Abstract
Objective
Bacteria have been identified in different regions of the placenta. Here, we tested the hypothesis that the maternal basal plate of the placenta harbors microbes which may be associated with adverse pregnancy outcomes.
Study Design
We performed a cross-sectional study of pregnancies from a single tertiary care hospital. Maternal medical and obstetric characteristics were obtained and pregnancies followed prospectively for outcomes and placental collection. After delivery, systematic random sampling of the placental basal plate was performed. Paraffin sections of basal plates were stained with four histological stains and scored for morphological evidence of bacteria.
Results
Of 195 total patients in the study, Gram positive and negative intracellular bacteria of diverse morphologies were documented in the basal plates of 27% of all placentas. 35% of the patients delivered preterm. No difference was noted between placental basal plates from preterm or term gestations. Intracellular bacteria were found in the placental basal plates of 54% spontaneous preterm deliveries before 28 weeks, and in 26% of term spontaneous deliveries (p=0.02). Intracellular bacteria were also documented in placentas without clinical or pathologic chorioamnionitis.
Conclusions
27% of placentas demonstrated intracellular bacteria in the placental basal plate using morphological techniques. Thus, the maternal basal plate is a possible source of intrauterine colonization and placental pathological examination could include examination for bacteria in this important maternal fetal interface.
Keywords: Infection, Preterm birth, chorioamnionitis
Introduction
Infection and inflammation are commonly associated with the risk for preterm birth (PTB). Predisposing factors for infection-related preterm delivery are diverse, including subclinical intrauterine infections1, intra-amniotic infections2,3 and pyelonephritis4. In addition, maternal history of prior PTB, especially multiple and/or early PTB(s) is a significant risk factor5. This recurrence risk suggests the presence of a risk factor that is presentfrom pregnancy to pregnancy. The specific mechanisms by which infectious insults trigger preterm parturition are poorly understood. Notably, randomized trials of antimicrobials for treatment of infection for prevention of PTB have been disappointing6–8.
Previous studies have shown that pathogenic microbes can establish occult intracellular reservoirs within the epithelium of the murine urinary tract, thereby evading immune recognition and allowing re-emergence of symptomatic infection9. In addition, prior studies have documented the presence of bacteria in various placental locations10–12 but have not examined the basal plate specifically. We hypothesized that the cells in the basal plate of the placenta which comprise the tissue layer directly at the maternal fetal interface may harbor occult microbes similar to the findings in previous placental studies as well as our studies in the murine urinary tract. We reasoned that occult microorganisms in the basal plate could lead to chronic or acute inflammation, and may be associated with adverse pregnancy outcomes such as preterm birth and chorioamniontis. The objective of this study is first, to examine the basal plate of a large group of placentas to diagnose the presence or absence of microbes at the maternal fetal interface. Second, when microbes were identified in the basal plate maternal fetal interface, we sought to investigate whether the presence of bacteria was associated with important clinical outcomes such as chorioamnionitis and preterm birth.
Materials and Methods
Study Design
This is a cross-sectional study of women from a single tertiary care hospital. The study was approved by the IRB of Washington University School of Medicine in St. Louis, MO. Women were enrolled during their antenatal course and followed until delivery. Clinical data and placental specimens were collected through an institutional core resource, the Women’s and Infant’s Health Specimen Consortium (WIHSC). Gestational age was assigned by the best data available from the last menstrual period (LMP) if consistent with ultrasound dating (±5 days in the first trimester or ±14 days in the second trimester). If LMP was unknown, or inconsistent with ultrasound dating, gestational age was assigned according to the earliest ultrasound available. Exclusion criteria were hepatitis B, hepatitis C, and HIV infection. In-depth chart reviews were performed by trained research personnel as the patient progressed through her antenatal and delivery course using close-ended data collection forms. This included maternal medical history, obstetric history, pregnancy diagnoses and outcomes as documented by the treating obstetric team.
Tissue harvest
Placentas (n= 195) were collected ≤12 h from delivery. Trained research assistants used sterile technique to harvest three random, 5–8 mm samples from the most superficial area of the basal plate equidistant from each other and without inclusion of the periphery of the placental disc13, 14. Specimens were fixed for 48 h at room temperature in 10% neutral buffered formalin prior to embedding in paraffin, as described9.
