Introduction
Age-related hearing loss, presbycusis, is one of the most common ailments of the elderly, affecting approximately 1/2 of those over the age of 74 (Corso, 1982). In addition to peripheral hearing loss, presbycusis is associated with central processing deficits involving trouble processing complex signals (including speech) in noise (Bergman et al., 1976; Konkle et al., 1977; Harris & Reitz, 1985; Gordon-Salant & Fitzgibbons, 1993, 1995, 1997; Turner et al., 1995; Stuart & Phillips, 1996; Divenyi & Haupt, 1997a, b; Frisina & Frisina, 1997; Snell, 1997; Frisina, 2001).
While hearing aid technology has made it possible for many elderly individuals with presbycusis to maintain (or regain) communication late in life (Weinstein, 1996), only a small proportion of older adults (18-20%) with hearing loss even use hearing aids (Kochkin, 1992, 1997). One possible reason for this is the perception held by some elderly that hearing aids do not work (Willott et al., 2001). This perception is likely generated by the lack of short term improvements noted in communication by simply amplifying some aspects of the acoustic environment. Some research suggests that patients would gain greater benefit by wearing hearing aids for a longer period of time to allow the auditory system to acclimate to the new input (Silman et al., 1984; Gatehouse, 1992; Arlinger et al., 1996; Ponton, 1996; Robinson & Gatehouse, 1996; Palmer et al., 1998; Willott et al., 2000; Philibert et al., 2002). This is supported by the evidence that some of the largest benefits of hearing aids occur between 3-18 weeks post-fitting (Gatehouse, 1992).
While there is considerable evidence that plastic brain changes occur as a result of hearing aid use (Arlinger et al., 1996; Syka, 2002), there is little evidence concerning the prevalence, time-course, magnitude, or conditions under which such changes occur. The exact nature of any peripheral and/or central changes, their effects on acoustic-signal-processing, and how acoustic experience might alter this process remains to be determined.
Previous research has addressed this situation in an animal model by rearing laboratory mice with partial hearing loss in an augmented acoustic environment (AAE), defined as exposure to augmented/amplified levels of controlled acoustic stimulation (Turner & Willott, 1998). Such studies have reared mice in an AAE (usually a 200ms, 70 dB SPL broadband noise presented at a pulsing 2 Hz rate) for as little as 10 days in some studies to well over a year in others in order to investigate the effects of chronic, low-level acoustic stimulation on the degenerating cochlea and central auditory system (Jeskey & Willott, 2000; Turner & Willott, 1998; Willott & Turner, 1999; Willott et al., 2000, 2005, 2006; Willott & Bross, 2004; Willott, 2009, Willott, Vandenbosche, & Shimizu, 2010). That work has shown that if mice with progressive hearing loss are reared in an AAE, their hearing (as measured by behavior, hair cell counts, ABR thresholds, electrophysiological responses of cells in the inferior colliculus) can be improved significantly. In some cases the improvements in hearing over controls can be dramatic. For example, raising C57/Bl6 mice in an AAE for one year can lead to ABR thresholds 25-35 dB better than controls at many frequencies (12, 16 and 24 kHz; Willott & Turner, 1999). Recent studies have also explored AAE effects in middle-aged rats and showed that a 13-wk nightly exposure to AAE slowed age related hearing loss (Tanaka et al., 2009a). In addition, cats reared in an AAE immediately after intense noise exposure demonstrated less severe permanent hearing loss (Norena & Eggermont, 2005). A similar finding was recently described in chinchillas, showing that rearing in an AAE immediately after intense noise exposure led to greater recovery of ABR thresholds and better hair cell survival (Tanaka et al., 2009b). Similar, relatively long-term exposures to non-traumatic noise has been shown to alter tuning in auditory cortex neurons (Norena & Eggermont, 2005; Norena et al., 2006), suppress responsiveness of auditory cortex (Pienkowski & Eggermont, 2009; 2010a,b) and alter spontaneous activity of neurons, which might have implications for tinnitus (Norena & Eggermont, 2006).
The purpose of the current study was to determine whether degenerative processes in the aged cochlea and central auditory system could be altered in a manner similar to exercise or increased neural activity in other sensory/neural systems. While it is clear that an AAE can have major ameliorative effects on the auditory system of young-to-middle-aged mice with progressive sensorineural hearing loss (Turner & Willott, 1998; Willott & Turner, 1999; Willott et al., 2005), the effects of AAEs on aged animals have not been fully investigated. The current study begins to address this gap in the literature by assessing the effects of AAE exposure on the peripheral and central auditory system in aged male and female CBA mice.
Materials & Methods
Subjects
Male (N=14) and female (N=12) aged CBA/CaJ mice (22-23 months of age) were obtained from Harlan through a contract with the National Institute on Aging. CBA/CaJ mice have been used as a model for late-onset presbycusis as they hear normally for much of their lifespan and demonstrate progressive deterioration of auditory function relatively late in life (e.g., Li, 1992; May, Kimar, & Prosen, 2006). This pattern is analogous to what many humans experience, making the CBA/CaJ a popular choice for studies looking at the impacts of normal aging processes on the auditory system. The male and female mice arrived at the study site in two separate shipments separated by several months of time. Mice were randomly assigned to either AAE or control conditions. The expected lifespan of the CBA/CaJ mouse is around 24 months so these mice could be considered equivalent to the human 70-80 year old in terms of their relative age. Due to normal aging, two of the 14 males died during the course of the 6-wk experiment (1 AAE, 1 control) and 3 of the 12 females died (2 AAE, 1 control), leaving 12 males (6 AAE, 6 control) and 9 females (4 AAE, 5 control) to finish the experiment. Animal use was approved by the Southern Illinois University School of Medicine's Laboratory Animal Care and Use Committee and conformed to NIH guidelines and the Society for Neuroscience's Policy on the Use of Animals in Neuroscience Research.
