male LDLRKO (open bar) and PON3 Tg-1/ LDLRKO mice (filled bar) are shown. For each genotype, the mean and SE of macrophage-positive areas (E) or of macrophage content (F) of 20 sections from 5 mice are shown. **P<0.01 vs LDLRKO.
Relationship Between PON3 Level and Obesity-Related Traits in an F2 Cross
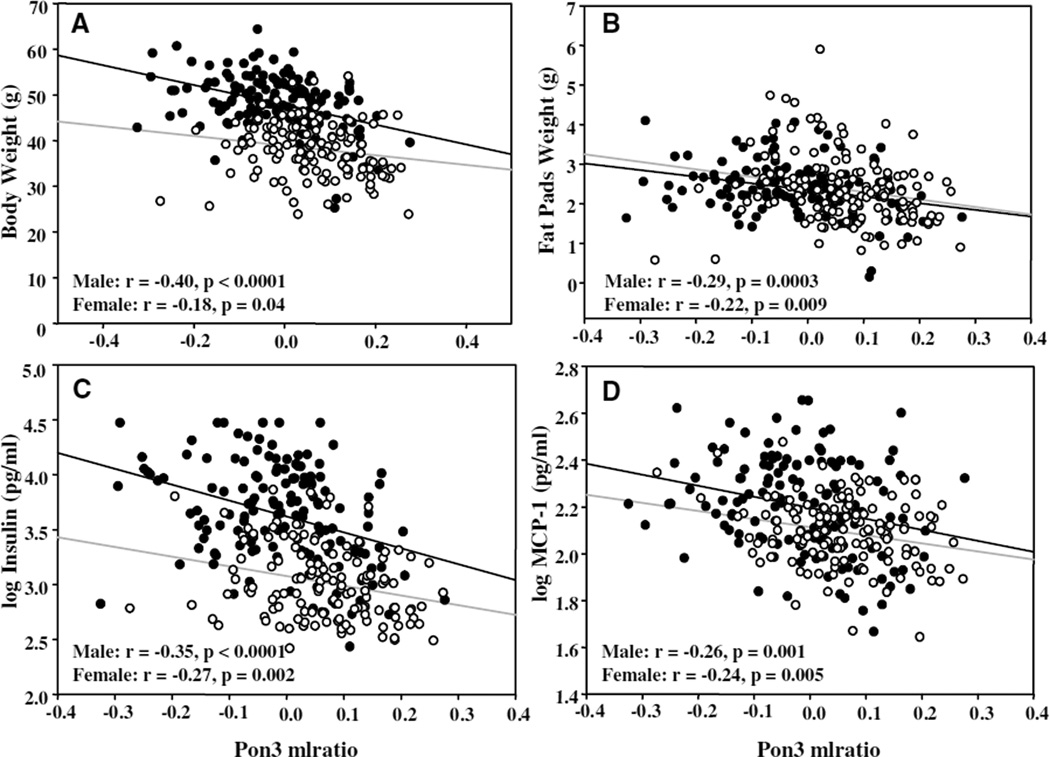
The observation that male PON3 Tg mice exhibited reduced adiposity as compared with non-Tg littermates was surprising. We further examined the relationship between PON3 expression and obesity-related traits in approximately 320 F2 mice derived from intercrossing of mouse strains B6 and C3H/HeJ on the apoE KO background. We observed that, in both sexes, mouse Pon3 mRNA levels in the adipose tissue correlated inversely with body weight (Figure 7A) and fat pad weight (Figure 7B), with more significant probability values observed in the males as compared with females. Adipose Pon3 mRNA level also correlated inversely with log(fasting insulin level) (Figure 7C), fasting glucose level (r=−0.26, P<0.001, both sexes included), and log(circulating MCP-1 level) (Figure 7D), with more significant probability values being observed in males as compared with females, except for fasting glucose, for which similar probability values were observed among males and females (data not shown). Interestingly, we also observed a trend of decreased plasma MCP-1 levels in the male PON3Tg-1/LDLRKO mice as compared with the male LDLRKO mice fed the Western diet (70±15 pg/mL versus 96±12 pg/mL, P=0.11). Thus, the data from the F2 cross suggest that elevated PON3 levels are associated with decreased adiposity, increased insulin sensitivity, and decreased inflammation.
Figure 7.
Relationship between adipose Pon3 mRNA level and obesity-related traits in an F2 intercross between B6 and C3H mice on the apoE KO background. Linear regression analysis was performed to calculate correlation coefficient and significance values between adipose Pon3 mRNA level (expressed as mlratio) and body weight (A), fat pad weight (including subcutaneous, mesenteric, and retroperitoneal fat) (B), log(insulin level) (C), and log(MCP-1 level) (D) of male F2 mice (closed circles, black line) and female F2 mice (open circles, gray line).
