Abstract
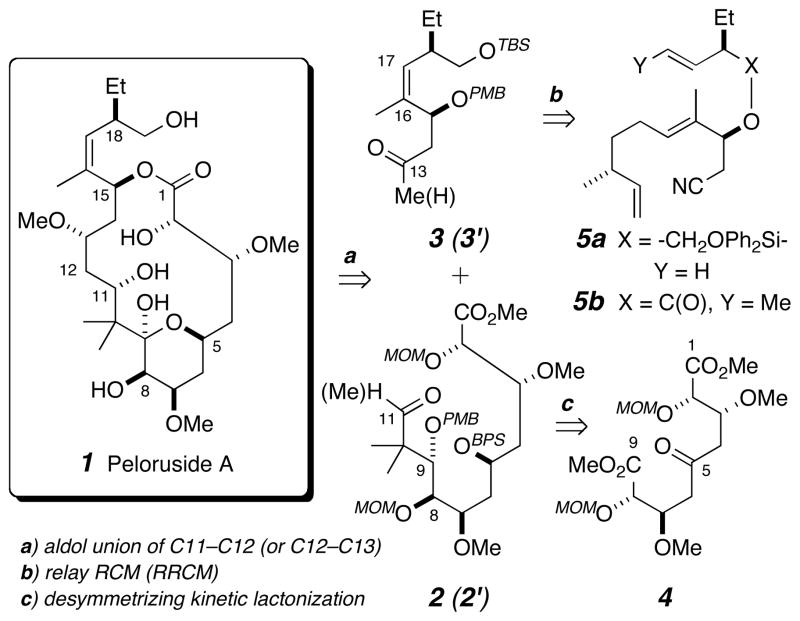
A convergent total synthesis of peloruside A (1) is described. The key strategic features are a diastereoselective lactonization to generate a C5-C9 valerolactone from the C2-symmetric ketone 4, which comprises C1–C9 of 1, and a relay ring closing metathesis (RRCM) reaction to produce a dehydrovalerolactone 20, which embodies C13–C19. A new isomer of 1, the valerolactone iso-peloruside A (iso-1), was identified.
Keywords: aldol reaction, lactones, metathesis, relay ring closing metathesis (RRCM), total synthesis
The highly cytotoxic marine macrolide (+)-peloruside A (1) was isolated from the New Zealand sponge, Mycale hentscheli, by Northcote and co-workers.[1] It showed LD50s ranging from 6–18 nM toward H441, SH-SY5Y, and P388 cancer cell lines[2] and has been further explored from a preclinical perspective. Like paclitaxel (cf. Taxol®), peloruside A is a microtubule-stabilizer, arresting cells in the G2–M phase of the cell cycle, but it targets a different tubulin binding site than paclitaxel.[3] As a consequence peloruside A (1) is a candidate for use against paclitaxel resistant cell lines.[4] The therapeutic potential, low natural abundance (3 mg/170 g wet sponge), and architectural complexity have generated significant interest in the development of chemical syntheses of 1. To date, four total syntheses of peloruside A have been reported (by the research groups of De Brabander, Taylor, Ghosh, and Evans)[5],[6],[7],[8] and related synthetic studies have been described.[9],[10],[11]
Our interest in peloruside A (1) as a target was greatly heightened when we identified a plan that seemed ideally matched with strategies and technologies developed earlier in our group. In particular, it was attractive to apply a diastereoselective kinetic lactonization of a pseudosymmetric azelaic acid derivative[12] and to capitalize on the versatility of relay ring-closing metathesis (RRCM)[13] reactions.
As summarized in Scheme 1 our plan for the synthesis was to effect a late stage aldol coupling (cf. a) between the aldehyde acceptor 2 and methyl ketone donor 3 (or the complementary ketone/aldehyde pair 2′/3′). We planned to install the Z-trisubstituted and doubly allylically branched Δ16,17-alkene in 3 (or its aldehyde analog 3′) via RRCM (cf. b) of the silaketal[14] 5a or ester[15] 5b (peloruside A skeleton numbering is used throughout). We envisioned the main aldehyde fragment 2 (or its ketone analog 2′) to arise from the C2-symmetric azelaic ester precursor 4 (cf. c). As further detailed below, reduction of the C5-ketone in 4 from either of its homotopic faces would give an alcohol that, through engagement of its pro-S rather than pro-R diastereotopic ester group, would selectively provide a valerolactone/monoester (i.e., 11 in Scheme 2).[16] Terminus differentiation and C9–C10 bond formation would then provide access to 2 (or 2′).
