Abstract
INTRODUCTION: The aim of this study was to compare the antimicrobial effects of MTAD, sodium hypochlorite (NaOCl) and their combination on endodontic micro-organisms.
MATERIALS AND METHODS: Zone of Inhibition (ZI) and Minimum Inhibitory Concentration (MIC) were the techniques used. In ZI technique blood agar plates were inoculated with organisms, paper discs were soaked with irrigants and maximum zones of bacterial inhibition were recorded. In the MIC technique the irrigants were serially diluted in TSB tubes and 0.1 mL of the tested microbe solutions were added. Results were obtained on the basis of turbidity and growth on agar plates. Statistical analyses were carried out using ANOVA and Tukey tests.
RESULTS: In ZI technique, we investigated 120 specimens including 5 microbial species, 3 irrigants and their control groups, each with 6 repetitions. The results demonstrated MTAD greater antimicrobial efficacy compared to NaOCl, and their mixture (M+N) against Staphylococcus (S) aureus, Enteric (E) bacteria and Enterococcus (E) faecalis (P<0.001). NaOCl was more effective in eradicating Candida (C) albicans than the others (P<0.01). MIC method (155 tubes) showed MTAD to be more effective against E. bacteria and S. aureus. MTAD and NaOCl were equally effective against E. faecalis; however, NaOCl was more effective against C. albicans.
CONCLUSION: Bacterial species were more susceptible to MTAD than NaOCl, C. albicans, however, was more susceptible to NaOCl. The advantage of NaOCl is that it has broad spectrum antimicrobial activity. The joint solution (M+N) did not prove to be more effective than their individual use.
Key Words: Candida albicans, Enteric bacteria, Enterococcus faecalis, MTAD, Sodium hypochlorite, Staphylococcus aureus
INTRODUCTION
It is well established that pulp and periapical disease as well as failed root canal therapy (RCT) are due to the presence of microbes in the root canal system (1). Eliminating microbes from the infected root canals and prevention of re-infection are one of the fundemental aims of RCT. Clinical investigations have shown that chemo-mechanical preparation with the use of anti microbial medicaments will effectively reduce the microbial content of the canals (2-4). Despite these meticulous efforts some micro-organisms stay within the canal (4-7). In a clinical study evaluating the effect of infection on the RCT outcome, Sjogren et al. cultured samples from the root canals after preparation and before obturation. When the cultures were negative, the success rate was 94%; when bacteria were found however, success rate was reduced to 68% after 5 year follow up (5).
Extensive research has discovered various microorganisms in root canal failures (Chronic Apical Periodontitis) such as Enterococci (8-15), Actinomyces (13), Streptococci (13), Enterobacter (11,12,16,17), Fungi (14,17-21) and Staphylococci (S) (11,12,15). Enterococcus (E) faecalis, the most prominent, is present in 30-70% of failure cases (10,14,17,22,23). Enteric (E) bacteria form approximately 5% (16) and fungi 7% (24) of the infected root canal micro flora.
Various Sodium Hypochlorite (NaOCl) concentrations have been used over the years as an irrigant in RCT. The main benefits of NaOCl are its ability to dissolve necrotic tissue (25), its broad spectrum antibacterial action (26,27) and lubricating effect during filing and irrigation of debris within the canals (28). However unpleasant taste and odor, toxicity (29), resorption (30), inability to remove smear layer and fully eradicate microbes from the infected canals (5,9,31) are the main disadvantages of this popular irrigant. To remove the remained bacteria, medicaments can be used between treatment sessions though their effectiveness in eradicating some microbes such as E. faecalis and Candida (C) albicans is questionable (4,17,32-35).
Recently a new irrigant called MTAD (a mixture of tetracycline isomer, an acid and a detergent) has been introduced as a final irrigant to be used after NaOCl for disinfecting the root canal into the market (36). The results of some investigations have shown that MTAD can effectively remove the smear layer and abolish E. faecalis (37-40) though some researchers are still adamant that NaOCl is more effective (41-44).
We decided to compare the antimicrobial effect of NaOCl, MTAD, and their mixture against resistant microbes in RCT with Zone of Inhibition (ZI) and Minimum Inhibitory Concentration (MIC) in-vitro.
