Abstract
Endosomal sorting complexes required for transport (ESCRTs) execute the biogenesis of late endosomal multivesicular bodies. The ESCRT pathway has traditionally been viewed as a means by which transmembrane proteins are degraded in vacuoles/lysosomes. More recent studies aimed at understanding the broader functions of ESCRTs have uncovered unexpected links with pathways that control cellular metabolism. Central to this communication is TORC1, the kinase complex that controls many of the catabolic and anabolic systems. The connection between TORC1 activity and ESCRTs allows cells to quickly adapt to the stress of nutrient limitations until the longer-term autophagic pathway is activated. Increasing evidence also points to ESCRTs regulating RNA interference pathways that control translation.
Introduction
Eukaryotic cells transport proteins and lipids to the vacuole/lysosome for degradation and recycling of amino acids and fatty acids that can be used for new protein and lipid synthesis. The importance of the vacuolar/lysosomal degradation system for normal metabolic function of the cell becomes evident when extracellular nutrients are scarce. Starvation dramatically increases the influx of cellular proteins into this system to harvest amino acid resources required for survival. The starvation-induced autophagy pathway is well established [1,2]. In this review, we focus on a second pathway in the starvation response whereby transmembrane proteins are targeted for vacuolar/lysosomal degradation via the multivesicular bodies. Recent studies indicate this degradation pathway is tightly linked to cellular metabolism, not only for stress response, but also as part of an overall regulatory system that balances anabolic and catabolic pathways. Because much of our understanding of the MVB pathway derives from studies in Saccharomyces cerevisiae, we will use yeast protein and organelle nomenclature unless stated otherwise.
Regulating the ubiquitination of MVB pathway cargoes
The topological problem of degrading transmembrane proteins is solved by the formation of MVBs, which package these proteins into intralumenal vesicles (ILVs) that are delivered into the hydrolytic lumen of the vacuole (Figure 1). The formation of ILVs and sorting of their transmembrane protein cargoes is executed by a set of five distinct cytosolic protein complexes called ESCRT-0, -I, -II, -III and the Vps4 complex [3]. These complexes transiently assemble on the endosomal membrane into a protein network that drives ILV formation by a poorly understood mechanism (reviewed in [4]).
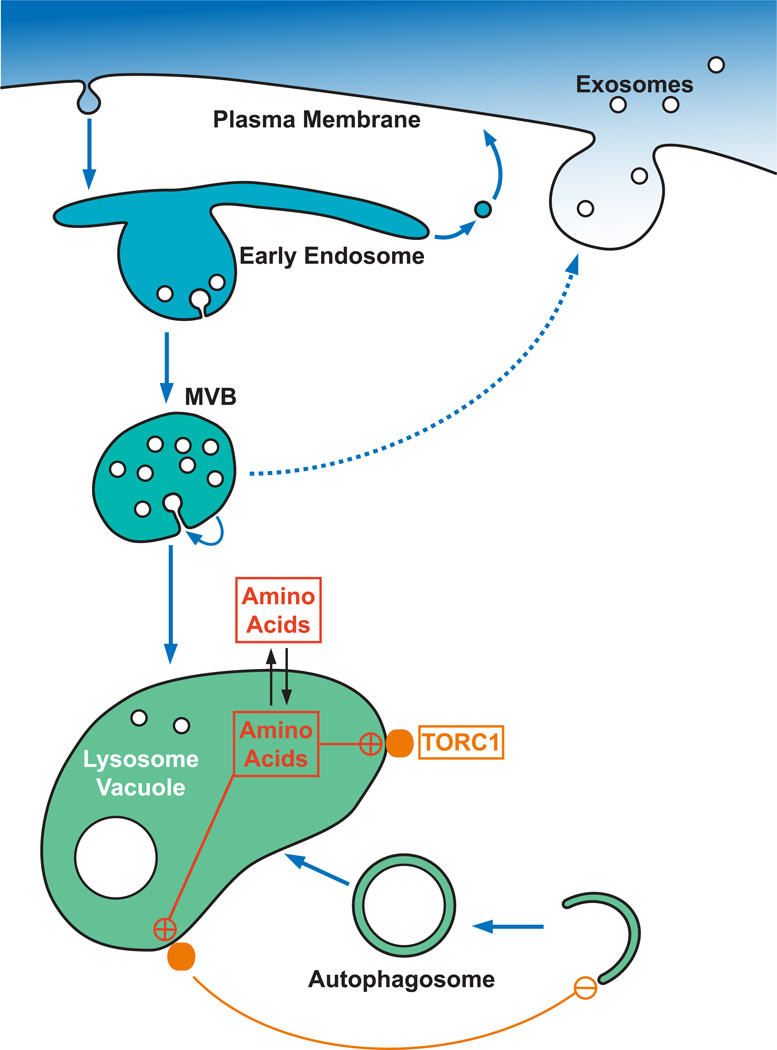
Figure 1.