Scheme 1.
Retrosynthesis outline for accessing peloruside A (1). BPS = tert-butyldiphenylsilyl; MOM = methoxymethyl; PMB = para-methoxybenzyl; RCM = ring-closing metathesis; TBS = tert-butyldimethylsilyl.
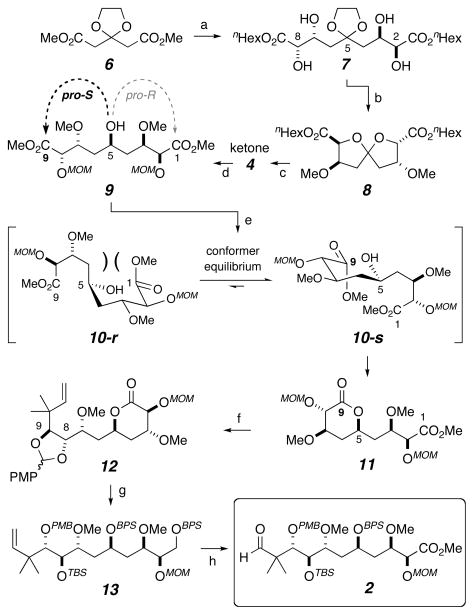
Scheme 2.
Synthesis of main fragment 2 via the key diastereoselective lactonization of pseudosymmetric carbinol 9. Reagents and conditions: (a) i) DIBAL-H, Et2O, −78 °C; then (EtO)2P(O)CH(Na)CO2nHex, 80%; ii) SAD, 0 °C, 88%; (b) i) 30 mol% HI, THF, 0 °C, 83%; ii) Me3OBF4, proton sponge, 0 °C to RT, 84%; (c) i) 1,2-ethanedithiol, BF3·OEt2, 0 °C; ii) MOMCl, i-Pr2NEt, DCM, RT, 90% over 2 steps; iii) I2, NaHCO3, acetone, H2O, 0 °C, 91%; iv) Otera’s catalyst,[22] MeOH, toluene, 90 °C, 77%; (d) Raney-Nickel, H2, EtOH, RT, 91%; (e) TMG, C6H6, RT; TFA, 98% (dr 12:1). (f) i) L-Selectride®, THF, −78 °C, 87%; ii) prenyl bromide, indium powder, DMF, 55 °C, 80%; iii) AlCl3, NaI, CH3CN, DCM, 0 °C, 92%; iv) (p-MeO)-PhCH(OMe)2, CSA, DCM, 4Å mol sieves, 87%; v) MOMCl, i-Pr2NEt, DCM, RT, 99%; (g) i) LAH, THF, 0 °C, 96%; ii) BPSCl, ImH, DMAP, DMF, RT, 92%; iii) DIBAL-H, DCM, −78 °C, 95%; iv) DMP, NaHCO3, DCM, RT; v) Zn(BH4)2,[23] DCM, 0 °C (dr 3:1), 62% (2 steps); vi) TBSOTf, 2,6-lutidine, DCM, RT, 89%; (h) i) HF·pyridine, THF, pyridine, RT, 91%; ii) DMP, NaHCO3, DCM, RT; iii) NaClO2, NaH2PO4, t-BuOH, H2O, Me2C=CHMe, RT; iv) CH2N2, RT, 87%, 3 steps; v) O3, pyridine, DCM, MeOH, −78 °C, 75%. CSA = camphorsulfonic acid; DCM = dichloromethane (CH2Cl2); DMAP = 4-dimethylamino-pyridine; DMF = dimethylformamide; DMP = Dess-Martin periodinane; dr = diastereomeric ratio; ImH = imidazole; LAH = lithium aluminum hydride; OTf = triflate; SAD = Sharpless asymmetric dihydroxylation; TFA = trifluoroacetic acid; THF = tetrahydrofuran; TMG = tetramethylguanidine.
The synthesis of fragment 2 is presented in Scheme 2. The main features are: i) synthesis of the C2-symmetric ketodiester 4, ii) diastereoselective conversion, via desymmetrizing kinetic lactonization, of the derived alcohol 9 to the δ-valerolactone 11, and iii) chemoselective elaboration (including prenylation) of the lactone vs. ester carbonyl groups [C9 vs. C1] in 11 to give fragment 2.