Histopathology and Histochemical analyses
Five micron sections of embedded tissues were deparaffinized with xylene and ethanol and stained with hematoxylin and eosin (H&E) and evaluated for histological evidence of basal plate components including stromal decidual cells and fetal epithelial trophoblasts. Since H&E stain is not sufficiently sensitive to detect bacteria (typically ≤1 µm), slides were stained for bacterial detection by three methods: Gram Stain (Acros Organics, Fair Lawn, NJ); Hema 3 modification of the Geimsa stain (Fisher Scientific, St. Louis, MO); and the Brown-Hopps modification of the Gram stain (Fisher Scientific, St. Louis, MO). Ten random fields of each specimen per 100 micron2 were examined for the presence or absence of bacteria by two independent observers blinded to clinical history. The histological location of microorganisms was recorded by use of a Nikon Eclipse microscope equipped with an Olympus DP71 color camera.
Exploration of Obstetric Outcomes and Statistical Analysis
Chorioamniontis and preterm birth were two clinical outcomes of interest in this study. A cohort analysis was performed to explore the association between these two clinical outcomes and bacterial colonization at the maternal fetal interface. Pregnancies with evidence of intracellular bacteria at the basal plate were compared to those without bacteria at the basal plate. PTB was defined broadly as delivery for any reason at less than 37 weeks of gestation. Spontaneous PTB sourced to preterm labor or preterm rupture of membranes as well as indicated PTB due to preeclampsia, abruptio placentae, or intrauterine growth restriction were included15–17. An analysis of spontaneous preterm birth alone (defined as preterm labor and preterm premature rupture of membranes) was performed. Chorioamnionitis was assigned based on the hospital derived placental pathology reports indicating the presence or absence of acute maternal or fetal inflammation or when antibiotics were administered in labor according to the institutional protocol for presumed chorioamnionitis.
GBS colonization was classified as positive if a vaginal/rectal swab was positive within 5 weeks of delivery, or if a urine culture grew GBS at anytime during the pregnancy. Per routine practice at our institution, all preterm patients receive antibiotic prophylaxis for neonatal Group B Streptococcus (GBS) colonization. Therefore, all women, independent of GBS carriage or treatment status were included in the main analysis. A secondary analysis was performed excluding women with GBS vaginal colonization or urinary infection.
Advanced maternal age was defined as maternal age at delivery ≥ 35 years old. Race, tobacco and alcohol use were based on self-report. Sexually transmitted infections were captured as “during pregnancy” (which included presence of infection at the time of delivery or any time during the current gestation) or “prior to pregnancy” (defined as anytime in the past but predating the current pregnancy). STIs included gonorrhea, chlamydia, genital herpes simplex, trichomonas, and syphilis. Urinary tract infection was assigned if a positive microbial culture was present, or if clinical suspicion was sufficient to treat the patient with antibiotics. All study related clinical information was entered into a secure database using Illume (DatStat, Inc, Seattle WA). Official pathology reports and diagnoses were also reviewed and added to the database.
Demographic information of women with and without intracellular bacteria was compared by t-test, chi-square or Fisher’s exact test as appropriate. Subsequently, univariate analysis was used to compare the incidence of intracellular bacteria in basal plate specimens from term compared to preterm gestations as well as from those with and without the diagnosis of chorioamnionitis. To compare each gestational age group to the reference group of term pregnancies, Poisson regression with robust error was used to estimate the relative risk and 95% confidence intervals. This analytic approach provides an unbiased estimate of the RR when the outcome is common (greater than 10%)18.