Model of the endocytic pathway of eukaryotic cells. Plasma membrane proteins are endocytosed and delivered to an early endosome from where they either recycle or continue on the way to the vacuole/lysosome. At the MVB, protein cargoes destined for degradation are packaged into ILVs. Upon fusion of the MVB with the vacuole/lyosome, the ILVs are exposed to the hydrolytic environment and both proteins and lipids are degraded. Alternatively, certain MVBs fuse with the plasma membrane and release the ILVs, termed exosomes, into the extracellular melieu. TORC1 localizes to the vacuolar membrane where it senses the amino acid content of the compartment. High amino acid levels activate TORC1 which in turn suppresses the autophagy pathway.
For most ILV cargoes, ubiquitination serves as the sorting signal that mediates their interaction with ESCRT machinery. The ubiquitination reaction in yeast is executed by the ubiquitin ligase Rsp5 and is a key regulatory step that decides the fate of plasma membrane proteins [5]. For many cell-surface proteins, ubiquitination occurs at the plasma membrane, which triggers their endocytosis and subsequent delivery to endosomes. However, the mechanism that determines when a particular protein undergoes ubiquitination is not always known and can vary, depending on the type of plasma membrane protein and the state of the cell. While many receptors that activate intracellular signaling cascades are ubiquitinated and degraded via the MVB pathway in response to their activation (e.g., pheromone receptors in yeast and growth factor receptors in metazoans) [6], the stability of most plasma membrane nutrient transporters is regulated by the concentration of the solute they pump (amino acids, saccharides, etc.). High solute concentration causes ubiquitination and degradation of the corresponding transporter, whereas low solute concentration stabilizes it [7]. In the case of the uracil transporter Fur4, a protein-intrinsic system triggers its ubiquitination when bound to substrate, indicating that the transporter regulates its own turnover rate to keep the cytoplasmic concentration of uracil within a physiological range [8] (Figure 2). Ubiquitination of many transporters is also regulated on a cellular level by arrestin-related trafficking adaptors (Art proteins). Yeast express at least 10 distinct Art proteins, each of which binds a specific set of transporters to recruit Rsp5 [9–13]. The activity of each Art is regulated by phosphorylation and ubiquitination, which allows the cell to modulate the turnover rate of nutrient transporters targeted by a specific Art [14,15]. Because the nutrients imported by transporters are integral to cellular metabolism, regulating Art proteins might allow adjustment of cell-surface transporter activity according to metabolic needs.
Figure 2.
Model of the regulatory interactions between endocytosis, MVB sorting and protein translation. The active TORC1 kinase suppresses ubiquitin-dependent endocytosis of nutrient transporters and increases translation efficiency. High translation efficiency, in turn, lowers ESCRT-mediated ILV formation at the MVB (via Ist1). ILV formation at the MVB is important for the assembly of active RISC which lowers expression of specific mRNAs by inhibiting translation or inducing mRNA degradation.
