Abstract
Scientific and policy interest in health disparities, defined as systematic, plausibly avoidable health differences adversely affecting socially disadvantaged groups, has increased markedly over the past few decades. Like other research, research in health disparities is strongly influenced by the underlying conceptual model of the hypothetical causes of disparities. Conceptual models are important and a major source of debate because multiple types of factors and processes may be involved in generating disparities, because different disciplines emphasize different types of factors, and because the conceptual model often drives what is studied, how results are interpreted, and which interventions are identified as most promising. This article reviews common conceptual approaches to health disparities including the genetic model, the fundamental cause model, the pathways model, and the interaction model. Strengths and limitations of the approaches are highlighted. The article concludes by outlining key elements and implications of an integrative systems-based conceptual model.
Keywords: health inequalities, social determinants, systems
INTRODUCTION
Scientific and policy interest in health disparities defined as “systematic, plausibly avoidable health differences adversely affecting socially disadvantaged groups” (10, p. e1) (note that the term health inequalities is often used with a similar meaning) has increased dramatically over the past few years. This increase has been reflected in publications, funding, training programs, and the mass media. Despite this increased interest, important unanswered questions remain, and even when proposed answers exist, they are contested with respect to both their validity and their implications for action. Like other research, health disparities research is strongly influenced by the conceptual model of the hypothetical causes of health disparities espoused by the researcher. The importance of the underlying conceptual model in guiding the formulation of research questions, the design of studies, and the analysis and interpretation of results is not unique to health disparities. However, it is of prime importance in this field because of the multifaceted factors (ranging from social to biological) that may contribute to disparities, because of disciplinary differences in the factors that are emphasized, and because models often have major implications for the interventions or policies that are identified as likely to be most effective in eliminating disparities.
Conceptual models involve the setting of bounds around a research problem and the specification of which constructs and relationships are fundamental to consider in understanding the problem and obtaining answers to related scientific questions. The answer that one obtains can be contingent on what else was considered (or was not considered) in the model. Most of the debate around conceptual models centers around which factors are included and which relationships are considered. Conceptual models often either implicitly or explicitly highlight one set of causal factors over another.
This article reviews major conceptual models utilized in health disparities research over the past decades. Examples are drawn from research on disparities by socioeconomic position and race/ethnicity because these are key domains linked to social disadvantage in many countries. [No attempt is made to differentiate race and ethnicity clearly given that both constructs may reflect combinations of social history, ancestry, and culture and the distinction is often arbitrary and blurred at least as the terms are commonly used in health research (89)]. The different conceptual models are categorized on the basis of what they emphasize. The review focuses on key differences across major schematic (and, to a certain extent, caricature) models. The boundaries across these different models however are fluid, and intermediate options that combine elements are not only plausible but common. Nevertheless, highlighting the key distinguishing features across various conceptual approaches can be useful in understanding the sometimes subtle distinctions across them. After reviewing each type of conceptual model, the article discusses emerging directions in conceptual models of health disparities.
THE GENETIC MODEL: GENETIC DIFFERENCES AS IMPORTANT DRIVERS OF HEALTH DISPARITIES
One strongly debated but nevertheless highly influential conceptual model of health disparities highlights the role of genetic factors. This model has been applied primarily to the study of race or ethic differences because of the perceived link between race or ethnicity and genetic ancestry, although genetic explanations for other forms of social disadvantage have not been uncommon in the past (32). There is a long history of debate in medicine, public health, and the social sciences on the extent to which self-reports of race or ethnicity correlate with genetic variants in such a way that the self-reported categories can serve as a proxy for genetic factors (7, 13, 80). If race and ethnic categories are substantially different in the distribution of genetic variants, genes become major candidates for the causes of health disparities. It is often stated, based on a classic study by Lewontin (45) as well as on subsequent replications (3, 23), that the proportion of total genetic variation that lies between commonly identified race groups is quite low (below 15%, often between 5% and 10%). This fact has led a number of scientists to state forcefully that race and ethnicity are very poor proxies for genetic background and, therefore, with the possible exception of selected rare conditions, genetic factors are unlikely to be major contributors to race or ethnic differences in health (7, 13, 37, 75, 80, 89).
Social and medical scientists have repeatedly noted that race and ethnic categories are socially and historically constructed and hence that any explanations of the causes of health disparities by race need to consider the social, historical, cultural, and environmental dimensions of race (7, 13). Arguments about genetic difference across races have been used inappropriately to establish hierarchies and to underplay the role of social factors and discrimination (7, 8). These arguments led to a discrediting of genetic explanation for health disparities, although articles continued explicitly or implicitly to interpret residual adjusted race differences as probably genetic despite admonitions to the contrary (8, 80), and genetic interpretations of disparities persisted in the popular press.
The Reemergence of Genetic Explanations
Recent years have witnessed a resurgence of the debate on the extent to which race and ethnic categories can reliably be used to proxy genetic background potentially relevant to a range of health outcomes (8, 37, 71). The methods used to apportion genetic variability across “races” (the basis for the often-cited 15% estimate) have been questioned (51), and some investigators have argued that even a between-race variability of under 15% is compatible with relevant genetic differences (50). The feasibility of measuring literally millions of markers of genetic variability in large population studies and the use of statistical techniques that allow the identification of patterns of segregation and clustering of these genetic markers have spurred the study of whether genetic markers can be used reliably to differentiate race or ethnic groups. Although it is true there is continuous variation in genetics across groups (78), that no race or ethnic group is homogeneous, and that sampling strategies may have an important impact on the groups or clusters identified (78, 84), a number of studies have shown that sets of appropriately chosen genetic markers can be used reliably to classify individuals into groups that often correlate quite well with self-identified race/ethnic groups (usually closely related to continental ancestry) (4, 58, 73, 83). Some researchers have argued that this correlation between genetically identified classifications and self reported race justifies the continued use of self-reported race and ethnicity as a proxy for genetic differences in epidemiologic studies (11, 71). Indeed Risch et al. (71) argue that self-reported race or ethnicity may be even more genetically informative than genetically identified clusters because it can identify genetic variability, which may not be detected by the clustering approaches.