Results
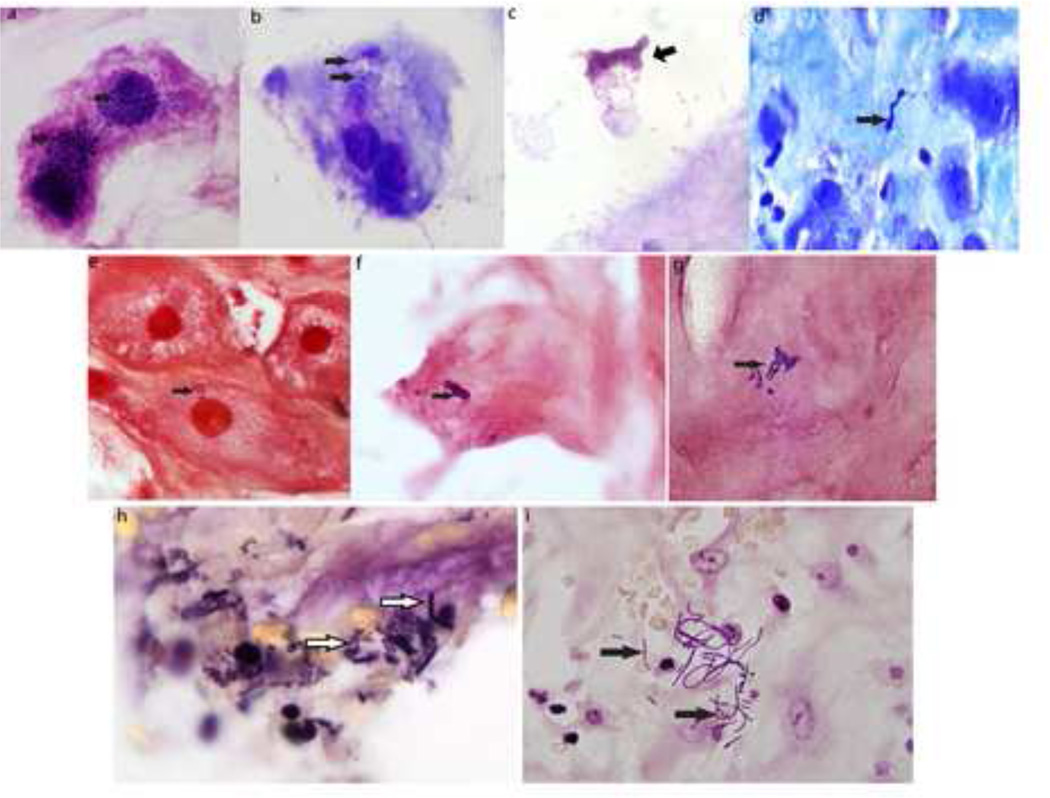
Of 195 pregnancies evaluated in this study, 27% had evidence of intracellular bacteria in the basal plate of the placenta. Demographic characteristics of the patients who participated are demonstrated in Table 1. The multiple histologic stains used in this study assured us that we could determine both morphology and location of bacteria within cells in the basal plate if they were present, as posed in our hypothesis. We identified individual bacteria and biofilm-like clusters of bacteria in the basal plate (Figure 1).
Table 1.
Baseline Maternal Demographic Information
| Characteristic | Intracellular Bacteria Present in Basal Plate n=53 |
Intracellular Bacteria Absent in Basal Plate n=142 |
p-value |
|---|---|---|---|
| Advanced Maternal Age (SD) | 29.8(5.9) | 28.4(5.8) | 0.12 |
| Black Race (%) | 47.2 | 47.2 | 0.99 |
| Tobacco Use (%) | 9.4 | 23.9 | 0.03 |
| Alcohol Use (%) | 5.8 | 11.4 | 0.29 |
| STI in current pregnancy (%) | 20.8 | 21.8 | 0.87 |
| History of STI prior to pregnancy (%) | 34.6 | 26.8 | 0.29 |
| UTI in current pregnancy (%) | 18.9 | 18.3 | 0.93 |
| Group B Streptococcus infection | 21.6 | 22.9 | 0.85 |
STI=sexually transmitted infection; UTI=urinary tract infection
Figure 1. Intracellular bacteria in basal plate.
(a–d) Hema 3 Geimsa stain (e–g) Gram stain (h–t) Brown and Hopps stain: all showing presence of single, clusters, chains, or filaments of intracellular bacteria (arrows).