Survival during starvation
The acute metabolic stress of starvation triggers rapid degradation of a large number of transporters in unison [16–21]. This stress response seems to be the result of upregulating both ubiquitin-dependent endocytosis from the plasma membrane and ESCRT-mediated sorting at endosomes. Gap1, the general amino acid permease, is an exception, in that starvation stabilizes Gap1 at the plasma membrane [13,22]. Therefore, cells respond to amino acid depletion by degrading specialized amino acid transporters and upregulating the general transporter Gap1.
Yeast ESCRT mutants die more readily than do wild-type cells when they reach stationary phase, a condition in which nutrients become limiting. Depriving individual amino acids is sufficient to expose this Achilles’ heel [21]. The reduction in viability of ESCRT-mutant yeast appears to stem from the central role MVBs have in transmembrane protein degradation at vacuoles. In the short term, their degradation can provide amino acids needed for producing proteins required for cells to immediately adapt to starvation stress. One such adaptation is the induction of autophagy, a long-term starvation response that requires new protein synthesis. Therefore protein degradation by the MVB pathway buys the cell time until autophagy kicks in to sustain cellular viability if starvation is prolonged. Such need for maintaining sufficient amino acid levels during acute starvation is shared by human cells [23].
Starvation-induced endocytosis
Both Rsp5 and the Art proteins are substrates for Npr1, a kinase that is a downstream effector of the Tor complex 1 (TORC1) [14,24]. TORC1 is a highly conserved protein complex containing the protein kinase known as target of rapamycin (Tor in yeast, mTor in mammalian cells). TORC1 monitors the metabolic state of the cell [25,26]. The presence of amino acids, glucose, and other nutrients activates TORC1, which in turn promotes anabolic functions such as protein production and suppresses starvation response pathways, including autophagy. Loss of TORC1 activity, induced either by starvation or the presence of the antibiotic rapamycin, increases endocytosis of nutrient transporters, in part by activating Npr1 (Figure 2).
Phosphorylation of Rsp5, Arts, and nutrient transporters by Npr1 could account for the increase in transporter ubiquitination and subsequent endocytosis [13,14,22,24,27,28] but the effect of these phosphorylation events is controversial. A recent study found rapamycin-induced phosphorylation of Art proteins suppresses recruitment of Rsp5 and concomitant ubiquitination and degradation of cell-surface transporters [14]. Other studies found, instead, that starvation induces degradation of cell-surface transporters. This discrepancy could originate from the use of rapamycin as a way to induce the starvation-response pathways. Although rapamycin blocks TORC1 activity to mimic starvation, gene expression profiling identified clear differences in the cellular response to rapamycin versus amino acid depletion [29]. Moreover, genetic disruption of Npr1 inhibits rapamycin-induced downregulation of transporters but not increased transporter degradation caused by amino acid deprivation [21], suggesting at least two regulatory systems, a TORC1-dependent and a TORC1-independent pathway.
Regulation of ESCRTs by starvation-response pathways
To handle the influx of transporters and other proteins targeted for degradation in response to starvation, ESCRT activity is upregulated through Ist1 [30,31]. Depending upon its cellular abundance, Ist1 can either promote or inhibit ESCRT activity. At low levels, Ist1 promotes recycling of the ESCRTs, which sustains MVB function by enabling further rounds of ESCRT activity, but at high levels, Ist1 stalls the cycle of ESCRT activity and thus impairs the MVB pathway [30]. The amount of Ist1 is high when wild-type yeast are grown in rich medium but drops precipitously when cells are starved, precisely when levels of intracellular amino acids plummet [21]. That Ist1 depletion is important for starvation survival is emphasized by the poor viability of starved cells when high Ist1 expression is artificially maintained [21].
