Abstract
Nucleosome positioning is critical for gene expression and most DNA-related processes. Here, we review the dominant patterns of nucleosome positioning that have been observed, and summarize current understanding of their underlying determinants. The genome-wide pattern of nucleosome positioning is determined by the combination of DNA sequence, ATP-dependent nucleosome remodeling enzymes, and transcription factors including activators, components of the preinitiation complex, and elongating RNA polymerase II. These determinants influence each other such that the resulting nucleosome positioning patterns are likely to differ among genes and among cells within a population, with consequent effects on gene expression.
Eukaryotic genomes are packaged into chromatin, whose basic repeating unit is a nucleosome that consists of a histone octamer wrapped around 147 bp of DNA1. Nucleosomes are arranged into regularly-spaced arrays, with the length of the linker region between nucleosomes varying among species and cell types. While nucleosomes were originally imagined to provide a universal, non-specific coating of genomic DNA, it has long been known that nucleosomes occupy favored positions throughout the genome. High-resolution, genome-wide analyses reveal a common pattern, in which nucleosomes are depleted at many (but not all) enhancer, promoter, and terminator regions, and they typically occupy preferred positions within genes and non-genic regions2-9. In yeast, the −1 and +1 nucleosomes flanking the promoter are strongly positioned, and the degree of nucleosome positioning gradually decreases from the 5’ to 3’ end of the coding region4,7.
This review will consider the mechanisms by which the genomic pattern of nucleosome positioning is achieved. Intense research efforts collectively indicate that nucleosomes are not determined by any single factor, but rather by the combined effects of several factors, including DNA sequence, DNA-binding proteins, nucleosome remodelers, and the RNA polymerase (Pol) II transcription machinery. One aim of this review is to resolve the apparent controversy (in large part generated by the authors and the late Jonathan Widom, to whom this review is dedicated) about the role of DNA sequence in establishing the genomic pattern. This controversy has reflected differences in interpretation and imprecision of terms, as the experimental observations have been remarkably consistent and are uncontested. This review will focus on nucleosome positioning in yeast, particularly the biochemical and genetic analyses that have been rarely performed in multicellular organisms. We suspect that many aspects of nucleosome positioning are conserved among eukaryotes, but one significant difference is that the linker histone H1 in yeast is structurally atypical and it is present at very low concentrations in comparison to core histones10.
Definition of nucleosome occupancy and positioning
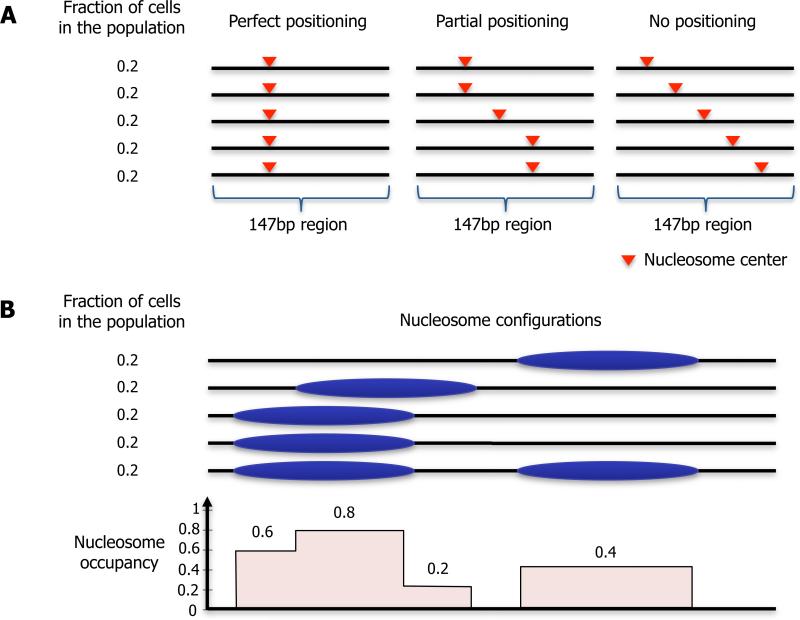
We define the term “nucleosome positioning” broadly to indicate where nucleosomes are located with respect to the genomic DNA sequence. Although nucleosome positioning is a dynamic process, sequencing-based mapping approaches identify the positions of individual nucleosomes in a single cell at a specific time. Nevertheless, nucleosome positions are typically discussed on a cell- and time-averaged basis. Thus, the degree of positioning can vary from perfect positioning, in which a nucleosome is located at a given 147 bp stretch in all DNA molecules in a population, to no positioning, in which nucleosomes across a cell population are located at all possible genomic positions equally (Figure 1A). Nucleosome positioning is related to, but distinct from, the concept of nucleosome occupancy, which reflects the fraction of cells from the population in which a given region of DNA is occupied by a histone octamer (Figure 1B). While most genomic DNA is occupied by nucleosomes, many functional regions (promoters, enhancers, terminators) are depleted of nucleosomes (i.e. have low occupancy) and some regions are largely nucleosome-free.
Figure 1. Illustration of the concepts of nucleosome positioning and nucleosome occupancy.
(A) Nucleosome positioning along every basepair in the genome is defined as the fraction of cells from the population in which that basepair is at the center of a 147bp nucleosome. Shown are illustrations of a 147bp region with perfect positioning (left), in which the nucleosome center is located at the same basepair across all cells; a 147bp region with partial positioning, in which there is a preference for some locations; and a 147bp region with no positioning, in which all locations have equal probability. (B) Nucleosome occupancy along every basepair in the genome is defined as the fraction of cells from the population in which the basepair is occupied by any histone octamer. Shown is an illustration of nucleosome locations across a cell population (top), and the resulting nucleosome occupancy per basepair (bottom).