Hema3 modification of the Giemsa stain permits detection of bacteria as well as inflammatory infiltrates. Using this stain, we noted biofilm-like clusters of bacteria stained dark blue/purple in the basal plate (Fig. 1a,b,c). Gram stains were conducted to determine whether the bacteria were Gram Positive or negative. Fig.1(e, f and g) illustrate examples of Gram positive bacteria. We further found that bacteria of all shape classes were recorded and were of diverse morphologies, including filamentous (Fig. 1e,i), diplococci (Fig. 1f) or streptococci (Fig. 1g), rods (Fig. 1b,c,h), spirochetes (Fig.1d). The biofilm-like clusters could be both coccoid as in Fig. 1a or rod shaped as in Fig. 1b or 1c. We used the Brown-Hopps stain which detects DNA content, thus rendering the bacteria and host nuclei a deep purple over a pale yellow background of cytosolic components to confirm the presence or absence of bacteria in all the basal plate tissues examined. This stain offers the advantage of rapid and reliable detection of bacteria even at 20× magnification due to the staining patterns. Fig.1 h and i panels depict bacteria evident in tissue stained with the Brown-Hopps stain.
We examined chorioamnionitis and PTB as two clinical outcomes of specific interest in this study. Thirty-three patients (17%) had either clinical or histologic diagnosis of chorioamnionitis. The finding of intracellular bacteria at the basal plate was no different in patients with or without clinical or histologic diagnosis of chorioamnionitis (Table 2). Sixty-eight patients were delivered preterm and the remainder (n=127) were delivered at term. PTB was no more common in placentas harboring intracellular bacteria at the maternal fetal interface than those without bacteria present (Table 2). As GBS carriage could potentially confound our analysis of intracellular bacteria incidence in basal plate, a secondary analysis was performed excluding women with GBS colonization. The results remained the same with similar rates of intracellular bacteria in preterm vs. term placentas (30.4% vs. 27.4%, p=0.7). Among the preterm deliveries, 38.2% (n=26) were delivered between 34–37 weeks’ gestation, 39.7% (n=27) between 28–34 weeks’ gestation, and 22.1% (n=15) at < 28 weeks’ gestation. No significant difference of intracellular bacteria in multiple gestational age strata was noted (Table 3). Fifty of the preterm birth cases (74%) were classified as spontaneous preterm birth. A significant 2-fold increased risk for intracellular bacteria in the basal plates of very preterm placentas (<28 weeks) in the spontaneous preterm birth subgroup was evident when compared to the term birth reference group (54.5% vs. 26.7%, p=0.02) (Table 4).
Table 2.
Comparison of Presence of Intracellular Bacteria in Preterm Birth and Chorioamnionitis
| Intracellular Bacteria Present in Basal Plate (%) |
Intracellular Bacteria Absent in Basal Plate |
p-value | |
|---|---|---|---|
| Preterm Birth < 37 weeks (n=68) | 35.9 | 34.5 | 0.86 |
| Chorioamnionitis (n=33) | 13.2 | 18.3 | 0.40 |
Table 3.
Presence of Intracellular Bacteria in Placentas Stratified by Gestational Age
| Gestational Age (weeks) |
Intracellular Bacteria Present in Basal Plate (%) |
Relative Risk (95%CI) | p-value |
|---|---|---|---|
| ≥37 | 26.7 | Reference | |
| 34–36 (n=26) | 15.4 | 0.6 (0.2–1.5) | 0.3 |
| 28–33 (n=27) | 33.3 | 1.3 (0.7–2.3) | 0.5 |
| <28 (n=15) | 40.0 | 1.5 (0.8–3.0) | 0.3 |
Table 4.