The tetrol 7 was prepared from the ethylene ketal of dimethyl acetone dicarboxylate (6)[17] by utilizing a one-pot DIBAL-H reduction of both esters to the intermediate 1,5-dialdehyde, followed by in situ double Horner-Wadsworth-Emmons homologation with (EtO)2P(O)CH(Na)CO2nHex.[18] Two-fold Sharpless asymmetric dihydroxylation (SAD)[19] of the bis-methyl ester analog was initially studied,[16] but this transformation was plagued by incomplete conversion to the bis-methyl ester variant of tetrol 7. NoD 1H NMR analysis[20] of the aqueous component of the biphasic mixture indicated the accumulation of the intermediate alkenediol. The polar nature of this half-oxidized intermediate likely led to its undesired partitioning into the aqueous layer and, consequently, away from the active hydroxylation species. The substantial water solubility of the desired tetrol was a further complication. This prompted our selection and use of the less polar and more well-behaved bis-n-hexyl ester series, which allowed isolation of 7 in 88% yield. Exposure to a catalytic amount of aqueous hydriodic acid then promoted ketal-metathesis by engagement (and protection) of the C2- and C8-hydroxyl groups to give a spirocyclic ketal as a single diastereomer. Installation of the methyl ethers found at C3 and C7 in peloruside A (1) was achieved with Meerwein’s salt to provide 8. Transketalization of 8 with ethanedithiol (BF3·OEt2),[21] bis-MOM ether protection of the C2/C8-diol, dithiolane removal (I2, aq NaHCO3), and transesterification with methanol (Otera’s catalyst) smoothly gave 4 (cf. Scheme 1). The sequence from 6 to 4 proceeded in over 30% yield.
While many reagents sufficed to chemoselectively reduce the C5-ketone in 4, most were complicated by premature lactonization of the resulting hydroxy bisester 9, with not only modest but also irreproducible levels of diastereocontrol. Hence, neutral conditions for this reduction that also minimized handling of 9 were sought. Use of hydrogen gas over Raney nickel provided a convenient solution; simple filtration and solvent removal provided the C1-symmetric alcohol 9 (91%), which contains the new non-stereogenic but chirotopic[24] C5-carbinol center. The key diastereoselective cyclization of this substrate[16] proceeded with preferential engagement of the pro-S ester group at C9 to give lactone 11 (98%, dr 12:1). Use of tetramethylguanidine (TMG, 2.0 equiv) to promote this lactonization gave reproducibly high levels of diastereoselectivity. Interestingly, a labile adduct of TMG with the product 11, perhaps involving a tetrahedral intermediate in which the lactone carbonyl group remained engaged with TMG, was generated. We found it best to cleave this adduct by treatment with anhydrous trifluoroacetic acid (NMR analysis) prior to aqueous workup. The sense of the kinetic diastereocontrol observed in this lactonization was anticipated[12] on the basis of conformational analysis; the equilibrium concentration of the reactive preclosure conformer 10-r, which could engage the C1-ester and lead to the minor (and undesired) C5-epimer of 11, should be lower than that of the reactive preclosure conformer 10-s, a necessary intermediate enroute to 11. Analogous considerations equally apply to the pair of tetrahedral intermediates derived from 10-r and 10-s, should it be the case that the rate-limiting step in the lactonization is not the initial closure implied by 10. A distinguishing feature of this symmetry-enabled approach is the rapidity and efficiency with which the C1-C9 portion of the peloruside skeleton was established.
Chemoselective reduction of the lactone rather than ester functional group in 11 was achieved with L-Selectride®[25] to provide a lactol, which was treated with prenyl bromide/indium.[26] This installed the gem-dimethylated C10-moiety, while simultaneously inducing relactonization, now of the C5-OH with the C1-ester.[25] Use of the prenyl unit was designed to permit potential access to either the aldehyde 2 or methyl ketone 2′, but as things later developed, implementation in the former role proved more valuable. Bis-MOM ether cleavage (AlCl3, NaI), PMP-acetal formation, and MOM reprotection of the C2-OH gave 12 as a single epimer at C9 (assigned to be of S configuration,[27] consistent with αMOM-chelation controlled addition). A series of functional/protecting group manipulations [cf. (g), Scheme 2] served to carry 12 efficiently to 13, the precursor to main fragment 2. Key among these was the oxidation/reduction of the C8-carbinol, which had just been revealed by a highly regioselective reductive cleavage by DIBAL-H of the PMP-acetal, to effect the required inversion of configuration at C8. Finally, but again efficiently, the C1-methyl ester was reinstated and the C11-aldehyde generated by ozonolysis [cf. (h), Scheme 2] to complete the synthesis of 2.