MATERIALS AND METHODS
The investigation was carried out in Shahid Beheshti University Dental School and approved by the ethical committee. The microbes tested were E. faecalis, Pseudomonas (P) aeruginosa, Escherichia (E) coli, S. aureus and C. albicans. E. faecalis was incubated in Bile Scoline Agar, P. aeruginosa and S. aureus in Blood Agar, E. coli in Esoin Methylen Blue (EMB), and C. albicans in Sabouraud Dextrose Agar plates. After 24 hours a suspension was prepared from each microbial species with a concentration of 1 McFarland (i.e. 3×108 Colony of bacteria in the Trypticase Soy Broth environment) and used in the investigation.
To assess the antimicrobial power of each irrigant ZI and MIC was employed. In the ZI technique 30 blood agar plates (batch no: 108860500, Merck KGaA, Darmstadt, Germany) were prepared according to manufacture’s instructions and the 125 mm Petri dishes were uniformly covered. These were then stored for 2 days in 37°C to make sure sterilized conditions were met and bacteria were not introduced. The sterile paper discs (batch no: 1288502, Padtan Teb, Tehran, Iran) diameter of 6 mm were divided into three groups. Each group was soaked either with 4 drops (0.2 mL) of NaOCl, MTAD or an equal mixture of MTAD and NaOCl (M+N).
Five mL from each 1 McFarland suspension was removed with a sterile swab next to a flame and under the ventilation hood and placed on a blood agar plate in a criss-cross fashion. Each culture area was then divided into 4 sections in the following manner: the first section contained disc soaked with BioPure MTAD (batch no: 050805, Dentsply Tulsa Dental, Tulsa, OK), the second section with NaOCl 5.25% (batch no: 28095, Pure Sodium Hypochlorite, Chemeen Company, Iran) and the third section contained disc soaked with equal amounts of MTAD and NaOCl (5.25%), the final section consisted of normal saline (batch no: 173885 A, 0.9% Sodium Chloride, Medicines and Drugs Company, Iran). This was repeated 6 times for each of the microbes; altogether 120 samples were taken. Samples were placed in the incubator (batch no: 0323212100, Farateb Tajheez Specialist Company, Iran) for 48 hours at 37°C and 10% CO2. The maximum microbial ZI around each disc was then measured and recorded in millimetres.
In the MIC technique 30 test tubes were prepared for each microbe. One mL of Trypticase Soy Broth (TSB) (batch no: 105459, Merck KgaA, Darmstadt, Germany) was poured into each test tube and then autoclaved (sterilization). The tubes were then divided into three groups of 10 test tubes each. One mL of the selected irrigant was placed in the first test tube of each group and shaken with Cenco instrument MIJ (batch no: 5444, Holland) to homogenise the fluid. Subsequently, 1 mL of the solution was taken from the first test tube and transferred into the next tube; this was then repeated for all the ten tubes. The irrigant was gradually diluted from 1:2 to 1:1024 in the last test tube. An extra test tube was used with normal saline as the control. Exactly 0.1 mL of the selected microbial suspension (1 McFarland) was added to each of the test tubes, and this was performed for all the tubes and investigated irrigants. Test tubes were placed in the incubator for 24 hours (37°C and 10% CO2).
MTAD and MTAD+NaOCl were partially opaque and therefore a culturing method was also employed. After measuring turbidity of the test tubes, 0.5 cc of each tube solution was placed on Nutrient Agar (batch no: 1054430500, Merck KGaA, Darmstadt, Germany) and placed in the incubator for 48 hours. One hundred and fifty five test tubes were used to test the 5 individual microbes, 5 of which were controls. The MIC of the irrigants for each individual microbe was recorded based on the degree of turbidity (with the naked eye) and the absence of microbial growth on the agar plates. The experiment was carried out in accordance with sterilisation principles (45).
The relevant results of ZI were added into SPSS for Windows V 11.5. Descriptive criteria (central values, measurements of dispersion and confidence interval levels) were calculated and the data was analysed with ANOVA and Tukey tests and P<0.05 was considered statistically significant.
RESULTS
The results for the ZI technique displayed that S. aureus was statistically the most sensitive micro-organism (maximum ZI) against investigated irrigants (P<0.002) (Table 1).
Table 1.