Discussion
In vitro studies demonstrate that PON3 protects against LDL oxidation and inflammation,23,24 suggesting that PON3 may be atheroprotective. The major finding of the present study is that overexpression of human PON3 decreases atherosclerotic lesion formation in 2 independent lines of transgenic mice in a male-specific fashion (Figure 5). Additionally, decreased adiposity and lower circulating leptin levels are observed in both lines of male PON3 Tg mice as compared with the male non-Tg mice (Figures 2 and 3). Lastly, our data show that adipose Pon3 mRNA levels inversely correlate with adiposity and related traits in an F2 cross (Figure 7).
We failed to detect human or mouse PON3 in mouse HDL or plasma. A previous study by Draganov et al23 found that lovastatinase activity in the mouse plasma was completely inhibited by the carboxylesterase inhibitors diisopropyl-phosphofluoridate or paraoxon, but not by EDTA, an inhibitor of PON3. Based on this observation, the authors suggest that the lovastatinase activity in mouse plasma is not caused by PON3 but results from the action of other carboxylesterase(s).23 In agreement, we observed that another carboxylesterase inhibitor, phenyl-methylsulfonyl fluoride, totally inhibited lovastatinase activity in mouse plasma (data not shown). Because human PON3 is detectable in human HDL (Figure 1), the reason for its absence in the HDL of PON3 Tg mice is not likely attributable to the structure of PON3 itself. A previous study showed that PON1 is located on the external membrane of producing cells and is released to HDL by a high-affinity, saturable, desorption mechanism.26 We postulate that PON3 may be released to HDL in humans in a similar mechanism as PON1. However, in mice, the mouse HDL may not be a good acceptor for either human or mouse PON3. Therefore, PON3 in mice remained cell-bound. Because human PON3 is not present in the circulation of PON3 Tg mice, it is not surprising that the abilities of HDL to protect against LDL oxidation were not different between HDLs isolated from PON3 Tg and non-Tg mice (Figure 4).
Interestingly, in males, but not females, PON3 overexpression reduced diet-induced atherosclerosis in mice on either the B6 or LDLRKO backgrounds (Figure 5A through 5C). We postulate that the abundant expression of human PON3 in the livers of transgenic mice may offer some protection against atherosclerosis. It has been suggested that oxidization of LDL leads to formation of LDL-associated cholesterol ester hydroperoxides (CEOOH). LDL-associated CEOOH can be transferred to HDL and converted to less-active cholesterol ester hydroxides (CEOHs). The HDL-associated CEOH is cleared by the liver via scavenger receptor BI-mediated selective uptake.27,28 We hypothesize that PON3 may be involved in inactivation and degradation of oxidized lipids in the liver and, therefore, decreases systemic inflammation and atherosclerosis. Although human PON3 mRNA was detected in the aorta samples of both lines of PON3 Tg mice (Figure 1A and 1B), its expression level was low as compared with the mouse PON3. Therefore, the aortic expression of human PON3 probably plays a minor, if any, role in protection against atherosclerosis.
Unexpectedly, we observed that the male PON3 Tg mice, maintained either on a low-fat chow diet or a high-fat Western diet, exhibited decreased adiposity as compared with the age and diet-matched, male non-Tg littermates (Figures 2 and 3). There was no significant difference in daily food consumption between PON3 Tg and non-Tg mice. Therefore, the decreased adiposity observed in the male PON3 Tg mice is not attributable to changes in food intake. It remains to be determined whether PON3 expression influences energy expenditure in mice.
Elevated leptin level is associated with arterial wall thickness, decreased vessel distensibility, and elevated C-reactive protein levels.29 Treatment of bovine aortic endothelial cells with leptin induces mitochondrial superoxide production and MCP-1 expression.30 A recent study further showed that recombinant leptin promoted atherosclerosis and thrombosis in apoE KO mice, providing direct evidence that leptin is proatherogenic.10 In the present study, the reduced circulating leptin levels observed in the male PON3 Tg-1/LDLRKO mice as compared with the male LDLRKO mice (Figure 3D) may explain in part the decreased aortic MCP-1 expression (Figure 5D) and atherosclerosis (Figure 5C) observed in the former.
We observed a male-specific protective effect of PON3 overexpression against atherosclerosis and obesity in both lines of PON3 Tg mice. Although human PON3 mRNA levels in the female PON3 Tg-1 mice were 50% lower as compared with male PON3 Tg-1 mice, no significant difference in human PON3 expression was observed between female and male PON3 Tg-2 mice. Thus, the male-specific protective effect is not likely attributable to differences in human PON3 levels between the sexes. In the F2 intercross, we also observed more significant inverse correlations between adipose Pon3 mRNA level and obesity-related traits among males as compared with females. We hypothesize that this sex-biased effect may be caused by interaction between PON3 and sex hormones, and/or interaction between PON3 and Y-linked gene(s). Previous genetic studies have identified atherosclerosis and obesity quantitative trait loci that are sex-dependent.31,32 For example, using the same F2 cross described in this study, we have previously identified 5 quantitative trait loci in the genome that are highly correlated with abdominal fat mass.31 Four of the five loci exhibited opposite effects on obesity in the two sexes, suggesting that interplay between gene and sex hormones determines phenotype. Among genetically altered mice, sex-dependent effects of gene deficiency or overexpression on susceptibility to atherosclerosis are common as well.33,34 Further investigations are required to examine the interactions among sex, sex hormones, and PON3.