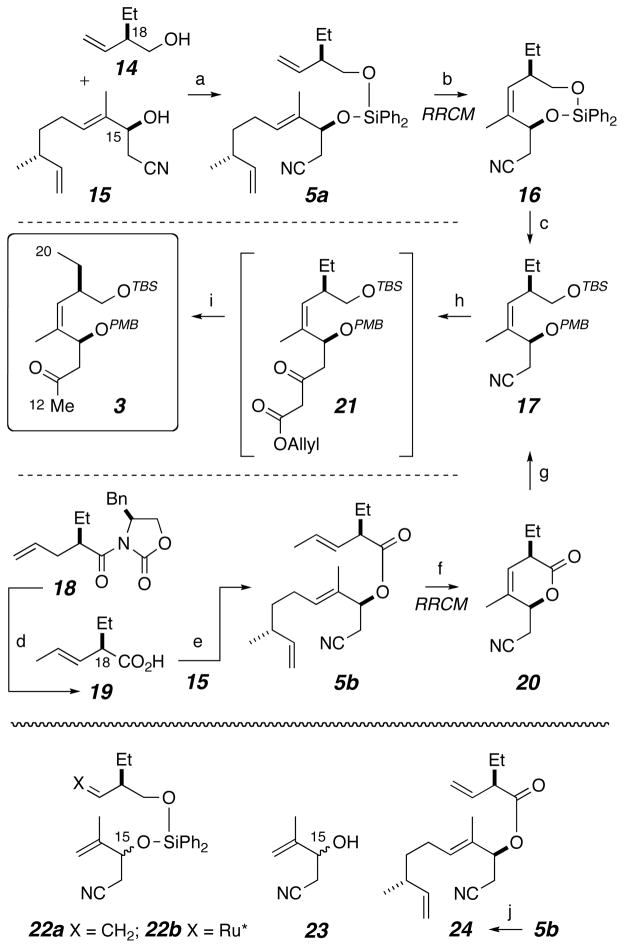
To explore a metathesis-based synthesis of the hindered Z-alkene in 3 (Scheme 3), we first prepared and examined the RCM reaction of the simple diene[14] 22a (Scheme 3), which was an epimeric mixture at C15. This proved to be a poor RCM substrate (e.g., <20% yield of 16 using 45 mol% of G2).[28] We turned to the relay RCM substrate 5a,[29] which was made by sequential loading of the alcohols 14[13] and 15 onto Ph2SiCl2. RRCM proceeded to give 16 in 92% yield. For the preparation of 5a as essentially one diastereomer, enantiomerically enriched β-hydroxynitrile 15 was accessed by addition of lithioacetonitrile to the enal derived from allylic oxidation (SeO2) of (R)-citronellene, followed by enzymatic (kinetic) resolution of the resulting C15-epimeric carbinols (Novozyme 435).[13],[30] An added advantage of adopting the RRCM strategy here was that the two epimeric carbinols (15 and C15-epi-15) were easily discriminated by the lipase, allowing for efficient resolution; the alternative substrate 23, the precursor to 22a, has two nearly isosteric groups and was a poor substrate for lipase resolution.
A complementary approach involving the ester 5b and its RRCM product, the lactone 20 was also studied. Preparation of 5b is inherently easier than that of the silaketal 5a, since it involves simple cross coupling of two functional groups with complementary reactivity, namely the alcohol 15 with the acid 19. Moreover, a sample of nonracemic 19 proved to be easier to access than one of 14. The terminal alkene in the 2-propenylated compound 18[31] was efficiently isomerized to the more stable, internal, 1-propenyl analog using RhCl3/EtOH;[32],[33] there was no evidence of erosion of the high dr, which implies that the branched α-carbon effectively impedes further isomerization by β-hydride elimination of the Cα methine hydrogen. This sequential allylation/2- to 1-propenyl isomerization/ethylene cross-metathesis degradation[34] resulted in net vinylation of, in this instance, an Evans acyloxazolidinone auxiliary (i.e., 24). We recommend consideration of this strategy in instances when ethenylation of carbanionic species is needed. Hydrolysis of the oxazolidinone 18 gave the acid 19, which upon DCC activation was coupled with 15 to provide 5b. This ester smoothly underwent RRCM to give lactone 20 in 70% yield.