General Procedures
Aged mice were either exposed to an AAE consisting of 6 weeks of low-level (70 dB SPL), broad-band noise stimulation (12 hours/night, 200 ms duration, 2/s rate) or normal vivarium conditions beginning at 22-23 months of age. The female mice in this study were considered to be of sufficient age to be “postmenopausal.” While there is variability between species, female mice gradually become acyclical and enter a non-reproductive, “postmenopausal” state between 12-20 months of age (Feicio, Nelson, & Finch, 1984). The broad-band noise AAE signal was created in Matlab, sampled at 96 kHz and played back to the animal via DVD through a RadioShack PA amplifier and high frequency Super Tweeter (model 40-1310). Mice were housed in a typical shoebox style polycarbondate cage with a wire mesh lid and the speaker sat in the middle top of the wire mesh lid and pointed into the cage. The AAE signal was calibrated to 70 dB SPL peak level directely below the speaker at typical mouse ear level using a Bruel & Kjaer ½” microphone (Type 4191) and Precision Sound Level Meter (Type 2203) fitted with a 1/3 octave filter set (Type 1616). Noise level within the cage varied by as much as 5-10 dB lower in the corners of the cage. For both the AAE signal and the behavioral testing, the 70 dB SPL peak level was calibrated by leaving the 1/3 octave filters out of the B&K meter and adjusting the amplifier gain of the acoustic output, such that the overall sound intensity level peaked at 70 dB SPL. While a broadband noise was played, due to characteristics of the speaker and amplifier used, most of the energy in the signal was likely in the 6-16 kHz range, with intensity levels on the low and high frequency side falling off until little energy was present below 4 kHz and above 24 kHz (estimated from previous measurements of the AAE signal using a similar speaker and amplifier.) AAE-treated and control mice were maintained in adjacent rooms in the vivarium. Routine measurements of background noise conditions in the vivarium indicate a background noise level below 43 dB SPL in the 4-24 kHz range, with additional energy in the low frequency range (below the range of mouse hearing) due to ventilation noise (<200 Hz). An automatic timer turned the AAE signal on at 7:30 pm and off at 7:30 am to coincide with the dark phase of the light/dark cycle in the vivarium. The effects of the treatment were assessed by measuring behavioral gap detection and prepulse inhibition (PPI), auditory brainstem response (ABR) thresholds, surface preparation cochleograms, and protein immunohistochemical analysis for GAD67 in inferior colliculus (IC) and primary auditory cortex (AI).
Auditory Brainstem Response (ABR) Thresholds
ABR thresholds for clicks and tone bursts at 5, 10, 20, and 40 kHz were obtained post AAE exposure to compare the degree of hearing loss between groups (3 ms duration, 1 ms rise/fall, 40 Hz presentation rate, 512 repetitions). Ketamine/xylazine anesthesia (50/9 mg/kg i.p.) was used during ABR threshold collection. Pretest ABR thresholds were planned but greater than expected mortality for the aged mice occurred under anesthesia, and given the difficulty in obtaining aged mice from the supplier the decision was made to discontinue pretest ABR thresholds. Intensity was stepped down in 10 dB steps from 85 dB until a repeatable threshold could be determined. In situations where a repeatable waveform was not detectable at 85 dB, an arbitrary ceiling threshold response of 90 dB was assigned. No single wave was used as the indicator wave for ABR threshold as robust responses would often be found at wave I, or wave V, but other waves (e.g., II/IV) were sometimes used. Relative to the human ABR, the small size of the mouse head results in changes in waveform due to very subtle differences in electrode placement. Tucker Davis Technologies (Alachua, FL) System III hardware and custom software written by Dr. Kenneth Hancock was used to obtain ABR thresholds. ABR thresholds were collected at the end of the experiment, within 1-2 weeks of the time of the final behavioral test. Presumably due to the same age-related instability under anesthesia seen in the pretest, animal stability under anesthesia was sometimes poor. As a result, we were only able to collect ABR thresholds from 6 AAE and 5 control males, and 3 AAE and 4 control females, thus limiting the statistical power of threshold measurements. ABR thresholds were determined by an experimenter who was blinded to the sex and AAE status of the animal.
Behavioral Testing
Behavioral testing was conducted before initiation of the AAE, and weekly thereafter for the 6 weeks of the AAE exposure. Testing was conducted with a 70 dB SPL peak level, broadband background noise on continuously (Hamilton-Kinder startle reflex hardware and software, Poway, CA). Control trials consisted of presentations of 20 ms, 110 dB SPL noise bursts, serving to elicit a startle reflex. On some trials, startle stimuli were presented alone and on other trials the startle stimulus was preceded by either a silent gap in the background or a prepulse stimulus. Gap trials included silent gaps ranging from 2-50 ms duration and prepulse trials consisted of a noise burst presented 5 or 10 dB above background. Each gap and prepulse stimulus was begun 100 ms before the onset of the startle stimulus. Gap and prepulse trials were collected in the same session. Behavioral testing was done in 10 blocks within a session, resulting in 10 replications of each gap/PPI trial condition. Each block included trials consisting of gap durations of 0, 2, 3, 4, 5, 10, 15, and 50 ms, and prepulse stimuli of 5 and 10 dB above background. A 20s mean variable inter-trial interval was used (range 15-25s). Startle only (control) trials were presented equally often as gap and prepulse trials throughout the approximately 45-min session, but were presented in a counterbalanced fashion. That is, in half of the trials the startle only trial preceded the gap or ppi trial, and the other half of the trials it followed. This counterbalancing helped control for order effects and habituation of the startle reflex within a test session. Responses to gap and prepulse trials are expressed as % response relative to startle only (control) trials. A response of 100% would suggest the presence of the gap or prepulse stimulus did not have an inhibitory effect on startle, while responses closer to zero were evidence of stronger inhibition. Responses were capped at 100% as the natural upper limit of the response (ceiling). The timing of the gap and prepulse stimuli used in the current study (50-100 ms lead times) are inconsistent with prepulse facilitation, a phenomenon whereby facilitation of the startle reflex can sometimes be observed when the preceding prepulse stimulus is within 10 ms of the startle stimulus (Ison, 2001). Because responses have a floor limit of 0%, no upper limit would result in a biased response greater than 100%. For example, in a condition where a prepulse or gap stimulus is not audible and does not inhibit the reflex, responses would be expected to vary around 100% of control startle only trials. While responses are not allowed to move below 0%, variability in the startle reflex often leads to individual trials where the relative response can be as high as 200%, 300%, or greater. Such outliers are due to the presence of a 0% lower limit and no upper limit, which biases non-responses to a prepulse cue to be greater than 100%. As expected, such capping occurred often in the 2ms condition, as would be expected, but rarely at longer gap durations or with prepulse conditions. Following is a count of the number of animals that had their data capped at 100% for each of the conditions: 2 ms gap (12/21), 3 ms gap (7/21), 4 ms gap (6/21), 5 ms gap (6/21), 10 ms gap (5/21), 15 ms gap (3/21), 50 ms gap (2/21), +5dB PPI (0/21), +10 dB PPI (2/21).
Surface Preparation Cochleograms
Cochleae were processed and surface preparation cochleograms were conducted as described in Viberg and Canlon (2004). Following post-test ABR thresholds, animals were transcardially perfused with phosphate buffered saline (PBS) and 4% paraformaldehyde (PF) in PBS (pH 7.4). Cochleae were immersed in 4% PF, the stapes was removed and a slit in the round window was made to gently circulate the fixative through the window with a pipette. The cochleae were maintained in the fixative for 1-2 hr then stored in 0.5% PF in PBS at 4°C and shipped to Sweden for further processing and analysis. Cochleae were given a blinded identification number such that all processing and analysis could be done without knowledge of the experimental condition or sex of the mouse. Cochleae were stained with phalloidin and dissected in half-turns in PBS. Each piece was further dissected in a drop of Citifluor AF1 (Agar Scientific) and as much as possible of the modiolus was removed. The pieces were placed separately on an 8-well microscopic slide (Histolab Products, Sweden) in a drop of Citifluor.
