Summary
Sympathetic neurons depend on target-derived neurotrophic cues to control their survival and growth. However, whether sympathetic innervation contributes reciprocally to the development of target tissues is less clear. Here, we report that sympathetic innervation is necessary for the formation of the pancreatic islets of Langerhans and for their functional maturation. Genetic or pharmacological ablation of sympathetic innervation during development resulted in altered islet architecture, reduced insulin secretion and impaired glucose tolerance in mice. Similar defects were observed with pharmacological blockade of β-adrenergic signaling. Conversely, the administration of a β-adrenergic agonist restored islet morphology and glucose tolerance in de-innervated animals. Furthermore, in neuron-islet co-cultures, sympathetic neurons promoted islet cell migration in a β-adrenergic dependent manner. This study reveals that islet architecture requires extrinsic inductive cues from neighboring tissues such as sympathetic nerves, and suggests that early perturbations in sympathetic innervation might underlie metabolic disorders.
Introduction
The sympathetic nervous system, a branch of the autonomic nervous system, is critical for organ homeostasis. Postganglionic sympathetic neurons innervate a variety of peripheral targets to regulate several physiological processes including blood glucose levels, cardiac output and body temperature. During development, the survival and growth of sympathetic neurons relies on diffusible cues released by their targets (Glebova and Ginty, 2005). However, whether sympathetic innervation contributes reciprocally to the morphogenesis of a target tissue remains unknown. The pancreas is a peripheral tissue that is richly innervated by adrenergic sympathetic nerves originating from the celiac and superior mesenteric ganglia (Woods and Porte, 1974). The islets of Langerhans in the endocrine pancreas regulate blood glucose homeostasis, and islet dysfunction underlies diabetes mellitus. In adult islets, sympathetic neural activity is well-known to inhibit insulin secretion and promote glucagon release to elicit an increase in circulating glucose during stress and exercise (Ahren, 2000; Woods and Porte, 1974). Developmentally, sympathetic innervation of the pancreas is promoted by the target-derived neurotrophin, nerve growth factor, NGF. Sympathetic innervation of the pancreas is incomplete in mice lacking NGF (Glebova and Ginty, 2004), while directed over-expression of NGF in β-cells results in hyper-innervation of the pancreatic islets (Edwards R. H. et al., 1989). The onset of sympathetic innervation is coincident with stages of rapid growth, differentiation and maturation in the embryonic pancreas (Burris and Hebrok, 2007). Previous studies indicate that pancreatic development is dependent on instructive signals derived from neighboring tissues including the mesenchyme and blood vessels (Golosow and Grobstein, 1962; Lammert et al., 2001). There is also growing evidence that dysfunction of the neuronal elements in the developing pancreas might be an instigating factor in the pathogenesis of pancreatic diseases. An early loss of sympathetic fibers and glial cells in the pancreas has been observed in diabetic animal models (Mei et al., 2002; Persson-Sjogren et al., 2005; Winer et al., 2003). However, whether sympathetic innervation contributes to pancreatic organogenesis remains unknown.
Pancreatic islets are highly organized functional micro-organs with defined size, shape, and a distinctive arrangement of endocrine cells. Islet architecture has been postulated to underlie effective inter-cellular coupling, synchronized insulin release, and paracrine regulation by the islet micro-vasculature (Bonner-Weir, 1988; Halban, 2004; Santos et al., 1991; Stagner and Samols, 1986; Valdeolmillos et al., 1989). Although inter-species differences in islet organization have been reported (Cabrera et al., 2006; Wieczorek et al., 1998), significant evidence suggests a unifying model that islet architecture, which optimizes cell-cell interactions between β-cells, is essential in the acquisition of an adult pattern of insulin secretion (Halban et al., 1982; Konstantinova et al., 2007; Ravier et al., 2005; Yamagata et al., 2002). Perturbed islet organization is also a characteristic feature in diabetic humans, and in animal models of the disease (Gepts and Lecompte, 1981; Gomez Dumm et al., 1990; Kilimnik et al., 2011; Tokuyama et al., 1995). In murine models, islet architecture has been shown to be established during late embryonic to early neonatal stages through a sequence of morphogenetic events, including delamination of endocrine precursors from a primordial ductal epithelium, endocrine cell migration into the surrounding mesenchyme and clustering to form nascent islets (Puri and Hebrok, 2010). While a cascade of transcription factors have been identified in the regulation of pancreatic cell specification and growth, relatively little is known about the signals responsible for the coordination of endocrine cell migration and establishment of islet organization.
By employing genetic and chemical ablation of sympathetic neurons in mice, we show that sympathetic innervation is necessary for establishing pancreatic islet shape and cytoarchitecture during development. Sympathectomized mice exhibit impaired glucose tolerance due to defects in glucose-stimulated insulin secretion later in life. Using neuron-islet co-cultures, we show that sympathetic neurons promote the directed migration of β-cells. We identify the nerve-derived signal as norepinephrine acting via pancreatic β-adrenergic receptors to influence islet cell migratory behavior. The effects of sympathetic de-innervation on islet architecture and glucose tolerance are recapitulated by the developmental blockade of β-adrenergic signaling. Remarkably, the pharmacological stimulation of β-adrenergic signaling during development is sufficient to restore islet morphologies and glucose metabolism in sympathectomized mice. Together, our results indicate that sympathetic neurons use noradrenergic signaling to establish the architecture of pancreatic islets, and that this inductive interaction at early stages of development is critical for mature islet function in regulating glucose metabolism.
Results
Sympathetic innervation is necessary for establishing islet architecture
To address the role of sympathetic innervation in pancreatic islet development, we visualized sympathetic nerves in the developing pancreas using immunofluorescent staining for tyrosine hydroxylase (TH), a sympathetic nerve marker, on tissue sections from MIP-GFP transgenic mice, which express green fluorescent protein (GFP) reporter under the insulin promoter (Hara et al., 2003). We found that sympathetic fibers innervate the developing pancreas as early as embryonic day 14.5 (E14.5), when GFP-labeled β-cells are scattered throughout the pancreas (Figure 1A). At E18.5, more GFP-labeled β-cells are found in the vicinity of TH-positive sympathetic fibers, where they begin to form aggregates (Figure 1B). By the first postnatal week (P6), discrete islet clusters are formed and wrapped by sympathetic fibers (Figure 1C). Strikingly, ablation of sympathetic innervation in neonatal mice using the neurotoxin, 6-hydroxydopamine (6-OHDA)(Kostrzewa and Jacobowitz, 1974), resulted in a profound disorganization of islets, with the islets appearing as large streak-like aggregates instead of the characteristic globular clusters (Figures 1D, E). Daily administration of 6-OHDA in newborn mice resulted in complete loss of sympathetic innervation by P6 (Supplemental Figures S1A and S1B).
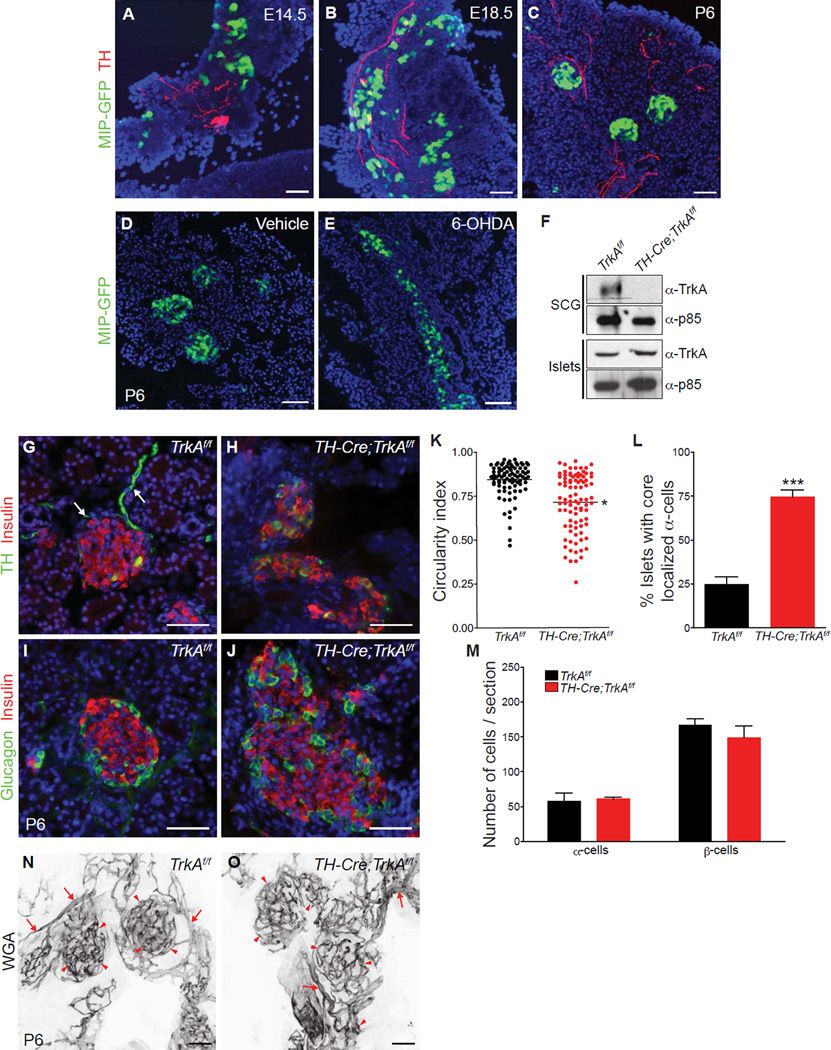
Figure 1. Sympathetic innervation establishes islet architecture and the spatial distribution of endocrine cells.