The implications of the clustering of genetic markers by race and ethnicity for causal explanations of health disparities are not as straightforward as may first appear. An important question is the extent to which groups identified using these markers of variability in the genome actually capture meaningful variability in the genes hypothesized to relate to the multiple conditions for which health disparities exist (2, 13, 37). This remains a debated question. Feldman et al. (24, p. 374) argue that “genes that are geographically distinctive in their frequencies are not typical of the human genome in general”; King & Motulsky (39) suggest that few genetic variants common enough to be of medical significance for an entire group are likely to be confined to one population, although this statement has been contested (2). Although persons of the same race/ethnicity are likely to share ancestral markers, there is still substantial genetic variability within the genetically identified clusters. Indeed most allelic variation remains within regions or “race” groups (73). [It is important to note that the presence of identifiable clusters is fully compatible with the fact that on average only 5–10% of genetic variation is between groups. This is because even small amounts of between-group variation can be useful to classify individuals if the variation is highly structured (2).] When health-relevant genetic variants are found to vary across race groups, race is often a poor proxy for their presence (69).
Even if variants relevant to disease are confined to certain groups or vary substantially across groups, it seems unlikely that ancestral clusters would capture variability in genetic susceptibility to the very large and diverse set of health outcomes for which disparities exist. In addition, the limited ability, at least to date, to consistently identify genetic variants that explain a substantial portion of the risk for common diseases (19, 54) makes it unlikely that genetic variants would explain the very large differences in major diseases that are observed across race groups. Last, and perhaps most important, even if these clusters are good proxies for health-relevant genetic variability, the fact that they are highly correlated with self-reported race and ethnicity means that they are also highly correlated with the social and environmental features that vary markedly across these groups. This correlation implies that any attempt to separate the causal impact of the genetic clusters from the causal impact of social and environmental variables correlated with race or ethnicity is extremely difficult.
The Concept of Population Stratification in Genetic Studies
Despite debate about the extent to which genetic clustering correlated with race and ethnicity is a good proxy for health-relevant genetic variability, the notion that the genetic variability captured by these markers is relevant to health is well accepted among geneticists, as evidenced by the concern with population stratification in genetic studies (66, 68). Population stratification refers to the presence of subgroups within a study population with shared ancestry that differ in genetic architecture, i.e., in the presence of genetic markers and in the interrelations between markers. A major concern in genetic epidemiology is that associations between variability in a given genetic marker (or single nucleotide polymorphism) and health may be confounded by population subgroups that are associated with the genetic marker under study but that also have other factors related to the outcome of interest. A simple example of the conceptual model underlying the notion of population stratification is shown in Figure 1a. Investigators often assume that the “confounders” proxied by population subgroups are other genetic variants, although environmental factors correlated with population strata could be much more important confounders. Another implication of the presence of genetically identifiable subgroups is that the correlations between various genetic markers may be different within the different groups. This has major implications for the interpretation of associations of genetic variants (most of which are assumed to be proxies for the true causal genetic variants rather than causal themselves) with health, given that the extent to which polymorphisms in a given Single Nucleptide Polymorphism (or SNP) are correlated with the causal genetic variability may vary across groups. Cooper et al. (14) argue that this differential clustering of genetic markers across subgroups can be exploited in ways that can help identify the true causal variants (which are likely to be universal across groups, in contrast to the proxy measures).
Figure 1.
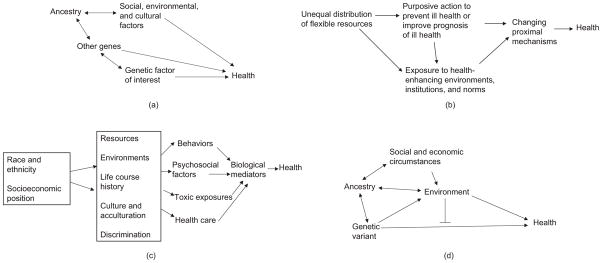
Essential elements of selected conceptual approaches to health disparities. A single-headed arrow from X to Y indicates that X is a cause of Y (e.g., social, environmental, and cultural factors are causally related to health (a), or that X causes increased exposure to Y (e.g., genetic factors cause exposures to certain environments (d ). A double-headed arrow indicates that both factors are associated (for example, genes and environments can become associated if persons of certain ancestry are more likely to live in certain areas as a result of institutional discrimination (a,d ). A line intersecting a one-headed arrow (in the form of a T) indicates that the factor modifies the relation between X and Y.
The concern with population stratification is the motivation for the call for race-specific analyses in genetic epidemiology and for the adjustment for population stratification even within analyses restricted to a single race or ethnic group. Substantial methodological work has focused on identifying the best ways to capture these “genetically defined” population subgroups (67, 90, 91). The notion that population subgroups identified through genetic clustering approaches are good proxies of health-related genetic variability (indeed for genetic variability relevant to all health outcomes since the same population stratification adjustment methods are used regardless of the outcome being studied) is a firm and ingrained assumption in most genetic epidemiology. The use of admixture mapping, in which the percent of a given ancestry (e.g., percent African ancestry) is examined in relation to health outcomes (58), is also heavily predicated on the notion that genetic markers of ancestry are likely to be good proxies for genetic variants related to a large number of health outcomes. These analyses are also subject to strong confounding by social and environmental factors linked to ancestry. Local admixture mapping (which focuses on ancestry for certain restricted sections of the genome) (14, 58) can be subject to less confounding as long as local admixture and general (genome-wide) admixture are not strongly correlated.
In summary, despite the longstanding debate and to some extent consensus in the late twentieth and early twenty-first century on the limitations of using self-reported race or ethnicity as a proxy for genetic factors (35), there has been resurgence of the idea that self-reported race and ethnic categorizations have a genetic basis and that genetic variability across race or ethnic groups may be highly relevant to health. Indeed this notion is strong and continually reinforced in genetic analyses through the concern with population stratification. As a result, the conceptualization of race and ethnic differences in health as substantially genetic remains strong among biomedical scientists and has served to reinforce this notion in the media and in the public at large. As noted by Duster (20), intended or not, the linkage of self-reported categories with genetic clusters and the growing use of admixture as a proxy for global genetic differences have to a certain extent served to reify race and reinforce stereotypical notions about innate race differences.
THE FUNDAMENTAL CAUSE MODEL: STRUCTURAL CONDITIONS AS FUNDAMENTAL CAUSES OF HEALTH DISPARITIES
A second influential conceptual model of health disparities highlights the importance of structural factors related to social and economic organization as fundamental causes of health disparities. This approach is exemplified by Link & Phelan’s (47) model of social conditions as fundamental causes of disease and by Williams’s (88) model of the causes of race differences in health. Both models explicitly allude to “basic causes” defined by Lieberson (46). Basic causes are defined as the factors responsible for generating a particular outcome. Changes in basic causes create a change in the outcome. Basic causes are distinguished from surface causes. Changes in surface causes do not necessarily result in changes in the outcome because new surface causes emerge to maintain the same outcome.