-
Efficient elaboration of either 16 or 20 to the differentially protected diol derivative 17 was straightforward following desilylation (of 16) or reduction (of 20) and sequential TBS and PMB installations on the 1° and 2° hydroxyls, respectively. The choice of sodium borohydride in acetic acid as the reagent for the reduction of 20 was dictated by its base lability, which otherwise led to epimeric mixtures of the reduced diol product. Base instability was an even bigger obstacle in the final conversion of the nitrile in 17 to the methyl ketone in fragment 3. A solution emerged in the form of a modified Blaise reaction, wherein the reagent formed in situ from BrCH2CO2allyl/Zn°/Cp2TiCl2[35] converted the nitrile to an intermediate β-aminoenoate, the hydrolysis of which was carefully monitored at pH 3 (ca. 2 days) to afford the β-ketoester 21. Attempts to effect hydrolysis or dealkylation and decarboxylation of the methyl ester analog of 21 to 3 were compromised by competitive β-elimination of PMB-OH. We solved this complication by use of the allyl ester 21, which could be smoothly decarbalkoxylated with Pd(PPh3)4 (HCO2H, Et3N).[36] The non-basic conditions associated with this conversion of nitrile to methyl ketone render this strategy applicable to other base-sensitive nitrile substrates.
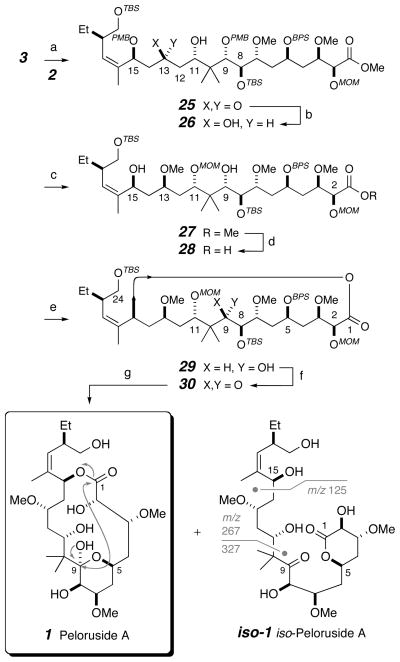
The final stage of the synthesis is presented in Scheme 4. The main features are vii) the Paterson boron aldol coupling[37] of fragments 2 and 3, viii) the regioselective macrocyclization of the diol acid 28, and ix) the removal of protecting groups from the penultimate intermediate 30, including our observation of a byproduct we name here iso-peloruside A (iso-1).
A number of model experiments designed to establish the feasibility of either the 2′/3′ or 3/2 aldol donor/acceptor pairing were carried out.[38] These eventually guided us to the use of the dicyclohexylboron enolate derived from ketone 3, which added to the aldehyde 2 (1.0 equiv, −78 to −20 °C) to form the C11–C12 bond and produce the ketoalcohol 25 (64%) with essentially full control of the new stereocenter at C11. Presumably, the 1,5-stereoinduction keyed by the β(C15)-stereocenter in the ketone donor 3[39] is enhanced by (i.e., matched with) that from the β(C9)-stereocenter of the α,α-gem-dimethylated aldehyde acceptor 2[40] to impart essentially perfect diastereoselection during this powerful cross-coupling reaction.[41] Reduction of the C13-ketone with Me4NBH(OAc)3 proceeded to give the 1,3-anti-diol 26 (dr 95:5). Methylation with Meerwein’s salt proceeded with near perfect regioselectivity. Installation of a MOM ether at C11 and removal of both PMB ethers at C9 and C15 proceeded smoothly to complete the preparation of 27.
Conversion of the methyl ester in 27 to the acid 28, in preparation for the required macrolactonization, was very slow with LiOH (aq MeOH) and was complicated by partial epimerization at C2 when KOSiMe3 (THF) was employed. These issues were solved by use of the more reactive and less basic LiOOH. Yamaguchi cyclization of diol acid substrate 28 was uneventful; the free C9-hydoxyl gave no evidence of interference (lactonization substrates in all previous peloruside A syntheses and studies have had the ketone oxidation state at C9). Oxidation of 29 to the C9-ketone 30 was smoothly effected with the Dess-Martin periodinane.