Ist1 control of ESCRT activity is indirectly regulated by TORC1. High levels of intracellular amino acids stimulate TORC1 signaling, which promotes protein synthesis, including that of Ist1 (Figure 2). This situation is reversed upon amino acid starvation or rapamycin treatment. The drop in Ist1 levels coincides precisely with phosphorylation of the eIF2 translation initiation factor α subunit [21], which is a well known consequence of reduced TORC1 activity that represses general protein synthesis to favor translation of mRNAs that encode proteins essential for cellular adaptation to amino acid starvation (reviewed in [32]). Together, the data suggest that lower Ist1 levels are a consequence of reduced translational activity during starvation, combined with a high turnover rate of Ist1. Therefore, Ist1 forms a regulatory link between the metabolic state of the cell and protein degradation via the MVB pathway: rich nutrient conditions promote cell growth by increasing synthesis of Ist1 and other proteins, thereby suppressing protein degradation, whereas starvation inhibits synthesis of Ist1 and non-essential proteins and also generally promotes protein turnover.
The ESCRTs are important for Gcn4 and TORC1 function
Amino acid starvation not only causes rapid shutdown of general translation but also induces translation of a set of starvation-response proteins, such as the Gcn4 transcription factor (see review [33]). Gcn4 regulates expression of numerous genes, including those of amino acid biosynthesis pathways. The goal of this regulatory system is to conserve amino acids and initiate pathways of amino acid synthesis. Surprisingly, mutations that either disrupt trafficking to endosomes or impair the MVB pathway inhibit transcriptional up-regulation of Gcn4 target genes [34]. Furthermore, the vacuolar delivery of proteins from the Golgi via the MVB pathway is critical for Gln3 mediated transcription of genes involved in nitrogen-starvation response [35]. In yeast mutants defective in Golgi-to-endosome trafficking, TORC1 regulated nuclear translocation of Gln3 is impaired, suggesting a link between endosomal trafficking and Tor signaling. This link is also observed in mammalian cells, where studies demonstrated the importance of a normal endosomal system for mTOR activity [36].
A likely reason for the close relationship between TORC1 and endosomal trafficking is the fact that both in yeast and higher eukaryotes, TORC1 localizes at least transiently to the cytosolic side of endosomal/lysosomal membranes [37,38] where it appears to monitor the amino acid pool stored within vacuoles/lysosomes (Figure 1). One explanation for how this sensation is achieved is based on the dependence of TORC1 activation on late endosomal/lysosomal localization of PAT1, a mammalian amino acid transporter [39]. PAT1 interacts with the Rag GTPases that function in the activation of TORC1, suggesting PAT1 functions not only as a transporter that pumps amino acids from the lysosome to the cytoplasm but also as a sensor for the presence of amino acids in the lysosomal lumen. In this regard, PAT1 seems to function as a “transceptor,” a transporter with signaling function (transporter and receptor). A well-studied transceptor in yeast is Gap1, which not only imports amino acids but, when active, also signals via protein kinase A the presence of extracellular amino acids [40]. The idea that PAT1 might signal to TORC1 the presence of lysosomal amino acid storage levels would also explain why the vacuolar ATPase (v-ATPase) is required for mTORC1 activation [41]. v-ATPase is the ATP-driven proton pump that acidifies the lumen of the endo-lysosomal compartments. Because amino acid transport by PAT1 is proton-driven, loss of the proton gradient would block PAT1 function and thus TORC1 activation.
Taken together, recent studies make a strong argument for a tight link between TORC1 signaling and the catabolic functions of the vacuole/lysosome. This link explains the importance of the MVB pathway, and thus ESCRT function, for proper TORC1 signaling and regulation of starvation-response pathways. The MVB pathway is responsible for the delivery of transmembrane proteins to the lumen of the vacuole for degradation. The resulting amino acids are transported to the cytoplasm for re-use in protein synthesis, thereby activating TORC1. Consistent with this idea, mutations in the yeast MVB pathway exhibit increased expression of the autophagy protein Atg8 even in presence of rich growth medium, a result that indicated decreased TORC1 activity in ESCRT-mutant cells [21].
