Abstract
Background
Posttraumatic stress disorder (PTSD) is a prevalent psychiatric disorder precipitated by exposure to extreme traumatic stress. Yet, most individuals exposed to traumatic stress do not develop PTSD and may be considered psychologically resilient. The neural circuits involved in susceptibility or resiliency to PTSD remain unclear, but clinical evidence implicates changes in the noradrenergic system.
Methods
An animal model of PTSD called Traumatic Experience with Reminders of Stress (TERS) was developed by exposing C57BL/6 mice to a single shock (2mA, 10sec) followed by exposure to six contextual1-minute reminders of the shock overa 25-dayperiod. Acoustic startle response (ASR) testing before the shock and after the last reminder allowed experimenters to separate the shocked mice into two cohorts: mice that developed a greatly increased ASR (TERS-susceptible mice) and mice that did not (TERS-resilient mice).
Results
Aggressive and social behavioral correlates of PTSD increased in TERS-susceptible mice but not in TERS-resilient mice or control mice. Characterization of c-Fos expression in stress-related brain regions revealed that TERS-susceptible and TERS-resilient mice displayed divergent brain activation following swim stress compared with control mice. Pharmacological activation of noradrenergic inhibitory autoreceptors or blockade of postsynaptic α1-adrenoreceptors normalized ASR, aggression, and social interaction in TERS-susceptible mice. The TERS-resilient, but not TERS-susceptible, mice showed a trend toward decreased behavioral responsiveness to noradrenergic autoreceptor blockade compared with control mice.
Conclusions
These data implicate the noradrenergic system as a possible site of pathological and perhaps also adaptive plasticity in response to traumatic stress.
Keywords: Amygdala, bed nucleus of stria terminalis, clonidine, locus coeruleus, norepinephrine, posttraumatic stress disorder, prazosin, resilience, stress, ventral tegmental area
The extreme psychological stress of severe trauma leads to posttraumatic stress disorder (PTSD) in susceptible persons. Others are resilient despite exposure to qualitatively and quantitatively similar trauma. Understanding the neurobiology of susceptibility and resiliency could lead to improved interventions to treat and even prevent PTSD (1–3). Although characteristics of the neural circuitry that determines PTSD susceptibility or resilience are unclear, increasing clinical evidence suggests brain noradrenergic system involvement in PTSD neurobiology (4–6). In persons with PTSD, pharmacological reduction of brain noradrenergic outflow (7–9) or responsiveness at postsynaptic α1-adrenoreceptors (ARs) (10–12) reduces some PTSD symptoms. In contrast, pharmacological enhancement of brain noradrenergic outflow precipitates PTSD symptoms in individuals with the disorder (6,13).
A better understanding of the neurobiology of individual differences in response to traumatic stress can be gained by examining animal models of PTSD (14). Pynoos et al. (15) developed a model of PTSD in which mice were exposed to a single footshock followed by contextual reminders of the shock. These weekly, brief situational reminders (SRs) phenomenologically mimicked the episodes of re-experiencing, which are characteristic of PTSD in people, and perpetuated the adverse effects of the shock (15). Our laboratory has refined and expanded this model by adding a baseline acoustic startle response (ASR) test along with posttests that measure behavioral correlates of PTSD symptoms. We refer to this model as traumatic experience with reminders of stress (TERS). Here, we demonstrate that susceptible and resilient mice from an inbred strain can be identified by measuring ASR before and after exposure to the TERS paradigm. In addition to an increase in ASR, TERS-susceptible mice develop other behavioral correlates of PTSD symptoms, whereas TERS-resilient mice behave similar to a no-shock control group on these tests. The TERS-susceptible and TERS-resilient mice may undergo different neurobiological adaptations following the shock, as they show different neural activation patterns in response to stress. We also report that the behavioral sequelae of PTSD we observe in TERS-susceptible mice can be normalized by pharmacologically blocking noradrenergic outflow or postsynaptic α1-ARs, suggesting that increased noradrenergic receptor activation may contribute to the behavioral correlates of PTSD symptoms we observe in TERS-susceptible mice.
Methods and Materials
Animals
A total of 615 adult male C57BL/6CRL mice (Charles River Laboratories, Hollister, California) were used for experiments. Mice were maintained under a 12-hour light/dark cycle with ad libitum access to food and water. Six-week-old mice were group-housed for at least 1 week after arrival. To avoid the confounding effects of aggressive behavior previously reported by researchers using a similar protocol (15), mice were single-housed at least 1 week before the first ASR test and remained so for the duration of experiments. All experiments were conducted in accordance with the guidelines of the Veterans Administration Puget Sound Health Care System Institutional Animal Care and Use Committee.
The TERS Paradigm
Mice were subjected to a single brief shock followed by six SRs (Figure 1). The day before TERS conditioning, mice were given an ASR pretest to determine baseline ASR. Control and shocked groups were balanced so that the average baseline ASRs of each group were similar at the start of the experiment. The day after the ASR pretest, mice were exposed to the shock apparatus, which consisted of two chambers separated by a remotely moveable door (Gemini Avoidance System, San Diego Instruments, San Diego, California). One chamber was brightly lit with an odorant that served as an additional contextual cue. The other chamber was dark with no odorant. Each mouse in the shocked group was placed in the well-lit chamber and allowed to acclimate to the environment (1.5 min). The door to the dark chamber was then opened remotely. When the mouse entered the dark chamber, the door was shut and the mouse was administered a footshock (2 mA, 10 sec) and then returned to its home cage. On the fourth day after the shock, mice were exposed to the first SR, during which each mouse was restricted to the light chamber for 1 minute. Situational reminders were repeated five times every following fourth day for six total SRs. The day after the last SR, mice were given a second ASR test (posttest 1). Mice in behavioral experiments then underwent behavioral testing over the course of about a month. To demonstrate persistence of behavioral changes, some mice underwent a third ASR test (posttest 2) 2 months after the shock. Control mice received the same treatment as shocked mice, including SRs, but were not shocked during their initial exposure to the box.