Presence of Intracellular Bacteria in Spontaneous Preterm Labor Compared to Term Labor
| Gestational Age (weeks) |
Intracellular Bacteria Present in Basal Plate (%) |
Relative Risk (95%CI) | p-value |
|---|---|---|---|
| ≥37 | 26.7 | Reference | |
| 34–36 (n=17) | 11.8 | 0.4 (0.1–1.7) | 0.2 |
| 28–33 (n=22) | 31.8 | 1.2 (0.6–2.3) | 0.6 |
| <28 (n=11) | 54.5 | 2.0 (1.1–3.8) | 0.02 |
Comment
Principal findings of the study
This study demonstrates the first morphological documentation of intracellular organisms in the decidual basal plate of human placentas. Microbial biofilms, defined as microbial communities encapsulated within polysaccharide matrices19 have been implicated in >80% of human infections such as periodontitis, urethritis, endocarditis, cystitis, and device-associated infections. Romero et al. demonstrated that there were biofilms in amniotic fluid3,20. Here, we show that 27% of all placentas harbor intracellular bacteria in the basal plate. These findings underscore our hypothesis that bacteria can colonize the maternal-fetal interface. Our data show that both term and preterm placentas can have intracellular bacteria present without overt infection while a substantial proportion of basal plate specimens even from preterm placentas do not demonstrate bacteria. However, we find a significant increase in incidence of detectable bacteria in the placentas from spontaneous preterm birth at very premature gestational ages. Our findings complement previous studies showing that bacteria are detectable in placentas10,11, 12 and recoverable from biopsies of chorion parenchyma from placentas at <28 gestational weeks21. Together, these findings are consistent with evidence that presence of microorganisms in placental tissue, whether via culture techniques or morphologically, convey biologically important information.
Clinical Implications
Our novel morphological findings add the maternal basal plate as a hitherto unexplored location for bacteria. The use of high magnification imaging with the use of histologic tissue stains described here to optimize the detection of bacteria may help to better identify the presence of bacteria in the basal plate while also pinpointing their location. The finding of intracellular bacteria of diverse morphologies in the basal plates of more than one-fourth of human placentas in the absence of a diagnosis of chorioamnionitis on the placental pathology report may explain why identification may be easily missed on routine placental pathologic examination and suggests that the basal plate may require more evaluation including special stains for bacteria during routine placental pathological exam. Our findings of the presence of intracellular bacteria, without overt chorioamnionitis or deciduitis, indicates that the microorganisms may, or may not, elicit a host-response that is identifiable as inflammation from the placental histopathology on examination. This is consistent with a recent report that histologic chorioamnionitis may not always indicate presence of an infection and in fact is associated with a reduced risk of sepsis in preterm infants22, 23. Chronic chorioamnionitis is commonly noted in unexplained preterm fetal death24 and may be more representative of maternal antifetal rejection rather than infection25.
Implications of the work for further research
Key findings from our previous studies show that pathogenic microbes can establish asymptomatic occult intracellular reservoirs within the urinary tract epithelium thus evading immune recognition and detection with culture techniques. Specifically, we have elucidated in the urinary epithelium, how uropathogenic E. coli bacteria establish occult infections, remain asymptomatic, and yet in the presence of certain stimuli re-emerge and initiate inflammatory cascades causing symptomatic infection9. Clinical dogma held that all UTIs, acute or recurrent, are due to bladder colonization by intestinal E. coli. However, given that a large percentage of recurrent UTIs are caused by the same strain of bacteria as the initial infection26,27,28 and recurrence occurs despite appropriate antibiotic treatment29, we and others established that the bladder itself as a source for bacteria that can seed new infections9. Similar to the urinary epithelium, we suggest that the endometrial epithelium of the non-pregnant uterus may harbor occult microbes which become incorporated into the basal plate at the time of placental implantation. Indeed, Andrews and co-workers have shown that micro-organisms can be found in the endometrium30, 31. In the urinary tract, injury to the tissue inducible by infectious or chemical means and consequent tissue remodeling serves as a trigger for re-emergence of the bacteria from their occult niches9. Women with a prior PTB are 2–3 times more likely to deliver preterm, and women with 2 prior PTBs have a six-fold increase in risk for another32. Our findings lead us to speculate that conceivably, such occult bacteria in the maternal basal plate could constitute a potential source of intrauterine colonization upon escape from this location33, 34. Our work also complements previous studies showing that the bacterial pathogen, L. monocytogenes can infect fetal epithelial cell cultures from the placental basal plate and survive intracellularly35. Both L. monocytogenes and E. coli are known to utilize specific receptors to invade intracellularly suggesting that unique bacterial ligand-host receptor interactions might govern bacterial localization36, 37. Genetic factors governing host tendency to harbor latent infections, host responses to microbes, and pregnancy related immune system changes all may further mediate the effect of these bacteria during pregnancy.