ESCRTs and translational regulation
In higher eukaryotes, ESCRT function at the MVB is also linked to RNA interference (RNAi) mediated by small interfering RNAs (siRNAs) and micro RNAs (miRNA). These RNAs originate from both intrinsic and external sources and are bound by Argonaute proteins (AGO), which then assemble with other factors to form RISCs (RNA-induced silencing complexes). RISC activity lowers expression of a specific protein by initiating degradation or inhibiting translation of its corresponding mRNA (reviewed in [42]). Studies suggest that siRNA-mediated silencing is affected by endosomal protein trafficking, which in part could be explained by the observation that RISC assembly occurs on MVBs [43–45]. Mutations in ESCRTs impair RISC assembly and thus cause inefficient RNAi. The precise role of ESCRTs in RISC formation/activity is unknown. It has been suggested that the observed packaging of the RISC component TNRC6/GW182 into ILVs might be important for the disassembly and recycling of the RISC proteins in order to re-engage new miRNAs ([43], Figure 2).
The localization of RISC components to MVBs could explain why certain mRNAs and miRNAs are packaged into exosomes, which are ILVs secreted from cells upon the fusion of MVBs with the plasma membrane [46]. The physiological role of exosomes is not fully understood, but increasing evidence suggests they represent a type of long distance signaling in which RNA packaged into exosomes in one cell can regulate protein expression in distant target cells (discussed in [47–49]). ESCRTs are not only essential in exosome formation but might also be responsible for sorting RNA molecules into ILVs that will become exosomes. In this regard, it is interesting to note that ESCRT-II has been shown in Drosophila to function as a RNA-binding complex [50].
Concluding remarks
In recent years an increasing number of studies have identified connections between the endosomal system and many regulatory pathways of the cell. The endosome seems to function as a hub for the convergence of cell signaling, environmental sensing, and metabolic control. Recent progress has revealed how the degradative function of the endosomal system, and thus the activity of the ESCRTs, is linked to regulatory systems that control metabolism and cell growth. This tight link between catabolic and anabolic activities of the cell is necessary because of the low storage capacity for energy and nutrients within cells. When extracellular nutrients become scarce, small metabolites such as sugars, amino acids, and fatty acids are rapidly depleted, and cells must rely on their existing macromolecules to remain viable and adapt to the new environmental condition.
Acknowledgements
The research conducted in the Odorizzi and Babst laboratories on the ESCRT proteins is supported by NIH Grants R01 GM065505 and R01 GM074171, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 5.Belgareh-Touze N, Leon S, Erpapazoglou Z, Stawiecka-Mirota M, Urban-Grimal D, Haguenauer-Tsapis R. Versatile role of the yeast ubiquitin ligase Rsp5p in intracellular trafficking. Biochem Soc Trans. 2008;36:791–796. doi: 10.1042/BST0360791. [DOI] [PubMed] [Google Scholar]
- 6.Wegner CS, Rodahl LM, Stenmark H. ESCRT Proteins and Cell Signalling. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 7.Lauwers E, Erpapazoglou Z, Haguenauer-Tsapis R, Andre B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010;20:196–204. doi: 10.1016/j.tcb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 8. Keener JM, Babst M. Quality control and substrate-dependent downregulation of the nutrient transporter fur4. Traffic. 2013;14:412–427. doi: 10.1111/tra.12039. ** This paper describes the molecular mechanism that triggers degradation of a transporter either by unfolding or high nutrient concentrations.
- 9. Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135:714–725. doi: 10.1016/j.cell.2008.09.025. ** This study characterizes the role of a group of arrestin-related proteins in the degradation of cell-surface transporters.