Figure 1.
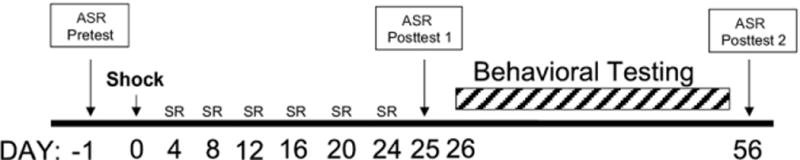
Time line of the traumatic experience with reminders of stress protocol. Mice undergo baseline acoustic startle response (ASR) testing and shock and no-shock groups are balanced so the average baseline ASR of the two groups does not differ. The following day, mice are exposed to the shock box. On the fourth day after the shock and every following fourth day for a total of six exposures, mice were exposed to the situational reminder, during which each mouse was restricted to the light chamber of the box for 1 minute. The day after the last situational reminder or 25 days after the shock, mice were subjected to a second ASR test (posttest 1). For about a month after posttest 1, mice in behavioral experiments underwent behavioral testing. In c-Fos experiments, mice were euthanized 3 days after ASR posttest 1. To demonstrate persistence of behavioral changes, some mice underwent a third ASR test (posttest2) 56 days or 2 months after the shock. SR, situational reminder.
Differentiation of TERS-Resilient and TERS-Susceptible Mice
The TERS-exposed mice were separated into cohorts: TERS-susceptible individuals (or mice that develop a PTSD-like syndrome) and TERS-resilient individuals (or mice that do not develop a PTSD-like syndrome). The TERS-susceptible mice were defined as those whose percentage increase in ASR between the ASR pretest and posttest 1 was greater than 70% (about 45.7% of shocked mice). The TERS-resilient mice were defined as those whose percentage change in ASR between the ASR pretest and posttest 1 was less than 30% (about 40.3% of shocked mice). Mice whose percentage change in ASR was between 30% and 70% (about 14% of shocked mice) displayed a mixed behavioral phenotype and were excluded from the study. The proportion of susceptible, resilient, and excluded mice varied across experiments.
Behavioral Protocols
Acoustic Startle Response
Acoustic startle response was measured using four startle chambers (SR-Lab System, San Diego Instruments). Each chamber included a ventilated, sound-attenuated cabinet with a speaker and contained a cylindrical Plexiglas enclosure with a piezoelectric accelerometer to detect movement. Chambers were regularly calibrated for both sensitivity to movement and sound level to ensure consistency between chambers and experiments. Testing took place 3 to 7 hours following the onset of light cycle. Mice were acclimated to the testing room for a minimum of 1 hour before ASR testing. Each ASR session began with a 5-minute acclimation period, during which 65 dBB white noise was played. Trials consisted of 10 consecutive presentations of a 40-millisecond, 100 dB white noise stimulus. Startle stimuli were presented for an average of 15 seconds at intervals of 21, 7, 20, 9, 14, 21, 11, 8, and 23 seconds. Interstimulus intervals were varied in this way to avoid anticipatory compensation by the mouse. Acoustic startle response data were calculated by averaging responses over the 10 trials. Between tests, each chamber was cleaned thoroughly. Mice with pretest ASR scores over 405 mV were not included because a high ASR pretest made it difficult to accurately classify them into susceptible and resilient groups by the percentage change in startle. For pharmacological experiments, mice underwent posttest 1, as described in the TERS protocol. After 3 days of rest, mice received an injection of saline, prazosin, or clonidine and were then given another ASR test. The ASR protocol was modified from methods previously described (16).
Locomotion during Sixth SR
Locomotor behavior during the last SR was quantified by dividing the chamber into quadrants. Movement from one quadrant to another was considered one locomotor count.
Light-Dark Box Test
Locomotor behavior during the light-dark box (LD) test was scored by a blinded observer as previously described (17). Mice were placed in the light side of a two-chambered box with one light chamber and one dark chamber. Mice were observed for 5 minutes, and the time spent in either chamber was recorded.
Resident Intruder
Resident intruder (RI) testing was conducted as previously described (18). Mice underwent three RI trials (up to 5 min, data are from third trial) over 3 days, during which a stress-naïve group-housed intruder mouse of equal or slightly lesser weight was introduced into the resident’s home cage. The trial was terminated if a mouse vocalized or if wrestling continued for more than 5 seconds, as these were indicators of attack biting that could cause injury.
Social Interaction
Mice were acclimated to the social interaction (SI) arena (circular, 1-m diameter) for 10 minutes a day over 2 days. On the third day, mice were placed in the arena with a stress-naïve group-housed companion mouse of equal or slightly lesser size that was marked to distinguish it from the experimental mouse. Mice were videotaped for 7 minutes. Total interaction time was recorded by SMART Video track system (San Diego Instruments) and a blinded observer. Trials during which aggressive interactions occurred, although rare, were discarded.
Drug Administration
For ASR, SI, and RI testing, mice were administered saline, prazosin, or clonidine 30 minutes before testing. A unique cohort of mice was used for each drug condition.