Hair cell loss was counted in the cochleae stained with phalloidin (TRITC, Molecular probes 1:80; 60 min). After several rinses in PBS the cochleae were dissected and placed on an 8-well slide as described in the previous section. The criterion for hair cell loss was scar formation. The hair cells were quantified using a Zeiss Axiovert light microscope with epifluorescence and a 40× oil objective. A 0.25-mm scale placed in the eyepiece was adjusted along the top of the pillar cells. The percent loss of hair cells for each 0.25 mm segment was calculated. Each 0.25 mm segment represents a certain percentage of the cochlear length depending on the total length. To make an average of the hair cell loss for cochleae of different lengths, the mm segments were converted into percent, and added into both 5% bins and bins corresponding to equal frequency steps for each cochlea. The average and SD for the hair cell loss were then calculated and plotted for each percent distance or frequency, as was suggested by Wang, Hirose, & Liberman (2002). Cochleograms were done on one ear from each animal. The ear determined to be in better shape by gross inspection (i.e., damaged the least by extraction) was used in the cochleograms. Cochleograms included 9 females (4 AAE, 5 control) and 12 males (6 AAE, 6 control).
Immunohistochemistry for GAD67 in AI and IC
Gad67 is a key marker for the production of gamma aminobutyric acid (GABA), which is the primary inhibitory neurotransmitter in the brain and plays a key role in experience-dependent plasticity (Caspary et al., 2008). Sections (30μm) were collected through the center of AI (Bregma -2.46mm, Paxinos & Franklin, 2001, Plate 51, to Bregma -2.70mm, Plate 53) and IC (Bregma -4.96, plate 72, to Bregma -5.20, plate 74). Free-floating sections were collected in ice-cold 0.1M PBS pH 7.4. Sections were incubated in blocking solution containing 10% goat serum and 3% BSA in PBS for 1 hr with agitation. Sections were transferred to an affinity purified polyclonal GAD67 rabbit antiserum (Santa Cruz) diluted 1:100 in blocking solution for 1 hr at room temperature and overnight at 4°C with agitation. Sections were labeled with a secondary biotinylated anti-rabbit immunoglobin diluted 1:200 in PBS using Vectastain ABC (Vector Laboratories). Peroxidase activity was visualized by exposing sections to 0.05% DAB and 0.0075%-0.001% hydrogen peroxide in PBS. Digital images of positively stained cells were captured using a CoolSnap digital camera interfaced with an Olympus light microscope that was attached to a computer with Scion Imaging software. Slides were blinded to the observer prior to acquisition of all measurements. Relative optical density measurements (ROD) were measured from 3 equal-sized fields of positively stained cells for the superficial and deep layers of AI, and the low, middle and high frequency regions of IC. Based on our previous tonotopic mapping studies in the mouse IC (e.g., Willott & Turner, 2000), we expect the low, middle and high frequency regions to roughly correspond to <12 kHz, 12-24 kHz, and >24 kHz. Values were standardized by subtracting the mean of 3 random background measurements taken from the ventricle visible on each section. Data files were imported to Excel spreadsheets for statistical analysis. Immunohistochemistry data represent means collected from 4 AAE females, 4 control females, 4 AAE males and 4 control males.
Results
ABR Thresholds and Cochleograms
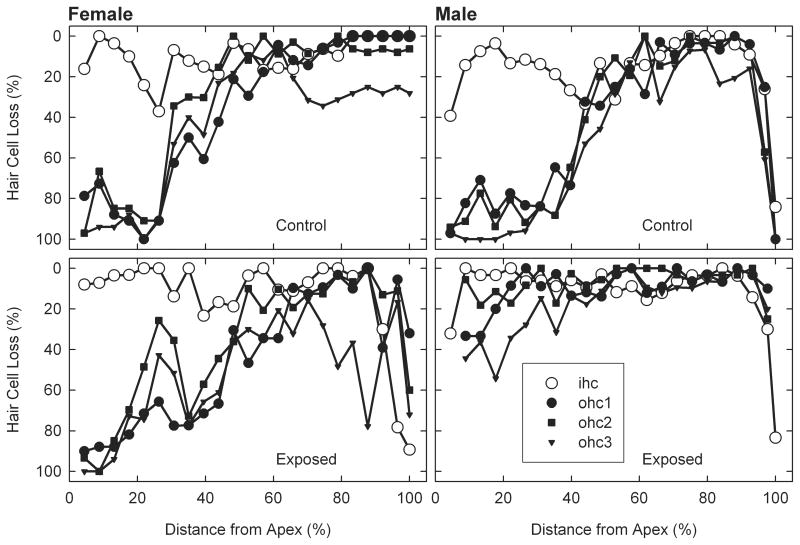
AAE treatment had a differential affect on ABR thresholds and cochlear hair cells in male and female aged mice. Males showed AAE-related improvements in both ABR thresholds (Figure 1) and hair cell counts (Figures 2 & 3) and females showed the opposite effect. The only frequency-specific ABR threshold differences were at 20 kHz in males, where AAE mice had significantly better thresholds than controls, t(9) p=0.02. However, trends were consistently present across nearly all frequencies and when averaged across ABR stimuli, thresholds were significantly worse in AAE exposed females relative to controls, t(5) p=0.03, and better in AAE exposed males relative to controls, t(9) p=0.04. As these results would predict, there was a significant Sex × Treatment interaction for BOTH ABR thresholds, F(1,14) = 8.82, p=0.01 (Figure 1), and for outer hair cell loss, F(1,17) = 4.09, p=0.05 (Figures 2 & 3), indicating that 6 weeks of AAE treatment was associated with greater outer hair cell loss in females but less loss in males, relative to controls. AAE had no significant effect on inner hair cell loss as the Treatment × Sex interaction was not significant for this measure, F(1,17) = 1.16, p=0.29. Mean ABR thresholds (averaged across ABR stimuli) were significantly worse in males controls than in female controls, t(7) p=0.001, suggesting a sex effect of aging alone on ABR thresholds irrespective of AAE treatment.
Figure 1.
