Abstract
Myocardial substrate metabolism provides the energy needed for cardiac contraction and relaxation. The normal adult heart uses predominantly fatty acids (FAs) as its primary fuel source. However, the heart can switch and use glucose (and to a lesser extent, ketones, lactate, as well as endogenous triglycerides and glycogen), depending on the metabolic milieu and superimposed conditions. FAs are not a wholly better fuel than glucose, but they do provide more energy per mole than glucose. Conversely, glucose is the more oxygen-efficient fuel. Studies in animal models of heart failure (HF) fairly consistently demonstrate a shift away from myocardial fatty acid metabolism and towards glucose metabolism. Studies in humans are less consistent. Some show the same metabolic switch away from FA metabolism but not all. This may be due to differences in the etiology of HF, sex-related differences, or other mitigating factors. For example, obesity, insulin resistance, and diabetes are all related to an increased risk of HF and may complicate or contribute to its development. However, these conditions are associated with increased FA metabolism. This review will discuss aspects of human heart metabolism in systolic dysfunction as measured by the noninvasive, quantitative method – positron emission tomography. Continued research in this area is vital if we are to ameliorate HF by manipulating heart metabolism with the aim of increasing energy production and/or efficiency.
Keywords: heart failure, systolic dysfunction, positron emission tomography, obesity, fatty acids, glucose
Heart failure (HF) is a major public health problem. It affects more than 5.8 million people in the United States, 14 million in Europe, and millions more worldwide (http://www.worldheartfailure.org/index.php?item=75). HF is the #1 reason for hospital admission in both men and women. Despite recent advances in medical and surgical therapy, patients with HF have a 5 y mortality rate of ∼50%, which is worse than most cancers. The cornerstone of medical treatment for HF traditionally has been pharmacological antagonism of the sympathetic nervous and renin-angiotensin-aldosterone systems. However, even with extensive blockade of these systems, the mortality rate for HF remains unacceptably high. Thus, new and different approaches to the treatment of HF are needed.
One attractive target for treatment is myocardial substrate metabolism. Heart substrate metabolism is needed for the generation of energy (in the form of adenosine triphosphate, ATP) that is required for both contractile and relaxation work. If this process can be made more efficient (i.e., more work/oxygen consumed), productive (i.e., more ATP made), and/or economical (i.e., less ATP required), then heart function in HF may improve. In addition, because excessive uptake and/or oxidation of certain substrates may actually contribute to the development of HF in certain conditions, the restoration of a more normal pattern of substrate utilization might potentially ameliorate HF.
In order to understand how heart metabolism in HF might be manipulated, we must first understand normal human heart metabolism. Next, our focus shifts to myocardial metabolism in some of the main conditions (such as diabetes) that can cause or accompany HF in humans. Finally, the research will examine the primary changes in myocardial fatty acid (FA) and glucose metabolism in systolic HF. Having reviewed the myocardial metabolic phenotypes of systolic HF, and the conditions that contribute to it, we will discuss the impact of standard HF therapies and metabolic modulator drugs on human heart metabolism.
Normal myocardial metabolism
The ever-beating heart has a continual need for energy in the form of ATP to fuel its contractile machinery and the ionic pumps that serve to regulate its function. A smaller, but still not insignificant, amount of ATP is also needed to support other cellular processes, e.g., protein synthesis, which proceeds at a rate 2- to 3-fold faster than in skeletal muscle [1] and accounts for ∼10% of the heart's energy requirement [2]. In total, the heart utilizes, and hence must also synthesize, more than 5 kg of ATP every day [3], or nearly 2 metric tons of ATP every year.