Neuroanatomical Analysis
Activation of stress-related brain regions was assessed by quantitating c-Fos expression in the central nucleus of the amygdala (CeA); the basal nucleus of the amygdala; the lateral nucleus of the amygdala; medial nucleus of the amygdala; the bed nucleus of the stria terminalis (BNST), ventral portion (vBNST); ventral tegmental area (VTA); and the locus coeruleus (LC), following a 15-minute forced swim stress. Three days after the last SR, mice were placed in a bucket of water (30°C) and forced to swim for 15 minutes. Mice were then removed from the water, towel dried, and returned to their home cage. One hour after the termination of the swim, mice were euthanized with an overdose of pentobarbital and perfused transcardially with cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in .1 mol/L phosphate buffer. Brains were removed and postfixed for 12 hours, followed by cryoprotection in 30% sucrose for3days. Brains were then cut coronally on a cryostat and stored in PBS with .05% sodium azide. Expression of c-Fos was detected using immunohistochemical techniques as previously described (19–21). Sections were washed in PBS and incubated in 3% hydrogen peroxide for 30 minutes, washed again, incubated in 3% normal goat serum for 1 hour, and then incubated in primary antibody (anti-c-Fos polyclonal, 1:2000) (H-125, #SC-7202, Santa Cruz Biotechnology, Santa Cruz, California) for 12hoursatroom temperature. Sections were washed and incubated in secondary antibody (biotinylated anti-rabbit, 1:200) (Vector Laboratories, Burlingame, California) for 1 hour and then washed again. Staining was visualized with Vector Laboratories ABC Kit, followed by incubation in diaminobenzidine (Sigma, St. Louis, Missouri). Sections from each region of interest were mounted in DPX Mountant for Histology (Sigma, St. Louis, Missouri), visualized on a Nikon Eclipse E-800 light microscope (Nikon Instruments, Melville, New York), and photographed using a QImaging Retiga 1300i digital camera (Q Imaging, Surrey, British Columbia, Canada). Images were then anatomically matched using two to four landmarks. An identical template demarcating the area of interest and a grid were laid over each image. Positive cells in each grid square were counted by a blinded investigator. Data constitute the sum of all grid square counts contained within the demarcated region.
Data Analysis
Data were analyzed using one-way analysis of variance (ANOVA) and post hoc testing was performed using Dunnett’s multiple comparison test, unless otherwise specified. Resident intruder data were analyzed by Pearson χ2 test.
Results
ASR in TERS-Exposed Mice
A shock followed by SRs has been demonstrated to increase the ASR in both mice (15) and rats (16). Thus, we hypothesized that mice exposed to the TERS protocol would develop an elevated ASR. To better characterize the change in ASR over the course of TERS treatment, we assessed baseline ASR in all mice 1 day before the shock (pretest) and 24 hours after the last SR (posttest 1). Shocked mice showed a higher ASR during the posttest than control mice (control n = 30, shocked n = 43, p < .05). However, the ASR of shocked mice during the posttest was variable. Some mice developed an increased ASR, whereas other mice showed a decrease below their baseline. We posited that these nonresponsive mice might be displaying an adaptive phenotype that could translate to other behavioral correlates of PTSD symptoms. To test this, shocked mice were separated into two cohorts based on individual change in ASR: a TERS-resilient group, which included mice that showed an increase in ASR of 30% or less, and aTERS-susceptible group, which included mice that showed an increase in ASR of 70% or more. Using ASR to separate mice into susceptible and resilient groups incorporates a number of desirable attributes: 1) it is a quantitative trait objectively measured by computer; 2) people with PTSD manifest exaggerated startle response; and 3) mice meeting this criterion also exhibit a PTSD-like profile in other behavioral assays (see below). The results of an ASR time-course study can be seen in Figure 2. When mice were separated into cohorts, differences among groups were observed [F(2,65) = 13.47, p < .0001; Figure 2]. Post hoc testing showed that TERS-susceptible mice showed a higher ASR during the posttest compared with control mice (p < .0001), whereas TERS-resilient mice did not differ from control mice. To determine if this difference in ASR persisted, mice were tested again 2 months after the shock. The TERS-susceptible mice maintained an elevated ASR compared with control mice at 2 months after the shock and 1 month after the last SR [F(2,65) = 4.425, p < .05; Figure 2]. In addition, we found that ASR in TERS-susceptible mice increased in an SR exposure-dependent manner (Figure S1 in Supplement 1).
Figure 2.
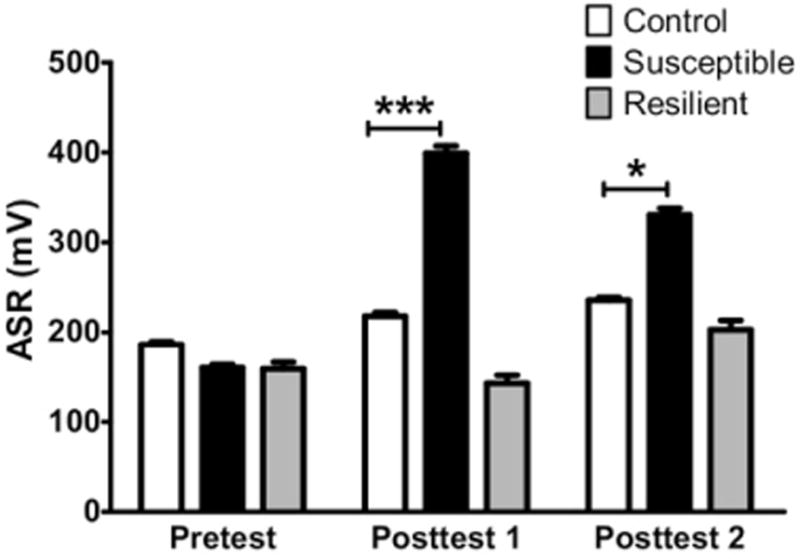
Traumatic experience with reminders of stress (TERS)-susceptible mice exhibit elevation of acoustic startle response (ASR) that persists for up to 2 months after the shock. After undergoing exposure to the TERS protocol, TERS-susceptible mice (black bars, n = 25) show a significantly higher ASR than control mice (white bars, n = 30) during posttest 1, almost 1 month following the shock. The TERS-resilient mice (gray bars, n = 13) did not differ from control mice. An additional startle test was performed at 2 months postshock to determine if the startle effect persisted over time. The TERS-susceptible mice showed a higher ASR than control mice during posttest 2 (2 months after the shock), whereas TERS-resilient mice did not differ from control mice. ***p < .001; *p < .05.