(A–C) TH immunohistochemistry and GFP fluorescence reveals the organization of β-cells into discrete clusters near sympathetic fibers during development in MIP-GFP mice. (D,E) Chemical sympathectomy with 6-OHDA administration disrupts islet clustering in neonatal MIP-GFP mice. GFP-positive β-cells (green) and DAPI (nuclei) staining are shown. (F) Genetic ablation of sympathetic innervation in TH-Cre;TrkAf/f mice. Immunoblotting shows depleted TrkA protein levels in superior cervical ganglia (SCGs) from TH-Cre;TrkAf/f mice at postnatal day 2 (P2), and intact TrkA expression in islets. The p85 subunit of phosphatidylinositol-3-kinase (PI-3K) was used to normalize for protein amounts. (G,H) Immunohistochemistry for TH and insulin shows that sympathetic axons (arrows) surrounding control TrkAf/f islets (G) are lost in TH-Cre;TrkAf/f islets (H) by postnatal day 6 (P6). (I,J) Immunostaining for islet hormones shows disrupted arrangement of endocrine cells within islets in neonatal (P6) TH-Cre;TrkAf/f mice. (K) Islet shape is quantified by circularity index. (L) Percentage of islets with mis-localized α-cells. (M) Normal endocrine cell numbers in P6 TH-Cre;TrkAf/f mutants. For all quantifications, n = 3 mice per genotype; mean ± SEM; *p < 0.05, ***p < 0.001, t test. (N,O) Intact islet vasculature in sympathectomized animals. The density and morphology of the pancreatic blood vessels (arrows) and islet capillaries (arrowheads) are not compromised, despite the alterations in islet architecture in TH-Cre;TrkAf/f pups. Blood vessels were fluorescently painted by perfusing Alexa-633-conjugated wheat germ agglutinin (WGA) into TH-Cre;TrkAf/f and control mice at P6. Scale bars, 50µm.
We further used a genetic approach to ablate sympathetic innervation. The survival and target innervation of sympathetic neurons depend on the expression of the TrkA receptor, which mediates trophic effects of the neurotrophin, NGF, secreted by target tissues (Glebova and Ginty, 2005). We crossed mice harboring a conditional (floxed) TrkA allele (Chen et al., 2005) with mice expressing TH-driven Cre recombinase (TH-Cre) (Gong et al., 2007). In TH-Cre;TrkAf/f mutant mice, we observed a selective depletion of TrkA protein in sympathetic neurons, whereas the expression of TrkA in islets was unaffected (Figure 1F). Previous studies have reported that in rodents, TrkA is be localized to β-cells in neonatal and adult islets (Kanaka-Gantenbein et al., 1995; Miralles et al., 1998). By the first week of birth (P6), while an enrichment of TH-positive sympathetic fibers was observed at the periphery of islets in control TrkAf/f animals (Figure 1G, arrows) (Burris and Hebrok, 2007; Rodriguez-Diaz et al., 2011), there was a complete loss of sympathetic innervation in the pancreas and several other peripheral tissues in TH-Cre;TrkAf/f mutant mice (Figure 1H and Supplemental Figures S1C–H).
In neonatal mice, immunostaining with the islet hormone markers, insulin and glucagon, revealed that wild-type islets had coalesced into globular clusters with the stereotypical arrangement of insulin-producing β-cells at the core surrounded by glucagon-producing α-cells at the periphery or mantle (Figure 1I). In contrast, in TH-Cre ;TrkAf/f mutants, significant disruptions in islet cytoarchitecture were evident with islets appearing as disorganized aggregates with an intermingling of α-cells and β-cells within the islet core (Figures 1J–L). The total numbers of α- and β-cells remained unaffected (Figure 1M). Despite the perturbation of islet organization in TH-Cre;TrkAf/f mice, the islets still retained a dense meshwork of micro-vasculature, indicating that ablation of innervation does not impair islet vascularization (Figures 1N, O). Significant disruptions in islet cytoarchitecture with mixed α-cells and β-cells within the islet core, in the absence of any significant changes in islet cell numbers, were also observed in the neonatal 6-OHDA-injected mice (Supplemental Figures S1I–M). These results suggest that sympathetic innervation is necessary for the establishment of islet shape and cytoarchitecture.
TH is also expressed in a subset of β-cells in the wild-type pancreas (Teitelman and Lee, 1987). Consistent with previous reports (Teitelman and Lee, 1987), we found 3% of insulin-positive β-cells showing TH immunoreactivity in wild-type islets (Supplemental Figures S1N, arrowheads, and S1P). We noticed there was a large increase in the number of TH-expressing β-cells in TH-Cre;TrkAf/f mice (Supplemental Figures S1O and S1P). Despite the increase in TH expression in TH-Cre;TrkAf/f mutant islets, no changes in TrkA levels were detected by immunoblotting (Figure 1F) or quantitative PCR analysis (Supplemental Figure S1Q), indicating that the islet deficits observed in TH-Cre;TrkAf/f mice were not due to changes in TrkA levels in the islets themselves. Interestingly, the number of TH-positive β-cells were unaffected by 6-OHDA treatment (Supplemental Figures S1R–T).
Sympathetic innervation is required for islet cell-cell contacts and glucose-stimulated insulin secretion
Given that perturbations in cytoarchitecture are associated with alterations in cell-cell interactions within islets (Jain and Lammert, 2009), we analyzed the expression of the major islet cell adhesion molecules, Neural Cell Adhesion Molecule (N-CAM) and E-cadherin (Dahl et al., 1996; Esni et al., 1999). In postnatal TH-Cre;TrkAf/f islets, we observed an overall reduction in N-CAM expression in β-cells (Figures 2A, B), with little immunoreactivity detected at the junctions between adjacent β-cells (Figures 2C, D). Immunostaining analyses also revealed a down-regulation in E-cadherin levels in mutant β-cells (Figures 2E, F). In mutant β-cells that still showed E-cadherin immunoreactivity, there were noticeable changes in its sub-cellular distribution. While wild-type β-cells showed a continuous distribution of E-cadherin at the sites of β-cell-cell contacts (Figure 2G), we observed a “patchy” uneven pattern of E-cadherin labeling between neighboring β-cells in mutant islets (Figure 2H). These analyses also revealed irregularly spaced clusters of β-cells in mutant islets, with “lesions” that were devoid of any cells (arrowheads in Figures 2B and 2F), compared to control islets where β-cells were closely packed (Figures 2A and 2E). Together, these results indicate that the loss of sympathetic innervation results in abnormalities in cell-cell contacts and cellular spacing of β-cells in TH-Cre;TrkAf/f islets.
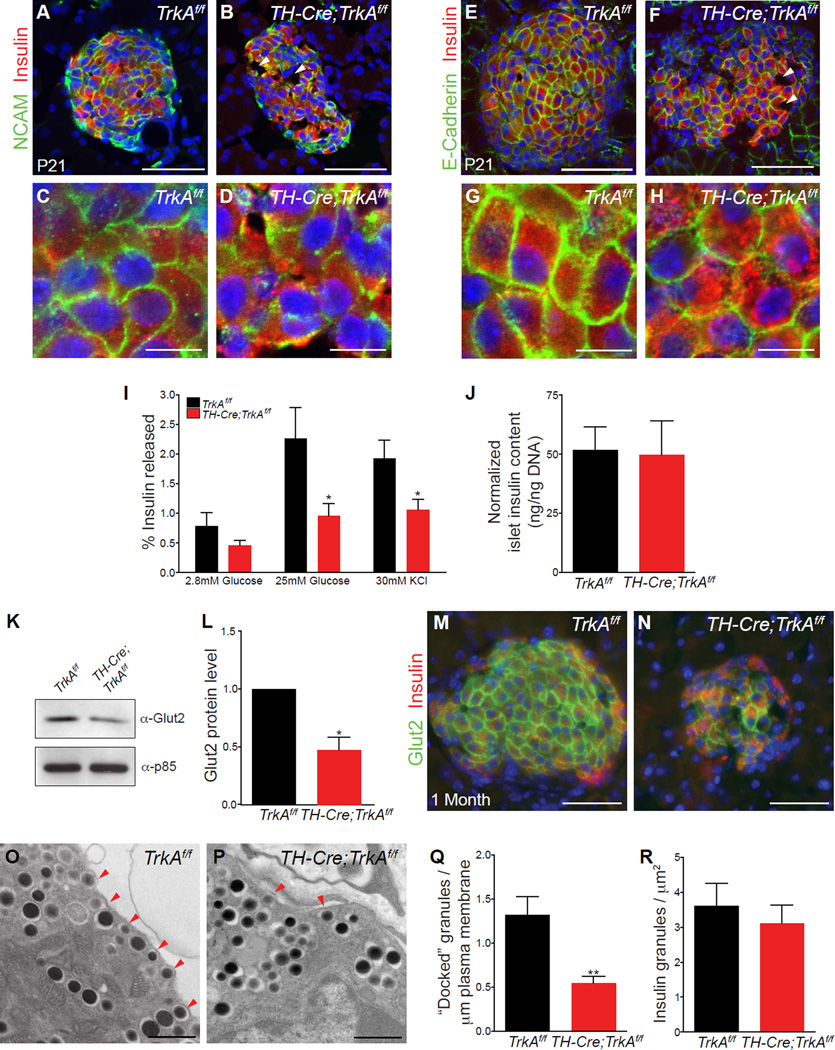
Figure 2. Defects in islet cell-cell contacts and glucose-stimulated insulin secretion in sympathectomized mice.
(A, B) Reduced N-CAM expression in islets from postnatal (P21) TH-Cre;TrkAf/f mice (P21). (C, D) Higher magnification images of regions from (A, B). (E, F) Reduced expression of E-cadherin in islets from P21 TH-Cre;TrkAf/f animals compared to control mice. (G, H) Higher magnification images of regions in (E, F) show an uneven distribution of E-cadherin at junctions between adjacent β-cells in mutant islets. Mutant islets also exhibit abnormalities in cellular spacing and gaps devoid of any cells (arrowheads in B and F). Scale bars 50µm for (A, B, E, F) and 10 µm for (C, D, G, H). (I) Isolated islets from one month-old TH-Cre;TrkAf/f mice secrete less insulin when challenged with high glucose or with potassium chloride treatment. Insulin released is expressed as a percentage of the total insulin content. n = 5 mice per genotype; mean ± SEM; *p<0.05, t test. (J) Total insulin content is normal in isolated islets from TH-Cre ;TrkAf/f mice. Insulin content is normalized to total islet DNA content. n=4 mice per genotype; mean ± SEM. (K–N) Reduced expression and surface localization of the glucose transporter, Glut2, in mutant β-cells as revealed by immunostaining and immunoblotting. n=3 mice per genotype; mean ± SEM; *p<0.05, t test. (O–Q) Ultrastructural analysis of β-cells reveals fewer insulin granules within 50nm of the plasma membrane (arrowheads), in mutant islets. Numbers in (Q) represent insulin granules docked per µm of the plasma membrane n=3 mice per genotype, mean ± SEM; **p < 0.01, t test. (R) The total number of insulin granules (per µm2) are not different between mutant and control islets. n=3 mice per genotype, mean ± SEM; t test.