Social Conditions as Fundamental Causes of Disease
In a series of papers (47, 48, 63–65), Link and Phelan posit that social conditions can be conceptualized as “fundamental causes” (or basic causes) of disease. They argue that health inequalities have been robust over time because new mechanisms for the health inequalities emerge when existing ones disappear or are blocked. This continual reemergence of new mechanisms arises because persons of higher socioeconomic status (SES) possess a wide range of flexible resources, including knowledge, money, prestige, power, and social connections that can be used to secure good health (65). These flexible resources ensure that persons of higher SES know about, have access to, can afford, and are motivated to engage in a broad range of health-enhancing activities, including behaviors and treatments and living or working in environments conducive to health. Under this model, the key driver of the continual reemergence of the social gradient is purposive action on the part of the higher SES groups based on access to information and the resources necessary to act on this information. This action results in improved health through the prevention of disease or through improved prognosis once disease occurs. This basic model has been amended to recognize that additional processes that are not the intended direct result of purposive action but that can be viewed as side benefits (such as health benefits of living or working in certain contexts and benefiting from social norms, cultural practices, and institutions linked to membership in certain social groups) may also play a role in the reemergence of inequalities (26, 65). The basic elements of the fundamental cause model are illustrated in Figure 1b.
Although the fundamental cause model was formulated largely to explain socioeconomic differences, some elements of the fundamental cause approach are also present in models of disparities by race and ethnicity. For example Williams (88) alludes to “culture, biology, racism, economic structures, and political and legal factors” as the “fundamental causes of racial differences in health” (p. 327). This model pays special attention to historical circumstances and posits that “[d]ifferent combinations of factors in the model may be more salient depending upon the context, historical period, health outcome, and the research question under consideration” (p. 327). Like Link and Phelan’s fundamental cause model, William’s model also alludes to basic causes (46). However, in contrast to Link and Phelan’s model, which alludes to one primary process for the continued generation of social differences in health (the differential distribution of flexible resources and their deployment to protect and improve health), Williams includes a number of factors among the basic causes, including not only economic structures (which capture at least some elements of the resources approach) and political and legal factors (which can influence, among other things, the access to and distribution of resources) but also biology and geographic origins as well as culture. Racism, in particular institutional racism (defined as the presence of societal structures that systematically constrain the opportunities of groups on the basis of their race or ethnicity) (9), plays a prominent role in this model and is explicitly posited as another fundamental cause of race/ethnic disparities. Through racism, race becomes linked to SES, which in turn has health consequences. The model explicitly cautions against reifying race (race itself is not the cause) and argues for the need to examine how basic causes contribute to race differences in specific surface causes, which in turn affect health. The surface causes include health practices, stress, psychosocial resources, and medical care. Further elaborations of this model also incorporate immigration history and acculturation (89).
Key Elements of the Fundamental Cause Model
One key element of fundamental cause approaches is the emphasis on distal causes. A full understanding of health disparities necessarily requires consideration of these distal causes, rather than the mechanisms or proximal causes that are changing and dictated by circumstances. However, fundamental or basic cause models are not just about the presence of distal antecedents connected to health through multiple mechanisms (26). Fundamental cause models explicitly allude to and emphasize the processes through which these distal factors generate the continual reemergence of health differences even under changing circumstances. In the case of Link & Phelan’s (65) fundamental cause model, this mechanism is defined quite specifically and involves purposive action in the presence of flexible resources. In the case of Williams’s (88) model for race differences, this mechanism is defined more generally and involves larger societal and historical structures and processes that create differential distribution of the surface causes across race groups. The mechanism that generates and perpetuates differential distribution of continuing reemerging surface causes has been referred to as the “metamechanism” (53) to distinguish it from the more proximal pathways (sometimes also referred to as mechanisms) through which the distal causes specifically affect health (although the term metamechanism was initially used in the context of the Link and Phelan model, it is also applicable to Williams’s model of race differences). The articulation of these metamechanisms, and indeed the emphasis placed on these metamechanisms, is the key distinguishing feature of fundamental cause models.
A second key element of the fundamental cause models is the notion that these distal factors can affect multiple health outcomes through similar or different pathways. Thus this model easily accounts for the fact that disparities are often present for multiple and often disparate health outcomes. In addition, this model also indicates (indeed explicitly posits) that these distal antecedents can affect a specific health outcome through multiple mechanisms. The notion of multiple mechanisms is fundamental to these conceptualizations because they posit that new mechanisms continually emerge. The overall effect on health is the combined net effect of all the mechanisms (some of which could conceivably have countervailing impacts). The metamechanisms tend to generate effects systematically in one direction, resulting for example in the health disadvantage of lower- compared with higher-SES persons or of minority groups compared with whites (26, 53). This asymmetry is systematically reproduced such that when new mechanisms emerge they tend to preserve the relationship.
Implications for Intervention
Both key elements (the presence of a metamechanism and the presence of multiple pathways that change over time) have implications for the interventions or policies that may reduce disparities. Intervening on the proximal mechanisms may temporarily reduce disparities, but differences will likely reemerge as new mechanisms emerge. Breaking the cycle requires affecting the metamechanism itself. For the fundamental cause model, this includes reducing inequalities in resources or developing interventions (preventive or curative) that do not depend on resources for their adoption or action (65). Williams’s model implies developing strategies that address the fundamental processes that generate and maintain differences in the distribution of surface causes across race groups.
THE PATHWAYS MODEL: UNDERSTANDING THE MULTIPLE PATHWAYS THAT CONTRIBUTE TO HEALTH DISPARITIES
A third conceptual approach to health disparities highlights the understanding of mediating pathways. The difference between this conceptual model and the fundamental cause model is largely one of emphasis because the fundamental cause model emphasizes the metamechanisms and deemphasizes the mediating mechanisms, whereas the pathways model emphasizes the mediating mechanisms. Although the presence of distal antecedents is fundamental to the pathways model, the pathways model itself does not necessarily require a particular theory regarding the perpetuation of social or race/ethnic differences. It does not require theory on metamechanisms, although such a theory can be incorporated, and many researchers who work in this tradition may have such a theory in mind.
For the pathways model, elucidating the specific mediating mechanisms is important both to understanding what the main drivers of health disparities are and to identifying interventions to eliminate disparities. For example, Wang & Sie (87) argue that understanding disparities by race requires deconstructing what race means for the more proximal mechanisms and focusing on the mediators of health differences. Although in its classic formulation the fundamental cause model emphasizes the metamechanisms rather than the pathways, the importance of understanding mediating mechanisms is explicitly recognized even in fundamental cause models. For example, Phelan et al. (65, p. S35) note that “much of the public health significance of fundamental cause theory may reside in understanding how the link between flexible resources and health-relevant risk and protective factors has been broken.” Williams (88) also argues that understanding mechanisms contributing to race differences is necessary to show how these processes are not purely individually determined but largely shaped by societal forces.