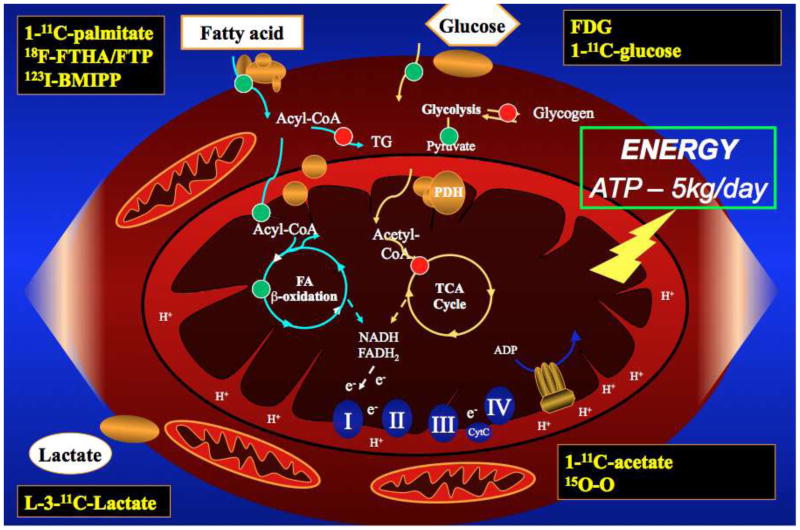
In the normal (i.e., well-perfused/oxygenated), adult heart, this high demand for ATP is met almost exclusively by mitochondrial oxidative phosphorylation. The reducing equivalents (i.e., nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2)) required to drive this process are generated primarily by degradation of acetyl coenzyme A (acetyl CoA) in the tricarboxylic acid (TCA) cycle and also by ß-oxidation of long-chain FAs. After an overnight fast, the heart obtains approximately two-thirds of its energy needs from circulating non-esterified (“free”) FAs (and also the FA moiety of plasma triglycerides (TG)), with the remainder coming from circulating glucose, lactate, and, to a much lesser extent, ketone bodies and amino acids [4]. A cartoon of the myocyte's substrate metabolism and the PET tracers that are used to quantify it is shown in Figure 1. Oxidation of 1 mole of a typical FA yields an estimated 106 to 129 mol of ATP, whereas oxidation of a mole of glucose yields only 36-38 mol of ATP. In the presence of hyperglycemia and hyperinsulinemia (e.g., the fed state), however, the utilization of FFA by the heart falls dramatically while that of glucose increases several-fold [5]. Utilization of lactate or ketone bodies becomes much more prominent during high intensity exercise [6, 7] or prolonged fasting [8], respectively, reflecting the increased arterial concentrations of these substrates. Data such as these illustrate the metabolic flexibility of the normal heart, i.e., its ability to meet its ongoing energy needs by constantly and rapidly altering its pattern of fuel utilization to adapt to the substrate and hormone environment. This differentiates the heart from some other tissues, especially the brain, which normally depends almost exclusively on glucose as an energy source. At the same time, however, the strong dependence of cardiac substrate utilization on extra-cardiac metabolism makes the heart potentially susceptible to deleterious changes originating in other tissues (e.g., skeletal muscle).
Figure 1.
Cardiomyocyte substrate metabolism pathways. 18F-FHTA/FTP = 18fluoro-6-thia-heptadecanoate/fluoro-4-thia-palmitate. 123I-BMIPP = Iodofilitic acid. FDG = fluorodeoxyglucose.
Conditions that may contribute to and/or coexist with HF: their impact on myocardial metabolism
While there are many possible contributors to HF, among the most prevalent are obesity, diabetes, hypertension, and ischemia. It is important to understand the effects that each of these conditions has on human myocardial metabolism as they may influence the heart metabolism of patients with these pre-existing conditions if they go on to develop HF. These co-existing conditions may also be reasonably expected to affect response to metabolic modulator treatment.
