Abstract
Tumor resection is recommended in anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis, however it is often difficult during an early stage of the disease. We report here the efficacy of early tumor removal in a patient with anti-NMDAR encephalitis. This 21-year-old woman was admitted to another hospital with rapidly progressive psychiatric symptoms, a decreased level of consciousness, and seizures. Abdominal CT showed a pelvic mass. On day 1 of admission to our center, she developed hypoventilation requiring mechanical support. She had orofacial dyskinesias with well-coordinated, pseudo-piano playing involuntary finger movements. Based on these clinical features, she was immediately scheduled for tumor resection on day 3. While awaiting surgery, she began to receive high-dose intravenous methylprednisolone. After tumor removal, she received plasma exchange, followed by intravenous immunoglobulin and additional high-dose methylprednisolone. Two weeks after tumor removal, she started following simple commands and progressive improvement, although she remained on mechanical ventilation for 10 weeks due to nocturnal central hypoventilation. Anti-NMDAR antibodies in serum/CSF were detected. Pathological examination showed immature teratoma with foci of infiltrates of B- and T-cells. Early tumor resection with immunotherapy facilitates recovery from this disease, but central hypoventilation may require long mechanical support. Non-jerky elaborate finger movements suggest antibody-mediated disinhibition of the cortico-striatal systems.
Keywords: paraneoplastic, encephalitis, ovarian tumor, NMDA receptor, early treatment
Introduction
Anti-N-methyl-D-aspartic acid receptor (NMDAR) encephalitis is an immune-mediated disorder caused by the antibodies against the NR1 subunit of the NMDAR (1, 2). Approximately 55% of adult women with this disorder have an underlying ovarian teratoma (2). In paraneoplastic cases tumor resection is recommended, but this is often delayed due to lack of early disease recognition, limited accessibility to antibody test, or disease-associated complications, including status epilepticus, septic shock, deep vein thrombosis or intractable dyskinesias. We previously reported the long-term outcome of 4 patients with anti-NMDAR encephalitis who improved without tumor removal (3), but two of these patients (cases 3 and 4) developed an extremely protracted course of the disease requiring several years for recovery.
We report here a patient with anti-NMDAR encephalitis whose teratoma was removed on day 3 of admission, before the antibody test result was available. With informed consent from the family, videos were obtained.
Case Report
A 21-year-old woman was transferred to our hospital in April 2009 with a possible diagnosis of ovarian teratoma-associated anti-NMDAR encephalitis. Two weeks earlier she had developed headache and fever. Seven days before transfer, she began to have memory loss, dizziness, speech deterioration, and behavioral problems with episodes of rage. Three days before transfer, she was admitted to a local hospital where she developed convulsive seizures followed by decreased level of consciousness. Brain MRI was unremarkable but abdominal CT showed a large calcified tumor in the pelvis. She was transferred to our hospital by helicopter for further evaluation and treatment.
On admission, her temperature was 38.1°C and the respiration rate was 22. The patient was obese (body mass index of 30.8) with an otherwise unremarkable general appearance. She was mute and unresponsive to verbal commands. During examination subtle oral dyskinesias were noted; she kept her eyes open but without response to visual or painful stimuli. Routine blood tests were unremarkable. Thyroid function tests, antinuclear antibodies, anti-thyroid peroxidase antibodies, anti-neutrophil cytoplasmic antibodies, and SSA/SSB antibodies, were negative. Lumbar puncture revealed mild pleocytosis (26/μL; mononuclear cells 100%) with a normal protein level, and the PCR for herpes simplex virus was negative. Samples of serum and CSF were archived for future studies. Brain MRI was normal and the EEG revealed diffuse delta slowing.