Nucleosome occupancy and positioning are critical to biological outcomes4,7,11,12, primarily because nucleosomes inhibit the access of other DNA-binding proteins to the DNA. Highly accessible regions within genomes were initially identified by preferential restriction endonuclease cleavage13 and DNase I hypersensitivity14. Quantitative analysis of HinfI cleavage15 or Leu3 binding16 in yeast cells indicates that access of factors to target sites in nucleosome-depleted promoter regions is ~10-20 fold higher than to identical sequences that are associated with nucleosomes. More generally, as a consequence of their modes of binding, transcription factors differ a great deal with respect to how much their binding is inhibited by nucleosomes. A special class of “pioneer” transcription factors (e.g. FoxA and GATA) can bind their target sites in the context of nucleosomal DNA17. Such pioneer factors, via recruitment of nucleosome remodelers, can open up the local chromatin, thereby facilitating the binding of other transcription factors that otherwise would be blocked by nucleosomes. The TATA-binding protein, and hence the entire basic RNA polymerase II transcription machinery, is virtually unable to bind nucleosomal DNA, and hence requires a nucleosome-free region to bind core promoters and initiate transcription18.
Nucleosome positioning is strongly affected by DNA sequence
The debate about the role of DNA sequence in nucleosome positioning in vivo revolves around the intrinsic sequence preferences of the histone octamer in the absence of any other component. The affinity of histone octamers for a given 147 bp sequence varies over more than 3 orders of magnitude19. As such, histone octamers show a considerable level of DNA sequence specificity, albeit below that of a classical specific DNA-binding protein. However, unlike DNA-binding proteins that achieve specificity by virtue of direct and strong interactions between a few base pairs and amino acids, the specificity of nucleosome formation largely reflects the overall ability of a given 147 bp sequence to bend around the histone octamer20. For optimal nucleosome formation, more bendable sequences are in contact with the histones, and less bendable sequences are solvent exposed.
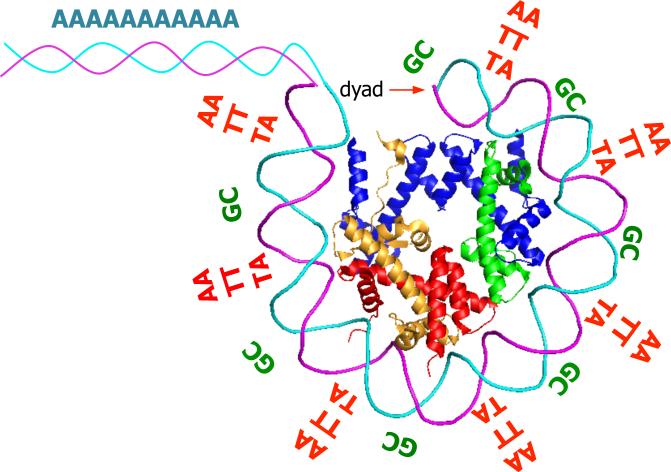
Two major sequence determinants affect bending, and hence nucleosome formation. First, di-nucleotides vary considerably with respect to their bending properties. Optimal nucleosome formation occurs when bendable di-nucleotides (AT, TA) occur on the face of the helical repeat (i.e. every 10 bp) that directly interacts with histones21,22. Recent mapping of nucleosomes in the yeast S. cerevisiae using a novel H4-S47C-mediated cleavage technique that allows precise mapping of nucleosomes with respect to the DNA sequence shows a striking 10-bp periodicity of bendable di-nucleotides throughout nearly the entire 147-bp region23. The exact position of the histone octamer with respect to the ~10 bp helical repeat is termed “rotational positioning”, and thus, DNA sequence is a critical determinant of how nucleosomes are rotationally positioned (Figure 2).
Figure 2. Illustration of nucleosome sequence preferences.
Within the 147bp that are wrapped around the histone octamer, there is a preference for distinctive di-nucleotides that recur periodically at the DNA helical repeat and are known to facilitate the sharp bending of DNA around the nucleosome. These include ~10-bp periodic AA/TT/TA dinucleotides that oscillate in phase with each other and out of phase with ~10-bp periodic GC dinucleotides. The linker regions exhibit a strong preference for sequences that resist DNA bending and thus disfavor nucleosome formation. Among these poly(dA:dT) tracts and their variants are most dominant and highly enriched in eukaryotic promoters.
Second, the homopolymeric sequences poly(dA:dT) and poly(dG:dC) are intrinsically stiff (for different structural reasons) and are strongly inhibitory to nucleosome formation24-27. Notably, poly(dA:dT) tracts are abundant in eukaryotic genomes28 and are particularly prevalent in promoters of certain organisms such as S. cerevisiae29,30. As will be discussed below, the intrinsic properties of poly(dA:dT) are important for nucleosome depletion, promoter accessibility, and transcriptional activity12,15,26.
By analogy to DNA-binding proteins, the above DNA sequence preferences for nucleosomes can be expressed as a position-weight matrix that can generate a nucleosome score for any 147-bp region of DNA22. Such nucleosome scores correspond to relative affinities of 147-bp sequences for histone octamers; such differences in affinities must affect nucleosome positioning in vivo. In particular, as dinucleotide preferences vary along the 147 bp region of the nucleosome, most DNA sequences will have considerably different nucleosome scores at neighboring positions within an individual helical repeat. As a consequence, there will usually be a preferred position(s) within a helical repeat, and such rotational positioning is clearly observed in eukaryotic genomes. However, if one considers a favored rotational position, genomic locations that are 10 bp apart (i.e. 1 helical turn) will have similar (although not identical) nucleosome formation scores, unless the nearby locations contain strongly inhibitory sequences31. Thus, in many genomic regions, DNA sequence alone is unlikely to strongly favor one nucleosome position over nearby positions that are rotationally equivalent.