The Notion of Embodiment
The notion of embodiment, defined as “how we, like any living organism, literally incorporate, biologically, the world in which we live, including our societal and ecological circumstances” (42, p. 351), is linked to the pathways model because it alludes to the importance of understanding the ways in which distal factors directly affect biologic structure and function with consequences for health. An important element of the notion of embodiment is that the proximal biological processes can be understood only if these are explicitly connected to their distal multilevel and historical/life course determinants. As such, it is closely linked to the fundamental cause model, although in contrast to this model, it is by definition explicitly interested in the proximal and biological factors. Detailed articulation of these mechanisms is viewed as important to demonstrating the causal effects of the distal causes on health outcomes.
Key Elements of the Pathways Model
An important characteristic of the pathways model is the consideration of various types of pathways or mediating mechanisms (Figure 1c). One often studied mechanism involves behavioral factors, such as smoking, dietary and physical activity patterns, and use of drugs, among others. Behaviors are explicitly placed in context and viewed as constrained by more distal factors such as resources (time and money), environments (neighborhood and work contexts), life course history (such as early-life factors), culture (social norms and traditions), and other social processes (such as discrimination). Another type of frequently hypothesized mechanism affected by these distal causes involves psychosocial processes. For example Matthews et al. (55) posit that life circumstances linked to socioeconomic position and race or ethnicity result in variable experiences of stress with consequences for a number of disease processes. A notable example is the focus on “allostatic load” (defined as the multisystem biological consequences of repeated attempts to adapt to stressors) as a contributor to health disparities (77). Although efforts have been made to determine the relative importance of behavioral and psychosocial factors as mediating pathways, definitive conclusions have remained elusive owing to difficulties in measurement, the absence of longitudinal data appropriate to investigate mediation, the presence of unmeasured confounders, and the likely bidirectional relationships between psychosocial and behavioral factors over time (5, 12, 29, 36). Other pathways that have been posited include traditional toxic exposures as well as institutional factors such as access to and quality of health care (89).
Environmental Context and Life Course Factors
Over the past 10–15 years, environmental context and life course factors (which can operate through any of the more proximal pathways described above) have become integral to many pathways approaches. Consideration of the impact of neighborhood social and physical environments (18) as well as work environments (49) has been viewed as increasingly important to understanding the mechanisms contributing to disparities. The unequal distribution of neighborhood social and physical features is increasingly viewed as a contributor to health disparities (18). Work environments may also contribute to disparities not only through toxic exposures at work but through a myriad of ways in which work affects individuals and communities (49, 59). Life course exposures, including factors during pregnancy, social conditions in childhood, and even factors affecting prior generations, have been viewed as increasingly important to understanding disparities in adult health. The incorporation of dimensions defined at different levels of organization and operating at variable time points has made the pathways model increasingly sophisticated (30). Another recent trend is the focus on more proximal pathways, including the effects of factors linked to social circumstances on processes related to differential gene expression (43).
Challenges in Pathways Models
The pathways model faces a number of challenges in articulating the detailed (including very proximal) mechanisms linking distal causes to health disparities. First, it can be very difficult to detect relations between very distal antecedents and biological factors that are likely to be affected by many interrelated factors in ways that are nonlinear and involve feedbacks and interactions. Second, methodologic difficulties persist in drawing firm conclusions regarding the presence and relative importance of mediating factors in observational studies, primarily owing to the presence of various forms of confounding (38, 86). Third, the focus on more proximal factors can lead to an unintended de-emphasis on the more distal antecedents. The study of the proximal biological mediators can become an end in itself and shift attention to intervening on proximal factors, which are viewed as more amenable to change (9). Some have expressed concern that this could result in a new reductionism to individual-level characteristics (21).
THE INTERACTION MODEL: THE ROLE OF GENE-BY-ENVIRONMENT INTERACTIONS
A fourth conceptual model highlights the importance of interactions between variables. Although the presence of interactions has long been hypothesized in the study of health, the interaction model has received increasing attention because of recent interest in the interplay between environments and genes. Geneticists hypothesize that gene-by-environment interactions may contribute to part of the “missing heritability” (the heritability that is unexplained by known genetic factors) (54) and that failure to consider interactions may explain why there is still limited evidence for a strong genetic basis to complex diseases (58). Researchers interested in social factors have found the gene-by-environment interaction model appealing because it emphasizes the continuing importance of broadly defined environmental factors (many of which can be conceptualized as social), even in the presence of genetic predictors. In addition, the study of gene-by-environment interactions could at least hypothetically facilitate the detection of new environmental factors. The recent interest in factors that affect gene expression (epigenetics) as contributors to health disparities (43) is closely linked to the gene-by-environment interaction approach because it explicates a mechanism through which environments can modify the expression of genes.
Typologies of Gene-by-Environment Interaction
Researchers have proposed a number of typologies of gene-by-environment interactions involving social factors (62, 76, 79). Shanahan & Hofer (79) propose four categories: (a) social context triggering the manifestation of an adverse genetic predisposition through exposure to stressors or personal/life circumstances that enhance the gene’s effect (b) social context neutralizing, compensating, or minimizing the manifestation of an adverse genetic predisposition through the absence of the trigger or through the provision of an enriched setting that compensates for the genetic predisposition; (c) social context exerting social control (through social norms or structural constraints) that affects the manifestation of the adverse genetic predisposition by limiting people’s behaviors and choices; and (d ) social context enhancing beneficial genetic predispositions (for example, genetic predispositions to higher educational attainment can be enhanced in the presence of a rich educational environment and opportunities). Although these four categories overlap, they differ in the process that is emphasized.
Contributions of Gene-by-Environment Interactions to Health Disparities
Despite the intellectual appeal of gene-by-environment interactions, there has been little progress in identifying replicable interactions. When reported, their presence has been contested (e.g., 72, 85). A number of challenges make the detection of interactions difficult (35, 79). Key among these is the need for very large sample sizes. The scale at which the interaction should be investigated (whether absolute differences or relative differences are used to quantify effects) remains an unresolved issue (31, 34). Many studies also lack appropriate environmental measures (57), and when available, measures often vary across studies, making much needed replication impossible. There is no consensus on whether the investigation of gene-by-environment interactions should be broadly exploratory or hypothesis-driven based on the specific mechanisms believed to play a role.