The silyl ether and MOM ether protecting groups were sequentially removed by treatment first with HF·pyridine in THF, buffered by additional pyridine, and second with 4N aq HCl in THF (RT). The first stage (removal of three silyl groups) was monitored by LC/MS analysis. The primary C24-OH was generated quickly at room temperature, the C5-tert-butyldiphenylsilyl ether (OBPS) was cleaved in about a day at 60 °C, and the hindered C8-OTBS required an additional day to completely release the C5/C8/C24-triol (having the C5-OH engaged to C9 as a hemiketal). Even though it required strongly acidic conditions (aq HCl, RT), the final MOM deprotection was planned with confidence, given the precedent from the earlier total syntheses. Without further purification, the C5/C8/C24-triol was treated with aq HCl at room temperature for 3 h to deliver (+)-peloruside A (1) in 49 % overall yield from 30 (over 2 steps). We also observed and isolated (via careful SiO2 chromatography, MeOH/DCM:5/95) a minor (5%) and slightly more polar byproduct formed during this experiment. We deduced its structure as that of the valerolactone derivative iso-1 based on 1H NMR and ESI MS/MS analyses. The masses of fragments observed from the latter (and indicated on the structure of iso-1 in Scheme 4) were informative in suggesting the absence of a macrolactone in this new isomeric substance. In particular, the fragmentation of the C10-C11 bond via retro-aldol reaction was evidenced by the complementary pair of sodiated ions at m/z 327 and 267 and further cleavage of the C14–C15 bond in the latter gave the ion observed at m/z of 125. Additionally, 1H NMR data of iso-1 bore strong similarities with the earlier described valerolactone 12. iso-Peloruside A (iso-1) presumably arose from intramolecular translactonization within 1 by the C5-hydroxyl in the minor [and unobserved (1H NMR)] open form of the C9-hemiketal to the C1-carbonyl group.
Scheme 3.
Synthesis of fragment 3 via the relay ring-closing metathesis reactions of substrates 5a and 5b. Reagents and conditions: (a) i) 15, Ph2SiCl2, pyridine, RT; ii) 14, pyridine, 41%; (b) G2, PhCH3, N2, 65 °C, 92%; (c) i) TBAF, THF, RT, 93%; ii) TBSCl, Et3N, DMF, RT, 84%; iii) Cl3CC(=NH)OPMB, CSA, DCM, RT, 76%; (d) i) RhCl3·3H2O, EtOH/H2O, 80 °C, 98% (15:1 = E:Z); ii) LiOH, H2O2, THF/H2O, 0 °C, 99%; (e) 15, DCC, DMAP, DCM, 0 °C to RT, 82%; (f) G2, DCM, 45 °C, 70%; (g) i) NaBH4, AcOH, EtOH, 0 °C to RT, 82%; ii) TBSCl, Et3N, DMAP, DCM, RT, 99%; iii) Cl3CC(=NH)OPMB, CSA, DCM, RT, 77%; (h) i) Zn°, Cp2TiCl2, allyl 2-bromoacetate, THF, 60 °C; ii) pH 3 buffer, i-PrOH/H2O/THF, RT; (i) Pd(PPh3)4, HCO2H, Et3N, THF, RT, 61% (over 3 steps). (j) CH2=CH2 (15 psi), G2, PhH, 65 °C, quantitative. G2 = second generation Grubbs initiator, [Ru=CHPh(Cl)2(PCy3)(H2IMes)]; [Ru*] = Ru(Cl)2(H2IMes)Ln; TBAF = tetrabutylammonium fluoride; DCC = dicyclohexylcarbodiimide.
Scheme 4.
Synthesis of peloruside A (1) via aldol coupling of fragments 2 and 3, macrolactonization of seco-acid 28, and deprotection. Reagents and conditions: (a) c-Hex2BCl, Et3N, 3, Et2O, −78 °C to −40 °C; 2, Et2O, −78 °C to −20 °C, 64% (along with 34% of recovered 3); (b) NMe4BH(OAc)3, AcOH/MeCN, −30 °C, 95% (dr 95:5); (c) i) Me3OBF4, 2,6-di-t-Bu-pyridine, DCM, 0 °C to RT, 82% (after two cycles); ii) MOMCl, i-Pr2NEt, DCM, 0 °C to RT, 98%; iii) DDQ, DCM/pH 7 buffer, 0 °C to RT, 94% (after two cycles); (d) LiOH, H2O2, THF/MeOH/H2O, 0 °C to RT, ca. 100%; (e) 2,4,6-trichlorobenzoyl chloride, i-Pr2NEt, PhCH3, 0 °C to RT; DMAP, PhCH3, RT, 54%; (f) DMP, NaHCO3, DCM, 0 °C to RT, 83%; (g) i) HF/pyr, THF, pyr, RT to 60 °C; ii) HCl (4 N), THF, RT; 49% 1 and 5 % iso-1 (over 2 steps).