In the stratified analysis by gestational age, the data are suggestive that intracellular bacteria occur more commonly in the earliest gestational ages particularly in association with spontaneous preterm birth and raise the question of whether such bacteria can play a role in spontaneous preterm labor and delivery under certain circumstances (for example, emergence from occult niches to contribute to infection). The specific types of organisms present may be different at the extremes of gestational age as prior studies have documented that earlier PTB is more likely to be associated with infection20, 38. However, given the presence of bacteria even in term placentas as well as the absence of bacteria in a large proportion of preterm placentas, one cannot exclude the possibility that such microbes in the basal plate are commensal and exert no pathologic effect, whereas others microbes may activate inflammatory cascades. Further investigations to elucidate the identity, origin, and precise functions of these microorganisms are needed.
Strengths and limitations of the study
Our study has several strengths. First, we employed histological stains optimized for detection of bacteria at other mucosal interfaces, for e.g, the urinary tract, which permit rapid and reliable scoring of bacteria in tissue sections at magnifications of 20×–100×. Morphological evidence of both Gram positive and negative species and of various sizes and shapes underscores that the basal plate is an important area for bacterial colonization at the maternal-fetal interface. Second, we focused on maternal basal plate, which has hitherto not been examined for presence of microbes relative to fetal membranes. Third, the researchers examining the basal plate tissue were not aware of the medical histories nor the gestational ages of the patients’ placentas they examined, thus minimizing any bias. Finally, multiple histological stains were used to confirm the presence or absence of bacteria in the basal plates.
A limitation of our study is that we did not employ culture techniques to determine whether bacteria in the basal plate were viable and could be identified with this methodology. Previous studies have indeed detected bacteria in subamniotic and chorion parenchyma, using culture-dependent techniques and culture-independent detection using PCR11, 21, 39. In addition, observational studies cannot address the problem of causality. Our data show that both term and preterm placentas can have intracellular bacteria present without overt infection while a substantial proportion of basal plate specimens even from preterm placentas do not demonstrate bacteria. The lack of significance between term and preterm placentas on the presence intracellular bacteria does not discount the potential pathologic effect of basal plate colonization as a contributor to PTB but raises multiple questions about how host responses to colonization by bacteria. Molecular techniques for detection of bacterial DNA in tissues have shown that bacterial culture methods underestimate the prevalence of bacteria in placentas and amniotic fluids and are themselves limited by their ability to amplify DNA40. Recently, it has been shown that species-specific RNA transcriptome signatures allow rapid identification of pathogens41. It has also been suggested that deep sequencing analysis would allow for the comprehensive characterization of the microbiome of a given body site such as the basal plate as well as detection of low-prevalence species42. Such sophisticated analyses are warranted in the future to definitively identify the basal plate bacterial species. Further investigations will collectively determine the outcome from the presence of intracellular bacteria as well as host-responses to the bacteria within cells in the basal plate.
In conclusion, we report the novel finding of intracellular bacteria in the basal plate in 27% of placental specimens. Our findings suggest that routine placental pathological examination would be optimized by expanding analyses to include a search for bacteria in this important area of maternal fetal interface.
Acknowledgments
We thank Fredrick Kraus MD and Phyllis Huettner MD for their expertise and assistance with histopathology interpretation. WIHSC was funded by grants from the Washington University ICTS (NIH UL1 RR024992) and the Children’s Discovery Institute of St. Louis Children’s Hospital.
Financial Support: Dr. Mysorekar is supported by a grant from the Burroughs Wellcome Fund. Dr. Stout is supported by NICHD T32 (5 T32 HD055172-02) and Washington University CTSA grant (UL1 RR024992)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human Subjects Approval: Washington University Institutional Review Board #09-1807; 11/30/2011
Disclosure: None of the authors have conflict of interest.