Islet morphology and cytoarchitecture have been postulated to be determinants in the acquisition of functional maturity (Bosco et al., 1989; Halban et al., 1982; Hopcroft et al., 1985; Ravier et al., 2005). We thus assessed glucose-stimulated insulin secretion (GSIS) in TH-Cre;TrkAf/f mice and wild-type litter-mates at one month of age, when islets have acquired functional maturity (Jermendy et al., 2011). Mutant islets exhibited significant reduction in GSIS (Figure 2I), although they had normal insulin content (Figure 2J). The attenuated response of de-innervated islets to a glucose challenge suggests that sympathetic innervation during development is necessary for functional maturation of the islets. To address the mechanisms underlying deficient GSIS in de-innervated islets, we assessed aspects of glucose sensing and insulin trafficking. The β-cell-specific glucose transporter, Glut2, is essential for glucose entry into β-cells and activation of the glycolytic pathway that subsequently fuels insulin secretion (Thorens, 2003). Glut2 deletion leads to severe defects in insulin secretion in mice (Guillam et al., 1997). Although it is reported that Glut2 is not the predominantly expressed glucose transporter in human β-cells (De Vos et al., 1995), new evidence points to recessive mutations in Glut2 in patients with neonatal diabetes (Sansbury et al., 2012; Yoo et al., 2002). TH-Cre;TrkAf/f islets exhibited reduced protein expression and surface localization of Glut2 at one month of age (Figures 2K–N), and these deficits were evident as early as P6 (Supplemental Figures S2A–D). The mutant islets also failed to secrete insulin in response to potassium chloride-mediated depolarization (Figure 2I), suggesting defects in the mobilization of a readily releasable pool of insulin (Seino et al., 2011). Ultrastructural analyses revealed fewer insulin granules at or very close (within 50 nm) to the plasma membrane in TH-Cre;TrkAf/f islets compared to control TrkAf/f islets (Figures 2O–Q). The total numbers of insulin granules were not significantly different between control and mutant β-cells (Figure 2R), consistent with the finding in Figure 2J. Together, these results indicate that the failure of mutant islets to respond to glucose arises from impaired glucose sensing and a reduction in the number of docked insulin granules. We also noticed that TH-Cre;TrkAf/f animals exhibited reductions in islet size that were only manifested by one month of age (Figure 2N and Supplemental Figures S2E–G). The decreased islet mass in mutant animals is likely, at least in part, due to an overall reduction in body weight in mature TH-Cre;TrkAf/f animals (Supplemental Figures S2H and S2I). The exocrine, ductal and vascular compartments in TH-Cre;TrkAf/f pancreata all appeared normal (data not shown).
Sympathectomized mice are glucose intolerant
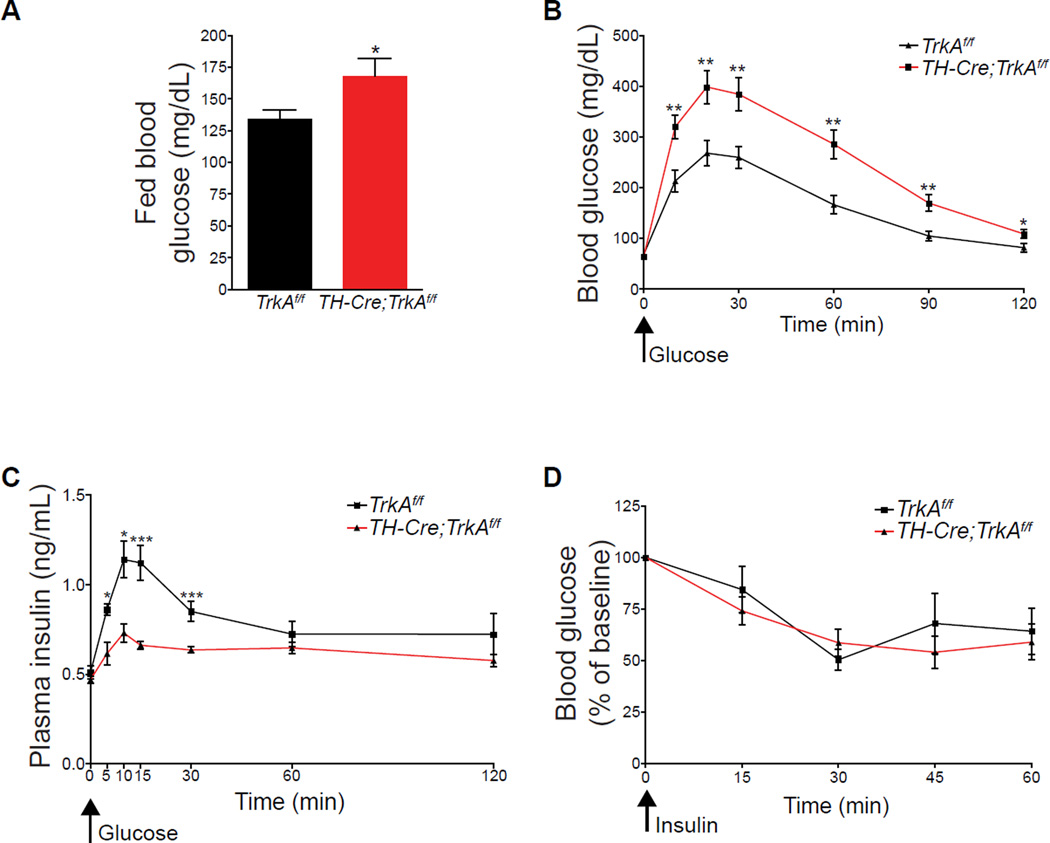
We then assessed metabolic parameters at the whole animal level in TH-Cre;TrkAf/f mice. At one month of age, TH-Cre;TrkAf/f mice showed normal fasting blood glucose levels (Supplemental Figure S3A), but were hyperglycemic when fed ad libitum (Figure 3A). Further, when TH-Cre;TrkAf/f mice were challenged in a glucose tolerance test, they had significantly elevated blood glucose levels as compared to control TrkAf/f mice (Figure 3B). Consistent with reduced glucose-stimulated insulin secretion in isolated TH-Cre ;TrkAf/f islets, plasma insulin levels were severely attenuated in response to the glucose challenge in TH-Cre;TrkAf/f mice (Figure 3C). However, the TH-Cre;TrkAf/f mice responded normally with a significant reduction in blood glucose levels when injected with insulin (Figure 3D), suggesting that the insulin sensitivity of muscle, adipose and liver tissues is not perturbed despite the loss of sympathetic innervation. In addition, the plasma levels of other pancreatic hormones, glucagon and ghrelin, as well as that of several non-pancreatic hormones known to influence β-cell biology, i.e., the gut-derived incretins (GIP and GLP-1), other gut-derived peptides (cholecystokinin, gastrin, and secretin) as well as adipose-derived leptin were all normal in TH-Cre;TrkAfl/fl mice (Supplemental Figures S3B–I). Together, these findings indicate that sympathetic innervation during development is necessary for glucose homeostasis later in life, and that the impaired glucose tolerance in the mutants arises primarily from islet dysfunction rather than broader physiological defects stemming from the loss of sympathetic innervation.
Figure 3. Sympathectomized mice exhibit impaired glucose tolerance but normal insulin sensitivity.
(A) Elevated blood glucose levels in one month-old TH-Cre;TrkAf/f compared to control mice when fed ad libitum. n=9 mice per genotype; mean ± SEM; *p < 0.05, t test. (B) Glucose intolerance in TH-Cre;TrkAf/f mice. Mice, at one month of age, were fasted overnight, then injected i.p with a bolus of 2g/kg glucose at t=0. Blood glucose measurements were made from tail vein at the indicated time intervals after glucose injection. n=10 mice per genotype; mean ± SEM; *p < 0.05, **p < 0.01, t test. (C) Reduced plasma insulin levels in response to a glucose injection (3g/kg glucose in TH-Cre;TrkAf/f mice compared to control mice. n=8 control, 10 mutant mice, mean ± SEM; *p<0.05, ***p<0.001, t test. (D) Normal insulin sensitivity in TH-Cre;TrkAf/f mice. A significant decrease (p<0.001, t test) in blood glucose levels was seen in both TH-Cre;TrkAf/f and control mice at 30 minutes after insulin injection compared to t=0). Mice were treated with 0.75U/kg of insulin, and blood glucose measurements made from tail blood at the indicated times post-injection. n=5 mice per genotype, t test, mean ± SEM,
Sympathetic de-innervation in mature animals does not affect islet architecture and glucose tolerance
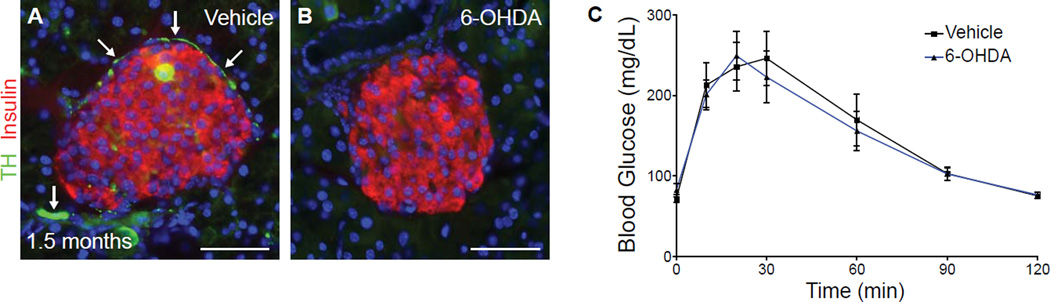
To determine whether sympathetic innervation is required during later postnatal stages of development for islet architecture and glucose responsiveness, we ablated sympathetic innervation in young animals at postnatal day 21 by 6-OHDA injection, after the mature organization of islets has been established. Examination of 6-OHDA treated animals at 1.5 months of age revealed depletion of sympathetic fibers in the pancreas (Figures 4A, B). However, no defects were observed in islet architecture or in glucose tolerance of sympathectomized mice (Figures 4B, C), indicating that sympathetic innervation is dispensable for the maintenance of islet architecture and glucose tolerance.
Figure 4. Sympathetic de-innervation in mature animals does not affect islet architecture and glucose tolerance.
(A–C) Normal islet morphology and glucose tolerance with 6-OHDA administration at a late postnatal stage (postnatal day 21). P21-old mice were serially injected with 6-OHDA or vehicle for 7 days, and then analyzed at 1.5 months of age. TH-positive sympathetic fibers (arrows in A) are sparser in mature animals, and are completely lost with 6-OHDA administration (B). n=6 mice for each treatment; t test, mean ± SEM.