In conclusion this total synthesis of peloruside A (1) capitalizes on the kinetic lactonization reaction of the pseudosymmetric azelaic acid derivative 9 (to 11, Scheme 2) and a relay ring closing metathesis reaction to access the trisubstituted Δ16.17-alkene subunit (5b to 20, Scheme 3). Modified Blaise and net enolate ethenylation sequences are also noteworthy. Formation of the isomeric valerolactone iso-1 during final HCl-catalyzed deprotection of the MOM-ethers at C2 and C11 was observed for the first time.
Supplementary Material
Acknowledgments
This investigation was supported by grants awarded by the DHHS (GM65597 and CA76497). We thank Mr. Joseph B. Katzenmeyer for assistance with the MS/MS measurement.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.West LM, Northcote PT, Battershill CN. J Org Chem. 2000;65:445–449. doi: 10.1021/jo991296y. [DOI] [PubMed] [Google Scholar]
- 2.West LM, Northcote PT. 2001010869. PCT Int Appl WO. 2001
- 3.Hood KA, West LM, Rouwe B, Northcote PT. Cancer Res. 2002;62:3356–3360. [PubMed] [Google Scholar]
- 4.Teesdale-Spittle P, Andreu JM, Miller JH. Cancer Res. 2004;64:5063–5067. doi: 10.1158/0008-5472.CAN-04-0771. [DOI] [PubMed] [Google Scholar]
- 5.Liao X, Wu Y, De Brabander J. Angew Chem Int Ed. 2003;42:1648–1652. doi: 10.1002/anie.200351145. [DOI] [PubMed] [Google Scholar]
- 6.Jin M, Taylor RE. Org Lett. 2005;7:1303–1305. doi: 10.1021/ol050070g. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh AK, Xu X, Kim JH, Xu CX. Org Lett. 2008;10:1001–1004. doi: 10.1021/ol703091b. [DOI] [PubMed] [Google Scholar]
- 8.Evans DA, Welch DS, Speed AWH, Moriz GA, Reichelt A, Ho S. J Am Chem Soc. 2009;131:3840–3841. doi: 10.1021/ja900020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams DR, Nag PP, Zorn N. Curr Opin Drug Discovery Dev. 2008;11:251–271. [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor RT, Zhao Z, Wünsch S. C R Chimie. 2008;11:1369–1381. [Google Scholar]
- 11.Smith AB, III, Cox JM, Furuichi N, Kenesky CS, Zheng J, et al. Org Lett. 2008;10:5501–5504. doi: 10.1021/ol8019132.and references therein Prantz K, Mulzer J. Angew Chem Int Ed. 2009;48:5030–5033. doi: 10.1002/anie.200901740.Ghosh AK, Xu X. Intl Pat Appl No PCT/US2009/030597, Pub No WO/2009/089450 Jonathan A, Xu CX, Xu X, West LM, Wilmes A, Chan A, Hamel E, Miller JH, Northcote PT, Ghosh AK. J Org Chem. 2010;75:2–10. doi: 10.1021/jo9021265.Wullschleger CW, Gertsch J, Altmann KH. Org Lett. 2010;12:1120–1123. doi: 10.1021/ol100123p.Prantz K, Mulzer J. Chem Eur J. 2010;16:485–506. doi: 10.1002/chem.200901567.