Poly(dA:dT) tracts are important for nucleosome depletion
The role of DNA sequence in establishing nucleosome positions in vivo has been addressed by experiments in which nucleosomes are assembled in vitro by salt dialysis using purified histones and genomic DNA32,33. Under these conditions, many yeast promoter and terminator regions are depleted for nucleosomes, indicating that the DNA sequences intrinsically disfavor nucleosome formation. Consistent with this view, nucleosome depletion at promoters is observed under a wide variety of conditions in vivo and is unaffected by transcriptional activity32,34. In addition, when artificial chromosomes containing large genomic regions from heterologous yeast species are analyzed in S. cerevisiae, nucleosome depletion at promoters is maintained in a manner that depends on the degree of poly(dA:dT) sequences35. Thus, nucleosome depletion at most promoter sequences is strongly influenced by the intrinsic properties of poly(dA:dT) sequences.
A variety of detailed functional analyses indicate that the intrinsic properties of poly(dA:dT) are important for nucleosome depletion, promoter accessibility, and transcriptional activity12,15,26,29,34-37. In principle, poly(dA:dT)-mediated depletion could be due to a poly(dA:dT)-binding activator protein, but the one known factor with such DNA-binding specificity (Datin) actually inhibits transcription15. Consistent with an earlier study15, expression measurements of a large-scale promoter library designed with systematic manipulations to the properties and spatial arrangement of poly(dA:dT) tracts showed that longer and more perfect tracts induce higher transcriptional levels regardless of the identity of the binding transcription factor12. This transcriptional effect of the tracts is mediated by the reduced nucleosome occupancy and thus increased accessibility that these tracts confer on nearby promoter elements, such as transcription factor sites.
Alterations in poly(dA:dT) tracts offers a general evolutionary mechanism, applicable to promoters regulated by different transcription factors, for tuning expression in a predictable manner, with resolution that can be even finer than that attained by altering transcription factor sites12. Indeed, genomes have likely utilized these tracts to regulate expression levels, for example, to partly compensate for gene copy number differences that exist among ribosomal protein genes in S. cerevisiae38, and to alter the expression level of cellular respiration genes across yeast species39. Moreover, partitioning yeast genes into two categories based on the degree to which their promoters favor nucleosome formation in a manner largely dependent on the occurrences of poly(dA:dT) tracts results in functionally distinct gene classes. Specifically, the class of genes whose promoters have a higher intrinsic preference for nucleosomes is enriched for stress-response genes, exhibits higher rates of histone turnover and transcriptional noise, and contains more targets of chromatin remodelers, consistent with an ongoing dynamic competition between nucleosome assembly and factor binding30,40. Thus, poly(dA:dT) tracts are important determinants of nucleosome depletion in vivo and have likely been utilized by organisms to obtain biological outcomes.
Yeast terminator regions are also depleted of nucleosomes both in vivo and in vitro. However, in contrast to the situation at promoters, nucleosome depletion at terminator regions is strongly correlated to the orientation of and distance to neighboring genes, and it is strongly affected by growth conditions and transcriptional elongation by Pol II41. Thus, the degree of contribution of DNA sequence to depletion at terminators requires further examination.
A minority of S. cerevisiae promoters are depleted for nucleosomes in vivo, yet lack poly(dA:dT) sequences and are not depleted in nucleosome assembly experiments in vitro. More generally, nucleosome depletion at promoters is observed in numerous species in which poly(dA:dT) sequences are less prevalent than in S. cerevisiae or are rare42-44. In these promoters, nucleosome depletion is not determined by DNA sequence, but rather is likely due to activator-mediated recruitment of nucleosome remodelers that evict histones in the promoter regions.
Aspects of positioning not determined by DNA sequence
Although nucleosome assembly experiments with purified histones and DNA can reconstitute nucleosome depletion at most promoter sequences, they do not reconstitute other aspects of the in vivo pattern of nucleosome positioning32,33. In particular, strong positioning of the +1 nucleosome is not observed, and indeed there is no evidence for any favored position within this region under conditions in which histone concentrations are either limiting or saturating. These observations suggest that the DNA sequence is not the main determinant of the +1 nucleosome. Furthermore, the positions of the +2 and further downstream nucleosomes are determined primarily by the length of the linker region, and hence by the position of the +1 nucleosome, and they are also not reconstituted in vitro.
Theoretically, the combination of a boundary constraint and high nucleosome concentrations along the DNA could generate an ordered array that begins with the +1 nucleosome and decays with increasing distance downstream. This “statistical positioning” phenomenon could permit nucleosome-depleted regions mediated by poly(dA:dT) tracts to act as barriers and indirectly contribute to the long-range positioning of nucleosomes flanking promoters. However, statistically positioned arrays flanking the poly(dA:dT) tracts were not observed even under in vitro conditions in which arrays were clearly generated33, providing evidence against this theoretical possibility.
Overall, the positions of in vitro-assembled nucleosomes resemble those observed in vivo significantly beyond random expectation, indicating that DNA sequence does contribute significantly to nucleosome positioning. However, in many cases, other factors can override the sequence preferences for nucleosome formation. Such overriding is likely to occur in the many genomic regions in which the dynamic range of affinities of the histone octamer to the underlying sequence is narrower than the >3 orders of magnitude observed for specific DNA sequences.
Role of nucleosome remodelers in nucleosome positioning
Several aspects of the in vivo nucleosome positioning pattern can be reconstituted in vitro if a yeast crude extract and ATP are added to purified histones and DNA45. Specifically, the combination of ATP-dependent nucleosome remodelers in a cell-free extract enhances nucleosome depletion at promoters to the extent observed in vivo, and it also generates positioned nucleosomes flanking the nucleosome-depleted regions (i.e., the +1 and−1 nucleosomes). In principle, remodeling enzymes may simply allow nucleosomes to sample alternative positions rapidly, resulting in a thermodynamic equilibrium that is not achieved when nucleosome assembly is performed by salt dialysis. However, nucleosome assembly reactions containing Drosophila ACF33 or the yeast RSC46 do not generate the in vivo pattern, even though they efficiently mobilize nucleosomes. These observations suggest that nucleosome-remodeling enzymes do not simply facilitate the movement of nucleosomes to preferred intrinsic positions, but rather play a critical role in the specificity of where nucleosomes are located.