Sympathetic neurons promote β-cell migration through norepinephrine signaling
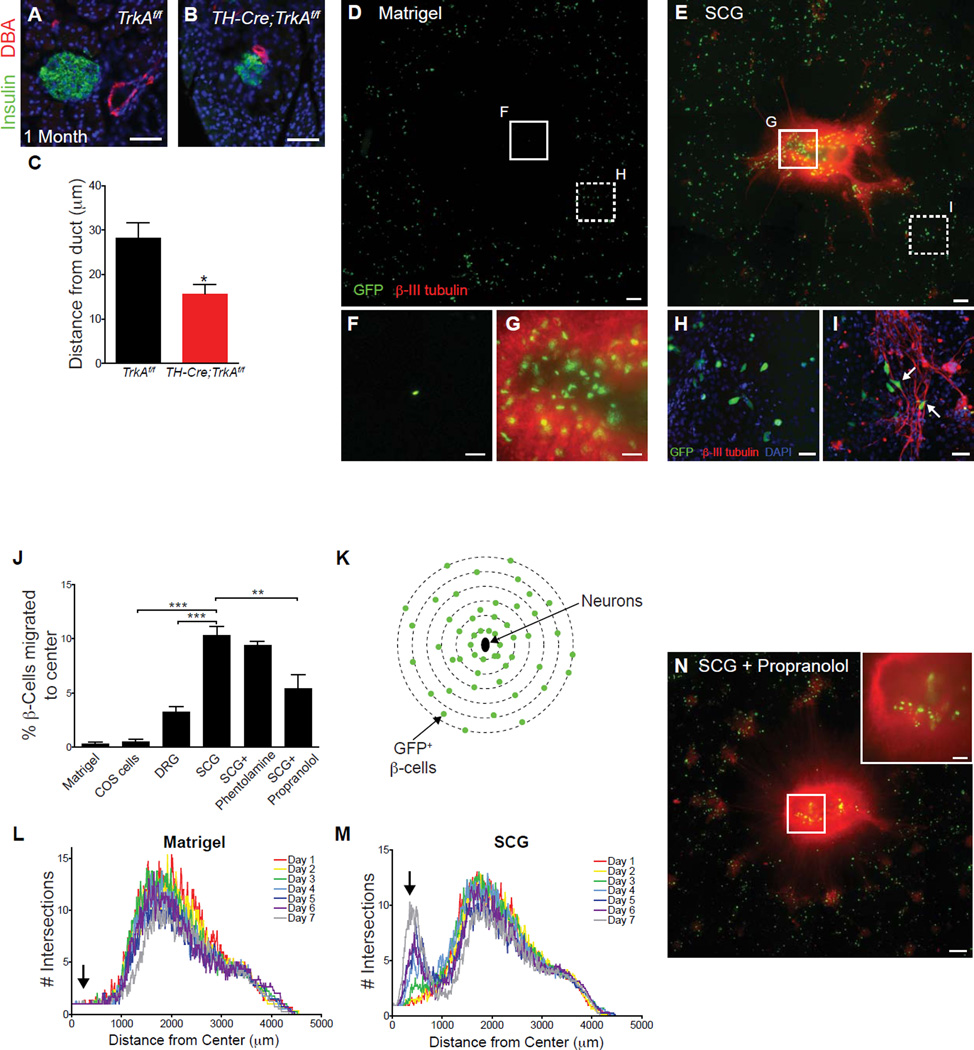
How does sympathetic innervation influence islet formation during development? The defects in islet architecture observed in TH-Cre;TrkAf/f mice (Figures 1E and J) were reminiscent of the phenotypes reported in mice where endocrine cell migration had been disrupted (Gannon et al., 2000; Kim et al., 2005; Miettinen et al., 2000). Endocrine progenitor cells migrate away from the ducts to form mature islets. The degree of islet-duct association is a well-established criterion of endocrine cell migratory behavior during development (Miettinen et al., 2000). The close association of islets with ducts is a feature commonly observed in neonatal mice compared to functionally mature animals. We found that one month-old TH-Cre;TrkAf/f mice exhibited a significantly smaller ductal-islet distance (Figure 5A–C), a hallmark of reduced endocrine cell migration. We established co-cultures of sympathetic neurons with fluorescently labeled β-cells isolated from MIP-GFP transgenic mice, and found that β-cells migrated toward explants of sympathetic ganglia when co-cultured in a three dimensional Matrigel matrix, while β-cells cultured alone exhibited little to no movement in the matrix over 7 days in culture (Figures 5D–G and Supplemental Figures S4A and S4B). Consistent with migratory behavior, β-cells in close proximity to sympathetic axons frequently exhibited polarized morphologies (Figures 5H, I). The β-cell migratory behavior was specific to the presence of sympathetic neurons, because little to no migration was observed in the presence of either non-neuronal COS cells or sensory neurons from dorsal root ganglia (DRGs) (Figure 5J). A modified Sholl analysis (Figure 5K) to quantify the overall distribution of the β-cells over the culture period revealed a gradual and directed movement of β-cells toward sympathetic explants (Figures 5L, M).
Figure 5. Sympathetic neurons promote β-cell migratory behavior through norepinephrine signaling.
(A–C) Ductal proximity of islets in one-month old TH-Cre;TrkAf/f mice. n=4 control and 3 mutant animals; mean ± SEM; *p < 0.05, t test. (D,E) Isolated β-cells exhibit directed migration towards sympathetic (SCG) tissue explants plated in the center of a Matrigel culture dish. Scale bars, 200µm. (F,G) Higher magnification images of the insets shown in D,E. Scale bars, 50 µm. (H,I) β-cells align along sympathetic axons and adopt polarized morphologies when cultured with neurons (arrows, I). Scale bars, 50 µm. (J) Percentage of β-cells that have migrated toward the center of Matrigel cultures containing COS cell aggregates, DRG or SCG explants with or without adrenergic antagonists after 7 days. n= at least 3 independent experiments; mean ± SEM; **p < 0.01, ***p < 0.001 by one-way ANOVA and Tukey’s post-hoc test. (K–M) Sholl analysis to quantify the distribution of GFP+ β-cells over 7 days in culture. The distribution of β-cells cultured alone does not change significantly over 7 days (L), while there is a gradual shift in the distribution of β-cells towards the center of the culture containing SCGs (M, arrow). Averaged profiles of four independent experiments are plotted. (N) β-cells exhibit decreased migration towards sympathetic explants in the presence of the β-adrenergic antagonist, propranolol (see J for quantification).
Sympathetic nerves innervating the pancreas release the neurotransmitter norepinephrine, which activates α- and β-adrenergic receptors (Ahren, 2000). Norepinephrine has been shown to influence the migration of peripheral cell types including hematopoietic stem cells and pancreatic cancer cells (Guo et al., 2009; Katayama et al., 2006). In the co-cultures, migration of β-cells towards sympathetic ganglia was significantly reduced by addition of the β-adrenergic receptor antagonist propranolol (Figures 5N, 5J and Supplemental Figure S4C). In contrast, the α-adrenergic antagonist phentolamine had no effect (Figure 5J and Supplemental Figure S4D). None of the treatments changed the total number of cultured β-cells (Supplemental Figure S4E). In addition, the effects of sympathetic ganglia were specific to β-cells. The ganglia explants had no effect on migration or numbers of glucagon-expressing α-cells in the co-cultures (Supplemental Figures S4F and S4G). These results suggest a model in which sympathetic neurons regulate islet formation during development by promoting β-cell migratory behavior through noradrenergic signaling.
Developmental β-adrenergic signaling regulates islet architecture and glucose metabolism
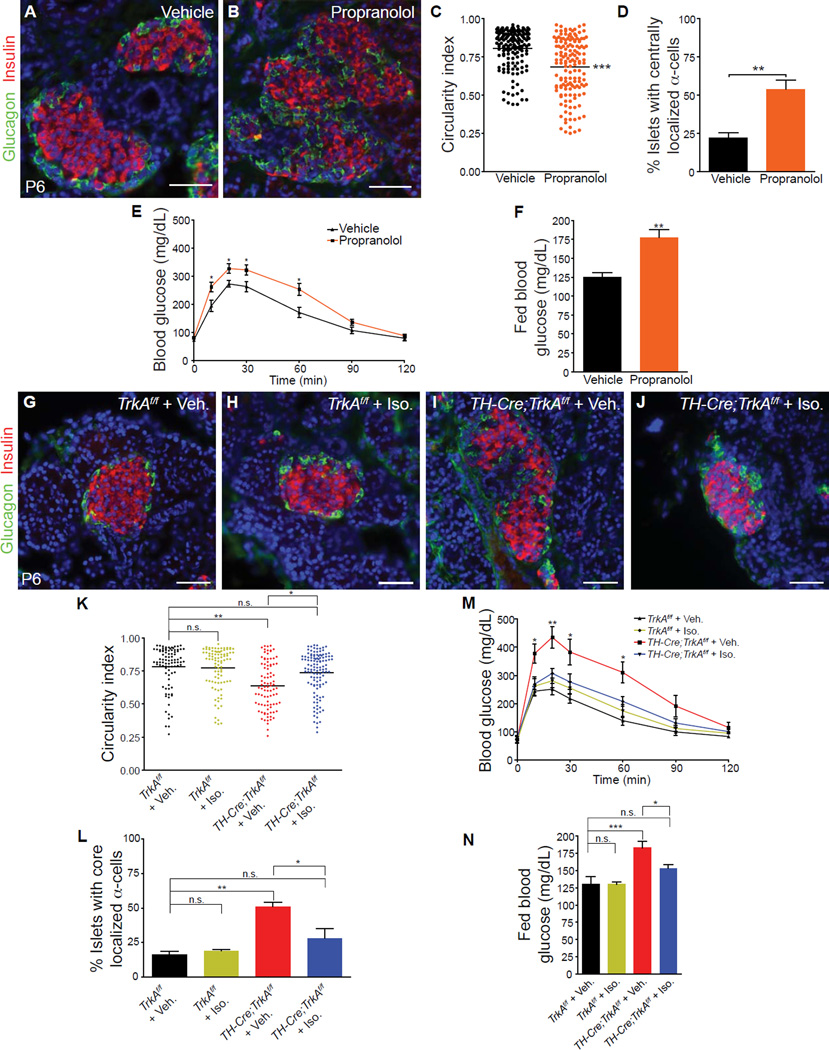
To test the hypothesis that sympathetic neurons use noradrenergic neurotransmission to regulate islet structure and function, we examined the effects of developmental blockade of β-adrenergic signaling on islet formation and functional maturation in vivo. We administered propranolol daily to pregnant wild-type mice from E18, and continued injections in the neonatal pups until P6. Propranolol injections did not affect islet sympathetic innervation (Supplemental Figures S5A and S5B) but resulted in disruptions to neonatal islet architecture (Figures 6A–D), which mimicked the phenotypes observed in TH-Cre;TrkAf/f pups. Additionally, when propranolol-injected animals were assessed six weeks after the treatment, they exhibited glucose intolerance and hyperglycemia in the fed state (Figures 6E, F). These results provide evidence that β-adrenergic blockade during the developmental window of E18 to P6 impairs glucose metabolism later in life.