References
- 1.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. American Journal of Obstetrics and Gynecology. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Schaudinn C, Kusanovic JP, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135 e1–135 e5. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schieve LA, Handler A, Hershow R, Persky V, Davis F. Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome. American journal of public health. 1994;84:405–410. doi: 10.2105/ajph.84.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth Project: placental histology in recurrent spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2006;195:792–796. doi: 10.1016/j.ajog.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal Fetal Medicine Units. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487–493. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 8.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD000262.pub3. CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leviton A, Allred EN, Kuban KC, et al. Microbiologic and histologic characteristics of the extremely preterm infant's placenta predict white matter damage and later cerebral palsy the ELGAN study. Pediatric research. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fichorova RN, Onderdonk AB, Yamamoto H, et al. Maternal microbe specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio. 2011;2:e00280–e00310. doi: 10.1128/mBio.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayhew TM. Stereology and the placenta: where's the point? -- a review. Placenta. 2006;27(Suppl A):S17–S25. doi: 10.1016/j.placenta.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Mayhew TM. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta. 2008;29:1–14. doi: 10.1016/j.placenta.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195:643–650. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Chaiworapongsa T, Gomez R, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 18.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American journal of epidemiology. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 19.Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 20.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. American Journal of Obstetrics and Gynecology. 2008;198:110 e1–110 e7. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 22.Roberts DJ, Celi AC, Riley LE, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7:e31819. doi: 10.1371/journal.pone.0031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strunk T, Doherty D, Jacques A, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012;129:e134–e141. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Romero R, Dong Z, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928–938. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Tarquini F, Romero R, et al. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. American journal of reproductive immunology. 2012;67:184–197. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo TA, Stapleton A, Wenderoth S, Hooton TM, Stamm WE. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. The Journal of infectious diseases. 1995;172:440–445. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 27.Brauner A, Jacobson SH, Kuhn I. Urinary Escherichia coli causing recurrent infections--a prospective follow-up of biochemical phenotypes. Clin Nephrol. 1992;38:318–323. [PubMed] [Google Scholar]
- 28.Foxman B, Zhang L, Tallman P, et al. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. The Journal of infectious diseases. 1995;172:1536–1541. doi: 10.1093/infdis/172.6.1536. [DOI] [PubMed] [Google Scholar]
- 29.Elliott TS, Reed L, Slack RC, Bishop MC. Bacteriology and ultrastructure of the bladder in patients with urinary tract infections. J Infect. 1985;11:191–199. doi: 10.1016/s0163-4453(85)92997-4. [DOI] [PubMed] [Google Scholar]
- 30.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Conner M, Goepfert AR. Endometrial microbial colonization and plasma cell endometritis after spontaneous or indicated preterm versus term delivery. American Journal of Obstetrics and Gynecology. 2005;193:739–745. doi: 10.1016/j.ajog.2005.02.128. [DOI] [PubMed] [Google Scholar]
- 31.Onderdonk AB, Hecht JL, McElrath TF, Delaney ML, Allred EN, Leviton A. Colonization of second-trimester placenta parenchyma. American Journal of Obstetrics and Gynecology. 2008;199:52 e1–52 e10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr-Hill RA, Hall MH. The repetition of spontaneous preterm labour. Br J Obstet Gynaecol. 1985;92:921–928. doi: 10.1111/j.1471-0528.1985.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev. 2002;60:S19–S25. doi: 10.1301/00296640260130696. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeldovich VB, Robbins JR, Kapidzic M, Lauer P, Bakardjiev AI. Invasive extravillous trophoblasts restrict intracellular growth and spread of Listeria monocytogenes. PLoS Pathog. 2011;7:e1002005. doi: 10.1371/journal.ppat.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lecuit M, Nelson DM, Smith SD, et al. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6152–6157. doi: 10.1073/pnas.0401434101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu XR, Sun TT, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Hecht JL, Onderdonk A, Delaney M, et al. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr Dev Pathol. 2008;11:15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 40.Jones HE, Harris KA, Azizia M, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4:e8205. doi: 10.1371/journal.pone.0008205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barczak AK, Gomez JE, Kaufmann BB, et al. RNA signatures allow rapid identification of pathogens and antibiotic susceptibilities. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6217–6222. doi: 10.1073/pnas.1119540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jefferson KK. The bacterial etiology of preterm birth. Adv Appl Microbiol. 2012;80:1–22. doi: 10.1016/B978-0-12-394381-1.00001-5. [DOI] [PubMed] [Google Scholar]