The mechanism by which nucleosome remodelers override intrinsic DNA sequence preferences of histone octamers is unclear. However, RSC47,48 and perhaps other nucleosome remodelers can bind specific DNA sequences, and they are likely to have other sequence preferences for positioning nucleosomes49,50. In addition, nucleosome remodelers may be influenced by the boundary of nucleosome-disfavoring sequences, such that in the course of moving nucleosomes, kinetic effects override thermodynamic equilibrium50. In this regard, nucleosome remodelers may use nucleosome-depleted regions as a measuring device to position the +1 and −1 nucleosomes. Interestingly, multiple nucleosome remodelers in the yeast cell-free extracts are necessary to reconstitute the genome-wide pattern observed in vivo. Reactions involving individual nucleosome remodelers reconstitute only part of the pattern, either being restricted to subsets of genes or generating less precise positioning46.
While yeast nucleosome remodeling activities per se can reconstitute some aspects of the in vivo pattern, there are two important aspects of the pattern that they do not faithfully generate. First, the precise locations of the +1 nucleosomes generated in vitro are poorly matched with the precise locations of the +1 nucleosomes that are generated in vivo. Second, the degree of positioning of more downstream nucleosomes (e.g. +3 and beyond) is significantly lower in the in vitro reactions than observed in vivo. As a consequence, the nucleosome arrays over coding regions are less pronounced and appear shorter. These observations indicate that nucleosome remodelers are necessary, but not sufficient for establishing the in vivo pattern of nucleosome positioning. As will be discussed later, aspects of Pol II transcription also play critical roles in establishing the in vivo pattern.
In addition to the in vitro reconstitution experiments, there is considerable genetic evidence for the critical role of nucleosome remodelers in establishing nucleosome positioning in vivo. In S. cerevisiae, a strain lacking the Isw2 remodeler shows altered nucleosome positioning adjacent to the promoter at the interface of genic and intergenic sequences51. Specifically, Isw2 helps to position the +1 nucleosome onto unfavorable DNA near the promoter in a directional manner, and it suppresses antisense transcription52. In addition, the RSC remodeling complex also mobilizes nucleosomes onto unfavorable sequences in the vicinity of the promoter53.
Role of nucleosome remodelers in nucleosome spacing
As nucleosomes are typically arranged in regularly-spaced arrays with a relatively constant linker length between nucleosomes, nucleosome spacing is a key aspect of nucleosome positioning. Drastic alteration of nucleosome positioning patterns is observed in an S. cerevisiae strain lacking both the Isw1 and Chd1 remodelers54 or in an S. pombe strain lacking two related CHD remodelers (Hrp1 and Hrp3)55-57. In each of these evolutionarily diverged yeast species, the degree of positioning of the +2 nucleosome is much lower than observed in the wild-type strain, and positioning of the +3 and more downstream nucleosomes is essentially lost. This dramatic alteration of nucleosome positioning within coding regions is not due to histone depletion, but rather to a defect in nucleosome spacing. Thus, a combination of the Isw1 and Chd1 (or Hrp1 and Hrp3) nucleosome remodeling enzymes is required for the correct nucleosome spacing that is the basis of positioned nucleosome arrays. Interestingly, a small subset of nucleosomes within downstream portions of coding regions are correctly positioned, presumably due to intrinsic DNA sequence preferences that facilitate or override the effect of nucleosome remodelers54. Importantly, the positions of the +1 and −1 nucleosomes are essentially unaffected by the combined loss of Isw1 and Chd1 function, indicating that their positioning occurs by a distinct mechanism that may involve Isw2.
Additional support for a role of nucleosome remodelers for nucleosome spacing and positioning comes from a functional evolutionary experiment35 that is based on the observation that nucleosome spacing varies among yeast species43,44. When large genomic regions from a foreign yeast species are introduced into S. cerevisiae, the distance between nucleosomes is characteristic of S. cerevisiae, not the donor yeast species35. As a consequence, the vast majority of nucleosomes on the foreign DNA are not located at the positions that occur when the same DNA is present in the endogenous organism. The change in spacing could be due to differences in histone concentrations between the species, as the number of nucleosomes on genomic DNA will necessarily affect the average spacing. However, depletion of H3 does not significantly alter nucleosome spacing, although some positioned nucleosomes are preferentially lost or maintained55,58,59. Alternatively, it may be due to species-specific differences in the spacing properties of the remodelers themselves, because nucleosome assembly in vivo requires remodelers, and remodelers have specific nucleosome spacing properties that are independent of histone concentration60.
In S. cerevisiae, histone H1 does not significantly affect nucleosome spacing, because H1 protein levels are much lower than those of the core histones10. However, H1 and its various subtypes play an important role in nucleosome spacing in multicellular organisms. Overexpression or depletion experiments in vivo indicate that H1 increases the spacing between adjacent nucleosomes61-64, and differences in linker histone subtypes might underlie cell-type specific differences in nucleosome spacing. In addition to H1, the HMG14, 17 proteins may also play a role in nucleosome spacing61.
Role of transcription factors and Pol II elongation
In addition to DNA sequence and ATP-dependent nucleosome remodelers, Pol II transcription also contributes to establishing the genomic pattern of nucleosome positioning. In this respect, nucleosome positioning, transcription and perhaps other DNA-based processes such as DNA replication should be viewed as processes that reciprocally affect each other. The effect of Pol II on nucleosome positioning is achieved via transcriptional activator proteins, general transcription factors that compose the preinitiation complex, and the elongating Pol II machinery.