Because so few gene-by-environment interactions have been confirmed, few if any studies have directly examined the contribution of these interactions to health disparities. Nevertheless it is important to consider under which circumstances gene-by-environment interactions could contribute to disparities in health. Table 1 summarizes these possibilities for a simple scenario involving comparison of two groups and synergism (a type of interaction in which the effect of one factor is magnified or is manifested only in the presence of the other) between a genetic and an environmental factor. In scenario I, neither the gene nor the environment varies across groups. The presence of gene-by-environment interaction will not contribute to health disparities regardless of whether one or both factors exert main effects (the term main effect refers to the effect that occurs regardless of the presence of the other factor; this is the effect that manifests itself even if the other factor is absent). In scenarios IIa and IIIa, none of the factors exerts a main effect, but either the gene (IIa) or the environment (IIIa) varies across groups. Under these circumstances, the presence of gene-by-environment interaction will be sufficient to generate health disparities, even in the absence of main effects of either factor. For example, in scenario IIa the gene differs across groups, but the environmental factor does not. The interaction will allow the genetic differences to manifest themselves (assuming that the prevalence of the environmental risk factor is not 0), even if there is no main effect of the gene. If in addition the gene or the environment exerts a main effect (IIb and IIIb), the presence of the interaction will magnify the health disparity that results from the main effect of the factor that varies across groups. In scenarios IVa–IVd, both the gene and the environmental factor differ across groups. For simplicity, assume that environments and genes covary in such a way that the group with higher prevalence of the genetic risk factor also has a higher prevalence of the environmental risk factor. In IVa, neither factor exerts a main effect. All of the health disparity will be attributable to the interaction between both factors. In scenario IVb, the disparity will be due partly to the gene alone and partly to the interaction as in IIb, but the contribution of the interaction to the disparity will likely be greater than in IIb because of the higher prevalence of the environmental factor in the group with higher prevalence of the gene. Analogous logic can be applied to IVc and IVd. This simplistic schematic illustration shows that the extent to which gene-by-environment interaction contributes to health disparities will be a function of the covariation of genetic and environmental factors across groups, of the main effects of both factors, and of the magnitude and type of interaction.
Table 1.
Hypothetical contributions of gene-by-environment (G by E) interactions to health disparities for different scenarios (all scenarios assume the presence of synergism between a genetic factor and an environmental factor)
| Gene | Environment | Contribution of gene by environment interaction to health disparities | |||
|---|---|---|---|---|---|
| Varies across groups | Main effect | Varies across groups | Main effect | ||
| I | No | No/yes | No | No/yes | None |
| Variation in gene OR environment across groups | |||||
| IIa | Yes | No | No | No | Generates health disparity |
| IIb | Yes | Yes | No | No | Magnifies disparity due to gene |
| IIIa | No | No | Yes | No | Generates health disparity |
| IIIb | No | No | Yes | Yes | Magnifies disparity due to environment |
| Variation in genes AND environment across groups | |||||
| IVa | Yes | No | Yes | No | Generates health disparity |
| IVb | Yes | Yes | Yes | No | Magnifies disparity due to gene |
| IVc | Yes | No | Yes | Yes | Magnifies disparity due to environment |
| IVd | Yes | Yes | Yes | Yes | Magnifies disparities due to gene AND environment |
Complex Interplay Between Genes and Environments
A major challenge in the study of gene-by-environment interactions is that they are often studied without clear reference to the broader causal model within which environments and genes are embedded (79). Environments can be defined at various levels and over the life course. In addition, they can be very proximal to the disease process (e.g., the physiologic milieu in which the genes operate as defined by levels of circulating markers in the blood) or very distal societal factors. Distal factors may in turn be causal antecedents to the more proximal factors that interact with the genes. Some gene-by-environment interactions may actually be phenotype-environment interactions (the gene encodes for a particular phenotype, which is what interacts with the environment) (25).
Another source of complexity is that the interrelations between genes and environments are likely to involve much more than the classic gene-by-environment interaction (25, 62, 74). Environments and genes may become correlated through the sharing of common antecedents (for example, geographic ancestry may be linked to genetic variation and to place of residence, creating an association between genes and placed-based environmental factors) or through processes by which genes affect exposures to environments (persons with a genetic predisposition to be more physically active choosing to live close to environments that facilitate physical activity, or persons with genetic predisposition to music being more likely to receive musical stimuli). The environmental factors correlated with the genes may, in turn, directly affect health (independently of genes) or may exacerbate the effects of genes. Some of these basic relations are summarized in Figure 1d. In an additional twist, genetic factors may also lead to phenotypic characteristics (e.g., certain aspects of physical appearance), which in turn have consequences for environmental exposures (e.g., discrimination) that affect health [genes operate through phenotypes, which have social and environmental implications) (25)]. In these situations, genetic and social or environmental factors have mutually reinforcing effects over time, and explanations that fail to consider these dynamics may result in mistaken attributions of variability (and causation) to genetic or environmental components. These complex interrelationships suggest the need for conceptual models that explicitly account for these dynamic relations in the study of how genes and environments may jointly contribute to health disparities.
TOWARD A DYNAMIC AND INTEGRATIVE SYSTEMS VIEW OF HEALTH DISPARITIES
Although the four schematic conceptual approaches described above differ in emphasis, they are interrelated and complementary in many ways. Health disparities researchers have increasingly emphasized the need to integrate and transcend false dichotomies (social and biologic factors, environments and genes, individual- and population-level factors, or behavioral and stress-related processes) in the understanding of the causes of health disparities. But this integration may require new ways of thinking as well as new analytical approaches and tools.
An emerging integrative paradigm in public health is that of complex systems. Although systems thinking has long been part of public health (especially in the area of infectious diseases) (15, 40, 41, 52, 56, 81), it has recently received attention beyond the field of infectious diseases as a way to address limitations in current approaches to population health (1, 16, 17, 27, 28, 60, 61, 70). The complex systems approach is appealing because it allows an integration of various important aspects emphasized by the different conceptual approaches outlined above. For example, a complex systems approach embraces the notion of structural causation expressed in the fundamental cause model but also emphasizes (and requires) the detailed articulation of mediating mechanisms highlighted in the pathways model. It recognizes the possibility that there are different causal pathways to the same outcome or that a single pathway can lead to different outcomes (79). It allows explicit articulation of the reciprocal and interacting relations between genes and environments over time. It does not artificially dichotomize individual- and population-level factors but shows how they can affect each other through feedbacks. More generally, it can help researchers explicitly articulate how group differences arise from the dynamic interactions between individuals as well as between individuals and environments (or populations) over time (1, 17). Several elements of a systems-based approach may be valuable to health disparities research.