- 12.Hoye TR, Peck DR, Swanson TA. J Am Chem Soc. 1984;106:2738–2739. [Google Scholar]
- 13.Hoye TR, Jeffrey CS, Tennakoon MA, Wang J, Zhao H. J Am Chem Soc. 2004;126:10210–10211. doi: 10.1021/ja046385t. [DOI] [PubMed] [Google Scholar]
- 14.Stocker BL, Teesdale-Spittle P, Hoberg JO. Eur J Org Chem. 2004:330–336. [Google Scholar]
- 15.Roulland E, Ermolenko MS. Org Lett. 2005;7:2225–2228. doi: 10.1021/ol050588k. [DOI] [PubMed] [Google Scholar]
- 16.Hoye TR, Ryba TD. J Am Chem Soc. 2005;127:8256–8257. doi: 10.1021/ja051604b. [DOI] [PubMed] [Google Scholar]
- 17.Jung M, Miller MJ. Tetrahedron Lett. 1985;26:977–980. [Google Scholar]
- 18.Hoye TR, Kopel LC, Ryba TD. Synthesis. 2006:1572–1574. [Google Scholar]
- 19.Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483–2547. [Google Scholar]
- 20.Hoye TR, Eklov BM, Ryba TD, Voloshin M, Yao LJ. J Org Lett. 2004;6:953–956. doi: 10.1021/ol049979+. [DOI] [PubMed] [Google Scholar]
- 21.Wong MYH, Gray GR. J Am Chem Soc. 1978;100:3548–3553. [Google Scholar]
- 22.Orita A, Sakamoto K, Hamada Y, Mitsutome A, Otera J. Tetrahedron. 1999;55:2899–2910. [Google Scholar]
- 23.Nakata T, Tanaka T, Oishi T. Tetrahedron Lett. 1983;24:2653–2656. [Google Scholar]
- 24.Mislow K, Siegel J. J Am Chem Soc. 1984;106:3319–3328. [Google Scholar]
- 25.Nakatsuka M, Ragan JA, Sammakia T, Smith DB, Uehling DE, Schreiber SL. J Am Chem Soc. 1990;112:5583–5601. [Google Scholar]
- 26.Paquette L, Mitzel T. J Am Chem Soc. 1996;118:1931–1937. [Google Scholar]
- 27.Kopel LC. PhD Thesis. University of Minnesota; Minneapolis, MN: 2008. [Google Scholar]
- 28.We surmised that loading of ruthenium to this diene occurred preferentially at the mono-substituted alkene to give an intermediate alkylidene 22b, whose rate of cyclization was simply too slow vis-à-vis catalyst lifetime. This result stands in contrast to successful RCM closures to medium-ring silaketals containing less hindered alkenes: Promo MA, Hoye TR. Tetrahedron Lett. 1999;40:1429–1432.Evans PA, Cui J, Buffone GP. Angew Chem, Int Ed. 2003;42:1734–1737. doi: 10.1002/anie.200250486.Gaich T, Mulzer J. Org Lett. 2005;7:1311–1313. doi: 10.1021/ol0500923.
- 29.RRCM within the substrate 5a would proceed via the more substituted ruthenium alkylidene analog of 22b, following metal loading and ejection of 3-methylcyclopentene from within the relay moiety.
- 30.Hoye TR, Ye ZX, Yao LJ, North JT. J Am Chem Soc. 1996;118:12074–12081. and references therein. [Google Scholar]
- 31.Evans DA, Rieger DL, Jones TK, Kaldor SW. J Org Chem. 1990;55:6260–6268. [Google Scholar]
- 32.Grieco PA, Nishizawa M, Marinovic N, Ehmann WJ. J Am Chem Soc. 1976;98:7102–7104. doi: 10.1021/ja00422a072. [DOI] [PubMed] [Google Scholar]
- 33.Morrill TC, D’Souza CA. Organometallics. 2003;22:1626–1629. [Google Scholar]
- 34.Karama U, Höfle G. Eur J Org Chem. 2003:1042–1049. [Google Scholar]
- 35.Ding Y, Zhao G. J Chem Soc, Chem Commun. 1992:941–942. [Google Scholar]
- 36.Tsuji J. Proc Japan Acad. 2004;80(B):349–358. [Google Scholar]
- 37.Cowden CJ, Paterson I. Org React. 1997;51:1–200. [Google Scholar]
- 38.Jeon J. PhD Thesis. University of Minnesota; Minneapolis, MN: 2009. [Google Scholar]
- 39.Paterson I, Di Francesco ME, Kühn T. Org Lett. 2003;5:599–602. doi: 10.1021/ol034035q. [DOI] [PubMed] [Google Scholar]
- 40.Meng D, Bertinato P, Balog A, Su DS, Kamenecka T, et al. J Am Chem Soc. 1997;119:10073–10092. [Google Scholar]
- 41.Interestingly, Evans et al. reported that the oxidation state of C9 strongly influenced the diastereoselectivity and yield of boron-mediated aldol coupling between substrates quite similar to acceptor aldehyde 2 and donor ketone 3. “For example, the reaction does not proceed if the C9-carbonyl group is reduced and protected.”[8] The contrasting behavior we observed in the successful aldol union of 2 with 3, the former containing a PMB-protected hydroxy group at C9, points to additional, likely yet longer-range, structure-reactivity subtleties in the powerful aldol cross-coupling[35] strategy.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.