Obesity
Obesity is now considered a major risk factor for the development of HF [9]. This is true even in the absence of other co-morbidities often associated with obesity (e.g., diabetes, hypertension). Obesity is very prevalent in patients with HF [[10]. In young women without HF, obesity has a characteristic myocardial metabolic pattern: increased myocardial blood flow (MBF), oxygen consumption (MVO2), and FA metabolism as quantified by positron emission tomography (PET) [11]. In fact, body mass index (BMI) was the only independent predictor of MBF and MVO2 in a study of young women [11]. BMI also directly related to myocardial FA metabolism, as did glucose area under the curve, a measure of insulin resistance [11]. This is likely at least in part due to the fact that with increasing BMI, there is increased fat mass, overall turnover of FAs, as well as increased MBF. Interestingly, men did not appear to respond to obesity in the same manner as women. In contrast to the women, men who were obese did not have higher MBF or MVO2 compared with nonobese men [12]. Obese men did have higher myocardial FA oxidation and oxidation than nonobese men, similar to what was seen in women. However, in multivariate analyses, sex was a much stronger independent predictor of myocardial FA metabolism than obesity [12]. Further highlighting the complexity of myocardial metabolism, sex and obesity interact in the prediction of both myocardial glucose uptake and glucose utilization/plasma insulin level [12]. Exactly how obesity (and sex) may affect myocardial metabolism when HF develops is an area of intense research currently.
Diabetes
Diabetes is also a major risk factor for the development of HF [13]. Epidemiologic evidence suggests that the unadjusted relative risk for the development of HF in diabetic men is ∼2.8, and it is even higher in diabetic women at ∼8 [13]. Even after adjustment for age, cigarette smoking, cholesterol level, left ventricular (LV) hypertrophy, and systolic blood pressure, diabetic men and women still have a much higher risk of HF than nondiabetic individuals. Animal and human autopsy studies suggest that excessive myocardial FA delivery and lipid deposition is linked with diabetes and cardiac dysfunction. Excessive FA delivery to and uptake by the heart in animals can directly cause dysfunction through a process known as “lipotoxicity.” In this process, excessive FA uptake by the heart contributes to ceramide and/or reactive oxygen species production, which can cause apoptosis [14, 15]. Alternatively, myocardial excessive reactive oxygen species production (and other processes) may cause dysfunction without causing cell death. The myocardial metabolic “fingerprint” of patients with type 1 diabetes (but without HF) shows that these patients have higher myocardial FA utilization, oxidation, and % FA oxidation than nondiabetic subjects [16]. This is in part due to higher plasma FA levels in type 1 diabetes. Myocardial glucose use as quantified by PET, on the other hand, is lower in these diabetic patients [16]. Congruent with these findings is the fact that myocardial oxygen consumption is also higher in the type 1 diabetic patients than controls (since FAs are a less oxygen efficient fuel than glucose) [16].
The hearts of patients with type 2 diabetes, like those with type 1 diabetes, have a higher consumption of FAs. Although many patients with type 2 diabetes are also obese, diabetes is associated with an even more pronounced myocardial metabolic derangement than obesity alone. For example, in one study of three groups of women, i.e., normal, obese, and diabetic, FA utilization and oxidation were higher in the diabetic patients than in either the normal or the obese subjects [17]. Conversely, fractional myocardial glucose uptake was lower in the patients with diabetes than in the other two groups [17]. In another PET study of both men and women, diabetes was again related to higher myocardial FA utilization, oxidation, and esterification rates compared to nondiabetic controls [18]. Thus, similar to the case with obesity and type 1 diabetes, type 2 diabetes is generally associated with increased myocardial use of FAs. Whether patients with these conditions maintain this characteristic shift towards increased reliance on myocardial FA metabolism if they develop HF is not yet completely clear.
Myocardial metabolism in systolic HF
The pathogenesis of HF includes alterations in hemodynamics, up-regulation of the neuro-hormonal axis (specifically the sympathetic nervous system and the renin-angiotensin- aldosterone system), and changes in metabolic substrate use. Whether changes in myocardial metabolism are adaptive or maladaptive likely depends upon the pathways by which the HF developed and the heart's milieu. Several animal models and in vitro studies of HF have shown a shift of myocardial metabolism from primarily FA use to glucose [19]. This has most particularly been shown in the progression from cardiac hypertrophy to ventricular dysfunction in animal models of pressure or volume overload-related HF. For example, elevations in glycolytic enzyme activation in cardiac hypertrophy and congestive HF were demonstrated in canine ventricular homogenates [20]. Guinea pigs with hypertrophied failing ventricles show defective oxidation of FAs, while maintaining their ability to oxidize glucose [21]. This shift in substrate preference mirrors that seen in the fetal heart, which relies primarily on glycolysis and lactate metabolism for energy, and in which rates of FA oxidation are very low. Newborn pigs induced with early-onset LV volume overload with hypertrophy show a delay in the normal development of FA oxidation in the newborn heart, supporting the shift from FA to glucose metabolism in HF [22]. These and other animal data have been extensively reviewed by others [19] and will not be discussed further here.