Formulating Dynamic Conceptual Models that are Bounded and Tractable
Perhaps the most important, but also the most challenging, element of a systems approach is the development of dynamic conceptual models of health disparities. These models may incorporate many aspects of existing conceptual models (multiple levels, distal and proximal factors, as well as factors operating at different times) but explicitly allude to feedbacks, dependencies between individuals, and nonlinear relations. Although these relations may be implicit in some existing conceptual models, systems conceptualizations require researchers to make these relations explicit and specific. They force researchers to move away from the focus on estimating associations or isolating the effects of a single factor to articulating how many factors operate together over time in a dynamic and nonlinear way to generate the outcome.
An important challenge in developing these dynamic conceptual models is setting the bounds on what should and should not be included. The setting of these bounds will be driven by the specific research problem that the model is intended to help address. To be useful, these models need to be bounded and tractable and, therefore, amenable to the development of formal models and simulation. The specificity and explicitness of the model also allow debate on whether the bounds that have been set are appropriate and facilitates discussion and input from scientists and various stakeholders (82). Once a conceptual model is developed, various types of formal models and simulations can be used to refine the model, to understand its functioning, and to evaluate the impact of various inputs.
Integrating Existing Knowledge and Data and Identifying New Data Needs
A key and appealing feature of systems approaches is that they allow integration of quantitative and qualitative information from various sources. This is important both in the development of the conceptual model and in the formulation of a formal model. The process of integrating various sources of data could be especially valuable in health disparities research because often the large amount of information available is not systematically integrated. In addition, the process of developing the model allows identification of novel and important areas in which data are lacking or the identification of new questions that need to be answered (often using the traditional approaches of experiments, observational studies, or qualitative studies) before the functioning of the system can be understood.
Explicit Link to Intervention and Policy
Another important component of the systems approach is the explicit link to policy or interventions. This is because the approach focuses on modeling processes and encourages investigators to consider how the outcomes of the functioning of the systems would change if different malleable inputs (or causes) were modified. The approach allows direct investigation of how factors distal to the final outcome may operate. It also allows for the detection of unintended effects (perhaps distant in space and time) as well as the identification of interventions or policies that might be useful in reducing disparities but would not have been identified using the standard approach. Systems approaches are especially valuable in situations of policy resistance (82), i.e., where policies have not had the expected effects, which may be a common situation in health disparities.
Integrating a Variety of Tools
Broadly speaking, a systems approach to health disparities would promote the integration of various methodological approaches and tools. These would include systems tools such as systems dynamic models or agent-based models (22, 33) as well as the traditional tools of observational studies, trials, and qualitative studies. Various aspects of dynamic conceptual models can be investigated using different tools where appropriate. For example, formal systems models (such as systems dynamic models or agent-based models) can be used to explore the functioning of the system as a whole, but the traditional tools of observational studies and trials and qualitative studies may be necessary to investigate specific aspects further or to provide input for the creation of formal systems models and simulations. A dynamic systems approach to health disparities does not require abandoning current methods or relying exclusively on formal systems models and simulation. It is more about the conceptualization of health disparities as the product of the functioning of a system rather than about the exclusive use of certain systems tools.
CONCLUSION
Conceptual models have a profound impact on what is studied, how it is studied, and how results are interpreted. The genetic, fundamental cause, pathways, and interaction models differ in the factors and relations they emphasize. Although sometimes subtle, these differences in emphasis influence the types of questions that are asked, the way data are collected, the analytical approaches used, and the interpretation of results.
Health disparities research is ripe for fresh approaches that allow us to rethink the conceptual models we utilize, combine data from various sources, identify new data needs, and integrate a variety of analytical approaches to improve our knowledge of the causes of health disparities and identify effective policies of interventions to eliminate them. A key challenge is recognizing the dynamic relations between factors at various levels, including the role of feedbacks, dependencies, and nonlinear relations that are absent from the most common approaches. Conceptualizing health disparities in a systems-inspired dynamic way may help integrate various aspects of existing conceptual models. It may also point to new questions or stimulate new ways of thinking about old questions and help transcend the false dichotomies that still plague the field.
Dynamic conceptual models are not necessarily very complicated. The development of conceptual models is about extracting essential elements and placing bounds on the problem so that it is tractable and so that fundamental understanding can be obtained. Some questions in health disparities may continue to be answerable using conceptualizations and tools already available, but others may require new ways of conceptualizing and studying dynamic relations. As in the story by Borges (6), where the map makers made such a detailed map that it became an actual representation of the land itself and was no longer useful as a map, the purpose of developing dynamic conceptual models is not to replicate reality exactly but to identify the elements essential for understanding. Health disparities researchers will have to use existing information, scientific insight, and simple intuition to extract what the essential elements of a model relevant to a particular problem might be. Systems-inspired conceptual models also do not imply abandoning traditional ways of collecting or analyzing data, but they may help integrate various sources of data, point to new data needs, and help understand the implications of the information we have in a new way.