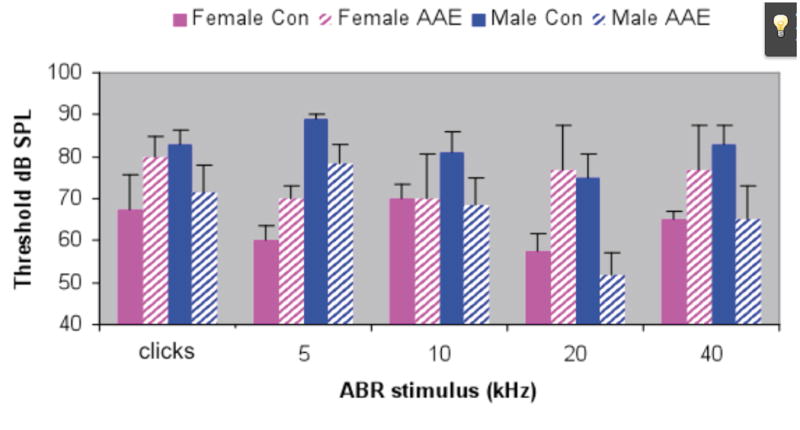
Auditory brainstem response (ABR) thresholds in aged male and female AAE treated and control mice. AAE treatment appeared to have opposite effects on male and female mice, making ABR thresholds better for AAE treated males (p=0.04) and worse for AAE treated females (p=0.03). Error bars = standard error of the mean.
Figure 2.
Outer and inner hair cell cochleograms as a function of location along the basilar membrane. Male AAE treated mice (bottom right panel) appear to have more outer hair cell savings than the Male Controls (upper right panel). This effect was especially prominent in more apical regions of the cochlea.
Figure 3.
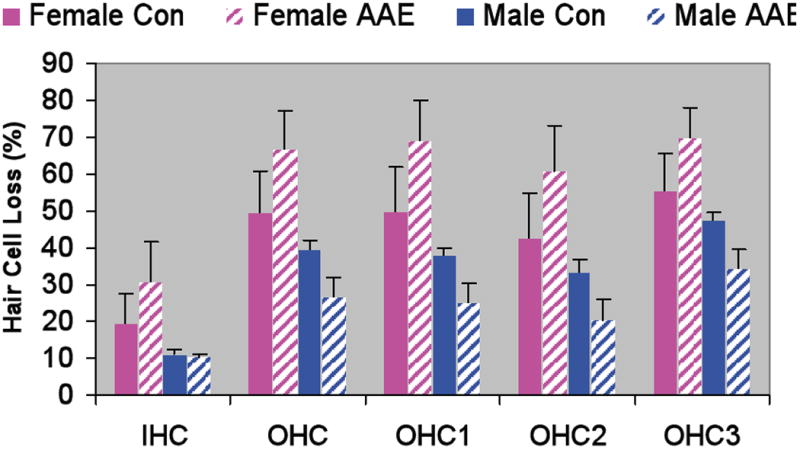
Cochleograms from male and female control and AAE-treated aged mice. AAE treatment also had a differential effect in male and female aged mice. Females given 6 wks of nightly AAE treatment showed greater outer hair cell loss whereas males given the same treatment showed less outer hair cell loss. Error bars = standard error of the mean.
Behavior
At the end of the 6 weeks of AAE exposure, neither gap detection responses nor PPI behavioral responses were significantly affected in either male or female mice (Figure 4). Startle amplitudes were also not significantly different between AAE and control animals in either males or females. While responses in males were generally better than females, no reliable AAE-related changes could be observed. There was in interesting trend for female AAE-treated mice to show worse gap and PPI responses relative to their controls. However, the relatively small sample size and inherent variability of behavior made it difficult to obtain enough statistical power to observe anything but very robust effects.
Figure 4.
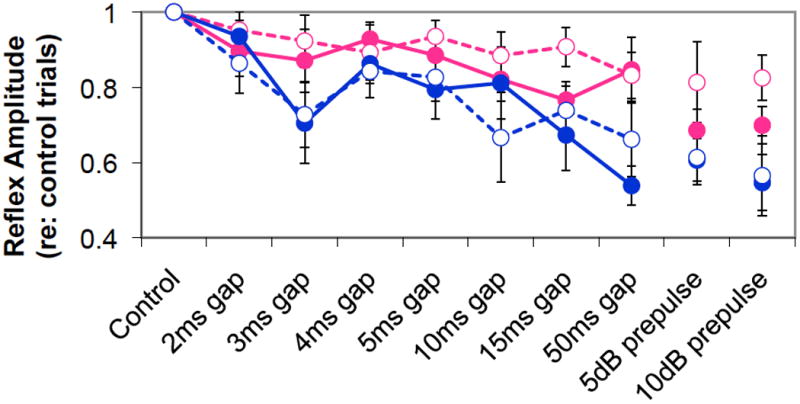
Behavioral gap detection and prepulse inhibition data from male and female control and AAE-treated aged mice at the end of the 6-week AAE exposure. While there were some interesting trends, especially for PPI, no significant changes related to sex or AAE treatment were found. (Error bars not included to minimize complexity of figure.)
GAD67 Neurochemistry
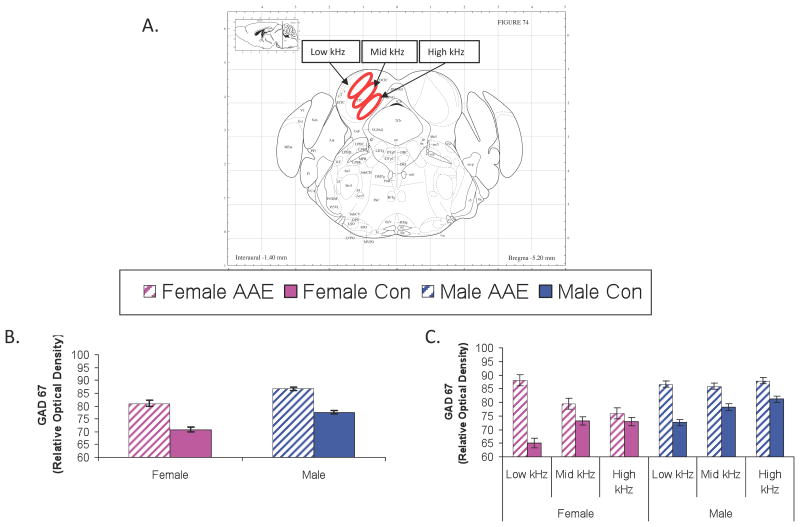
AAE exposure significantly altered GAD67 levels in the inferior colliculus (IC; Figure 5). The IC of both male and female mice showed increased GAD67 levels following AAE treatment, F(1,6836) = 119.77, p<0.0001. No significant Treatment X Sex interaction was present, suggesting AAE treatment was associated with increased GAD67 in both sexes (Figure 5b). A significant three-way interaction was found between Tonotopic Location (high, middle, low-frequency), Treatment (AAE, Control) and Sex (male, female). This result suggests a relatively greater impact of AAE on the more dorsal, low frequency regions of the IC in females and a more uniform increase in GAD levels across all tonotopic locations in the male AAE-treated IC, F(2,6828) = 4.60, p= 0.01 (Figure 5c). In AI (Figure 6), AAE Treatment interacted significantly with Sex, leading to a pattern more similar to the peripheral effects, F(1,2654) = 41.53, p<0.0001. Females exposed to the AAE showed an increase in GAD levels in AI while males, surprisingly showed a significant decrease (Figure 6b). There was no Significant Treatment (AAE, control) X Sex (male, female) × Depth (superficial, deep layers) or Treatment X SEX X Cellular Location (soma, neuropil) interactions, suggesting that the changes occurred regardless of whether GAD67 was measured in the deep or superficial layers, or in the soma or neuropil of AI (Figure 6c). As expected from previous work, regardless of Treatment and Sex, GAD67 immunolabeling was stronger in both the superficial layers (vs deep layers) and in the soma (vs neuropil), as evidenced by the significant Depth X Cellular Location interaction, F(1,2654) = 10.54, p=0.001 (Figure 6c).