Nucleosome depletion can generate positioning in vivo
Transcriptional activators, via targeted recruitment of nucleosome remodelers, can generate nucleosome-depleted regions. Some activator proteins can bind nucleosomal DNA fairly well, whereas others rely on intrinsic histone destabilizing sequences or cooperativity with other activators to access their sites. Under standard growth conditions, the Rap1, Abf1, and Reb1 activators are important for generating nucleosome-depleted regions at subsets of S. cerevisiae genes32,47,53. Interestingly, when foreign DNA is introduced into S. cerevisiae, nucleosome-depleted regions often occur in coding regions, unlike the case in the native organisms35. Such de novo nucleosome-depleted regions presumably arise from the fortuitous binding of S. cerevisiae activators to evolutionarily irrelevant target sites. Notably, many of these fortuitous nucleosome-depleted regions are associated with a positioned nucleosome array that strongly resembles the standard nucleosome positioning pattern of endogenous S. cerevisiae genes. Thus, generation of a nucleosome-depleted region, even in the absence of intrinsic nucleosome-destabilizing sequences, is sufficient to generate the in vivo pattern of nucleosome positioning.
Basal transcription factors help position the +1 nucleosome
The precise location of the +1 nucleosome is a critical determinant of the nucleosome positioning pattern, because nucleosome spacing constraints are major determinants of the downstream nucleosome positions. The inability of the collection of nucleosome remodelers in crude extracts to accurately reconstitute the in vivo positions of the +1 and −1 nucleosomes suggests that some other factor(s) is involved. The striking relationship between the position of the +1 nucleosome and the transcription start site (TSS) suggests that the general transcription factors play a role33. Furthermore, the locations of the +1 nucleosome and TSS shift in concert when foreign yeast DNA is introduced into S. cerevisiae, such that TSS on the foreign DNA shifts to a S. cerevisiae-like location35. Given the strong in vivo positioning of both the preinitiation complex and the +1 nucleosome, a spacing relationship between these two entities requires that at least one of these be anchored to a specific location, thereby permitting a defined location for the second entity. While the limited sequence specificity of nucleosome remodelers makes it unlikely that they can provide such an anchor, preinitiation complexes bound at core promoters may be sufficient, with the location of the TBP bound to the TATA element or TATA-related sequence being the major determinant of the anchor point65.
These considerations strongly suggest that the preinitiation complex plays a role in fine-tuning the position of the +1 nucleosome. One speculative possibility is that a component(s) of the preinitiation complex is important for transiently recruiting the Isw2 and/or RSC complex that overrides inherent DNA sequence preferences to precisely position the +1 nucleosome. In addition, for organisms in which Pol II is often paused just downstream of the promoter66, there is a strong distance relationship between the presence of paused Pol II and the NELF pausing factor and the position of the +1 nucleosome67.
Pol II elongation and generating nucleosome arrays
Nucleosome arrays emanating from promoter regions occur unidirectionally in the transcribed direction, even though the +1 and −1 nucleosomes are well positioned. In addition, the decay of nucleosome positioning towards the center of genes displays a 5’/3’ asymmetry68. These initial observations suggested that the elongating Pol II machinery plays an important role in establishing the pattern of nucleosome positioning. In accord with this idea, the Chd1 and Isw1 nucleosome remodelers that are critical for positioning within coding regions have genome-wide association patterns that strongly resemble that of elongating Pol II. Conversely, nucleosome assembly in transcriptionally incompetent, cell-free extracts poorly reconstitutes positioned nucleosome arrays in the downstream portions of coding regions, even though the +1 and −1 nucleosomes are strongly positioned. Lastly, the length of nucleosome arrays emanating from fortuitous nucleosome-depleted regions in foreign yeast DNA that act as functional promoters is strongly correlated both in direction and length with the mRNA35.
These observations suggest that Pol II elongation strongly affects nucleosome positioning. In particular, the unidirectionality of nucleosome arrays can be easily explained by the unidirectionality of transcription, whereas it is unclear how such unidirectionality could be imposed only by nucleosome deleted regions and nucleosome remodelers. Furthermore, the inefficiency of yeast cell extracts to reconstitute downstream nucleosome positions in the coding region suggests that recruitment of the Chd1 and Isw1 remodeling enzymes is not the sole mechanism by which elongating Pol II affects nucleosome positioning. Nevertheless, the mechanistic connection between Pol II elongation and nucleosome arrays within coding regions remains to be established.
Summary
The combined genetic, biochemical, and informatics analyses performed by many laboratories demonstrate that the genome-wide pattern of nucleosome positioning is determined by the combination of DNA sequence, nucleosome remodelers, and transcription factors including activators, components of the preinitiation complex, and elongating Pol II (Figure 3). Importantly, although each of these components has discernible effects in isolation, they also reciprocally affect each other and hence affect the nucleosome positioning pattern in ways that are potentially complex. The DNA sequence is critical for rotational positioning along the DNA helix, and it also is an important determinant for nucleosome occupancy. In particular, poly(dA:dT) and poly(dG:dC) tracts are intrinsically inhibitory to nucleosome formation, whereas non-homopolymeric GC-rich regions favor nucleosome formation. DNA sequence also contributes to the nucleosome positioning pattern, but several aspects of the in vivo pattern cannot be accounted for by intrinsic histone-DNA interactions.
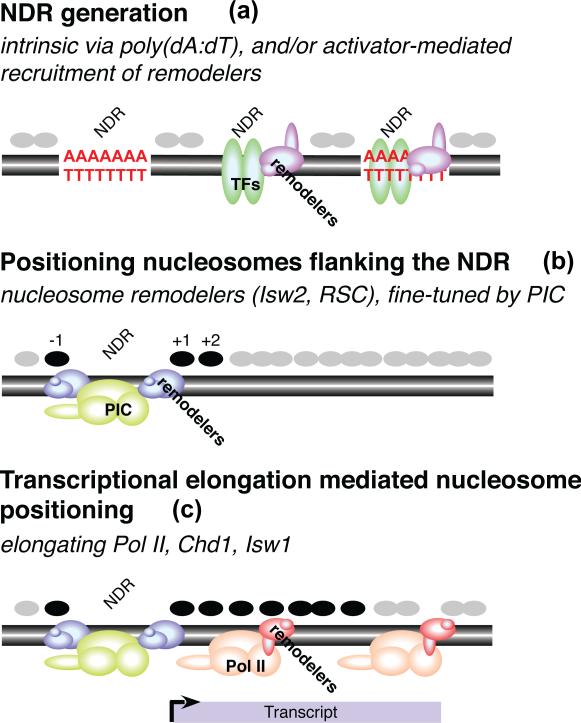
Figure 3.