Human data
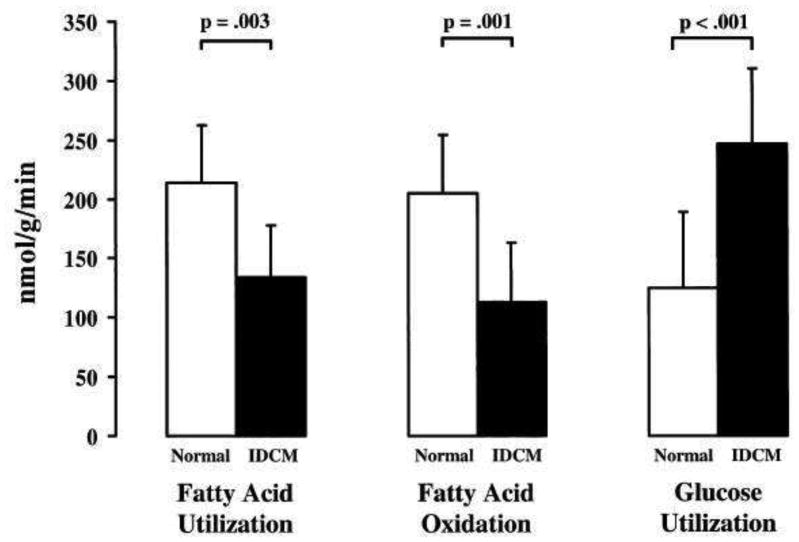
Despite the consensus of a metabolic shift from FA to glucose use in animal studies of HF, studies in humans have been fewer and more conflicting. A study in adult humans with idiopathic dilated cardiomyopathy (IDCM) using PET for determination of substrate utilization showed that FA utilization, including FA oxidation, was significantly decreased, while rates of glucose utilization were increased compared to normal controls, see Figure 2 [23]. In contrast, another PET study examining a mixed population of ischemic and non-ischemic cardiomyopathy subjects found increased rates of FA uptake compared to glucose [24]. Although the majority of the patients in this study had ischemic cardiomyopathy, the PET analyses used only non-ischemic regions of interest as assessed by blood flow and normal contractility on echocardiogram. Given that these regions have higher relative workload compared to regions with decreased contractility, it is unclear if extrapolations can be made to metabolism in global LV dysfunction, potentially explaining the conflicting results from these two studies. In addition, there is a study of mostly non-ischemic (and 1 ischemic) cardiomyopathy patients and normal controls using invasive arterio-coronary sinus measurements that demonstrated no difference in FA uptake between the groups [25]. FA oxidation was not measured in this study.
Figure 2.