Figure 5.
GAD67 in the inferior colliclus (IC). Figure 5a depicts the approximate location of GAD67 measurements collected from the IC of aged mice using the coordinates of Paxinos and Watson. Figures 4b and 4c depict the relative optical density of GAD67 labeling in the IC in male and female mice either collapsed (5b) across tonotopic location or broken down by this feature (5c). A significant Main Effect for Treatment was found (5b) as well as a significant Sex × Treatment × Tonotopic Location (5c). Error bars = standard error of the mean.
Figure 6.
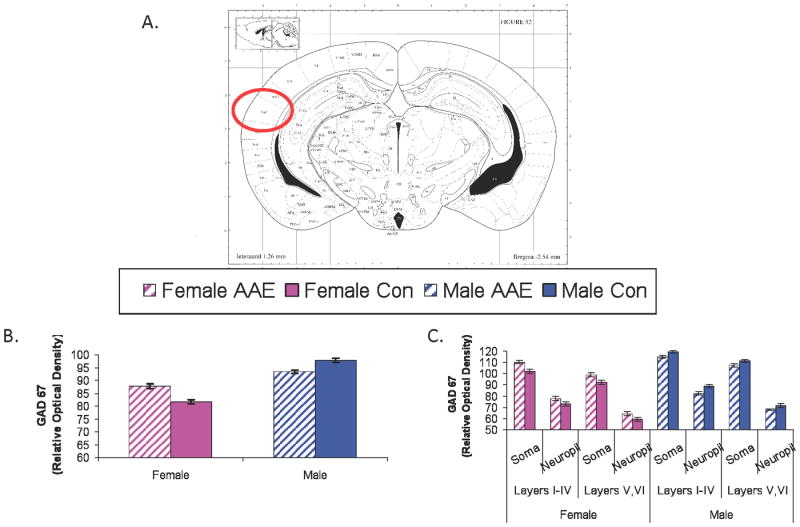
GAD67 in primary auditory cortex (AI). Figure 6a depicts the approximate coordinates of Paxinos and Watson. Figure 6b and 6c depict the relative optical density of GAD67 labeling in the AI in male and female mice either collapsed (6b) across anatomical layer and cellular location or broken down by these features (6c). A significant Sex × Treatment interaction was found (6b) but there were no significant effects or interactions with Depth (superficial, deep) or Cellular Location (soma, neuropil). Error bars = standard error of the mean.
Discussion
AAE was associated with significant structural and functional plasticity in aged mice. These effects were observed with multiple measures probing the activity of both peripheral and central auditory structures. Female and male aged mice were affected in an inverse manner by the treatment as measured by outer hair cell loss, ABR threshold, and GAD67 levels in AI. However, both sexes exhibited increased GAD67 levels in IC following AAE exposure. Female cochlear pathology appeared to be worsened by the treatments and male cochlear pathology appeared to be somewhat slowed. These results suggest that exposure to low-level, non-traumatic noise in aged mice has the potential to alter auditory structure and function along the auditory neuraxis, from outer hair cells, to ABR thresholds to GAD67 levels in the inferior colliculus and primary auditory cortex.
Hormonal systems might have played a key role in the current findings. For example, female mice in the current study were well beyond menopause (Felicio, Nelson & Finch, 1984) by the time of initiation of AAE treatment, likely leading to levels of estrogen that were well below those of males. It could be that estrogen normally plays a protective role in the auditory system (Meltser et al., 2008) and that once such protection has been removed, the system is more fragile and susceptible to sound exposure. Consistent with this hypothesis, Guimaraes et al. (2004) demonstrated that female CBA mice had better hearing than males until menopause, but that after menopause females underwent a more rapid age-related decline in hearing which eliminated much of their advantage. While the 70 dB SPL AAE exposure used in the current study was chosen so as not to be loud enough to damage the auditory system, it is possible that the exposure (12 hours on/12 hours off, 200 ms duration, 2/s rate) long-term exposure over-stimulated the fragile, unprotected female auditory system. However, recent work with AAE has demonstrated sex differences suggestive of the opposite effect of female sex hormones. In a series of studies exploring sex effects and AAE treatment, Willott and colleagues (Willott & Bross, 2004; Willott et al., 2006; Willott, 2009) provide evidence that ovarian sex hormones have a negative effect on the female C57 auditory system. Ovariectomy in females somewhat mitigated this negative impact of AAE exposure while orchidotomy data in males suggest the presence of androgens had beneficial effects for AAE treatment (Willott, 2009). Willott & Bross (2004) demonstrated that in C57 mice raised in an AAE from 25 days to 12-14 months of age, females exhibited less severe loss of spiral ganglion cells and anteroventral cochlear nucleus (AVCN) neurons than males. Males in this same study demonstrated greater AAE-related savings of inner hair cells than females and their AAE-related savings in outer hair cells and ABR thresholds included a wider range of middle-to-higher frequencies than in females, suggesting a greater benefit. While the Willott and Bross study focused on changes in middle age for mice reared from 25 days of age, and the current study focused on old age and only a 6-week exposure, this previous work does suggest sex can play a key role in determining the outcome of AAE exposure. An additional study focused on AAE-related sex effects in adult C57 mice found that a higher frequency AAE (centered at 20 kHz) led to greater ABR threshold elevations and hair cell damage in females (Willott et al., 2006), consistent with the negative peripheral effects of AAE on females in the current study. It is important to consider that while these previous studies on sex effects and AAE were done using adult and middle-aged C57 mice with early onset presbycusis, the current study used very old CBA/CaJ mice that exhibit a much later onset of presbycusis than previous work. (It should be noted that CBA/CaJ mice demonstrate more age-related hearing loss than their CBA/J counterparts, which can lead to confusion in the literature when this strain difference is not noticed, Ohlemiller, Dahl, & Gagnon, 2010). It was also the case in the current study that the control mice displayed sex differences as well, with female controls showing significantly better thresholds than males. This difference at the outset of AAE exposure between male and female mice should be noted as it might help explain the differential response to AAE exposure. Henry (2004) and Guimaraes et al. (2004) demonstrated a similar sex effect in aged CBA/CaJ mice, with males showing worse age-related hearing than females. Nevertheless, this difference in controls should be considered when interpreting the effects of AAE treatment on males and females as previous work has shown that AAE effects are relatively greater in animals that have a more rapidly degrading auditory system (Willott, Turner, & Sundin, 2000). Future studies will attempt to determine the mechanisms underlying the apparent sex effect by systematically manipulating age, hearing loss, estrogen and androgen levels, as well as stimulus features at the time of AAE initiation.