Three hours after arrival (day 1), she rapidly developed hypoxia due to hypersalivation, dysphagia and hypoventilation, requiring emergent endotracheal intubation and ventilatory support. Given that this clinical picture was highly suggestive of anti-NMDAR encephalitis, she was immediately scheduled for tumor resection on day 3. While awaiting surgery she began to receive intravenous high-dose methylprednisolone (IVMP) (1,000 mg/day, 5 days), and continuous infusion of propofol and heparin. On day 2, a pelvic MRI confirmed the presence of a large cystic tumor in the left ovary. On day 3, she developed well-coordinated involuntary finger-movements with the right hand as if playing piano (see supplementary video). She underwent unilateral salpingo-oophorectomy, and four days later (day 7) she started treatment with plasma exchange (PE) (7 changes in 2 weeks). On day 9, propofol was discontinued. On day 25, she started treatment with intravenous immunoglobulin (IVIg) (0.4 g/kg/day, 5 days). During hospitalization she also received topiramate (100-300 mg/day), clonazepam (1.5-6 mg/day), and diazepam (3-6 mg/day) to suppress involuntary movements.
Two weeks after tumor resection she started following simple commands and afterwards she continued with progressive improvement. The orofacial dyskinesias resolved one month after admission, but they re-emerged after amantadine (150 mg/day, 4 days) was added to increase self-motivation. Discontinuation of amantadine resulted in rapid resolution of dyskinesias. Subsequently, she had full recovery of consciousness, muscle power, and volitional ventilation, but remained on mechanical support due to nocturnal central hypoventilation. On day 31, she started an additional course of IVMP followed by gradual taper over 5 weeks. On day 70, she became free of mechanical support. On day 79, she was discharged to the referral hospital. On discharge, she was able to walk with a walker, and the revised Hasegawa Dementia Scale (HDS-R) that is equivalent to the mini-mental state examination was 26/30. At the last followup 4 months later, the HDS-R was 30/30 and the neurological examination was normal.
Both serum and CSF were obtained on day 1, 25, 44 and 72, and archived for subsequent analysis of anti-NMDAR antibodies, which were measured at the University of Pennsylvania. Antibodies were first determined using side-by-side paired serum and CSF at the same dilution (1 : 40). These studies showed antibodies in all samples of CSF while the antibodies in serum were detected on days 1 and 25, but not detected on days 44 and 72. When measured by a more sensitive ELISA test (2), serum antibodies decreased from 26,256 reference fluorescence units (day 1) to 2,530 (day 72), and the CSF antibodies decreased from 74,125 (day 1) to 6,525 (day 72).
Pathological examination of the tumor revealed a grade 2 immature teratoma containing both immature and mature components with extensive infiltrates of B- and T lymphocytes (Fig. 1). A few deposits of IgG and plasma cells were seen.
Figure 1.
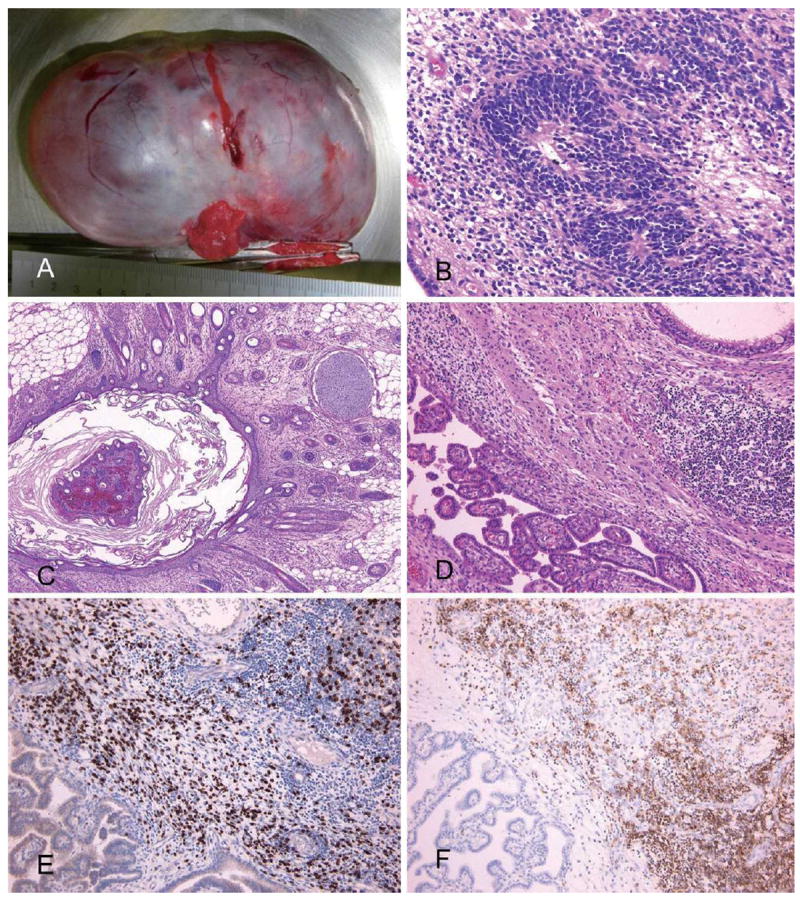
Pathology of ovarian tumor. Panel A shows the macroscopic appearance of the ovarian tumor (10×7×5 cm in diameter). Pathological examination demonstrates cystic immature teratoma containing neural tubes, neurons, choroid plexus, cartilage, fat tissue, and hair follicles (B: ×20, C: ×4, D: ×10, Hematoxylin and Eosin staining). Panel D shows focal infiltrates of lymphocytes in the neural tissue. Panel E (×10), immunostaing with CD3 (a marker of T cells), and panel F (×10), immunostaining with CD20 (B cells) demonstrates accumulation of both T cells and B cells.