Reductionist approaches have prospered for a reason: They radically simplify complexity and make it tractable. The problem is that sometimes this simplification can be obfuscating rather than illuminating (44). Systems approaches also simplify, but they simplify in a different way than the approaches commonly used in disparities research today. Part of the challenges facing health disparities research may be related to what we have been abstracting in our models and what we have chosen to ignore. Exploring alternate ways of simplifying could yield new insights. But these approaches will also present a whole new set of challenges that will become increasingly apparent as they are applied to concrete problems.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Auchincloss AH, Diez Roux AV. A new tool for epidemiology: the usefulness of dynamic-agent models in understanding place effects on health. Am J Epidemiol. 2008;168:1–8. doi: 10.1093/aje/kwn118. [DOI] [PubMed] [Google Scholar]
- 2.Bamshad M, Wooding S, Salisbury BA, Stephens JC. Deconstructing the relationship between genetics and race. Nat Rev Genet. 2004;5:598–609. doi: 10.1038/nrg1401. [DOI] [PubMed] [Google Scholar]
- 3.Barbujani G, Magagni A, Minch E, Cavalli-Sforza LL. An apportionment of human DNA diversity. Proc Natl Acad Sci USA. 1997;94:4516–19. doi: 10.1073/pnas.94.9.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastos-Rodrigues L, Pimenta JR, Pena SD. The genetic structure of human populations studied through short insertion-deletion polymorphisms. Ann Hum Genet. 2006;70:658–65. doi: 10.1111/j.1469-1809.2006.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–93. [PubMed] [Google Scholar]
- 6.Borges J. In: A Universal History of Infamy. de Giovanni Norman Thomas., translator. London: Penguin; 1975. [Google Scholar]
- 7.Braun L. Race, ethnicity, and health: Can genetics explain disparities? Perspect Biol Med. 2002;45:159–74. doi: 10.1353/pbm.2002.0023. [DOI] [PubMed] [Google Scholar]
- 8.Braun L, Fausto-Sterling A, Fullwiley D, Hammonds EM, Nelson A, et al. Racial categories in medical practice: How useful are they? PLoS Med. 2007;4:e271. doi: 10.1371/journal.pmed.0040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 10.Braveman P, Kumanyika S, Fielding J, Laveist T, Borrell L, et al. Health disparities and health equity: The issue is justice. Am J Public Health. 2011 doi: 10.2105/AJPH.2010.300062. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348:1170–75. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 13.Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348:1166–70. doi: 10.1056/NEJMsb022863. [DOI] [PubMed] [Google Scholar]
- 14.Cooper RS, Tayo B, Zhu X. Genome-wide association studies: implications for multiethnic samples. Hum Mol Genet. 2008;17:R151–55. doi: 10.1093/hmg/ddn263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diez-Roux AV. On genes, individuals, society, and epidemiology. Am J Epidemiol. 1998;148:1027–32. doi: 10.1093/oxfordjournals.aje.a009578. [DOI] [PubMed] [Google Scholar]
- 16.Diez Roux AV. Integrating social and biologic factors in health research: a systems view. Ann Epidemiol. 2007;17:569–74. doi: 10.1016/j.annepidem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Diez Roux AV. Can complex systems help us transcend current impasses in health disparities reserach? Am J Public Health. 2011;101:1627–34. doi: 10.2105/AJPH.2011.300149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–45. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly P. Progress and challenges in genome-wide association studies in humans. Nature. 2008;456:728–31. doi: 10.1038/nature07631. [DOI] [PubMed] [Google Scholar]
- 20.Duster T. Medicine. Race and reification in science. Science. 2005;307:1050–51. doi: 10.1126/science.1110303. [DOI] [PubMed] [Google Scholar]
- 21.Duster T. Comparative perspectives and competing explanations: taking on the newly configured reductionist challenge in sociology. Am J Sociol. 2006;71:1–15. [Google Scholar]
- 22.Epstein JM. Generative Social Science: Studies in Agent-Based Computational Modeling. Princeton, NJ: Princeton Univ. Press; 2007. [Google Scholar]
- 23.Excoffier L, Hamilton G. Comment on “Genetic structure of human populations”. Science. 2003;300:1877. doi: 10.1126/science.1083411. author reply 77. [DOI] [PubMed] [Google Scholar]
- 24.Feldman MW, Lewontin RC, King MC. Race: a genetic melting-pot. Nature. 2003;424:374. doi: 10.1038/424374a. [DOI] [PubMed] [Google Scholar]
- 25.Freese J. Genetics and the social science explanation of individual outcomes. Am J Sociol. 2008;114:S1–35. doi: 10.1086/592208. [DOI] [PubMed] [Google Scholar]
- 26.Freese J, Luftey K. Fundamental causality: challenges of an animating concept for medical sociology. In: Pescosolido B, Martin J, McLeod J, Rogers A, editors. Handbook of Medical Sociology. New York: Springer; 2011. pp. 67–81. [Google Scholar]
- 27.Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. Int J Epidemiol. 2010;39:97–106. doi: 10.1093/ije/dyp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62:1650–71. doi: 10.1016/j.socscimed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Gravlee CC. How race becomes biology: embodiment of social inequality. Am J Phys Anthropol. 2009;139:47–57. doi: 10.1002/ajpa.20983. [DOI] [PubMed] [Google Scholar]
- 31.Greenland S. Basic problems in interaction assessment. Environ Health Perspect. 1993;101(Suppl 4):59–66. doi: 10.1289/ehp.93101s459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtzman NA. Genetics and social class. J Epidemiol Community Health. 2002;56:529–35. doi: 10.1136/jech.56.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homer JB, Hirsch GB. System dynamics modeling for public health: background and opportunities. Am J Public Health. 2006;96:452–58. doi: 10.2105/AJPH.2005.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6:287–98. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- 35.Haynes MA, Smedley BD, editors. Inst. Med. Comm. Cancer Res. Among Minor. Medically Underserved. The Unequal Burden of Cancer: An Assessment of NIH Reserach and Programs for Ethnic Minorities and the Medically Underserved. Washington, DC: Natl. Acad. Press; 1999. http://www.nap.edu/catalog.php?record_id=6377. [PubMed] [Google Scholar]
- 36.Kahn RS, Wilson K, Wise PH. Intergenerational health disparities: socioeconomic status, women’s health conditions, and child behavior problems. Public Health Rep. 2005;120:399–408. doi: 10.1177/003335490512000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman JS, Cooper RS. Race in epidemiology: new tools, old problems. Ann Epidemiol. 2008;18:119–23. doi: 10.1016/j.annepidem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov. 2004;1:4. doi: 10.1186/1742-5573-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King MC, Motulsky AG. Human genetics. Mapping human history Science. 2002;298:2342–43. doi: 10.1126/science.1080373. [DOI] [PubMed] [Google Scholar]
- 40.Koopman J. Modeling infection transmission. Annu Rev Public Health. 2004;25:303–26. doi: 10.1146/annurev.publhealth.25.102802.124353. [DOI] [PubMed] [Google Scholar]
- 41.Koopman JS, Lynch JW. Individual causal models and population system models in epidemiology. Am J Public Health. 1999;89:1170–74. doi: 10.2105/ajph.89.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krieger N. Embodiment: a conceptual glossary for epidemiology. J Epidemiol Community Health. 2005;59:350–55. doi: 10.1136/jech.2004.024562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- 44.Levins R. Ten propositions on science and antiscience. Soc Text. 1996;14:101–11. [Google Scholar]
- 45.Lewontin R. The apportionment of human diversity. Evol Biol. 1972;6:381–98. [Google Scholar]