The cochleograms and ABR thresholds suggest the 6-wk regimen of nightly, 12-hr exposure to an AAE instituted in aged (2-yr-old) CBA mice has the potential to alter peripheral auditory structure and central auditory function. GABA levels in the brain were also measured (via GAD67) following AAE exposure. These results suggested that the aged auditory system retains significant neurochemical plasticity of the GABA system. In both males and females, AAE exposure was associated with increased GABA immunolabeling in the midbrain inferior colliculus. This is consistent with prior work suggesting that acoustic stimulation can upregulate GABA neurotransmission in the IC (Lorke et al., 2003). As with many of the other findings, the GABA changes in AI exhibited an interaction with sex. Females, who showed worse ABR thresholds and cochleograms following AAE treatment, showed significantly increased levels of GABA in AI. Males, who showed better ABR thresholds and cochleograms following AAE treatment, showed significantly decreased levels of GABA in AI. While a clear explanation for the differential effect of AAE exposure on AI neurochemistry is presently lacking, these results suggest that the experience with sound has the potential to alter the aged mammalian ear and brain.
The inhibitory neurotransmitter GABA is found in all sensory systems (Hendry et al., 1987) where it plays a critical role in shaping the responses of neurons (e.g., Foeller et al., 2001; Wang, McFadden, Caspary, & Salvi, 2002). A strong body of literature now suggests that sensory system experience can alter the GABA system. The results of the current study suggest that one way in which experience with acoustic stimuli can alter stimulus detection/coding, which might up or down-regulate the GABA system as required. Across sensory systems, reduced peripheral input is associated with reductions in GABA function (Caspary et al., 2005). Experiments that damage sensory afferents generally cause a down-regulation of normal GABAergic function. In the visual system, retinal lesions lead to a reduction of GABA in cortical regions receiving projections from the damaged area (Rosier et al., 1995). Tetrodotoxin blockade of peripheral visual input activity results in a reversible (50%) reduction in the number of glutamic acid decarboxylase (GAD) immunoreactive neurons in primary visual cortex (Jones, 1990). Whisker trimming in adult rats results in a down-regulation of GAD and muscimol binding in somatosensory cortex (Akhtar & Land, 1991; Fuchs & Salazar, 1998). Recent work in the primary auditory cortex of rats suggests that experience with sound via operant conditioning can be enough to reverse some of the age-related deficits in the GABA system (de Villers-Sidani et al., 2010). Interestingly, recent work by Pienkowski & Eggermont (2009, 2010) suggests that rearing adult cats in a passive acoustic noise environment (of similar intensity to the current study) leads to a reduction of responsiveness in the auditory cortex for the exposure frequencies (Pienkowski & Eggermont 2009, 2010a,b; Pienkowski, Munguia, & Eggermont, 2011). It is not clear whether such reductions in responsiveness are associated with commensurate changes in the GABA system in AI. However, the Pienkowski et al. work does suggest an additional tool for helping to understand the dissociation between IC and AI activity seen in the current study.
The auditory system appears to respond in a similar fashion to reduced peripheral input. Aging, which can be modeled as a slow, progressive degradation of auditory input to the brain, alters GABA systems along the auditory neuraxis. At the level of the inferior colliculus (IC), neurochemical and anatomical aging studies demonstrate decreased GABA levels, GABA release, GAD levels, and binding by GABAB receptors, as well as, a rearrangement of axon terminals (Banay-Schwartz et al., 1989a, b; Caspary et al., 1990; Raza et al. 1994; Gutiérrez et al., 1994; Helfert et al., 1999). Reductions in GABA neurotransmission have also been found in primary auditory cortex (AI) when looking at the GABA synthetic enzyme GAD (Caspary et al., 2005). Age-related reductions of GAD65&67 mRNA and GAD67 protein (GAD65 not measured) in AI were found. These findings are similar to the age-related GABA changes observed in the IC and suggest that age-related changes in GABA neurochemistry could result in altered coding of acoustic signals in AI of older individuals. Age-related changes in ascending input activity into AI could serve as a signal for altered GAD production in aging. The findings of the current study suggest the GABA system retains considerable experience-dependent plasticity, even in aged mice. While the differential effect of AAE on males and females awaits a clear explanation, it appears possible that in some animals acoustic experience might alter, even reverse, age-related down regulation of the GABA system.
The current findings suggest that exposure to an augmented acoustic environment during old age can have widespread implications for the peripheral and central auditory system. While there are a number of limitations of the current study (e.g., relatively small sample size with limited statistical power concerning sex effects, use of an inbred strain that might not generalize to other strains or species, use of an augmented acoustic environment that may have little relevance for hearing aid use), these results provide evidence that the aged auditory system retains a great deal of experience-dependent anatomical, electrophysiological, and neurochemical plasticity. These results also suggest the need for more research into the complex variables (age, sex, hormone levels, prior hearing loss, etc.) that might be interacting with sensory stimulation late in life as a way to better understand the frustration and variability so common in hearing aid use in the elderly. Much more research needs to be done on the plastic changes that occur subsequent to reintroduction of sound late in life, so plastic processes can be utilized to allow maximal benefit to hearing aid users. Future studies will attempt to further elucidate these changes and much remains to be learned. However, these results begin to provide evidence that the ear and brain change as a result of experience with sound.
Acknowledgments
Supported by NIH grants AG023910 and DC008357 (JGT), DC00151 (DMC), The Swedish Research Council, Tysta Skolan, and the Karolinska Institutet. Behavioral equipment donated by Kinder Scientific in the memory of SIU graduate Dorothy Jean Kinder (Walker).