Discussion
The patient reported here is remarkable for the following features: 1) the development of well-coordinated pseudopiano playing movements despite the low level of consciousness, 2) the transient worsening of the dyskinesias induced by amantadine, 3) the persistence of nocturnal central hypoventilation during the process of recovery and 4) the presence of extensive infiltrates of lymphocytes in the tumor.
Anti-NMDAR encephalitis can occur with or without tumor association (2). Since the first two reports suggesting that limbic encephalitis (most likely, anti-NMDAR encephalitis) may improve after resection of an underlying ovarian teratoma (4, 5), the efficacy of tumor removal has been emphasized in this disorder (1, 2). However, no clinical trials have been conducted yet, and the outcome of individual patients ranges from spontaneous recovery without tumor removal (3, 6) to severe residual deficits or death, with a mortality rate of 7% (2). Since the establishment of the concept of the disease in 2007, we have constructed the framework for early tumor removal. This is due to our previous experience with patients (cases 3 and 4) whose tumor was not removed during the acute stage of the disease, resulting in a long-lasting decreased level of consciousness for 7-20 months, prolonged ventilatory support for 6-9 months, and a recovery process that took 4-5 years (3). In the current patient, the early recognition of the syndrome by physicians of the referring hospital and the close collaboration with gynecologists lead to prompt tumor removal on day 3 (9 days after symptom onset).
The clinical presentation of this patient was in all respects similar to that of our previous patients who fell into an unresponsive state within 3-5 days of symptom onset followed by rapid development of central hypoventilation. However, in contrast to those patients who did not have tumor removal, our current patient had a dramatic recovery of consciousness that started 2 weeks after tumor removal (3 weeks after presentation). Additionally, this patient received IVMP, PE, and IVIg. Although it remains unclear which modality of treatment (tumor removal or immunotherapy) was more effective, the experience with this patient supports the concept of prompt tumor removal (2).
The patient is a piano player and her involuntary movements suggested a display of learned finger motions playing piano. Bizarre involuntary movements despite a decreased level of consciousness are characteristic of anti-NMDAR encephalitis (1-3). Some of the involuntary movements are semi-rhythmic, repetitive bulbar and limb movements that have been attributed to disinhibition of the brainstem pattern generators by the antibodies (7). However, the non-jerky, elaborate finger movements of the present patient would not be explained by the release of brainstem pattern generators, rather suggesting complex learned sequential finger movements (8). Given that in normal conditions GABAergic neurons contain high levels of NMDAR, and the cortico-striatal systems are under tonic inhibitory control by the GABAergic system (9), silencing of this system by an antibody-induced decrease of NMDAR would result in the release of complex bulbar and limb motions, as those of our patient.