TERS-Resilient Phenotype Is Not due to Extinction of Fear Response
The nonresponsiveness of TERS-resilient mice could be due to factors unrelated to positive adaptive change and therefore not indicative of a resilient phenotype. For example, it is possible that TERS-resilient mice do not feel the shock as much as susceptible mice, that they forget the shock, or that they extinguish their fear response during the SR period. To test this possibility, fear responses in TERS-exposed and control mice were examined by monitoring locomotion during the sixth SR and by assessing their responses on the light-dark box test. A significant decrease in locomotor activity in TERS-susceptible and TERS-resilient mice was observed (p < .0001) compared with control mice during the last SR [ANOVA F(2,54) = 19.03, p < .0001; Figure 3A]. Qualitatively, the behavior of the TERS-exposed mice was very different from that of control mice. Control mice freely explored the SR chamber, whereas most TERS-susceptible and TERS-resilient mice remained immobile in a corner, with the exception of a few mice that jumped, an escape behavior. Several shocked mice displayed piloerection, indicating sympathetic nervous system activation.
Figure 3.
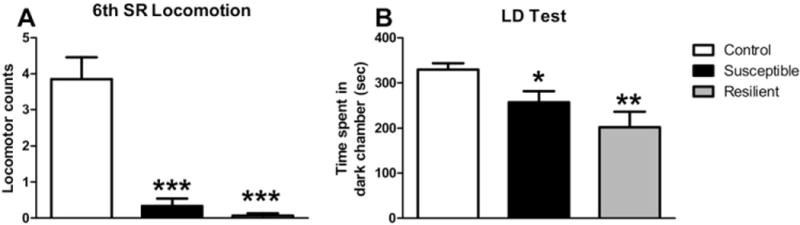
Traumatic experience with reminders of stress (TERS)-exposed mice do not extinguish fear response. We assessed the conditioned fear response in TERS-susceptible (black bars), TERS-resilient (gray bars), and control (white bars) mice by quantifying freezing behavior during the last situational reminder (SR) and by measuring avoidance of an environment similar to the shock chamber. (A) We indirectly measured freezing behavior in TERS-exposed mice by quantifying locomotor activity during the sixth SR. Both TERS-susceptible (n = 15) and TERS-resilient (n = 15) mice showed a significant decrease in locomotor activation during the last SR compared with control mice (n = 27). Experimenter observation confirmed that mice were engaged in freezing behavior during the SR, often huddled in the corner opposite the door to the shock chamber. (B) Mice were exposed to a light-dark box, a rectangular apparatus divided into a light and a dark chamber and therefore similar to the shock box. Both TERS-susceptible (n = 29) and TERS-resilient (n = 14) mice spent less time in the dark chamber than control mice (n = 27). *p < .05;**p < .01; ***p < .001. LD, light-dark box.
We used the LD test as a second measure of fear response. The LD apparatus is similar to that of the shock box in that it consists of two chambers, one light and one dark. However, its location in the testing room, dimensions, and smell differed from the shock apparatus. Mice were placed in the light chamber of the LD apparatus and time spent in either chamber was recorded. Analysis of variance demonstrated a difference among groups [F(2,67) = 6.632, p < .0024; Figure 3B]. Both TERS-resilient (p < .01) and TERS-susceptible (p < .05) mice spent less time in the dark chamber than control mice. Taken together, these data indicate that both TERS-resilient and TERS-susceptible mice maintain a robust fear response for over a month after the shock.
TERS-Susceptible Mice Show Increased Aggressive Behavior Compared with Control Mice
We next sought to assess other behavioral correlates of PTSD symptoms. People with PTSD can manifest both increased aggressive behavior and social withdrawal (22). To quantify aggressive behavior in TERS-exposed mice, mice underwent the RI test. To determine if mice showed an increased propensity toward extreme aggression (prolonged wrestling and attack bites), we quantified how many mice in each group were separated before the end of the 5-minute testing period. Compared with control mice, more TERS-susceptible mice engaged in violent attacks that required separation before the end of the testing period, whereas TERS-resilient mice did not differ from control mice (Pearson χ2 = 8.009, df = 2, p < .0182; Figure 4A).
Figure 4.
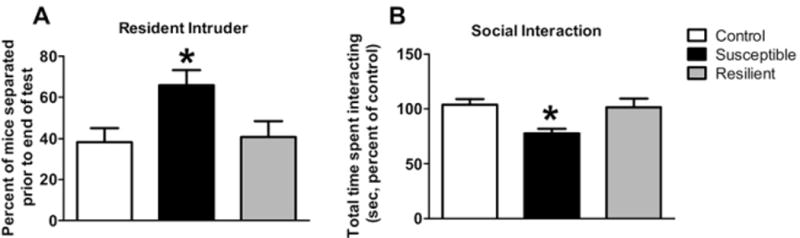
Traumatic experience with reminders of stress (TERS)-susceptible mice show other behavioral correlates of posttraumatic stress disorder symptoms. (A) We assessed aggressive behavior in TERS-susceptible, TERS-resilient, and control mice using the resident-intruder test. To measure extreme aggression, we quantified the frequency of violent attacks that required separation to prevent physical injury before the end of the testing period. The TERS-susceptible mice (black bars) showed an increased frequency of violent attacks compared with control mice (white bars), whereas TERS-resilient mice (gray bars) did not (Pearson χ2 = 8.009, df = 2) *p < .0182. (B) We assessed social behavior in TERS-susceptible, TERS-resilient, and control mice using the social interaction test. The amount of time the experimental mouse spent interacting with a stress-naïve, group-housed, novel companion mouse in a neutral environment was quantified. The TERS-susceptible mice (black bars) showed decreased social interaction time compared with control mice (white bars), whereas TERS-resilient mice (gray bars) did not differ from control mice, *p < .05.