Figure 6. β-adrenergic signaling is necessary during development to regulate islet architecture and glucose metabolism.
(A,B) In vivo administration of propranolol (20 mg/kg body weight) during development (E18-P6) results in defects in islet architecture in neonatal (P6) mice, compared to saline-injected controls. (C,D) Quantification of islet shape and endocrine cell compartmentalization in mice injected with saline or propranolol. n=5 animals per genotype; mean ± SEM; **p < 0.01, ***p < 0.001, t test. (E) Glucose intolerance in one month-old animals injected with propranolol during development (E18.5-P6), compared to vehicle-treated controls. n=7,9 mice for vehicle and propranolol respectively;, mean ± SEM; *p < 0.05, t test. (F) Developmental administration of propranolol results in elevated fed blood glucose levels in mature animals at one month. n=7,9 mice for vehicle and propranolol respectively; mean ± SEM; **p < 0.01, t test. (G–L) Restoration of islet morphologies in neonatal TH-Cre;TrkAf/f mice treated with a β-adrenergic receptor agonist, isoproterenol, from E18-P6. Isoproterenol administration (Iso, 30 mg/kg body weight) has no effect on islet architecture in control TrkAf/f mice (G,H), but normalizes islet structure and cytoarchitecture in TH-Cre;TrkAf/f mice (J) compared to saline-injected TH-Cre;TrkAf/f mice (I). (K,L) Quantification of islet shape and endocrine cell compartmentalization in TH-Cre;TrkAf/f and control mice injected with saline or isoproterenol. n = 3 mice for all treatments except for TH-Cre;TrkAf/f + Iso where n=4 mice; mean ± SEM; *p < 0.05, **p<0.01 one-way ANOVA with Tukey’s post-hoc test. (M) Developmental administration of isoproterenol administration (E18-P6) rescues the glucose intolerance in one-month old TH-Cre;TrkAf/f mice, compared to vehicle-injected TH-Cre;TrkAf/f mice. Isoproterenol had no effect on glucose metabolism in control TrkAf/f mice. n=5 mice for vehicle- and Iso-injections in TrkAf/f mice; n=6 for vehicle-injected TH-Cre;TrkAf/f mice and n=7 for Iso-injected TH-Cre;TrkAf/f mice; mean ± SEM; *p < 0.05, **p<0.01 as determined by one-way ANOVA and Tukey’s post-hoc test. (N) Normalization of fed blood glucose levels in in one-month old TH-Cre;TrkAf/f mice treated with isoproterenol between E18-P6, compared to saline-treated TH-Cre;TrkAf/f mice. n=5 mice for vehicle- and Iso-injections in TrkAf/f mice; n=6 for vehicle-injected TH-Cre;TrkAf/f mice and n=7 for Iso-injected TH-Cre;TrkAf/f mice; mean ± SEM; *p < 0.05, ***p<0.001 one-way ANOVA with Tukey’s post-hoc test.
Next, we asked whether adrenergic stimulation during development would be sufficient to restore islet morphologies and functional maturity in the sympathectomized animals? Treatment of TH-Cre;TrkAf/f mice with the β-adrenergic agonist, isoproterenol, from E18 to P6 resulted in a significant improvement in islet architecture by P6, in the absence of sympathetic innervation (Figures 6G–L and Supplemental Figure S5C and S5D). Since sympathetic nerves are usually aligned with the pancreatic vasculature (Burris and Hebrok, 2007; Chiu et al., 2012), these results imply that, despite the absence of innervation in TH-Cre;TrkAf/f mice, isoproterenol released from the islet vasculature may elicit directional migration of endocrine cells, similar to the effects of norepinephrine released from nerve terminals. Isoproterenol treatment during E18 to P6 also improved glucose tolerance and significantly lowered fed blood glucose levels in TH-Cre;TrkAf/f mice, when these metabolic parameters were examined 6 weeks later (Figures 6M, N). Sympathetic innervation, islet architecture and glucose tolerance in control TrkAf/f animals were unaffected by the isoproterenol administration during development (Figures 6H, K, L, M, N and Supplemental Figure S5C). Together, the loss and gain-of function analyses indicate that islet architecture and subsequent function relies on β-adrenergic signaling during development.
Discussion
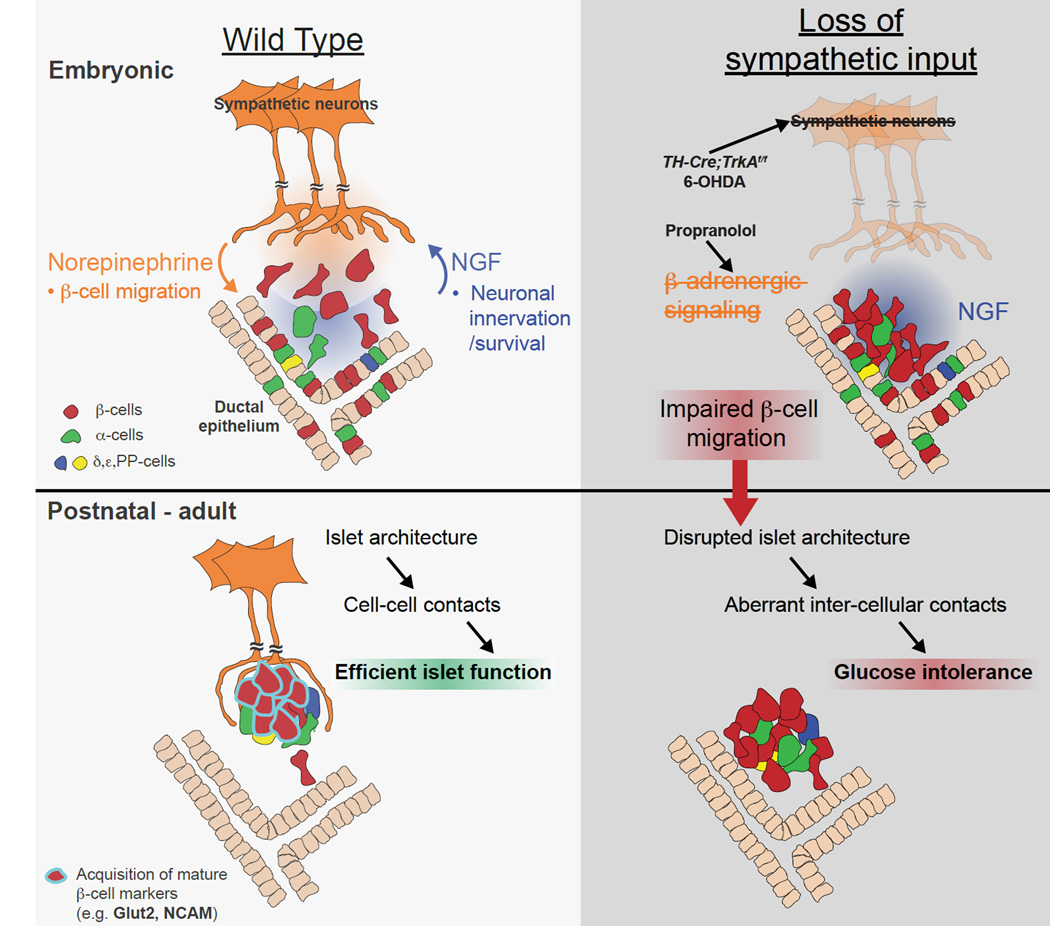
To date, all studies on sympathetic innervation of the pancreas have focused on the modulatory functions in adult islets during stress and exercise (Ahren, 2000; Woods and Porte, 1974). Here, we provide the first evidence for sympathetic innervation in organizing islet architecture during normal development. Further, we demonstrate that this inductive interaction at early stages is critical for mature islet function in regulating glucose metabolism. It is well-established that sympathetic innervation of the embryonic pancreas occurs in response to target-derived NGF, which then supports survival of innervating neurons (Glebova and Ginty, 2005). Our findings support the model that, during development, sympathetic nerves, in turn, release norepinephrine that activates β-adrenergic signaling in migrating β-cells to influence islet architecture and the acquisition of functional maturity (Figure 7). Similar to TH-Cre;TrkAf/f animals,β-adrenergic receptor knockout mice are glucose intolerant and have reduced insulin secretion due to perturbations in Glut2 levels when analyzed at adult stages (Asensio et al., 2005; Santulli et al., 2012), although a detailed analysis of islet development remains to be done in these mice. Together, these findings highlight reciprocal signaling between sympathetic neurons and the pancreas that results in the co-development of these two physiological systems.
Figure 7. Developmental interactions between sympathetic neurons and pancreatic endocrine cells.
Sympathetic innervation of the embryonic pancreas occurs in response to target-derived NGF, which then supports survival of innervating neurons (Glebova and Ginty, 2005). Sympathetic nerves release norepinephrine, which acts through β-adrenergic receptors on β-cells to contribute to endocrine cell migration and formation of discrete islet clusters. Nerve-dependent establishment of islet architecture is essential for intercellular contacts within islets, β-cell maturation (i.e. Glut2 levels) and optimal glucose-stimulated insulin secretion.