Statistics of inhibition zones and all the 5 microorganisms tested
| Groups | N | Mean | SD |
|---|---|---|---|
| Enterococcus Faecalis | 18 | 22.111 | 5.189 |
| Escherichia Coli | 18 | 21.500 | 3.535 |
| Pseudomonas aeroginosa | 18 | 22.722 | 5.210 |
| Candida albicans | 18 | 25.555 | 19.281 |
| Staphylococcus aureus | 18 | 33.277 | 5.798 |
| Total | 90 | 25.033 | 10.454 |
MTAD was more effective than 5.25% NaOCl and NaOCl was more effective than MTAD+NaOCl against E. faecalis, S. aureus and E. bacteria (P<0.001) (Table 2).
Table 2.
Statistics of inhibition zones and the 3 irrigants for 4 microorganisms tested
| Irrigants | N | Mean | SD |
|---|---|---|---|
| MTAD | 24 | 28.500 | 6.036 |
| NaOCl | 24 | 25.916 | 5.740 |
| MTAD/NaOCl | 24 | 20.291 | 6.457 |
| Total | 72 | 24.902 | 6.920 |
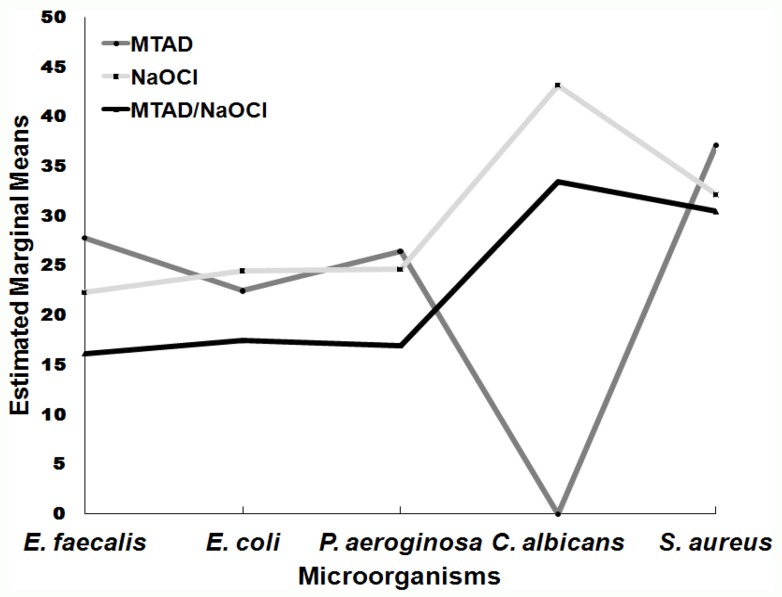
Overall NaOCl showed statistically greater antimicrobial power against E. faecalis, S. aureus, E. bacteria and C. albicans: NaOCl>MTAD=MTAD+NaOCl (P<0.019) (Table 3 and Figure 1).
Table 3.
Statistics of inhibition zones and the 3 irrigants for 5 microorganisms
| Irrigants | N | Mean | SD |
|---|---|---|---|
| MTAD | 30 | 22.800 | 12.780 |
| NaOCl | 30 | 29.366 | 8.825 |
| MTAD/NaOCl | 30 | 22.933 | 8.064 |
| Total | 90 | 25.033 | 10.454 |
Figure 1.
Estimated Marginal means of inhibition zones for the 5 bacteria
C. albicans was the most resistant micro-organism to MTAD (P<0.001).
MIC results illustrated that MTAD and NaOCl were strong antimicrobials and may be equally effective even when diluted. In this study MTAD was more effective against S. aureus and E. bacteria, however, NaOCl 5.25% and MTAD were equally effective against E. faecalis (Table 4).
Table 4.
MIC test of the 5 different microorganisms
| Microorganism | MTAD | NaOCl | MTAD/NaOCl |
|---|---|---|---|
| E. faecalis | 1:32 | 1:32 | 1:8 |
| P. aeroginosa | 1:64 | 1:8 | 1:8 |
| E. coli | 1:128 | 1:32 | 1:32 |
| S. aureus | 1:512 | 1:32 | 1:16 |
| C. albicans | 1:4 | 1:16 | 1:8 |
DISCUSSION
The results of this in vitro investigation reiterate the superiority of MTAD compared to NaOCl and M+N against S. aureus, E. bacteria and E. faecalis by the ZI method. The poorest antimicrobial agent was shown to be the mixture of M+N.