TERS-Susceptible Mice Show Decreased SI Compared with Control Mice
To examine social behavior, mice were assessed with the SI test while interacting with an unknown, stress-naïve companion mouse in a neutral environment. Time spent interacting differed significantly among groups [F(2,46) = 3.762, p < .0307; Figure 4B]. Mice in the TERS-susceptible group spent less time interacting with the companion mouse than control mice (p < .05), whereas TERS-resilient mice did not differ from control mice.
TERS-Resilient and TERS-Susceptible Mice Show Different Patterns of Neural Activation Following a Stressor
To determine if TERS-susceptible and TERS-resilient mice activate different neural circuits in response to a stress, we examined c-Fos activation in the brain regions implicated in the fear and/or stress response, including the LC, several nuclei of the amygdala, the vBNST, and the VTA following a swim stress. The TERS-susceptible mice showed an increase in c-Fos activation in LC, the CeA, and the VTA compared with control mice, whereas TERS-resilient mice did not [LC F(2,29) = 4.411, p < .0213; CeA F(2,29) = 4.310, p < .023; VTA F(2,19) = 7.001, p < .0053; Figure 5A–C]. However, TERS-resilient mice showed a significant increase in c-Fos activation in the basal nucleus of the amygdala compared with control mice, whereas activation in this region did not differ from control mice in TERS-susceptible mice [F(2,30) = 4.821, p < .0153]. Finally, TERS-resilient mice showed decreased activation in the vBNST compared with control mice, whereas TERS-susceptible mice did not differ from control mice in activation of this region [F(2,28) = 8.859, p < .001; Figure 5D]. No differences were observed between groups in the lateral nucleus of the amygdala or medial nucleus of the amygdala (Figure 5B) or in prefrontal cortex (data not shown).
Figure 5.
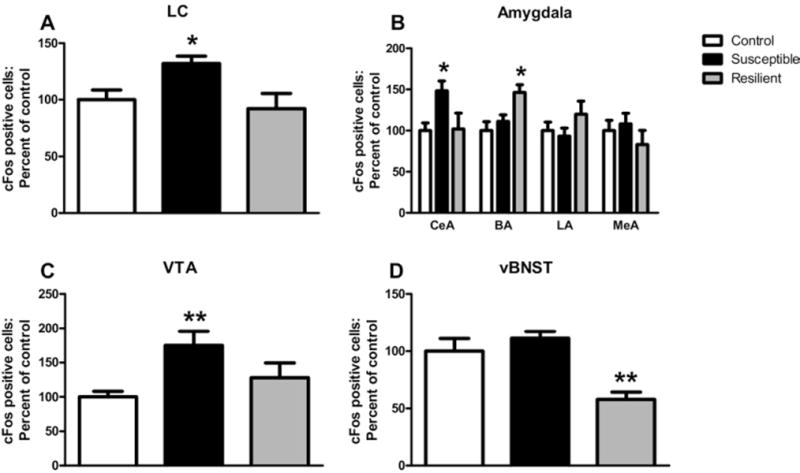
Traumatic experience with reminders of stress (TERS)-resilient and TERS-susceptible mice show different patterns of neural activation following a stressor. We measured c-Fos activation in stress-related brain regions following a 15-minute forced swim stressor in TERS-exposed and control mice. (A) The TERS-susceptible mice (black bars, n = 10), but not TERS-resilient mice (gray bars, n = 10), show elevated levels of c-Fos in the locus coeruleus compared with control mice (white bars, n = 12). (B) The TERS-susceptible mice show increased c-Fos activation in the central nucleus of the amygdala (n = 9–12), while TERS-resilient mice show increased levels of c-Fos in the basal nucleus of the amygdala (n = 8–14), compared with control mice. (C) The TERS-susceptible mice (n = 6), but not TERS-resilient mice (n = 5), show elevated levels of c-Fos in the ventral tegmental area compared with control mice (n = 11). (D) The TERS-resilient mice (n = 6), but not TERS-susceptible mice (n = 15), show decreased levels of c-Fos in the ventral portion of the bed nucleus of the stria terminalis compared with control mice (n = 10). *p < .05, **p < .01. BA, basal nucleus of the amygdala; CeA, central nucleus of the amygdala; LA, lateral nucleus of the amygdala; LC, locus coeruleus; MeA, medial nucleus of the amygdala; vBNST, ventral portion of the bed nucleus of the stria terminalis; VTA, ventral tegmental area.
The Role of Norepinephrine in the TERS-Susceptible Phenotype
Because we observed increased c-Fos activation in the LC, the largest noradrenergic nucleus in the brain, in TERS-susceptible mice, we hypothesized that increased noradrenergic activation may contribute to the behavioral sequelae of PTSD symptoms in these mice. Totestthis, we pharmacologically decreased noradrenergic activation in TERS-exposed and control mice and assessed their ASR, aggressive behavior, and SI.