In conclusion, this study has demonstrated that elevated PON3 expression attenuates atherosclerosis and obesity in male mice. Therefore, PON3 is an attractive candidate for further studies of its involvement in cardiovascular disease and obesity.
Supplementary Material
Acknowledgments
We thank Yi-Shou Shi, Janet Danciger, Lawrence Castellani, Sarada Charugundla, Judy Wu, Zhiqiang Zhou, and Victor Grijalva for excellent technical assistance. We thank Janet Yu for providing the mouse PON3 antibody.
Sources of Funding
This work was supported by NIH grants RO1 HL71776 (to S.T.R. and D.M.S.) and PO1 HL30568 (to D.M.S.).
Footnotes
Disclosures
A.M.F. is an officer of Bruin Pharma.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth J, Qiang X, Marban SL, Redelt H, Lowell BC. The obesity pandemic: where have we been and where are we going? Obes Res. 2004;12(suppl 2):88S–101S. doi: 10.1038/oby.2004.273. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 6.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 7.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 10.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:e119–e122. doi: 10.1161/01.ATV.0000173306.47722.ec. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adi-ponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 12.Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 1996;33:498–507. doi: 10.1006/geno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 13.Blatter MC, James RW, Messmer S, Barja F, Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45 Identity of K-45 with paraoxonase. Eur J Biochem. 1993;211:871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- 14.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 15.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation, preserves its functions A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Navab M. Protective effect of high density lipoprotein associated paraoxonase Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih DM, Reddy S, Lusis AJ. CHD and Atherosclerosis: Human Epidemiological Studies and Mouse Models. Norwell, Mass: Kluwer Academic Publishers; 2002. [Google Scholar]
- 18.Ng CJ, Shih DM, Hama SY, Villa N, Navab M, Reddy ST. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med. 2005;38:153–163. doi: 10.1016/j.freeradbiomed.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, Mackness M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 20.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 21.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ, Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 22.Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 23.Draganov DI, Stetson PL, Watson CE, Billecke SS, La Du BN. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J Biol Chem. 2000;275:33435–33442. doi: 10.1074/jbc.M004543200. [DOI] [PubMed] [Google Scholar]
- 24.Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gangopadhyay A, Shih DM, Lusis AJ, Navab M, Fogelman AM. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21:542–547. doi: 10.1161/01.atv.21.4.542. [DOI] [PubMed] [Google Scholar]
- 25.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Deakin S, Leviev I, Gomaraschi M, Calabresi L, Franceschini G, James RW. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J Biol Chem. 2002;277:4301–4308. doi: 10.1074/jbc.M107440200. [DOI] [PubMed] [Google Scholar]
- 27.Fluiter K, Vietsch H, Biessen EA, Kostner GM, van Berkel TJ, Sattler W. Increased selective uptake in vivo and in vitro of oxidized cholesteryl esters from high-density lipoprotein by rat liver parenchymal cells. Biochem J. 1996;319(pt 2):471–476. doi: 10.1042/bj3190471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fluiter K, Sattler W, De Beer MC, Connell PM, van der Westhuyzen DR, van Berkel TJ. Scavenger receptor BI mediates the selective uptake of oxidized cholesterol esters by rat liver. J Biol Chem. 1999;274:8893–8899. doi: 10.1074/jbc.274.13.8893. [DOI] [PubMed] [Google Scholar]
- 29.Kougias P, Chai H, Lin PH, Yao Q, Lumsden AB, Chen C. Effects of adipocyte-derived cytokines on endothelial functions: implication of vascular disease. J Surg Res. 2005;126:121–129. doi: 10.1016/j.jss.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–25100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Yehya N, Schadt EE, Wang H, Drake TA, Lusis AJ. Genetic and genomic analysis of a fat mass trait with complex inheritance reveals marked sex specificity. PLoS Genet. 2006;2:e15. doi: 10.1371/journal.pgen.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teupser D, Tan M, Persky AD, Breslow JL. Atherosclerosis quantitative trait loci are sex- and lineage-dependent in an intercross of C57BL/6 and FVB/N low-density lipoprotein receptor−/− mice. Proc Natl Acad Sci U S A. 2006;103:123–128. doi: 10.1073/pnas.0509570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD. Absence of P-selectin delays fatty streak formation in mice. J Clin Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101:17795–17800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.