We found that both chemical and genetic ablation of sympathetic innervation during development resulted in similar alterations in islet shape and cellular organization. In ablating sympathetic innervation, it would have been ideal to accomplish pancreas-selective deletion of sympathetic fibers. However, a potential genetic approach of pancreas-specific deletion of the sympathetic neuron survival factor, NGF, would be expected to perturb NGF signaling within the pancreas and thus affect pancreas development. Moreover, surgical lesioning of sympathetic projections to the pancreas cannot be accomplished in embryonic or neonatal pups. Despite that our approaches for de-innervation were not pancreas-specific, we provided multiple lines of evidence that indicate a direct action of sympathetic innervation on islet architecture and function. First, in neuron-islet co-cultures, sympathetic neurons directly promote β-cell migratory behavior in a manner dependent on noradrenergic signaling. Second, isolated islets from sympathectomized mice show attenuated glucose-stimulated insulin secretion in vitro, suggesting islet-intrinsic defects with de-innervation. Third, when injected with insulin, sympathectomized mice responded normally with a significant reduction in blood glucose levels, indicating normal insulin sensitivity in peripheral tissues despite the loss of sympathetic innervation. Fourth, in TH-Cre;TrkAf/f animals, we did not observe any changes in the plasma levels of several intestinal hormones and leptin that are known to influence β-cell physiology. Finally, consistent with recent findings showing that mutant mice lacking sympathetic innervation due to genetic deletion of a key transcription factor, Phox2b, have normal vascular patterning of the heart (Nam et al., 2013), we found that vascular organization was intact in TH-Cre;TrkAf/f islets. The preservation of the vasculature further supports that the structural and functional deficits in TH-Cre;TrkAf/f islets indeed arises from the loss of sympathetic nerves.
We did observe a difference between the genetic and chemical approaches of sympathectomy. We found an increase in the number of TH-positive β-cells in TH-Cre;TrkAf/f mice, but not in 6-OHDA-treated animals. An up-regulation in TH expression has been reported in functionally immature or stressed islets (Teitelman et al., 1988; Teitelman and Lee, 1987). The reasons for TH expression in β-cells, and the functional consequences of increased TH-positive cells in TH-Cre;TrkAf/f islets remain unclear. To our knowledge, there are no reports of β-cells synthesizing or releasing catecholamines, raising the intriguing possibility of TH functions in the pancreatic cells that are independent of catecholamine biosynthesis. It is also important to note that 6-OHDA is unlikely to have a direct effect on islets since rodent β-cells, unlike human β-cells, lack the dopamine re-uptake transporter used by 6-OHDA (Schafer et al., 2013).
Our findings imply a causal relationship between the early developmental perturbations in islet architecture and the later functional deficits in sympathectomized animals. Both sympathectomy and β-adrenergic antagonism resulted in defects in islet architecture during development and glucose intolerance later in life. Importantly, the restoration of islet architecture, with β-adrenergic stimulation in sympathectomized animals, correlated with improved function. In line with previous studies implicating islet architecture in homogeneous and synchronous secretory responses, our results suggest that the disorganized islet clusters in the TH-Cre;TrkAf/f mice with the peripheral α-cells interspersed among the core β-cells compromises the homotypic inter-cellular interactions between β-cells that is required for optimal insulin secretion (Halban et al., 1982; Konstantinova et al., 2007; Smolen et al., 1993). Consistent with this notion, in de-innervated islets, we observed abnormalities in the expression and localization of N-CAM and E-cadherin that are known mediators of β-cell-cell contacts and function. Similar to our observations in TH-Cre;TrkAf/f mice, N-CAM−/− mutant mice show abnormal islet architecture with intermingling of α- and β-cells within the islet core as well as impaired glucose tolerance in vivo (Esni et al., 1999; Olofsson et al., 2009). E-cadherin is necessary for β-cell clustering during pancreas development and glucose-stimulated insulin secretion (Dahl et al., 1996; Jaques et al., 2008; Yamagata et al., 2002). The relevance of inter-cellular contacts in normal β-cell function is underscored by animal and human studies suggesting that perturbations in islet architecture, due to injury or disease, are closely correlated with metabolic dysfunction (Gepts and Lecompte, 1981; Gomez Dumm et al., 1990; Kilimnik et al., 2011; Tokuyama et al., 1995). In addition, it is recognized that, in the current translational efforts for diabetes treatment, preserving the three-dimensional arrangement of transplanted β-cells could contribute to the long-term survival and optimal function of the transplants (Halban, 2004; Van Hoof et al., 2011). It will be of interest, in future studies, to identify the signaling pathways and transcriptional programs disrupted by loss of cell-cell contacts in the de-innervated islets. The reduced Glut2 levels and defects in docked insulin granules in TH-Cre;TrkAf/f mice suggest that the signaling pathways impinge on the glucose sensing and insulin trafficking machinery.
Recent studies have reported that human and murine islets have distinct architecture, with the different endocrine cell types being interspersed within human islets (Brissova et al., 2005; Cabrera et al., 2006). These findings imply that, for human islet function, heterotypic rather than homotypic cellular interactions within islets predominate. However, other findings suggest that, like rodent models, human islets, also require β-cell-cell contacts for efficient glucose-stimulated insulin secretion (Wojtusciszyn et al., 2008). In particular, at higher glucose concentrations, homotypic contacts between human β-cells were better at facilitating insulin secretion than heterotypic contacts (Wojtusciszyn et al., 2008), pointing to a requirement for β-cell-cell interactions in potentiating insulin secretion especially under high metabolic demand. Additionally, limited studies performed on human islets at embryonic stages have reported that fetal islets are initially formed with a segregated architecture typical of murine islets, compared to the mixed architecture seen in adult human islets (Jeon et al., 2009; Piper et al., 2004). These findings imply a significant plasticity in islet structure that may reflect the metabolic requirements at a particular stage.
Recent findings have also pointed to differences in the innervation patterns between mouse and human islets (Rodriguez-Diaz et al., 2011). In human islets, sympathetic fibers do not directly contact α- and β-cells as in murine islets, but instead innervate blood vessels within the islets. Thus, in human islets, sympathetic nerve-mediated modulation of hormone release is likely to be indirectly via release of norepinephrine into the islet circulation or by altering the blood flow. However, in both mice and humans, neuronal activity has similar physiological effects on inhibiting insulin and enhancing glucagon release in adult islets despite the differences in innervation patterns and architecture (Ahren, 2000; Gilliam et al., 2007; Taborsky, 2011). Thus, our study raises the possibility that during development, sympathetic innervation and neuronal activity may have similar effects in mice and humans in coordinating islet organization and subsequent function. In support of this prediction, we note that children with Congenital Insensitivity to Pain with Anhidrosis (CIPA), an inherited neural disorder characterized by mutations in the TrkA gene, show a deficit in glucose responsiveness (Schreiber et al., 2005), along with reduced peripheral innervation (Indo et al., 1996), reminiscent of that seen in TH-Cre;TrkAf/f mice. It is now recognized that β-cell dysfunction, inadequate insulin secretion and impaired glucose tolerance are early hallmarks of type 2 diabetes (DeFronzo and Abdul-Ghani, 2011; Meece, 2007). Although autonomic neuropathy is an established complication of diabetes (Kuehl and Stevens, 2012), it is important to note that autonomic dysfunction in patients is already evident at the time of diagnosis of diabetes or even during a clinically normal range of blood glucose levels (Lehtinen et al., 1989; Pfeifer et al., 1984). Indeed, epidemiological studies suggest that individuals with impaired autonomic functions are at greater risk for developing type 2 diabetes (Carnethon et al., 2003). Together with our findings, these studies suggest that reduced or dysfunctional sympathetic innervation of the pancreas during development may pose a risk factor for developing diabetes later in life.
Experimental Procedures
Mice
TrkAF592A (TrkAf/f) and TH-Cre mice were generous gifts from Dr. D. Ginty (JHMI) and Dr. C. Gerfen (NIH), respectively. MIP-GFP mice were obtained from Jackson Laboratories. All procedures relating to animal care and treatment conformed to institutional and NIH guidelines.
Morphometric analyses of islets
10µm thick sections of pancreata were collected every 200µm and immunofluorescently labeled for insulin and glucagon. For cell counting, 10–20 representative sections of P6 pancreata were chosen and imaged on a Zeiss AxioImager microscope using a 20× objective. The number of α- and β-cells in islets were counted and divided by the number of sections to determine endocrine cell numbers. Islet circularity was determined using the ImageJ software (NIH) shape descriptors tool, calculated by the equation: circularity index=4 ダ (area/perimeter2) with ‘1’ and ‘0’ denoting a perfect circle and an increasingly elongated polygon, respectively. For cytoarchitecture analyses, an islet was considered to have increased α-cell core localization if more than three α-cells were localized to greater than two cell layers inside of the outer islet perimeter. For measurement of islet-ductal distance, 25 islets in proximity to ducts were imaged per animal. ImageJ software was used to measure the distance between the edge of the insulin-positive area and the closest adjacent duct.
In vitro islet migration assays
Pancreata, superior cervical ganglia (SCGs), and dorsal root ganglia (DRGs) were harvested from litters of 8–12 MIP-GFP mice on the day of birth. Pancreata were digested in 1mg/mL collagenase (Type 2, Worthington Biochemical) solution in HBSS, washed with HBSS, and islets picked under an inverted fluorescent microscope. Islets were then dissociated into single cells by gentle trituration in 4mM EDTA in HBSS. Cell suspensions were resuspended in DMEM with 10% fetal bovine serum and 5U/L penicillin/streptomycin, Invitrogen. To plate the co-cultures, a 3µL drop of Matrigel (BD Biosciences) was placed in the center of a collagen coated well in a 4-well culture dish (Thermo Scientific). One SCG or DRG explant was embedded in this drop. The MIP-GFP islet cell suspension was then diluted 1:1 with Matrigel, and 10µL of this cell suspension was plated around the central drop containing the ganglia. Culture media containing 100ng/mL NGF was added and the cultures maintained for 7 days at 37°C under 10% CO2, with fresh media added every other day. For experiments with COS cells, a dense suspension of COS cells was mixed 1:1 with Matrigel, and plated in the center of the culture dish. In experiments using adrenergic antagonists, after plating, cultures were allowed to recover overnight in culture media containing NGF alone. The next day, fresh media containing NGF and 10µM of propranolol (Sigma) or phentolamine (Sigma) was added. Cultures were plated in duplicates or triplicates for each condition.
The cultures were imaged each day with day 1 being the day after plating, using a Zeiss Axiovert microscope and a 2.5× objective. Four images were taken of each well and the images stitched together using Adobe Photoshop. Sholl analyses were performed using ImageJ software and the Sholl analysis plugin (Ghosh Lab). To perform the analysis, a complete image of the culture was first subjected to a threshold to only include the GFP+ cells using the same settings for each image. The analysis plugin was then centered on the location of the ganglia, and the following plugin parameters used: starting radius, 10µm; ending radius, 5000µm; radius step size, 10µm; radius span, 1µm; span type, median. The Sholl analysis plots for each well were obtained and the plots of replicate samples averaged together.