- 46.Lieberson S. Making It Count: The Improvement of Social Research and Theory. Berkeley: Univ. Calif. Press; 1985. [Google Scholar]
- 47.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;(80–94) [PubMed] [Google Scholar]
- 48.Link BG, Phelan JC. Understanding sociodemographic differences in health—the role of fundamental social causes. Am J Public Health. 1996;86:471–73. doi: 10.2105/ajph.86.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipscomb HJ, Loomis D, McDonald MA, Argue RA, Wing S. A conceptual model of work and health disparities in the United States. Int J Health Serv. 2006;36:25–50. doi: 10.2190/BRED-NRJ7-3LV7-2QCG. [DOI] [PubMed] [Google Scholar]
- 50.Long JC. Update to Long and Kittles’s “Human genetic diversity and the nonexistence of biological races” 2003: fixation on an index. Hum Biol. 2009;81:799–803. doi: 10.3378/027.081.0622. [DOI] [PubMed] [Google Scholar]
- 51.Long JC, Kittles RA. Human genetic diversity and the nonexistence of biological races. 2003. Hum Biol. 2009;81:777–98. doi: 10.3378/027.081.0621. [DOI] [PubMed] [Google Scholar]
- 52.Loomis D, Wing S. Is molecular epidemiology a germ theory for the end of the twentieth century? Int J Epidemiol. 1990;19:1–3. doi: 10.1093/ije/19.1.1. [DOI] [PubMed] [Google Scholar]
- 53.Luftey K, Freese J. Toward some fundamentals of fundamental causality: socioeconomic status and health in the routine clinic visit for diabetes. Am J Sociol. 2005;110(5):1326–72. [Google Scholar]
- 54.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matthews KA, Gallo LC, Taylor SE. Are psychosocial factors mediators of socioeconomic status and health connections? A progress report and blueprint for the future. Ann N Y Acad Sci. 2010;1186:146–73. doi: 10.1111/j.1749-6632.2009.05332.x. [DOI] [PubMed] [Google Scholar]
- 56.McMichael AJ. Prisoners of the proximate: loosening the constraints on epidemiology in an age of change. Am J Epidemiol. 1999;149:887–97. doi: 10.1093/oxfordjournals.aje.a009732. [DOI] [PubMed] [Google Scholar]
- 57.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–81. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 58.Mountain JL, Risch N. Assessing genetic contributions to phenotypic differences among ‘racial’ and ‘ethnic’ groups. Nat Genet. 2004;36:S48–53. doi: 10.1038/ng1456. [DOI] [PubMed] [Google Scholar]
- 59.Muntaner C, Solar O, Vanroelen C, Martinez JM, Vergara M, et al. Unemployment, informal work, precarious employment, child labor, slavery, and health inequalities: pathways and mechanisms. Int J Health Serv. 2010;40:281–95. doi: 10.2190/HS.40.2.h. [DOI] [PubMed] [Google Scholar]
- 60.Ness RB, Koopman JS, Roberts MS. Causal system modeling in chronic disease epidemiology: a proposal. Ann Epidemiol. 2007;17:564–68. doi: 10.1016/j.annepidem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Pearce N, Merletti F. Complexity, simplicity, and epidemiology. Int J Epidemiol. 2006;35:515–19. doi: 10.1093/ije/dyi322. [DOI] [PubMed] [Google Scholar]
- 62.Pescolido B, Perry B, Long S, Martin J, Nurnberger J, Hesselbrock V. Under the influence of genetics: how transdisciplinarity leads us to rethink social pathways to illness. Am J Sociol. 2008;114:S171–201. doi: 10.1086/592209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phelan JC, Link BG. Controlling disease and creating disparities: a fundamental cause perspective. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec 2):27–33. doi: 10.1093/geronb/60.special_issue_2.s27. [DOI] [PubMed] [Google Scholar]
- 64.Phelan JC, Link BG, Diez-Roux A, Kawachi I, Levin B. “Fundamental causes” of social inequalities in mortality: a test of the theory. J Health Soc Behav. 2004;45:265–85. doi: 10.1177/002214650404500303. [DOI] [PubMed] [Google Scholar]
- 65.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(Suppl):S28–40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 66.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 67.Price AL, Zaitlen NA, Reich D, Patterson N. New approaches to population stratification in genome-wide association studies. Nat Rev Genet. 2010;11:459–63. doi: 10.1038/nrg2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pritchard JK, Stephens M, Rosenberg NA, Donnelly P. Association mapping in structured populations. Am J Hum Genet. 2000;67:170–81. doi: 10.1086/302959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramos E, Rotimi C. The A’s, G’s, C’s, and T’s of health disparities. BMC Med Genomics. 2009;2:29. doi: 10.1186/1755-8794-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Resnicow K, Page SE. Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98:1382–89. doi: 10.2105/AJPH.2007.129460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: genes, race and disease. Genome Biol. 2002;3:comment2007–2007.12. doi: 10.1186/gb-2002-3-7-comment2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, et al. Genetic structure of human populations. Science. 2002;298:2381–85. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 74.Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–61. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz RS. Racial profiling in medical research. N Engl J Med. 2001;344:1392–93. doi: 10.1056/NEJM200105033441810. [DOI] [PubMed] [Google Scholar]
- 76.Seabrook JA, Avison WR. Genotype-environment interaction and sociology: contributions and complexities. Soc Sci Med. 2010;70:1277–84. doi: 10.1016/j.socscimed.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–39. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 78.Serre D, Paabo S. Evidence for gradients of human genetic diversity within and among continents. Genome Res. 2004;14:1679–85. doi: 10.1101/gr.2529604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec 1):65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- 80.Shields AE, Fortun M, Hammonds EM, King PA, Lerman C, et al. The use of race variables in genetic studies of complex traits and the goal of reducing health disparities: a transdisciplinary perspective. Am Psychol. 2005;60:77–103. doi: 10.1037/0003-066X.60.1.77. [DOI] [PubMed] [Google Scholar]
- 81.Stallones R. The epidemiologist as environmentalist. Int J Health Serv. 1973;3:29–33. doi: 10.2190/AM3A-AW5N-5W12-BCUH. [DOI] [PubMed] [Google Scholar]
- 82.Sterman JD. Learning from evidence in a complex world. Am J Public Health. 2006;96:505–14. doi: 10.2105/AJPH.2005.066043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet. 2005;76:268–75. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tayo BO, Teil M, Tong L, Qin H, Khitrov G, et al. Genetic background of patients from a university medical center in Manhattan: implications for personalized medicine. PLoS ONE. 2011;6:e19166. doi: 10.1371/journal.pone.0019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- 86.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172:1339–48. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang VA, Sie S. In the eye of the storm: race and genomics in research and practice. Am Psychol. 2005;60:37–45. doi: 10.1037/0003-066X.60.1.37. [DOI] [PubMed] [Google Scholar]
- 88.Williams DR. Race and health: basic questions, emerging directions. Ann Epidemiol. 1997;7:322–33. doi: 10.1016/s1047-2797(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 89.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu C, DeWan A, Hoh J, Wang Z. A comparison of association methods correcting for population stratification in case-control studies. Ann Hum Genet. 2011;75:418–27. doi: 10.1111/j.1469-1809.2010.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang F, Wang Y, Deng HW. Comparison of population-based association study methods correcting for population stratification. PLoS ONE. 2008;3:e3392. doi: 10.1371/journal.pone.0003392. [DOI] [PMC free article] [PubMed] [Google Scholar]