Experiments carried out by Davis et al. (40) and Krause et al. (43) in 2007 both proved MTAD to be more effective against E. faecalis than NaOCl 5.25%. This study also demonstrates that MTAD is effective against a range of bacteria.
Tay et al. carried out a similar study that compared MTAD, NaOCl and the consecutive use of the irrigants (instead of combination) (46). They used a concentration of 1.3% NaOCl; their results confirmed that MTAD was the most effective irrigant in eliminating E. faecalis. However, they found no difference between NaOCl and N+M; possibly due to the different concentration of NaOCl, bacterial species and/or slightly different incubation conditions employed.
Torabinejad et al. has compared the effectiveness of MTAD and NaOCl (5.25%) using the ZI technique and discovered their similar antibacterial action against E. faecalis (38). However, in this study after dilution MTAD was shown to have a significantly greater ZI than NaOCl.
Interestingly the antimicrobial effect of the mixture (M+N) was less than MTAD or NaOCl alone. A recent study showed that MTAD used alone has greater substantivity than other irrigants (47). NaOCl reduces the antimicrobial power of MTAD; both irrigants are stronger antimicrobials when used independently. Attention must be paid to the oxidation of MTAD by NaOCl which reduces the substantivity and antimicrobial power of MTAD; similar to the peroxidation of tetracycline with Reactive Oxygen Species (ROS), confirmed in Tay’s study (46).
There have been reports that antioxidant such as ascorbic acid rinse following NaOCl irrigation will remove remnants of hypochlorite (48).
C. albicans distorts the findings as NaOCl is stronger agent than MTAD and M+N; MTAD showed no antifungal effects. Ruff et al’s study also supports our results; they demonstrated that Chlorohexidine 2% and NaOCl 6% were most effective in reducing the CFU of C. albicans (49).
There is a large body of evidence including this current study that find bacteria such as E. faecalis, strands of E. bacteria (P. aeruginosa, E. coli) and types of C. albicans to have greater resistance to irrigation when compared to S. aureus (8-21). These microorganisms have also been known to be resistant to antibacterials and intracanal medicaments such as calcium hydroxide (50-52).
MIC results illustrated that MTAD and NaOCl were strong antimicrobials even after dilution. In this study, MTAD was more effective against S. aureus and E. bacteria, thought NaOCl 5.25% and MTAD were equally effective against E. faecalis.
A similar study using MIC, Torabinejad et al. demonstrated that MTAD was effective when diluted 200 times, and NaOCl (5.25%) was effective when diluted 32 times against E. faecalis (38), concurring with our results (MTAD was found effective when diluted 32 times). This may be due to the various strands of E. faecalis, different amounts of doxycycline in MTAD (43), conditions of incubation used.
The present study used Bio Pure MTAD (Dentsply, Tulsa Dental, Tulsa, OK) with 150 mg of Doxycycline i.e. 3%. MTAD is naturally opaque and therefore measuring its turbidity may cause discrepancy in the results. MTAD instructions require NaOCl to be used as the final irrigant (36). The results in this study and Tay et al’s indicate that an interaction may occur altering the antimicrobial efficacy of MTAD (46). The advocacy of using these irrigants consequetively may be questioned.
We suggest further investigations using irrigants in a polymicrobial environment to mirror endodontics infections.
CONCLUSION
MTAD is ineffective against C. albicans and its substantivity may be altered when used in conjunction with NaOCl. C. albicans is often present in resistant and secondary endodontic infections as well as in peri-radicular lesions. NaOCl is an inexpensive and readily available chemical with broad spectrum action which has stood the test of time.
ACKNOWLEDGEMENT
We would like to thank Shahid Beheshti Laboratory and Grant Committee as well as Dr Salmasi, Mrs Taheri and Miss M. Zamani for their continual assistance.
Conflict of Interest: ‘None declared’.