Clonidine is an α2-AR agonist, which, at low doses, activates LC inhibitory auto-receptors and thereby decreases norepinephrine (NE) release. We assessed the efficacy of clonidine in normalizing the behavior of TERS-susceptible mice (Figure 6A). A treatment effect was observed in TERS-susceptible mice [F(3,130) = 19.28, p < .0001) but not in control or TERS-resilient mice. Clonidine decreased the ASR in TERS-susceptible mice at both .005 mg/kg (p < .05) and .01 mg/kg (p < .01).
Figure 6.
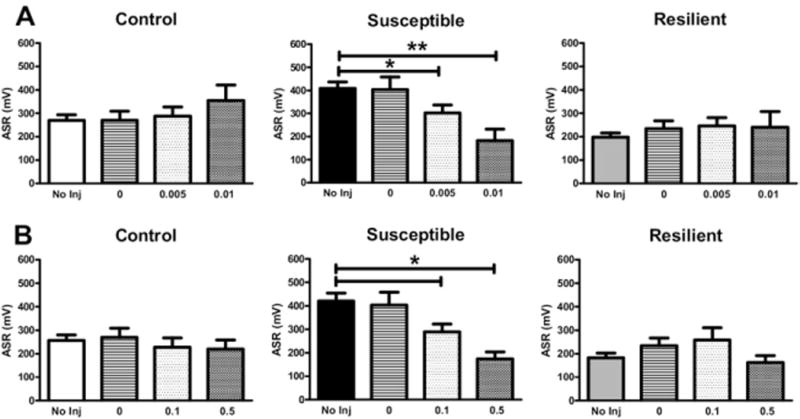
Administration of either clonidine or prazosin decreases the acoustic startle response (ASR) in traumatic experience with reminders of stress (TERS)-susceptible mice. (A) Pretreatment with clonidine (.005 or .01 mg/kg, subcutaneous), which activates noradrenergic α2-adrenoreceptor autoreceptors, significantly decreased the ASR in TERS-susceptible mice (n = 10–62) but had no effect on TERS-resilient (n = 10–58) or control (n = 11–57) mice at either dose tested. (B) Blockade of postsynaptic α1-adrenoreceptors via administration of the drug prazosin (.1 or .5 mg/kg, intraperitoneal) significantly decreased the ASR in TERS-susceptible mice (n = 6–47) but had no effect on TERS-resilient (n = 10–43) or control (n = 13–49) mice. *p < .05, **p < .01. inj, injection.
Multiple studies have demonstrated that prazosin is an effective treatment for trauma-related nightmares in PTSD (11,12,23), suggesting that increased signaling through the α1-AR may mediate some symptoms of PTSD. We assessed the ability of prazosin to normalize the behavior of TERS-susceptible mice (Figure 6B). A significant treatment effect was observed in TERS-susceptible mice [F(3,90) = 19.28, p < .05] but not in control or TERS-resilient mice. Prazosin decreased the ASR in TERS-susceptible mice at both .1 mg/kg (p < .05) and .5 mg/kg (p < .05).
The ability of clonidine and prazosin to normalize the hyperaggressive behavior in TERS-susceptible mice was also assessed. Upon injection of saline (a control; Figure 7A), TERS-exposed and control mice behaved similarly to their uninjected counterparts. That is, TERS-susceptible mice showed a greater propensity to attack during RI test (Pearson χ2 = 8.731, df = 2, p < .0127). However, after administration of either clonidine (.005 mg/kg; Figure 7B) or prazosin (.5 mg/kg; Figure 7C), this difference was no longer evident. We observed similar results in the SI test. As in uninjected mice, saline-injected TERS-susceptible mice showed a decrease in SI compared with control mice [F(2,65) = 4.044, p < .05; Figure 7D]. Administration of clonidine (.005 mg/kg; Figure 7E) or prazosin (.5 mg/kg; Figure 7F) eliminated this difference. These data suggest that increased noradrenergic activity, specifically signaling at the α1-AR, may lead to the increase in ASR and aggression and the decrease in SI observed in TERS-susceptible mice.
Figure 7.
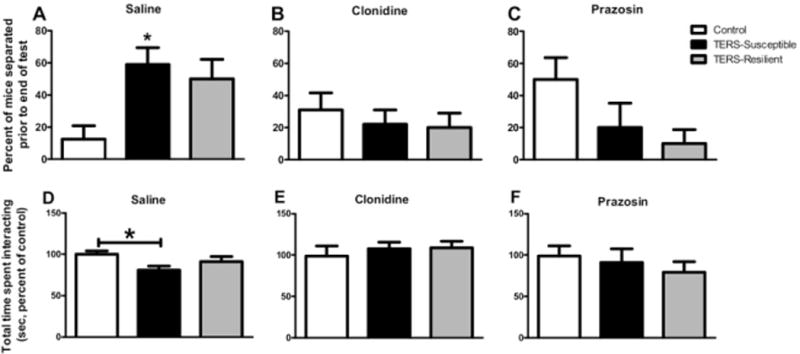
Administration of either clonidine or prazosin normalizes aggressive behavior in traumatic experience with reminders of stress (TERS)-susceptible mice. (A) Similar to uninjected TERS-susceptible mice (Figure 3A), TERS-susceptible mice (black bars, n = 22) treated with saline before the resident intruder test showed increased aggressive behavior compared with control mice (white bars, n = 16) treated with saline, whereas TERS-resilient mice (gray bars, n = 15) did not differ from control mice. *p < .05. In groups treated with either clonidine (B) (.005 mg/kg) or prazosin (C) (.5 mg/kg), neither TERS-susceptible (n = 6–22) nor TERS-resilient (n = 11–20) mice differed from control mice (n = 12–19). Neither drug affected the behavior of control mice. (D) Similar to uninjected TERS-susceptible mice (Figure 3B), TERS-susceptible mice (black bars, n = 26) treated with saline before the social interaction test showed decreased social interaction compared with control mice (white bars, n = 23) treated with saline, whereas TERS-resilient mice (gray bars, n = 19) did not differ from control mice *p < .05. In groups treated with either clonidine (E) (.005 mg/kg) or prazosin (F) (.5 mg/kg), neither TERS-susceptible (n = 6–22) nor TERS-resilient (n = 11–19) mice differed from control mice (n = 12). Neither drug affected the behavior of control mice.