PET-determined rates of myocardial FA utilization and oxidation and glucose utilization in patients with idiopathic dilated cardiomyopathy (IDCM) vs. normal controls. From Ref. [23]
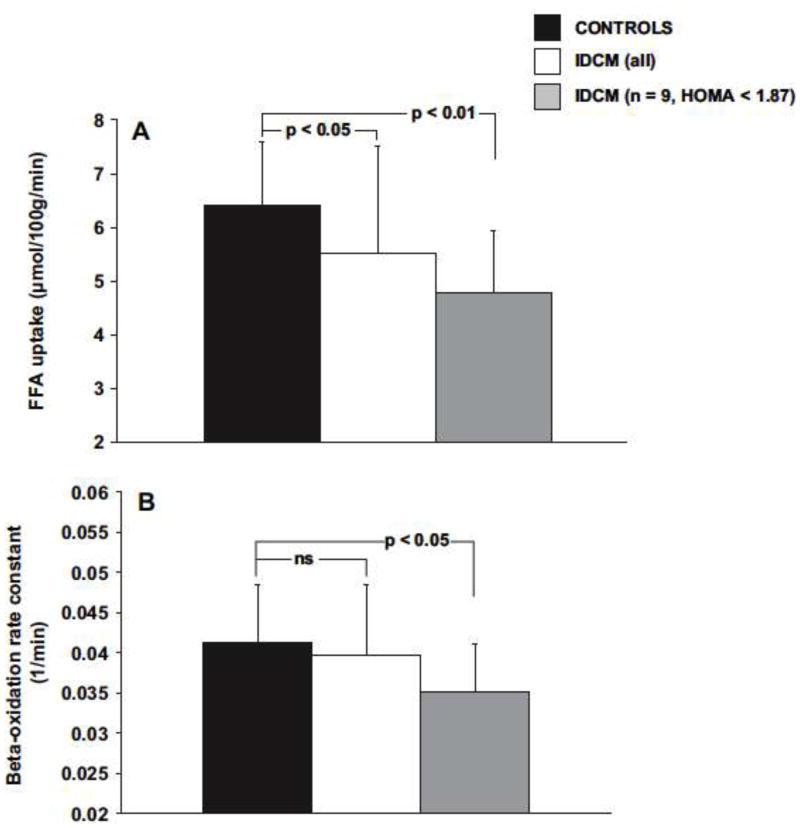
These discrepancies in substrate preference may also be explained by the degree of LV dysfunction, and/or the development of insulin resistance. In one human PET study, myocardial FA metabolism was reduced compared to controls, but further reductions in LV function were associated with insulin resistance and an up-regulation of FA uptake and oxidation (see Figure 3) [26]. As HF progresses, increased adrenergic tone causes increased lipolysis and free FA levels [27]. Differences in serum substrate levels affecting the metabolic milieu may also explain some of the inconsistencies in human HF studies. While Dávila et al. [23] and Tuunanen et al. [26] reported similar FA levels between IDCM patients and controls; Taylor et al. reported an increase in serum FA levels compared to reported normal values, and their levels were much higher than that in either of the other studies.
Figure 3.
PET-derived rates of myocardial FA uptake and oxidation in normal controls (dark bars) and patients with IDCM. Those patients with IDCM and greater insulin resistance (white bars) oxidize a similar amount of FAs compared with normal controls. Patients without insulin resistance take up and oxidize fewer FAs than controls.
Whichever substrate the heart prefers in failure, it likely needs both glucose and FAs. When the failing heart is acutely depleted of FAs, cardiac work and efficiency are compromised [28]. Reduced substrate selection flexibility and myocardial metabolic reserve in HF are also seen in response to stress. During pacing-induced tachycardia, subjects with IDCM were unable to increase their glucose uptake unlike control subjects. [29] (Neither the IDCM group nor the controls displayed an increase in FA uptake or oxidation during stress).
Changes in substrate preference are also affected by the etiology of HF and consequent changes in substrate availability. Although in acute, severe ischemia glucose (from glycogen) is favored over FA [30], the degree of ischemia also affects substrate preference. In moderate ischemia, FAs still provide the majority of acetyl CoA for ATP production [30]. In one study in which 10 of 12 patients with decreased LV function had ischemic cardiomyopathy, the hearts displayed increased FA uptake and decreased glucose use [24]. In that study, plasma FFA levels were high, suggesting that peripheral insulin resistance that often accompanies long-standing HF may play a role in determining the myocardial metabolic profile [24]. Although there are no studies of myocardial metabolism in patients with hypertension-induced LV dysfunction, there is a study comparing myocardial metabolism in patients with hypertension-induced LV hypertrophy to patients with IDCM and to normal controls [31]. In that study, myocardial metabolism in the patients with hypertension and LV hypertrophy is characterized by reduced rates of myocardial FA metabolism – similar to patients with IDCM – but in contrast to normal controls [31]. Thus it is reasonable to expect that in patients with both hypertension and decreased LV function that there would be a metabolic shift favoring glucose relative to FA. To our knowledge, there are no studies of myocardial metabolism in patients with diabetic cardiomyopathy and decreased LV systolic function. In those with diabetic cardiomyopathy and normal ejection fraction (but with diastolic dysfunction, which often precedes systolic dysfunction), FA utilization and oxidation are increased and glucose uptake decreased [32]. Thus, it appears that the conditions accompanying or causing HF likely have a significant impact on the heart's substrate preference.