References
- 1.Siqueira JF Jr. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34:1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35–40. doi: 10.1111/j.1365-2591.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 3.Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24:119–25. doi: 10.1111/j.1365-2591.1991.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 4.Peters LB, van Winkelhoff AJ, Buijs JF, Wesselink PR. Effects of instrumentation, irrigation and dressing with calcium hydroxide on infection in pulpless teeth with periapical bone lesions. Int Endod J. 2002;35:13–21. doi: 10.1046/j.0143-2885.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 5.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 6.Molander A, Reit C, Dahlén G. The antimicrobial effect of calcium hydroxide in root canals pretreated with 5% iodine potassium iodide. Endod Dent Traumatol. 1999;15:205–9. doi: 10.1111/j.1600-9657.1999.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 7.Chávez De Paz LE, Dahlén G, Molander A, Möller A, Bergenholtz G. Bacteria recovered from teeth with apical periodontitis after antimicrobial endodontic treatment. Int Endod J. 2003;36:500–8. doi: 10.1046/j.1365-2591.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- 8.Gomes BP, Lilley JD, Drucker DB. Variations in the susceptibilities of components of the endodontic microflora to biomechanical procedures. Int Endod J. 1996;29:235–41. doi: 10.1111/j.1365-2591.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 9.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 10.Dahlén G, Samuelsson W, Molander A, Reit C. Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral Microbiol Immunol. 2000;15:309–12. doi: 10.1034/j.1399-302x.2000.150507.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheung GS, Ho MW. Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral Microbiol Immunol. 2001;16:332–7. doi: 10.1034/j.1399-302x.2001.160603.x. [DOI] [PubMed] [Google Scholar]
- 12.Rolph HJ, Lennon A, Riggio MP, Saunders WP, MacKenzie D, Coldero L, Bagg J. Molecular identification of microorganisms from endodontic infections. J Clin Microbiol. 2001;39:3282–9. doi: 10.1128/JCM.39.9.3282-3289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock HH 3rd, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:579–86. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 14.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 15.Adib V, Spratt D, Ng YL, Gulabivala K. Cultivable microbial flora associated with persistent periapical disease and coronal leakage after root canal treatment: a preliminary study. Int Endod J. 2004;37:542–51. doi: 10.1111/j.1365-2591.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 16.Haapasalo M, Ranta H, Ranta KT. Facultative gram-negative enteric rods in persistent periapical infections. Acta Odontol Scand. 1983;41:19–22. doi: 10.3109/00016358309162299. [DOI] [PubMed] [Google Scholar]
- 17.Peciuliene V, Reynaud AH, Balciuniene I, Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J. 2001;34:429–34. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 18.Nair PN, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990;16:580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 19.Waltimo TM, Sirén EK, Torkko HL, Olsen I, Haapasalo MP. Fungi in therapy-resistant apical periodontitis. Int Endod J. 1997;30:96–101. doi: 10.1046/j.1365-2591.1997.00058.x. [DOI] [PubMed] [Google Scholar]
- 20.Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31:1–7. [PubMed] [Google Scholar]
- 21.Siqueira JF Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:632–41. doi: 10.1016/S1079210404000046. [DOI] [PubMed] [Google Scholar]
- 22.Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–8. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro ET, Gomes BP, Ferraz CC, Teixeira FB, Zaia AA, Souza Filho FJ. Evaluation of root canal microorganisms isolated from teeth with endodontic failure and their antimicrobial susceptibility. Oral Microbiol Immunol. 2003;18:100–3. doi: 10.1034/j.1399-302x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 24.Waltimo TM, Ørstavik D, Meurman JH, Samaranayake LP, Haapasalo MP. In vitro susceptibility of Candida albicans isolates from apical and marginal periodontitis to common antifungal agents. Oral Microbiol Immunol. 2000;15:245–8. doi: 10.1034/j.1399-302x.2000.150406.x. [DOI] [PubMed] [Google Scholar]
- 25.Harrison JW. Irrigation of the root canal system. Dent Clin North Am. 1984;28:797–808. [PubMed] [Google Scholar]
- 26.Siqueira JF Jr, Batista MM, Fraga RC, de Uzeda M. Antibacterial effects of endodontic irrigants on black-pigmented gram-negative anaerobes and facultative bacteria. J Endod. 1998;24:414–6. doi: 10.1016/S0099-2399(98)80023-X. [DOI] [PubMed] [Google Scholar]