It is also of interest that amantadine which is a weak NMDAR antagonist exaggerated the dyskinesias. Amantadine-induced exaggeration of dyskinesias implies the presence of hypersensitivity to dopamine or a synergistic effect with the antibody-induced decrease of NMDAR, as demonstrated in in vitro and in vivo studies (10). These studies show that patient’s antibodies cause a selective and reversible reduction in NMDAR surface density and synaptic localization through complement-independent, antibody-induced internalization of the receptors (10, 11). These data suggest that the antibodies directly contribute to schizophrenia-like symptoms (12), catatonia (3), and stereotyped movements, as occur with pharmacological antagonists of the NMDAR (11).
Hypoventilation occurs frequently in patients with anti-NMDAR encephalitis (1-3) however, the development of nocturnal hypoventilation has not been previously reported. In our patient, this problem was noted after recovering the level of consciousness and partial improvement of the continuous requirement of ventilatory support. Therefore, in addition to autonomic and neuroleptic malignant-like symptoms related to the encephalitis (tachycardia, bradycardia, cardiac pauses, rhabdomyolysis, renal failure), and systemic complications derived from prolonged stays in intensive care units (sepsis, thromboembolic events (3)), nocturnal hypoventilation should be considered as a potential life threatening complication during the process of recovery.
Although approximately 50% of patients with anti-NMDAR encephalitis show abnormal MRI findings and 25% of those have severe residual deficits (2), the resolution of symptoms and reversibility of chronic brain atrophy (13) suggest that this disorder results from a functional alteration of synaptic transmission rather than structural damage of the neurons. Moreover, the demonstration of infiltrates of B and T lymphocytes in the tumor, 2 days after IVMP, suggests a role of the ovarian teratoma in triggering or sustaining the immune activation. In patients with a typical picture of anti-NMDAR encephalitis and a radiologically demonstrated ovarian tumor, early tumor resection along with immunotherapy should be performed without waiting for antibody test results.
Supplementary Material
Acknowledgments
We are grateful to all participants and staffs for their contribution to this study and for clinical data collection. This study was supported in part by RO1CA89054 (JD).
Footnotes
The authors state that they have no Conflict of Interest (COI).
References
- 1.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iizuka T, Sakai F, Ide T, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nokura K, Yamamoto H, Okawara Y, Koga H, Osawa H, Sakai K. Reversible limbic encephalitis caused by ovarian teratoma. Acta Neurol Scand. 1997;95:367–373. doi: 10.1111/j.1600-0404.1997.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 5.Okamura H, Oomori N, Uchitomi Y. An acutely confused 15-year-old girl. Lancet. 1997;350:488. doi: 10.1016/S0140-6736(97)06208-9. [DOI] [PubMed] [Google Scholar]
- 6.Shindo A, Kagawa K, Ii Y, Sasaki R, Kokubo Y, Kuzuhara S. Anti-N-methyl-D-aspartate receptor-related grave but reversible encephalitis with ovarian teratoma in 2 Japanese women presenting with excellent recovery without tumor resection. Eur Neurol. 2009;61:50–51. doi: 10.1159/000175122. [DOI] [PubMed] [Google Scholar]
- 7.Kleinig TJ, Thompson PD, Matar W, et al. The distinctive movement disorder of ovarian teratoma-associated encephalitis. Mov Disord. 2008;23:1256–1261. doi: 10.1002/mds.22073. [DOI] [PubMed] [Google Scholar]
- 8.Doyon J, Bellec P, Amsel R, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Boyes J, Bolam JP. Localization of GABA receptors in the basal ganglia. Prog Brain Res. 2007;160:229–243. doi: 10.1016/S0079-6123(06)60013-7. [DOI] [PubMed] [Google Scholar]
- 10.Tüzün E, Zhou L, Baehring JM, Bannykh S, Rosenfeld MR, Dalmau J. Evidence for antibody-mediated pathogenesis in anti-NMDAR encephalitis associated with ovarian teratoma. Acta Neuropathol. 2009;118:737–743. doi: 10.1007/s00401-009-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iizuka T, Yoshii S, Kan S, et al. Reversible brain atrophy in anti-NMDA receptor encephalitis: a long-term observational study. J Neurol. 2010;257:1686–1691. doi: 10.1007/s00415-010-5604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