Determinants of nucleosome positioning. (a) Nucleosome-depleted regions (NDRs) are generated either by poly (dA:dT) tracts and/or by transcription factors and their recruited nucleosome remodeling complexes. Gray circles indicate nucleosomes. (b) Nucleosomes located at highly preferred positions (black circles) flanking the NDR are generated by nucleosome-remodeling complexes (for example, Isw2 and RSC, likely in a transcription-independent manner), and fine-tuned by the Pol II preinitiation complex (PIC) and associated factors. (c) Positioning of the more downstream nucleosomes depends on transcriptional elongation, and the recruitment of nucleosome-remodeling activities (for example, Chd1 and Isw1) and histone chaperones by the elongating Pol II machinery. This figure, which was modified from ref. 36, does not include DNA sequence determinants of rotational positioning (Fig. 2) or contributions to nucleosome spacing by histone H1.
As demonstrated by the cross-species experiments, nucleosome-depleted regions are largely sufficient to generate the standard pattern of ordered nucleosome arrays35. At native promoters, nucleosome-depleted regions can be generated intrinsically via poly(dA:dT) tracts and/or activator-mediated recruitment of nucleosome remodelers that evict histones in the vicinity of the activator binding site. The use of poly(dA:dT) sequences varies considerably among organisms; they are very common in S. cerevisiae, but not in the fission yeast S. pombe and multicellular organisms42-44.
In addition to their role in creating nucleosome-depleted regions, ATP-dependent remodelers play an important role in nucleosome positioning. In a manner independent of transcription, they can assemble nucleosomes flanking the depleted region to generate the +1 and −1 nucleosomes. However, these remodeling enzymes cannot accurately position the +1 nucleosome to the in vivo location, nor can they generate properly positioned nucleosomes at more downstream locations. Precise positioning of the +1 nucleosome is strongly influenced by the location of the preinitiation complex, although the mechanistic basis for this spacing relationship is poorly understood. Lastly, generation of positioned nucleosome arrays throughout the coding region is coupled to Pol II elongation, and it involves Pol II-dependent recruitment of nucleosome remodelers and perhaps some other aspect of Pol II elongation. The putative roles of Isw2 and RSC for positioning the +1 nucleosome and of Isw1 and Chd1 for transcription-coupled positioning and spacing of nucleosomes within the coding region are strongly supported by genome-wide mapping of nucleosome remodelers on positioned mononucleosomes69.
In some situations where a gene is activated, it is likely that establishment of the nucleosome pattern occurs step-wise- activator-mediated generation of a nucleosome depleted region; positioning of the +1 and −1 nucleosomes; elongation-coupled assembly of positioned and properly spaced nucleosome arrays over the coding region. However, such a step-wise process is unnecessary, and probably irrelevant, for steady-state maintenance of the nucleosome positioning pattern. Instead, the combined effects of DNA sequence, nucleosome remodelers, and transcription factors make independent contributions to the pattern.
Lastly, it is important to mention that the nucleosome positioning pattern described here is gene-averaged, and hence represents a typical pattern. Thus, although the positioning mechanisms discussed here apply to all genes, the precise pattern at individual genes may differ from the gene-averaged pattern. Such variations will depend on the DNA sequences, the nucleosome remodelers that act at the gene, and the complexity of transcription units within the gene (e.g. antisense or unstable transcripts). In addition, the relative contributions of these mechanisms may differ among genes, which is likely to result in differential regulation of these genes. Furthermore, even when the typical pattern predominates at a given gene, it is highly likely that the pattern is not present in all cells. For this reason, differences in the nucleosome positioning pattern among cells within a population are likely to confer distinct transcriptional properties in these cells. Nevertheless, although several questions remain open, we believe that there is now a good understanding of how many aspects of the nucleosome positioning patters are generated in vivo.
ACKNOWLEDGMENTS
This review is dedicated to the memory of Jon Widom, our dear friend and colleague, who is sorely missed by the authors and the scientific community. We thank Daphne Schneider for encouraging us to write this review, and Zarmik Moqtaderi, Tali Raveh-Sadka, Michael Levo, Naom Kaplan, and Yair Field for comments on the manuscript. This work was supported by grant GM 30186 from the National Institutes of Health to K.S. and grants to E.S. from the European Research Council and the US National Institutes of Health.