- 27.Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int Endod J. 1999;32:99–102. doi: 10.1046/j.1365-2591.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 28.Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992;18:605–12. doi: 10.1016/S0099-2399(06)81331-2. [DOI] [PubMed] [Google Scholar]
- 29.Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:446–50. doi: 10.1067/moe.2001.116812. [DOI] [PubMed] [Google Scholar]
- 30.Busslinger A, Sener B, Barbakow F. Effects of sodium hypochlorite on nickel-titanium Lightspeed instruments. Int Endod J. 1998;31:290–4. doi: 10.1046/j.1365-2591.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 31.Shuping GB, Orstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000;26:751–5. doi: 10.1097/00004770-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 33.Siqueira JF Jr, Sen BH. Fungi in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:632–41. doi: 10.1016/S1079210404000046. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson JW, Hatton JF, Gillespie MJ. Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J Endod. 2002;28:68–71. doi: 10.1097/00004770-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Waltimo TM, Sirén EK, Orstavik D, Haapasalo MP. Susceptibility of oral Candida species to calcium hydroxide in vitro. Int Endod J. 1999;32:94–8. doi: 10.1046/j.1365-2591.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 36.Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003;29:576–9. doi: 10.1097/00004770-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, Kim J, Shabahang S. A new solution for the removal of the smear layer. J Endod. 2003;29:170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Torabinejad M, Shabahang S, Aprecio RM, Kettering JD. The antimicrobial effect of MTAD: an in vitro investigation. J Endod. 2003;29:400–3. doi: 10.1097/00004770-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Shabahang S, Pouresmail M, Torabinejad M. In vitro antimicrobial efficacy of MTAD and sodium hypochlorite. J Endod. 2003;29:450–2. doi: 10.1097/00004770-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Davis JM, Maki J, Bahcall JK. An in vitro comparison of the antimicrobial effects of various endodontic medicaments on Enterococcus faecalis. J Endod. 2007;33:567–9. doi: 10.1016/j.joen.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Clegg MS, Vertucci FJ, Walker C, Belanger M, Britto LR. The effect of exposure to irrigant solutions on apical dentin biofilms in vitro. J Endod. 2006;32:434–7. doi: 10.1016/j.joen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AL. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J Endod. 2006;32:527–31. doi: 10.1016/j.joen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Krause TA, Liewehr FR, Hahn CL. The antimicrobial effect of MTAD, sodium hypochlorite, doxycycline, and citric acid on Enterococcus faecalis. J Endod. 2007;33:28–30. doi: 10.1016/j.joen.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Johal S, Baumgartner JC, Marshall JG. Comparison of the antimicrobial efficacy of 1.3% NaOCl/BioPure MTAD to 5. 25% NaOCl/15% EDTA for root canal irrigation. J Endod. 2007;33:48–51. doi: 10.1016/j.joen.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Finegold S, Baron M, Ellen Jo. Bailey and Scotts diagnostic microbiology. 8th Edition. Finegold-USA: Mosby; 1990. pp. 171–84. [Google Scholar]
- 46.Tay FR, Hiraishi N, Schuster GS, Pashley DH, Loushine RJ, Ounsi HF, Grandini S, Yau JY, Mazzoni A, Donnelly A, King NM. Reduction in antimicrobial substantivity of MTAD after initial sodium hypochlorite irrigation. J Endod. 2006;32:970–5. doi: 10.1016/j.joen.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Mohammadi Z, Shahriari S. Residual antibacterial activity of chlorhexidine and MTAD in human root dentin in vitro. J Oral Sci. 2008;50:63–7. doi: 10.2334/josnusd.50.63. [DOI] [PubMed] [Google Scholar]
- 48.Tay FR, Mazzoni A, Pashley DH, Day TE, Ngoh EC, Breschi L. Potential iatrogenic tetracycline staining of endodontically treated teeth via NaOCl/MTAD irrigation: a preliminary report. J Endod. 2006;32:354–8. doi: 10.1016/j.joen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Ruff ML, McClanahan SB, Babel BS. In vitro antifungal efficacy of four irrigants as a final rinse. J Endod. 2006;32:331–3. doi: 10.1016/j.joen.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Radcliffe CE, Potouridou L, Qureshi R, Habahbeh N, Qualtrough A, Worthington H, Drucker DB. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii. A naeslundii Candida albicans and Enterococcus faecalis. Int Endod J. 2004;37:438–46. doi: 10.1111/j.1365-2591.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 51.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari PH, Cai S, Bombana AC. Effect of endodontic procedures on enterococci, enteric bacteria and yeasts in primary endodontic infections. Int Endod J. 2005;38:372–80. doi: 10.1111/j.1365-2591.2005.00947.x. [DOI] [PubMed] [Google Scholar]