To analyze the percentage of α- and β-cells that migrated to the center of the cultures at the end of the experiment, the cultures were fixed in 4% PFA and immunostained with anti-glucagon, anti-GFP or anti-β-III tubulin. After imaging, a 1mm diameter circle was inscribed in the middle of the image centering on the ganglia and the number of GFP+ or glucagon+ cells within this circle was compared to the total number of endocrine cells using ImageJ software.
Electron microscopy
Pancreata from one month old control and mutant animals were minced and fixed in buffer containing 3% formaldehyde, 1.5% glutaraldehyde, 5mM CaCl2, 2.5% sucrose, and 0.1M sodium cacodylate for 1hr at room temperature. The tissue was post-fixed in 1% Palade’s OsO4 for 1hr on ice, followed by incubation in Kellenberger’s uranyl acetate overnight at room temperature. After dehydration through graded alcohols, the tissue was embedded in Epon and ultra-thin (~90nm) sections were collected onto EM grids. These grids were then imaged using an FEI Tecnai-12 TWIN transmission electron microscope operating at 100kV. Three islets were chosen at random per animal and three representative images of β-cells within each islet were taken. For each image, the insulin granule density (granules/µm2 of cytoplasm) and number of granules within 50nm of the plasma membrane were determined using ImageJ software.
Animal studies
Glucose tolerance and in vivo analysis of glucose-stimulated insulin secretion and insulin sensitivity
For glucose tolerance tests, 1–1.5 month old mice were fasted overnight, with a blood glucose reading the evening before the assay serving as a fed blood glucose measurement. The next morning, mice were injected i.p. with 2g/kg glucose (Sigma) dissolved in water, and blood glucose measurements made from tail blood using a OneTouch Ultra glucometer at the indicated times (Gu et al., 2010). For in vivo insulin secretion assays, 1–2 month old mice were fasted overnight before being injected i.p. with 3g/kg of glucose. Blood was collected from the tail at the times indicated, spun down, and the resulting plasma fraction subjected to insulin ELISA (Crystal Chem). For insulin sensitivity tests, 1–2 month old mice were separated into individual cages with food the evening prior to the assay. The next morning, mice were treated with 0.75U/kg of insulin (Novolin-R, NovoNordisk) and blood glucose measurements made from tail blood at the indicated times (Bruning et al., 1997).
Analysis of glucose stimulated insulin secretion and total islet insulin content in isolated islets
Islets were isolated as previously described (Wollheim et al., 1990). Briefly, following euthanasia of mice, pancreata were distended using a 0.375mg/mL collagenase solution (Collagenase P, Roche), dissolved in Hanks Buffered Salt Solution (HBSS, Mediatech) and digested at 37°C. The digested pancreata were then washed with HBSS and subjected to discontinuous density gradient centrifugation using different Ficoll densities (Mediatech). The islet layers were collected, washed with HBSS, and islets hand-picked under an inverted microscope for subsequent analyses. For total insulin content assays, islets were immediately lysed in acid ethanol solution, and the amount of insulin determined by ELISA. Insulin values were normalized to islet DNA content from the same lysates using a Picogreen kit (Invitrogen).
For insulin secretion assays in isolated islets, harvested islets were allowed to recover overnight in RPMI-1640 media containing 10% fetal bovine serum and 5U/L penicillin/streptomycin (Invitrogen). Assays were performed in Krebs-Ringer HEPES buffer (KRBH) containing low (2.8mM) glucose, high (25mM) glucose, or 30mM potassium chloride (Sigma). Islets were washed in low glucose KRBH before being pre-incubated for 1hr in low glucose KRBH. After pre-incubation, groups of 10 islets were hand picked into a 24 well plate and allowed to incubate in the conditions indicated for 1hr. After incubation the supernatant fraction was removed and the islets were lysed in acid ethanol, followed by ELISA to determine the insulin concentration in both supernatant and islet fractions.
Measurement of plasma glucagon, ghrelin and non-pancreatic hormones
Tail vein blood was collected from 1–1.5 month old mice in the evening prior to an overnight fast (fed ad lib sample). After fasting, a second blood sample was collected (fasted sample). Blood collected in capillary tubes was transferred to tubes containing a protease inhibitor cocktail (Roche) on ice. Samples were spun down, and the plasma fraction acidified to 0.05M HCl. Plasma samples were then subjected to a panel of ELISA assays, following the respective manufacturers instructions: Glucagon, GLP-1, Cholecystokinin, Secretin (Phoenix Pharmaceuticals), Gastrin-1 (RayBiotech), GIP (total, Millipore), Ghrelin (active, Millipore), Leptin (Crystal Chem).
6-OHDA treatment
Beginning at day of birth (P0), TrkAf/f mice were injected intraperitoneally (i.p) once daily with 250mg/kg of 6-OHDA (Sigma) freshly dissolved in 0.1% ascorbic acid (Sigma) in 0.9% saline (Sigma), or with ascorbic acid-saline solution as a control. Pups were sacrificed at P6 and pancreata processed for TH, insulin and glucagon immunofluorescence. For late postnatal treatments, mice were injected beginning at postnatal day 21(P21) twice a week for 2 weeks. At 1.5 months of age, mice were subjected to glucose tolerance measurements, and sacrificed to process their pancreata for immunofluorescence.
Propranolol injections
Beginning at embryonic day 18 (E18), pregnant TrkAf/f mice were injected i.p. daily with 20mg/kg of propranolol freshly dissolved in saline, or saline alone as a control. After birth, pups were injected using the same dosing scheme until P6 when they were sacrificed and pancreata processed for TH, insulin and glucagon immunofluorescence. In parallel experiments, animals injected with propranolol or saline during E18 to P6 were allowed to recover for 6 weeks and glucose tolerance tests and blood glucose levels assessed at 1.5 months of age.
Isoproterenol injections
Beginning at E18, pregnant TH-Cre;TrkAf/f and control TrkAf/f and mice were injected daily with 30mg/kg of isoproterenol (Sigma) freshly dissolved in saline, or saline alone as a control. After birth, pups were injected until P6 when they were sacrificed and pancreata processed for immunofluorescence. Isoproterenol- and saline-injected animals were also allowed to grow to adulthood (1.5 months) for glucose metabolism tests.
Statistical Analyses
All graphs and statistical analyses were done using GraphPad Prism software. Unless otherwise noted in the figure legends, statistical significance was determined using an unpaired, two-tailed Student’s t test.
Supplementary Material
Highlights.
Sympathetic innervation during development is necessary for islet architecture
Sympathectomized mice exhibit defects in insulin secretion and glucose tolerance
Sympathetic neurons promote endocrine cell migration via β-adrenergic signaling
Developmental β-adrenergic signaling regulates islet shape and glucose metabolism
Acknowledgements
We thank H. Zhao, A. Riccio and M. Van Doren for comments on this manuscript, and D. Ginty and C. Gerfen for sharing mice. This work was supported by NIH grants R21 DK090624 to R.K., and R01 DK056211 to S.D.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Asensio C, Jimenez M, Kuhne F, Rohner-Jeanrenaud F, Muzzin P. The lack of beta-adrenoceptors results in enhanced insulin sensitivity in mice exhibiting increased adiposity and glucose intolerance. Diabetes. 2005;54:3490–3495. doi: 10.2337/diabetes.54.12.3490. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes. 1988;37:616–621. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- Bosco D, Orci L, Meda P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Experimental cell research. 1989;184:72–80. doi: 10.1016/0014-4827(89)90365-0. [DOI] [PubMed] [Google Scholar]
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- Burris RE, Hebrok M. Pancreatic innervation in mouse development and beta-cell regeneration. Neuroscience. 2007;150:592–602. doi: 10.1016/j.neuroscience.2007.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation. 2003;107:2190–2195. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Hua TE, Fu YY, Pasricha PJ, Tang SC. 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia. 2012;55:3252–3261. doi: 10.1007/s00125-012-2699-6. [DOI] [PubMed] [Google Scholar]
- Dahl U, Sjodin A, Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895–2902. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. The Journal of clinical investigation. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Abdul-Ghani MA. Preservation of beta-cell function: the key to diabetes prevention. The Journal of clinical endocrinology and metabolism. 2011;96:2354–2366. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Rutter WJ, D a.H. Directed expression of NGF to pancreatic b cells in transgenetic mice leads to selective hyperinnervation of the islets. Cell. 1989;58:161–170. doi: 10.1016/0092-8674(89)90412-1. [DOI] [PubMed] [Google Scholar]
- Esni F, Taljedal IB, Perl AK, Cremer H, Christofori G, Semb H. Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. The Journal of cell biology. 1999;144:325–337. doi: 10.1083/jcb.144.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon M, Ray MK, Van Zee K, Rausa F, Costa RH, Wright CV. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of beta cell function. Development. 2000;127:2883–2895. doi: 10.1242/dev.127.13.2883. [DOI] [PubMed] [Google Scholar]
- Gepts W, Lecompte PM. The pancreatic islets in diabetes. The American journal of medicine. 1981;70:105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Gilliam LK, Palmer JP, Taborsky GJ., Jr. Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. The Journal of clinical endocrinology and metabolism. 2007;92:4035–4038. doi: 10.1210/jc.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annual review of neuroscience. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Golosow N, Grobstein C. Epitheliomesenchymal interaction in pancreatic morphogenesis. Developmental biology. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- Gomez Dumm CL, Semino MC, Gagliardino JJ. Sequential morphological changes in pancreatic islets of spontaneously diabetic rats. Pancreas. 1990;5:533–539. doi: 10.1097/00006676-199009000-00007. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Stein GH, Pan N, Goebbels S, Hornberg H, Nave KA, Herrera P, White P, Kaestner KH, Sussel L, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell metabolism. 2010;11:298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillam MT, Hummler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, Schmidt A, Deriaz N, Thorens B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nature genetics. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D, Zhang M. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncology reports. 2009;22:825–830. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- Halban PA. Cellular sources of new pancreatic beta cells and therapeutic implications for regenerative medicine. Nature cell biology. 2004;6:1021–1025. doi: 10.1038/ncb1104-1021. [DOI] [PubMed] [Google Scholar]