- 10.Nikko E, Pelham HR. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic. 2009;10:1856–1867. doi: 10.1111/j.1600-0854.2009.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9:1216–1221. doi: 10.1038/embor.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatakeyama R, Kamiya M, Takahara T, Maeda T. Endocytosis of the aspartic acid/glutamic acid transporter Dip5 is triggered by substrate-dependent recruitment of the Rsp5 ubiquitin ligase via the arrestin-like protein Aly2. Mol Cell Biol. 2010;30:5598–5607. doi: 10.1128/MCB.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merhi A, Andre B. Internal amino acids promote Gap1 permease ubiquitylation via TORC1/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol Cell Biol. 2012;32:4510–4522. doi: 10.1128/MCB.00463-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacGurn JA, Hsu PC, Smolka MB, Emr SD. TORC1 regulates endocytosis via Npr1-mediated phosphoinhibition of a ubiquitin ligase adaptor. Cell. 2011;147:1104–1117. doi: 10.1016/j.cell.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 15. Becuwe M, Vieira N, Lara D, Gomes-Rezende J, Soares-Cunha C, Casal M, Haguenauer-Tsapis R, Vincent O, Paiva S, Leon S. A molecular switch on an arrestin-like protein relays glucose signaling to transporter endocytosis. J Cell Biol. 2012;196:247–259. doi: 10.1083/jcb.201109113. * The study demonstrates how the regulation of an Art protein affects the turnover rate of a nutrient transporter.
- 16.Galan JM, Volland C, Urban-Grimal D, Haguenauer-Tsapis R. The yeast plasma membrane uracil permease is stabilized against stress induced degradation by a point mutation in a cyclin-like "destruction box". Biochem Biophys Res Commun. 1994;201:769–775. doi: 10.1006/bbrc.1994.1767. [DOI] [PubMed] [Google Scholar]
- 17.Penalver E, Lucero P, Moreno E, Lagunas R. Catabolite inactivation of the maltose transporter in nitrogen-starved yeast could be due to the stimulation of general protein turnover. FEMS Microbiol Lett. 1998;166:317–324. doi: 10.1111/j.1574-6968.1998.tb13907.x. [DOI] [PubMed] [Google Scholar]
- 18.Beck T, Schmidt A, Hall MN. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J Cell Biol. 1999;146:1227–1238. doi: 10.1083/jcb.146.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omura F, Kodama Y, Ashikari T. The N-terminal domain of the yeast permease Bap2p plays a role in its degradation. Biochem Biophys Res Commun. 2001;287:1045–1050. doi: 10.1006/bbrc.2001.5697. [DOI] [PubMed] [Google Scholar]
- 20.Krampe S, Boles E. Starvation-induced degradation of yeast hexose transporter Hxt7p is dependent on endocytosis, autophagy and the terminal sequences of the permease. FEBS Lett. 2002;513:193–196. doi: 10.1016/s0014-5793(02)02297-4. [DOI] [PubMed] [Google Scholar]
- 21. Jones CB, Ott EM, Keener JM, Curtiss M, Sandrin V, Babst M. Regulation of membrane protein degradation by starvation-response pathways. Traffic. 2012;13:468–482. doi: 10.1111/j.1600-0854.2011.01314.x. ** This paper demonstrates the importance of the MVB pathway for the survival of the cell during amino acid starvation.
- 22.O'Donnell AF, Apffel A, Gardner RG, Cyert MS. Alpha-arrestins Aly1 and Aly2 regulate intracellular trafficking in response to nutrient signaling. Mol Biol Cell. 2010;21:3552–3566. doi: 10.1091/mbc.E10-07-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science. 2005;310:1960–1963. doi: 10.1126/science.1121925. ** The study indicates an important role for the proteasomal pathway in the recycling of amino acids during acute starvation.