Abbreviations
- AAE
Augmented Acoustic Environment
- GABA
Gamma-aminobutyric acid
- GAD67
glutamic acid decarboxilase
- IC
Inferior colliculus
- AI
Primary auditory cortex
- ABR
Auditory Brainstem Response
- PBS
phosphate buffered saline
- PF
4% paraformaldehyde
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtar ND, Land PW. Activity-dependent regulation of glutamic acid decarboxylase in the rat barrel cortex: effects of neonatal versus adult sensory deprivation. J Comp Neurol. 1991;307:200–213. doi: 10.1002/cne.903070204. [DOI] [PubMed] [Google Scholar]
- Arlinger S, Gatehouse S, Bentler RA, Byrne D, Cox RM, Dirks DD, Humes L, Neuman A, Ponton C, Robinson K, Silman S, Summerfield AQ, Turner CW, Tyler RS, Willott JF. Report of the Eriksholm Workshop on auditory deprivation and acclimatization. Ear Hear. 1996;7(3 Suppl):87S–98S. doi: 10.1097/00003446-199617031-00009. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. I. Glutamate and related amino acids. Neurochem Res. 1989;14:555–562. doi: 10.1007/BF00964918. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. II. Taurine and small neutral amino acids. Neurochem Res. 1989;14:563–570. doi: 10.1007/BF00964919. [DOI] [PubMed] [Google Scholar]
- Bergman M, Blumenfeld VG, Cascardo D, Dash B, Levitt H, Margulies MK. Age-related decrement in hearing for speech. Sampling and longitudinal studies. J Gerontol. 1976;31(5):533–558. doi: 10.1093/geronj/31.5.533. [DOI] [PubMed] [Google Scholar]
- Carabellese C, Appollonio I, Rozzini R, Bianchetti A, Frisoni GB, Frattola L, Trabucchi M. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc. 1993;41(4):401–407. doi: 10.1111/j.1532-5415.1993.tb06948.x. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Hughes LF. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132:1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt 11):1781–91. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Lawhorn-Armour B, Pippin J, Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus. J Neurosci. 1990;10:2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso JF. Sensory processes and perception in aging. In: Vudik A, editor. Lectures on Gerontology. Lond: Academic Press; 1982. pp. 441–479. [Google Scholar]
- De Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RC, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci USA. 2010;107(31):13900–5. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divenyi PL, Haupt KM. Audiological correlates of speech understanding deficits in elderly listeners with mild-to-moderate hearing loss. II. Correlation analysis. Ear Hear. 1997a;18(2):100–113. doi: 10.1097/00003446-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Divenyi PL, Haupt KM. Audiological correlates of speech understanding deficits in elderly listeners with mild-to-moderate hearing loss. III. Factor representation. Ear Hear. 1997b;18(3):189–201. doi: 10.1097/00003446-199706000-00002. [DOI] [PubMed] [Google Scholar]
- Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol of Repro. 1984;31:446–453. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kossl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. JARO. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD. Anatomical and neurochemical bases of presbycusis. In: Hof PR, Mobbs CV, editors. Functional Neurobiology of Aging. New York: Academic Press; 2001. pp. 531–547. [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106(1-2):95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Salazar E. Effects of whisker trimming on GABA(A) receptor binding in the barrel cortex of developing and adult rats. J Comp Neurol. 1998;395:209–216. [PubMed] [Google Scholar]
- Gatehouse S. The time course and magnitude of perceptual acclimatization to frequency responses: evidence from monaural fitting of hearing aids. J Acoust Soc Am. 1992;92(3):1258–1268. doi: 10.1121/1.403921. [DOI] [PubMed] [Google Scholar]
- Goŕdon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36(6):1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Goŕdon-Salant S, Fitzgibbons PJ. Recognition of multiply degraded speech by young and elderly listeners. J Speech Hear Res. 1995;38(5):1150–1156. doi: 10.1044/jshr.3805.1150. [DOI] [PubMed] [Google Scholar]
- Goŕdon-Salant S, Fitzgibbons PJ. Selected cognitive factors and speech recognition performance among young and elderly listeners. J Speech Lang Hear Res. 1997;40(2):423–431. doi: 10.1044/jslhr.4002.423. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192(1-2):83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Gutièrrez A, Khan ZU, Morris SJ, DeBlas AL. Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J Neurosci. 1994;14:7469–7477. doi: 10.1523/JNEUROSCI.14-12-07469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harless EL, McConnell F. Effects of hearing aid use on self concept in older persons. J Speech Hear Disord. 1982;47(3):305–309. doi: 10.1044/jshd.4703.305. [DOI] [PubMed] [Google Scholar]
- Harris RW, Reitz ML. Effects of room reverberation and noise on speech discrimination by the elderly. Audiology. 1985;24(5):319–324. doi: 10.3109/00206098509078350. [DOI] [PubMed] [Google Scholar]
- Helfert RH, Sommer TJ, Meeks J, Hofstetter PL, Hughes LF. Age-related synaptic changes in the central nucleus of the inferior colliculus of Fischer-344 rats. J Comp Neurol. 1999;406:285–298. [PubMed] [Google Scholar]
- Hendry SH, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KR. Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hear Res. 2004;190:141–148. doi: 10.1016/S0378-5955(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Ison JR. The acoustic startle response: Reflex elicitation and reflex modification by preliminary stimuli. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. CRC Press; Boca Raton, FL: 2001. [Google Scholar]
- Jeskey JE, Willott JF. Modulation of prepulse inhibition by an augmented acoustic environment in DBA/2J mice. Behavioral Neuroscience. 2000;114(5):991–997. doi: 10.1037//0735-7044.114.5.991. [DOI] [PubMed] [Google Scholar]
- Jones EG. The role of afferent activity in the maintenance of primate neocortical function. J Exp Biol. 1990;153:155–176. doi: 10.1242/jeb.153.1.155. [DOI] [PubMed] [Google Scholar]
- Kochkin S. MarkeTrak III: The billion dollar opportunity in the hearing instrument market. The Hearing Journal. 1992;45:2–7. [Google Scholar]
- Kochkin S. MarkeTrak IV: What is the viable market for hearing aids? The Hearing Journal. 1997;50:31–39. [Google Scholar]
- Konkle DF, Beasley DS, Bess FH. Intelligibility of time-altered speech in relation to chronological aging. J Speech Hear Res. 1977;20(1):108–115. doi: 10.1044/jshr.2001.108. [DOI] [PubMed] [Google Scholar]
- Li HS. Genetic influences on susceptibility of the auditory system to aging and environmental factors. Scand Audiol Suppl. 1992;36:1–39. [PubMed] [Google Scholar]
- Lorke DE, Wong LY, Lai HW, Poon PW, Zhang A, Chan WY, Yew DT. Early postnatal sound exposure induces lasting neuronal changes in the inferior colliculus of senescence accelerated mice (SAMP8): a morphometric study on GABAergic neurons and NMDA expression. Cell Mol Biol. 2003;23:143–164. doi: 10.1023/A:1022993704617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BJ, Kimar S, Prosen CA. Auditory filter shapes of CBA/CaJ mice: behavioral assessments. J Acoust Soc Am. 2006;120(1):321–30. doi: 10.1121/1.2203593. [DOI] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118(4):1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrow CD, Aguilar C, Endicott JE, Velez R, Tuley MR, Charlip WS, Hill JA. Asociation between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc. 1990;38(1):45–50. doi: 10.1111/j.1532-5415.1990.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci. 2005;25(3):699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport. 2006;17(6):559–63. doi: 10.1097/00001756-200604240-00001. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Gourevitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006;9(7):932–9. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Dahl AR, Gagnon PM. Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J Assoc Res Otolaryngol. 2010;11:605–623. doi: 10.1007/s10162-010-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CV, Nelson CT, Lindley GA. The functionally and physiologically plastic adult auditory system. J Acoust Soc Am. 1998;103(4):1705–1721. doi: 10.1121/1.421050. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd. San Diego: Academic Press; 2001. [Google Scholar]