Discussion
Why some individuals develop psychopathology while others maintain normal psychological functioning following exposure to psychological trauma is unknown. Animal models of PTSD may facilitate understanding of individual differences in response to stress. Here we describe the TERS mouse model of PTSD in which resilient and susceptible mice can be identified by differences in ASR. Mice that develop an increase in ASR (susceptible mice) also manifest other behavioral correlates of PTSD, including social withdrawal and increased aggression. By contrast, resilient mice behave similarly to control mice on most tests. The fact that TERS-resilient mice do not develop PTSD behavioral sequelae is not likely due to extinction or forgetting the trauma, as they maintain a robust fear response. Analysis of c-Fos expression suggests that TERS-susceptible and TERS-resilient mice manifest divergent activation of stress neural circuits upon exposure to a swim stress. Increased c-Fos expression in the LC in conjunction with results from pharmacological studies support the idea that increased noradrenergic outflow and α1-AR activation may underlie the behavioral changes observed in susceptible mice.
In developing the TERS model, we sought to emulate the clinical syndrome. Posttraumatic stress disorder is distinct from other psychiatric stress-related disorders because it persists for long periods (sometimes years to decades) and is characterized by symptoms from three distinct categories or clusters: 1) episodes of re-experiencing the traumatic event; 2) avoidance of trauma-associated stimuli and social withdrawal/emotional numbing; and 3) symptoms of hyperarousal, including outbursts of anger and an exaggerated startle response. In the TERS paradigm, SRs may phenomenologically mimic episodes of re-experiencing. To determine if mice manifest behavioral correlates of symptoms from the other two clusters, we assessed social interaction, ASR, and aggression in TERS-exposed mice. Although TERS-susceptible mice developed all of these behavioral sequelae of PTSD, we observed the strongest differences between groups in ASR, and we also found that ASR increased in an SR exposure-dependent manner (Figure S1 in Supplement 1). Startle testing has a number of advantages as a methodology for translational research. It can be objectively measured by a computer with great accuracy; it can be repeatedly tested to assess the efficaciousness of therapies or disease progression (24); and it is an endophenotype that can be measured in humans and mice that is mediated by conserved neural circuitry (25,26). Thus, ASR testing can serve as an integrative research tool, allowing more direct comparison between clinical and preclinical studies (26). For these reasons, we suggest that the use of ASR in the TERS paradigm greatly increases the utility of the model.
The neurobiological substrates of PTSD and resilience to traumatic stress remain unclear, but the noradrenergic system has been proposed as a substrate for stress vulnerability (27,28). Clinical studies demonstrate that PTSD patients manifest increased noradrenergic outflow and/or receptor responsiveness that may contribute to symptomatology (5–9,11,12,23,29–32). Similarly, TERS-susceptible mice may have elevated brain noradrenergic release due to increased LC activation during stress. Both clonidine and prazosin normalize the ASR and aggressive and social behaviors in these mice, further supporting this idea. We do not know which noradrenergic projection regions contribute to the behavioral correlates of PTSD symptoms in TERS-susceptible mice, but several of these regions may mediate stress vulnerability, including the BNST (33), the VTA (34), and the hippocampus (35). We found stress-induced c-Fos expression in TERS-susceptible and TERS-resilient mice to be divergent in a number of brain areas, including the vBNST, VTA, CeA, and LC. Presumably, prazosin and clonidine could exert their therapeutic effects in any or all of these regions or in other areas not included in our study, such as the hippocampus (35).
Adaptations in the noradrenergic system may also contribute to resilience (5–9,11,12,23,29–32). The TERS-resilient mice show a trend toward decreased responsiveness to increased noradrenergic outflow caused by yohimbine administration (Figure S4 in Supplement 1), supporting the idea that an attenuated noradrenergic response may play a role in resiliency. Further supporting this idea is the fact that TERS-resilient mice show decreased activation in the vBNST, a brain region that receives one of the densest noradrenergic innervations in the brain (36,37). Stress elevates NE release in the BNST (38), and activation of this brain region has been linked to increased ASR and anxiety-related behaviors (39,40). Blockade of NE receptors in this region has been shown to block the anxiogenic effects of stress (39). One possible mechanism by which TERS-resilient mice could moderate noradrenergic outflow in this and other regions is upregulation of neuropeptide Y and/or galanin activity. Both of these neuropeptides are co-expressed in noradrenergic neurons (41), can inhibit LC activity (42,43), and have been linked to resiliency in preclinical models of PTSD (3,17,33) and in the case of neuropeptide Y, in clinical studies as well (44,45).
Animal models of PTSD offer the opportunity to elucidate the neurobiological mechanisms that lead to psychopathology or resilience in response to traumatic stress (1). The data presented here suggest that the TERS model may be a valuable tool in understanding both of these complex processes and provide further preclinical evidence for the dysregulation of the noradrenergic system in the neuropathology of PTSD.
Supplementary Material
Acknowledgments
This material is based upon work supported by the Office of Research and Development Medical Research Service, Department of Veterans Affairs (VA). This work was supported by a VA Career Development Award (VGO), VA Research Enhancement Award Program Grant (MAR), a University of Washington Royalty Research Fund Grant (VGO), and the US National Institutes of Health, National Institute on Drug Abuse (T32 DA07278) (VAR).