REFERENCES
- 1.Richmond TJ, Davey CA. The structure of DNA in the nucleosome core. Nature. 2003;423:145–150. doi: 10.1038/nature01595. [DOI] [PubMed] [Google Scholar]
- 2.Yuan G-C, et al. Genome-scale identification of nucleosome positions in S. cerevisiae. Science. 2005;309:626–630. doi: 10.1126/science.1112178. [DOI] [PubMed] [Google Scholar]
- 3.Lee W, et al. A high-resolution atlas of nucleosome occupancy in yeast. Nat. Genet. 2007;39:1235–1244. doi: 10.1038/ng2117. [DOI] [PubMed] [Google Scholar]
- 4.Mavrich TN, et al. A barrier nucleosome model for statistical positioning of nucleosome throughout the yeast genome. Genome Res. 2008;18:1073–1083. doi: 10.1101/gr.078261.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavrich TN, et al. Nucleosome organization in the Drosophila genome. Nature. 2008;453:358–362. doi: 10.1038/nature06929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schones DE, et al. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132:887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shivaswamy S, et al. Dynamic remodeling of individual nucleosomes across a eukaryotic genome in response to transcriptional perturbation. PLoS Biol. 2008;6:e65. doi: 10.1371/journal.pbio.0060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valouev A, et al. A high-resolution, nucleosome position map of C. elegans reveals a lack of universal sequence-dictated positioning. Genome Res. 2008;18:1051–1063. doi: 10.1101/gr.076463.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valouev A, et al. Determinants of nucleosome organization in primary human cells. Nature. 2011;474:516–520. doi: 10.1038/nature10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freidkin I, Katcoff DJ. Specific distribution of the Saccharomyces cerevisiae linker histone HHO1 in the chromatin. Nucl. Acids Res. 2001;29:4043–4051. doi: 10.1093/nar/29.19.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam FH, Steger DJ, O'Shea EK. Chromatin decouples promoter threshold from dynamic range. Nature. 2008;453:246–250. doi: 10.1038/nature06867.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raveh-Sadka T, et al. Manipulating nucleosome disfavoring sequences allows fine-tune regulation of gene expression in yeast. Nat. Genet. 2012;44:743–750. doi: 10.1038/ng.2305. [DOI] [PubMed] [Google Scholar]
- 13.Varshavsky AJ, Sundin O, Bohn M. A stretch of “late” SV40 viral DNA about 400 bp long which induces the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979;16:453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- 14.Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNaseI. Nature. 1980;286:854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- 15.Iyer V, Struhl K. Poly(dA:dT), a ubiquitous promoter element that stimulates transcription via its intrinsic structure. EMBO J. 1995;14:2570–2579. doi: 10.1002/j.1460-2075.1995.tb07255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Lee CK, Granek JA, Clarke ND, Lieb JD. Whole-genome comparison of Leu3 binding in vitro and in vivo reveals the importance of nucleosome occupancy in target site selection. Genome Res. 2006;16:1517–1528. doi: 10.1101/gr.5655606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 19.Thastrom A, et al. Sequence motifs and free energies of selected natural and non-natural nucleosome positioning DNA sequences. J. Mol. Biol. 1999;288:213–229. doi: 10.1006/jmbi.1999.2686. [DOI] [PubMed] [Google Scholar]
- 20.Drew HR, Travers AA. DNA bending and its relation to nucleosome positioning. J. Mol. Biol. 1985;186:773–790. doi: 10.1016/0022-2836(85)90396-1. [DOI] [PubMed] [Google Scholar]
- 21.Satchwell SC, Drew HR, Travers AA. Sequence periodicities in chicken nucleosome core DNA. J. Mol. Biol. 1986;191:659–675. doi: 10.1016/0022-2836(86)90452-3. [DOI] [PubMed] [Google Scholar]
- 22.Segal E, et al. A genomic code for nucleosome positioning. Nature. 2006;442:772–778. doi: 10.1038/nature04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brogaard KR, Xi L, Wang JP, Widom J. A map of nucleosome positions in yeast at base-pair resolution. Nature. 2012;486:496–501. doi: 10.1038/nature11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson HCM, Finch JT, Luisi BF, Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987;330:221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- 25.Suter B, Schnappauf G, Thoma F. Poly(dA-dT) sequences exist as rigid DNA structures in nucleosome-free yeast promoters in vivo. Nucl. Acids Res. 2000;28:4083–4089. doi: 10.1093/nar/28.21.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal E, Widom J. Poly(dA:dT) tracts: major determinants of nucleosome organization. Curr. Opin. Struct. Biol. 2009;19:65–71. doi: 10.1016/j.sbi.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCall M, Brown T, Kennard O. The crystal structure of d(G-G-G-G-C-C-C-C): a model for Poly(dG).Poly(dC). J. Mol. Biol. 1985;185:385–396. doi: 10.1016/0022-2836(85)90009-9. [DOI] [PubMed] [Google Scholar]
- 28.Dechering KJ, Cuelenaere K, Konings RN, Leunissen JA. Distinct frequency-distributions of homopolymeric DNA tracts in different genomes. Nucl. Acids Res. 1998;26:4056–4062. doi: 10.1093/nar/26.17.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc. Natl. Acad. Sci. U.S.A. 1985;82:8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field Y, et al. Distinct modes of regulation by chromatin encoded through nucleosome positioning signals. PLoS Comput. Biol. 2008;4:e100216. doi: 10.1371/journal.pcbi.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaffney DJ, et al. Controls of nucleosome positioning in the human genome. PLoS Genet. 2012;8:e1003036. doi: 10.1371/journal.pgen.1003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan N, et al. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature. 2009;458:362–366. doi: 10.1038/nature07667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Intrinsic histone-DNA interactions are not the major determinant of nucleosome positions in vivo. Nat. Struct. Mol. Biol. 2009;16:847–852. doi: 10.1038/nsmb.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekinger EA, Moqtaderi Z, Struhl K. Intrinsic histone-DNA interactions and low nucleosome density are important for preferential accessibility of promoter regions in yeast. Mol. Cell. 2005;18:735–748. doi: 10.1016/j.molcel.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Hughes A, Jin Y, Rando OJ, Struhl K. A functional evolutionary approach to identify determinants of nucleosome positioning: A unifying model for establishing the genome-wide pattern. Mol. Cell. 2012;48:5–15. doi: 10.1016/j.molcel.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Tabor S, Struhl K. Distinguishing between mechanisms of eukaryotic transcriptional activation with bacteriophage T7 RNA polymerase. Cell. 1987;50:1047–1055. doi: 10.1016/0092-8674(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Thiele DJ. A specialized nucleosome modulates transcription factor access to a C. glabrata metal responsive promoter. Cell. 1996;87:459–470. doi: 10.1016/s0092-8674(00)81366-5. [DOI] [PubMed] [Google Scholar]