- Philibert B, Collet L, Vesson JF, Veuillet E. Intensity-related performances are modified by long-term hearing aid use: a functional plasticity? Hear Res. 2002;165(1-2):142–151. doi: 10.1016/s0378-5955(02)00296-4. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ. Long-term, partially-reversible reorganization of frequency tuning in mature cat primary auditory cortex can be induced by passive exposure to moderate-level sounds. Hear Res. 2009;257(1-2):24–40. doi: 10.1016/j.heares.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ. Intermittent exposure with moderate-level sound impairs central auditory function of mature animals without concomitant hearing loss. Hear Res. 2010a;261(1-2):30–35. doi: 10.1016/j.heares.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ. Passive exposure of adult cats to moderate-level tone pip ensembles differentially decreases AI and AII responsiveness in the exposure frequency range. Hear Res. 2010b;268(1-2):151–62. doi: 10.1016/j.heares.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Munguia R, Eggermont JJ. Passive exposure of adult cats to bandlimited tone pip ensembles or noise leads to long-term response suppression in auditory cortex. Hear Res. 2011 Feb 18; doi: 10.1016/j.heares.2011.02.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ponton CW. Possible application of functional imaging of the human auditory system in the study of acclimatization and late onset deprivation. Ear Hear. 1996;17(3 Suppl):78S–86S. doi: 10.1097/00003446-199617031-00008. [DOI] [PubMed] [Google Scholar]
- Raza A, Milbrandt JC, Arneric SP, Caspary DM. Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hear Res. 1994;77:221–230. doi: 10.1016/0378-5955(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Robinson K, Gatehouse S. The time course of effects on intensity discrimination following monaural fitting of hearing aids. J Acoust Soc Am. 1996;99(2):1255–1258. doi: 10.1121/1.414637. [DOI] [PubMed] [Google Scholar]
- Rosier AM, Arckens L, Demeulemeester H, Orban GA, Eysel UT, Wu YJ, Vandesande F. Effect of sensory deafferentation on immunoreactivity of GABAergic cells and on GABA receptors in the adult cat visual cortex. J Comp Neurol. 1995;359:476–489. doi: 10.1002/cne.903590309. [DOI] [PubMed] [Google Scholar]
- Silman S, Gelfand SA, Silverman CA. Late-onset auditory deprivation: effects of monaural versus binaural hearing aids. J Acoust Soc Am. 1984;76(5):1357–1362. doi: 10.1121/1.391451. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. J Acoust Soc Am. 1997;101(4):2214–2220. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Stuart A, Phillips DP. Word recognition in continuous and interrupted broadband noise by young normal-hearing, older normal-hearing, and presbyacusic listeners. Ear Hear. 1996;17(6):478–489. doi: 10.1097/00003446-199612000-00004. [DOI] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82(3):601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Bielefield EC, Chen G, Li M, Henderson D. Ameliorative effects of an augmented acoustic environment on age-related hearing loss in middle-aged fischer 344/NHsd Rats. The Laryngoscope. 2009a;119:1374–9. doi: 10.1002/lary.20244. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Chen G, Hu B, Chi L, Li M, Zheng G, Bielefield EC, Jamesdaniel S, Coling D, Henderson D. The effects of acoustic environment after traumatic noise exposure on hearing and outher hair cells. Hear Res. 2009b;250:10–18. doi: 10.1016/j.heares.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Thomas A, Herbst KG. Social and psychological implications of acquired deafness for adults of employment age. Br J Audiol. 1980;14(3):76–85. doi: 10.3109/03005368009078906. [DOI] [PubMed] [Google Scholar]
- Turner CW, Souza PE, Forget LN. Use of temporal envelope cues in speech recognition by normal and hearing-impaired listeners. J Acoust Soc Am. 1995;97(4):2568–2576. doi: 10.1121/1.411911. [DOI] [PubMed] [Google Scholar]
- Turner JG, Willott JF. Exposure to an augmented acoustic environment alters auditory function in hearing-impaired DBA/2J mice. Hear Res. 1998;118:101–113. doi: 10.1016/s0378-5955(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Viberg A, Canlon B. The guide to plotting a cochleogram. Hear Res. 2004;197:1–10. doi: 10.1016/j.heares.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noiseinduced cellular injury and repair in the mouse cochlea. JARO. 2002;03:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Weinstein BE. Treatment efficacy: hearing aids in the management of hearing loss in adults. J Speech Hear Res. 1996;39(5):S37–S45. [PubMed] [Google Scholar]
- Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25(4):593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- Willott JF. Effects of sex, gonadal hormones, and augmented acoustic environments on sensorineural hearing loss and the central auditory system: Insights from research on C57BL/6J mice. 2009 doi: 10.1016/j.heares.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Bross LS. Effects of prolonged exposure to an augmented acoustic environment on the auditory system of middle-aged C57BL/6J mice: cochlear and central histology and sex differences. J Comp Neurol. 2004;472:358–370. doi: 10.1002/cne.20065. [DOI] [PubMed] [Google Scholar]
- Willott JF, Bross LS, McFadden S. Ameliorative effects of exposing DBA/2J mice to an augmented acoustic environment on histological changes in the cochlea and anteroventral cochlear nucleus. JARO. 2005;6(3):234–243. doi: 10.1007/s10162-005-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Chilsom TH, Lister JJ. Modulation of presbycusis: current status and future directions. Audiol Neurotol. 2001;6(5):231–249. doi: 10.1159/000046129. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory changes in C57BL/6J and DBA/2J mice. Hear Res. 1999;135:78–88. doi: 10.1016/s0378-5955(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG. Neural plasticity in the mouse inferior colliculus: relationship to hearing loss, augmented acoustic stimulation, and prepulse inhibition. Hear Res. 2000;147:275–281. doi: 10.1016/s0378-5955(00)00137-4. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG, Sundin VS. Effects of exposure to an augmented acoustic environment on auditory function in mice: roles of hearing loss and age during treatment. Hear Res. 2000;142:79–88. doi: 10.1016/s0378-5955(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Willott JF, Vandenbosch J, Shimizu T. Effects of a high-frequency acoustic environment on parvalbumin immunolabeling in the anteroventral cochlear nucleus of DBA/2J and C57BL/6J mice. Hear Res. 2010;261(1-2):36–41. doi: 10.1016/j.heares.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Vandenbosch J, Shimizu T, Ding Da-Lian, Salvi R. Effects of exposing gonadectomized and intact C57BL/6J mice to a high-frequency augmented acoustic environment: Auditory brainstem response thresholds and cytocochleograms. Hear Res. 2006;221:73–81. doi: 10.1016/j.heares.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]