- Halban PA, Wollheim CB, Blondel B, Meda P, Niesor EN, Mintz DH. The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology. 1982;111:86–94. doi: 10.1210/endo-111-1-86. [DOI] [PubMed] [Google Scholar]
- Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. American journal of physiology Endocrinology and metabolism. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- Hopcroft DW, Mason DR, Scott RS. Structure-function relationships in pancreatic islets: support for intraislet modulation of insulin secretion. Endocrinology. 1985;117:2073–2080. doi: 10.1210/endo-117-5-2073. [DOI] [PubMed] [Google Scholar]
- Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nature genetics. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- Jain R, Lammert E. Cell-cell interactions in the endocrine pancreas. Diabetes, obesity & metabolism. 2009;11(Suppl 4):159–167. doi: 10.1111/j.1463-1326.2009.01102.x. [DOI] [PubMed] [Google Scholar]
- Jaques F, Jousset H, Tomas A, Prost AL, Wollheim CB, Irminger JC, Demaurex N, Halban PA. Dual effect of cell-cell contact disruption on cytosolic calcium and insulin secretion. Endocrinology. 2008;149:2494–2505. doi: 10.1210/en.2007-0974. [DOI] [PubMed] [Google Scholar]
- Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2009;57:811–824. doi: 10.1369/jhc.2009.953307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermendy A, Toschi E, Aye T, Koh A, Aguayo-Mazzucato C, Sharma A, Weir GC, Sgroi D, Bonner-Weir S. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia. 2011;54:594–604. doi: 10.1007/s00125-010-2036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka-Gantenbein C, Tazi A, Czernichow P, Scharfmann R. In vivo presence of the high affinity nerve growth factor receptor Trk-A in the rat pancreas: differential localization during pancreatic development. Endocrinology. 1995;136:761–769. doi: 10.1210/endo.136.2.7835308. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, Hara M. Altered islet composition and disproportionate loss of large islets in patients with type 2 diabetes. PloS one. 2011;6:e27445. doi: 10.1371/journal.pone.0027445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Schleiffarth JR, Jessurun J, Sumanas S, Petryk A, Lin S, Ekker SC. Wnt5 signaling in vertebrate pancreas development. BMC biology. 2005;3:23. doi: 10.1186/1741-7007-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129:359–370. doi: 10.1016/j.cell.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacological reviews. 1974;26:199–288. [PubMed] [Google Scholar]
- Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nature reviews Endocrinology. 2012;8:405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lehtinen JM, Uusitupa M, Siitonen O, Pyorala K. Prevalence of neuropathy in newly diagnosed NIDDM and nondiabetic control subjects. Diabetes. 1989;38:1307–1313. doi: 10.2337/diab.38.10.1307. [DOI] [PubMed] [Google Scholar]
- Meece J. Pancreatic islet dysfunction in type 2 diabetes: a rational target for incretin-based therapies. Current medical research and opinion. 2007;23:933–944. doi: 10.1185/030079906x167336. [DOI] [PubMed] [Google Scholar]
- Mei Q, Mundinger TO, Lernmark A, Taborsky GJ., Jr. Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes. 2002;51:2997–3002. doi: 10.2337/diabetes.51.10.2997. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Huotari M, Koivisto T, Ustinov J, Palgi J, Rasilainen S, Lehtonen E, Keski-Oja J, Otonkoski T. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development. 2000;127:2617–2627. doi: 10.1242/dev.127.12.2617. [DOI] [PubMed] [Google Scholar]
- Miralles F, Philippe P, Czernichow P, Scharfmann R. Expression of nerve growth factor and its high-affinity receptor Trk-A in the rat pancreas during embryonic and fetal life. The Journal of endocrinology. 1998;156:431–439. doi: 10.1677/joe.0.1560431. [DOI] [PubMed] [Google Scholar]
- Nam J, Onitsuka I, Hatch J, Uchida Y, Ray S, Huang S, Li W, Zang H, Ruiz-Lozano P, Mukouyama YS. Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development. 2013;140:1475–1485. doi: 10.1242/dev.087601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson CS, Hakansson J, Salehi A, Bengtsson M, Galvanovskis J, Partridge C, SorhedeWinzell M, Xian X, Eliasson L, Lundquist I, et al. Impaired insulin exocytosis in neural cell adhesion molecule−/− mice due to defective reorganization of the submembrane F-actin network. Endocrinology. 2009;150:3067–3075. doi: 10.1210/en.2008-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson-Sjogren S, Holmberg D, Forsgren S. Remodeling of the innervation of pancreatic islets accompanies insulitis preceding onset of diabetes in the NOD mouse. Journal of neuroimmunology. 2005;158:128–137. doi: 10.1016/j.jneuroim.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Pfeifer MA, Weinberg CR, Cook DL, Reenan A, Halter JB, Ensinck JW, Porte D., Jr. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes care. 1984;7:447–453. doi: 10.2337/diacare.7.5.447. [DOI] [PubMed] [Google Scholar]
- Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. The Journal of endocrinology. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- Puri S, Hebrok M. Cellular plasticity within the pancreas--lessons learned from development. Developmental cell. 2010;18:342–356. doi: 10.1016/j.devcel.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravier MA, Guldenagel M, Charollais A, Gjinovci A, Caille D, Sohl G, Wollheim CB, Willecke K, Henquin JC, Meda P. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell metabolism. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansbury FH, Flanagan SE, Houghton JA, Shuixian Shen FL, Al-Senani AM, Habeb AM, Abdullah M, Kariminejad A, Ellard S, Hattersley AT. SLC2A2 mutations can cause neonatal diabetes, suggesting GLUT2 may have a role in human insulin secretion. Diabetologia. 2012;55:2381–2385. doi: 10.1007/s00125-012-2595-0. [DOI] [PubMed] [Google Scholar]
- Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M. Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Archiv : European journal of physiology. 1991;418:417–422. doi: 10.1007/BF00550880. [DOI] [PubMed] [Google Scholar]
- Santulli G, Lombardi A, Sorriento D, Anastasio A, Del Giudice C, Formisano P, Beguinot F, Trimarco B, Miele C, Iaccarino G. Age-related impairment in insulin release: the essential role of beta(2)-adrenergic receptor. Diabetes. 2012;61:692–701. doi: 10.2337/db11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MK, Hartwig NR, Kalmbach N, Klietz M, Anlauf M, Eiden LE, Weihe E. Species-specific vesicular monoamine transporter 2 (VMAT2) expression in mammalian pancreatic beta cells: implications for optimising radioligand-based human beta cell mass (BCM) imaging in animal models. Diabetologia. 2013;56:1047–1056. doi: 10.1007/s00125-013-2847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Levy J, Loewenthal N, Pinsk V, Hershkovitz E. Decreased first phase insulin response in children with congenital insensitivity to pain with anhidrosis. Journal of pediatric endocrinology & metabolism : JPEM. 2005;18:873–877. doi: 10.1515/jpem.2005.18.9.873. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. The Journal of clinical investigation. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen P, Rinzel J, Sherman A. Why pancreatic islets burst but single beta cells do not. The heterogeneity hypothesis. Biophysical journal. 1993;64:1668–1680. doi: 10.1016/S0006-3495(93)81539-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagner JI, Samols E. Retrograde perfusion as a model for testing the relative effects of glucose versus insulin on the A cell. The Journal of clinical investigation. 1986;77:1034–1037. doi: 10.1172/JCI112356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky GJ., Jr. Islets have a lot of nerve! Or do they? Cell metabolism. 2011;14:5–6. doi: 10.1016/j.cmet.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelman G, Alpert S, Hanahan D. Proliferation, senescence, and neoplastic progression of beta cells in hyperplasic pancreatic islets. Cell. 1988;52:97–105. doi: 10.1016/0092-8674(88)90534-x. [DOI] [PubMed] [Google Scholar]
- Teitelman G, Lee JK. Cell lineage analysis of pancreatic islet development: glucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Developmental biology. 1987;121:454–466. doi: 10.1016/0012-1606(87)90182-5. [DOI] [PubMed] [Google Scholar]
- Thorens B. A gene knockout approach in mice to identify glucose sensors controlling glucose homeostasis. Pflugers Archiv : European journal of physiology. 2003;445:482–490. doi: 10.1007/s00424-002-0954-2. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, Tang J, Sun X, Polonsky KS, Bell GI. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–1457. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M, Santos RM, Contreras D, Soria B, Rosario LM. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS letters. 1989;259:19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Van Hoof D, Mendelsohn AD, Seerke R, Desai TA, German MS. Differentiation of human embryonic stem cells into pancreatic endoderm in patterned size-controlled clusters. Stem cell research. 2011;6:276–285. doi: 10.1016/j.scr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Wieczorek G, Pospischil A, Perentes E. A comparative immunohistochemical study of pancreatic islets in laboratory animals (rats, dogs, minipigs, nonhuman primates) Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 1998;50:151–172. doi: 10.1016/S0940-2993(98)80078-X. [DOI] [PubMed] [Google Scholar]
- Winer S, Tsui H, Lau A, Song A, Li X, Cheung RK, Sampson A, Afifiyan F, Elford A, Jackowski G, et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nature medicine. 2003;9:198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51:1843–1852. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- Wollheim CB, Meda P, Halban PA. Isolation of pancreatic islets and primary culture of the intact microorgans or of dispersed islet cells. Methods in enzymology. 1990;192:188–223. doi: 10.1016/0076-6879(90)92071-k. [DOI] [PubMed] [Google Scholar]
- Woods SC, Porte D., Jr. Neural control of the endocrine pancreas. Physiological reviews. 1974;54:596–619. doi: 10.1152/physrev.1974.54.3.596. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Nammo T, Moriwaki M, Ihara A, Iizuka K, Yang Q, Satoh T, Li M, Uenaka R, Okita K, et al. Overexpression of dominant-negative mutant hepatocyte nuclear fctor-1 alpha in pancreatic beta-cells causes abnormal islet architecture with decreased expression of E-cadherin, reduced beta-cell proliferation, and diabetes. Diabetes. 2002;51:114–123. doi: 10.2337/diabetes.51.1.114. [DOI] [PubMed] [Google Scholar]
- Yoo HW, Shin YL, Seo EJ, Kim GH. Identification of a novel mutation in the GLUT2 gene in a patient with Fanconi-Bickel syndrome presenting with neonatal diabetes mellitus and galactosaemia. European journal of pediatrics. 2002;161:351–353. doi: 10.1007/s00431-002-0931-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.