- 38.Zeevi D, et al. Compensation for differences in gene copy number among yeast ribosomal proteins is encoded within their promoters. Genome Res. 2011;21:2114–2128. doi: 10.1101/gr.119669.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Field Y, et al. Gene expression divergence in yeast is coupled to evolution of DNA-encoded nucleosome organization. Nat. Genet. 2009;41:438–445. doi: 10.1038/ng.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirosh I, Barkai N. Two strategies for gene regulation by promoter nucleosomes. Genome Res. 2008;18:1084–1091. doi: 10.1101/gr.076059.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan X, et al. Nucleosome depletion in yeast terminator regions is not intrinsic and can occur by a transcriptional mechanism linked to 3' end formation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17945–17950. doi: 10.1073/pnas.1012674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lantermann AB, et al. Schizosaccharomyces pombe gene-wide nucleosome mapping reveals positioning mechanisms distinct from those of Saccharomyces cerevisiae. Nat. Struct. Mol. Biol. 2010;17:251–257. doi: 10.1038/nsmb.1741. [DOI] [PubMed] [Google Scholar]
- 43.Tsankov AM, Thompson DA, Socha A, Regev A, Rando OJ. The role of nucleosome positioning in the evolution of gene regulation. PLoS Biol. 2010;8:e1000414. doi: 10.1371/journal.pbio.1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsankov A, Yanagisawa Y, Rhind N, Regev A, Rando OJ. Evolutionary divergence of intrinsic and trans-regulated nucleosome positioning sequences reveals plastic rules for chromatin organization. Genome Res. 2011;21:1851–1862. doi: 10.1101/gr.122267.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, et al. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science. 2011;332:977–980. doi: 10.1126/science.1200508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wippo CJ, et al. The RSC chromatin remodelling enzyme has a unique role in directing the accurate positioning of nucleosomes. EMBO J. 2011;30:1277–1288. doi: 10.1038/emboj.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badis G, et al. A library of yeast transcription factor motifs reveals a widespread function for Rsc3 in targeting nucleosome exclusion at promoters. Mol. Cell. 2008;32:878–887. doi: 10.1016/j.molcel.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Floer M, et al. A RSC/nucleosome complex determines chromatin architecture and facilitates activator binding. Cell. 2010;141:407–418. doi: 10.1016/j.cell.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rippe K, et al. DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15635–15640. doi: 10.1073/pnas.0702430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Vugt JJ, et al. Multiple aspects of ATP-dependent nucleosome translocation by RSC and Mi-2 are directed by the underlying DNA sequence. PloS one. 2009;4:e6345. doi: 10.1371/journal.pone.0006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitehouse I, Tsukiyama T. Antagonistic forces that position nucleosomes in vivo. Nat. Struct. Mol. Biol. 2006;13:633–640. doi: 10.1038/nsmb1111. [DOI] [PubMed] [Google Scholar]
- 52.Whitehouse I, Rando OJ, Delrow J, Tsukiyama T. Chromatin remodelling at promoters suppresses antisense transcription. Nature. 2007;450:1031–1035. doi: 10.1038/nature06391. [DOI] [PubMed] [Google Scholar]
- 53.Hartley PD, Madhani HD. Mechanisms that specify promoter nucleosome location and identity. Cell. 2009;137:445–458. doi: 10.1016/j.cell.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gkikopoulos T, et al. A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organization. Science. 2011;333:1758–1760. doi: 10.1126/science.1206097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hennig BP, Bendrin K, Zhou Y, Fischer T. Chd1 chromatin remodelers maintain nucleosome organization and repress cryptic transcription. EMBO Rep. 2012;13:997–1003. doi: 10.1038/embor.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pointer J, et al. CHD1 remodelers regulate nucleosome spacing in vitro and align nucleosomal arrays over gene coding regions in S. pombe. EMBO J. 2012;31:4388–4403. doi: 10.1038/emboj.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shim YS, et al. Hrp3 controls nucleosome positioning to suppress non-coding transcription in eu- and heterochromatin. EMBO J. 2012;31:4375–4387. doi: 10.1038/emboj.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Celona B, et al. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011;9:e1001086. doi: 10.1371/journal.pbio.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gossett AJ, Lieb JD. In vivo effects of histone H3 depletion on nucleosome occupancy and position in Saccharomyces cerevisiae. PLoS Genet. 2012;8:e1002771. doi: 10.1371/journal.pgen.1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito T, Bulger M, Pazin MJ, Kobayashi R, Kadonaga JT. ACF, an ISW1-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell. 1997;90:145–155. doi: 10.1016/s0092-8674(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 61.Drew HR. Reconstitution of short-spaced chromatin from the histone octamer and either HMG14,17 or histone H1. J. Mol. Biol. 1993;230:824–836. doi: 10.1006/jmbi.1993.1204. [DOI] [PubMed] [Google Scholar]
- 62.Fan Y, et al. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell. 2005;123:1199–1212. doi: 10.1016/j.cell.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto H, et al. Histone H1 null vertebrate cells exhibit altered nucleosome architecture. Nucl. Acids Res. 2010;38:3533–3545. doi: 10.1093/nar/gkq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oberg C, Izzo A, Schneider R, Wrange O, Belikov S. Linker histone subtypes differ in their effect on nucleosome spacing in vivo. J. Mol. Biol. 2012;419:183–197. doi: 10.1016/j.jmb.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Rhee HS, Pugh BF. Genome-wide structure and organization of eukaryotic preinitiation complexes. Nature. 2012;483:295–301. doi: 10.1038/nature10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nature Rev. 2012;13:720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang GS, et al. Unusual combinatorial involvement of poly-A/T tracts in organizing genes and chromatin in Dictyostelium. Genome Res. 2012;22:1098–1106. doi: 10.1101/gr.131649.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaillant C, et al. A novel strategy of transcription regulation by intragenic nucleosome ordering. Genome Res. 2010;20:59–67. doi: 10.1101/gr.096644.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yen K, Vinayachandran V, Batta K, Koerber RT, Pugh BF. Genome-wide nucleosome specificity and directionality of chromatin remodelers. Cell. 2012;149:1461–1473. doi: 10.1016/j.cell.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]