- 24.Breitkreutz A, Choi H, Sharom JR, Boucher L, Neduva V, Larsen B, Lin ZY, Breitkreutz BJ, Stark C, Liu G, et al. A global protein kinase and phosphatase interaction network in yeast. Science. 2010;328:1043–1046. doi: 10.1126/science.1176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Proud CG. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 2009;19:260–267. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaouass M, Gamache I, Ramotar D, Audette M, Poulin R. The spermidine transport system is regulated by ligand inactivation, endocytosis, and by the Npr1p Ser/Thr protein kinase in Saccharomyces cerevisiae. J Biol Chem. 1998;273:2109–2117. doi: 10.1074/jbc.273.4.2109. [DOI] [PubMed] [Google Scholar]
- 28.Martin Y, Gonzalez YV, Cabrera E, Rodriguez C, Siverio JM. Npr1 Ser/Thr protein kinase links nitrogen source quality and carbon availability with the yeast nitrate transporter (Ynt1) levels. J Biol Chem. 2011;286:27225–27235. doi: 10.1074/jbc.M111.265116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimaano C, Jones CB, Hanono A, Curtiss M, Babst M. Ist1 regulates vps4 localization and assembly. Mol Biol Cell. 2008;19:465–474. doi: 10.1091/mbc.E07-08-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rue SM, Mattei S, Saksena S, Emr SD. Novel Ist1-Did2 complex functions at a late step in multivesicular body sorting. Mol Biol Cell. 2008;19:475–484. doi: 10.1091/mbc.E07-07-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 34. Zhang F, Gaur NA, Hasek J, Kim SJ, Qiu H, Swanson MJ, Hinnebusch AG. Disrupting vesicular trafficking at the endosome attenuates transcriptional activation by Gcn4. Mol Cell Biol. 2008;28:6796–6818. doi: 10.1128/MCB.00800-08. * The paper describes an unexpected link between the activity of the transcription factor Gcn4 and endosomal function.
- 35.Puria R, Zurita-Martinez SA, Cardenas ME. Nuclear translocation of Gln3 in response to nutrient signals requires Golgi-to-endosome trafficking in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2008;105:7194–7199. doi: 10.1073/pnas.0801087105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flinn RJ, Yan Y, Goswami S, Parker PJ, Backer JM. The late endosome is essential for mTORC1 signaling. Mol Biol Cell. 2010;21:833–841. doi: 10.1091/mbc.E09-09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturgill TW, Cohen A, Diefenbacher M, Trautwein M, Martin DE, Hall MN. TOR1 and TOR2 have distinct locations in live cells. Eukaryot Cell. 2008;7:1819–1830. doi: 10.1128/EC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ogmundsdottir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DC. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS One. 2012;7:e36616. doi: 10.1371/journal.pone.0036616. * The study demonstrates a regulatory link between the amino acid transporter PAT1 and TORC1. This observation is able to explain how TORC1 senses amino acids stored in the lumen of lysosomes.
- 40. Donaton MC, Holsbeeks I, Lagatie O, Van Zeebroeck G, Crauwels M, Winderickx J, Thevelein JM. The Gap1 general amino acid permease acts as an amino acid sensor for activation of protein kinase A targets in the yeast Saccharomyces cerevisiae. Mol Microbiol. 2003;50:911–929. doi: 10.1046/j.1365-2958.2003.03732.x. * This study reveals that Gap1 functions as a transceptor in that it not only imports amino aicds but also signals the presence of amino acids.
- 41.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Czech B, Hannon GJ. Small RNA sorting: matchmaking for Argonautes. Nat Rev Genet. 2011;12:19–31. doi: 10.1038/nrg2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. * The paper describes the endosomal localization of components of the miRNA effector complex and demonstrates the importance of normal endosomal funciton for effective RNAi.
- 44. Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ, Li X, Lubell K, Lim do H, Cho IS, et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009;11:1150–1156. doi: 10.1038/ncb1930. * The paper demonstrates that endosomal trafficking affects siRNA-mediated silencing.
- 45.Harris DA, Kim K, Nakahara K, Vasquez-Doorman C, Carthew RW. Cargo sorting to lysosome-related organelles regulates siRNA-mediated gene silencing. J Cell Biol. 2011;194:77–87. doi: 10.1083/jcb.201102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 47.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 48.Gibbings D, Voinnet O. Control of RNA silencing and localization by endolysosomes. Trends Cell Biol. 2010;20:491–501. doi: 10.1016/j.tcb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 49.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 50. Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–558. doi: 10.1038/nature05503. * The study demonstrates that ESCRT-II is able to bind to a specific mRNA and affect its subcellular localization.