We thank Jane Shofer, M.S., for her assistance with some of the statistical analyses.
Footnotes
Drs. Olson, Szot, Redila, and Hague and Ms. Rockett, Venkov, Tran, Reh, and DeFino report no biomedical financial interests or conflicts of interest. Dr. Peskind reports no conflict of interest. Dr. Peskind receives research support from Bristol-Myers Squibb, Lilly, and Novartis and is on the speakers bureau for Forest Labs and Novartis. Dr. Raskind reports no conflict of interest. Dr. Raskind receives research support from Elan/Johnson and Johnson, Bristol-Myers Squibb, Lilly, and Novartis and is on the speakers bureau for Forest Labs and Schering Plough/Merck.
Supplementary material cited in this article is available online.
References
- 1.Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 2.Yehuda R. Risk and resilience in posttraumatic stress disorder. J Clin Psychiatry. 2004;65(suppl 1):29–36. [PubMed] [Google Scholar]
- 3.Charney DS. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 4.Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 5.Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 6.Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, et al. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related post-traumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 7.Kinzie JD, Sack RL, Riley CM. The polysomnographic effects of clonidine on sleep disorders in posttraumatic stress disorder: A pilot study with Cambodian patients. J Nerv Ment Dis. 1994;182:585–587. doi: 10.1097/00005053-199410000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Kinzie JD, Leung P. Clonidine in Cambodian patients with post-traumatic stress disorder. J Nerv Ment Dis. 1989;177:546–550. doi: 10.1097/00005053-198909000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: The case for clonidine and prazosin. J Psychiatr Pract. 2007;13:72–78. doi: 10.1097/01.pra.0000265763.79753.c1. [DOI] [PubMed] [Google Scholar]
- 10.Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: A placebo-controlled study. Biol Psychiatry. 2008;63:629–632. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raskind MA, Peskind ER, Kanter ED, Petrie EC, RadantA, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 12.Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Southwick SM, Morgan CA, 3rd, Bremner AD, Grillon CG, Krystal JH, Nagy LM, Charney DS. Noradrenergic alterations in posttraumatic stress disorder. Ann N Y Acad Sci. 1997;821:125–141. doi: 10.1111/j.1749-6632.1997.tb48274.x. [DOI] [PubMed] [Google Scholar]
- 14.Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry. 1993;33:479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- 15.Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry. 1996;39:129–134. doi: 10.1016/0006-3223(95)00088-7. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen DD, Crites NJ, Burke BL. Acoustic startle amplitude predicts vulnerability to develop post-traumatic stress hyper-responsivity and associated plasma corticosterone changes in rats. Psychoneuroendocrinology. 2008;33:282–291. doi: 10.1016/j.psyneuen.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumstein LK, Crawley JN. Further characterization of a simple, automated exploratory model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1983;18:37–40. doi: 10.1016/0091-3057(83)90247-2. [DOI] [PubMed] [Google Scholar]
- 18.Gammie SC, Seasholtz AF, Stevenson SA. Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience. 2008;157:502–512. doi: 10.1016/j.neuroscience.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, et al. Regulation of drug reward by cAMP response element-binding protein: Evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- 21.Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: A report of 4 cases. J Clin Psychiatry. 2000;61:129–133. doi: 10.4088/jcp.v61n0208. [DOI] [PubMed] [Google Scholar]
- 24.Siegelaar SE, Olff M, Bour LJ, Veelo D, Zwinderman AH, van Bruggen G, et al. The auditory startle response in post-traumatic stress disorder. Exp Brain Res. 2006;174:1–6. doi: 10.1007/s00221-006-0413-y. [DOI] [PubMed] [Google Scholar]
- 25.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: A translational research perspective. Horm Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 27.Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 28.Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Yehuda R, Southwick S, Giller EL, Ma X, Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. J Nerv Ment Dis. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 30.McFall ME, Murburg MM, Ko GN, Veith RC. Autonomic responses to stress in Vietnam combat veterans with posttraumatic stress disorder. Biol Psychiatry. 1990;27:1165–1175. doi: 10.1016/0006-3223(90)90053-5. [DOI] [PubMed] [Google Scholar]
- 31.Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA. Neuroendocrine and psychophysiologic responses in PTSD: A symptom provocation study. Neuropsychopharmacology. 1999;21:40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 32.Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 33.Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 37.Moore RY, Bloom FE. Central catecholamine neuron systems: Anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 38.Ma S, Morilak DA. Norepinephrine release in medial amygdala facilitates activation of the hypothalamic-pituitary-adrenal axis in response to acute immobilisation stress. J Neuroendocrinol. 2005;17:22–28. doi: 10.1111/j.1365-2826.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- 39.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 40.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holets VR, Hokfelt T, Rokaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24:893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 42.Sevcik J, Finta EP, Illes P. Galanin receptors inhibit the spontaneous firing of locus coeruleus neurones and interact with mu-opioid receptors. Eur J Pharmacol. 1993;230:223–230. doi: 10.1016/0014-2999(93)90806-s. [DOI] [PubMed] [Google Scholar]
- 43.Illes P, Finta EP, Nieber K. Neuropeptide Y potentiates via Y2-receptors the inhibitory effect of noradrenaline in rat locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:546–548. doi: 10.1007/BF00173217. [DOI] [PubMed] [Google Scholar]
- 44.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: Relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Morgan CA, 3rd, Wang S, Southwick SM, Rasmusson A, Hazlett G, Hauger RL, Charney DS. Plasma neuropeptide-Y concentrations in humans exposed to military survival training. Biol Psychiatry. 2000;47:902–909. doi: 10.1016/s0006-3223(99)00239-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