Studies have demonstrated that other modulating factors such as age, sex, and obesity alter myocardial metabolism in the normal heart. There is generally a decline in FA utilization and oxidation with advancing age [33], but higher levels of FA utilization and oxidation in females and the obese [11, 34]. All of the subjects in the study by Taylor et al. and the majority of the IDCM group in the paper by Dávila et al. were men. Precisely how age, sex, and obesity affect myocardial metabolism in patients with HF remains unknown.
Impact of standard HF therapy on myocardial metabolism
Beta-blocker therapy
One of the most effective medical therapies for improving survival in HF is beta-adrenergic blockade. In general, beta-blockers decrease cardiac oxygen demand by decreasing heart rate, blood pressure, and dp/dt. They can also decrease renin secretion, thereby decreasing preload. Some beta-blockers also have antioxidant properties. The myocardial metabolic effects of beta-blockers are not extensively studied but are fairly consistent. In one study, patients with ischemic cardiomyopathy treated with carvedilol for ∼4 months resulted in a lowering of myocardial uptake of the FA analog, 14(R,S)-[18]fluoro-6-thia-heptadecanoic acid [35]. Despite the usual inverse relationship between glucose and FA use, mean myocardial glucose uptake (as traced by the glucose analog [18F]-fluoro-2-deoxy-glucose) did not change with beta-blocker treatment in this study. Given that FA oxidation requires more oxygen consumption/mole than glucose, it is not surprising that other studies of beta-blocker treatment (with metoprolol) have shown a decrease in MVO2 [36,37]. Beta-blockers are well-known to inhibit catecholamine-driven lipolysis, and thus, may decrease FA delivery to the heart in HF. There is also evidence from a study in a dog model that beta-blockers also directly decrease FA oxidation by inhibiting carnitine palmitoyl transferase I activity [38].
Angiotensin converting enzyme inhibition (ACE-I)
The other medication that is standard of care for HF therapy is ACE-I. Unlike beta-blockers, these drugs have been shown to increase FA uptake and utilization. For example, in one study in which most patients had IDCM, 6 months of enalapril therapy increased FA uptake and decreased FA wash-out, as measured using a FA analog tracer and single photon emission (SPECT) imaging [39].
Impact of metabolic modulator therapy
There are a few medical therapies that are designed to modulate myocardial metabolism for the improvement of HF. In general, these therapies are aimed at increasing glucose oxidation in the failing heart. The rationale put forth is that glucose is a more oxygen efficient fuel. Since glucose and FA metabolism in the heart generally oppose one another in a “yin yang” fashion, these drugs generally enhance glucose oxidation via decreasing FA delivery or metabolism [40,41].
Among the treatments that decrease plasma free FA levels are: 1) a regimen of intravenous glucose, insulin, and potassium, 2) nicotinic acid and its derivatives, and 3) thiazolidinediones. The latter are not recommended for the treatment of HF, in part because of the black box warning of increased cardiac events with thiazolidinediones and because of the fluid retention they can induce. Glucose, insulin, and potassium treatment acutely increased cardiac systolic function in one small, unblinded, uncontrolled study of men with ischemic cardiomyopathy [42]. It is not possible to determine from this study whether these subjects may have obtained benefit from the enhanced uptake of a more oxygen efficient fuel (glucose) because they had ischemia or because they had HF. Nevertheless, the difficulty of administering this regimen, maintaining glucose homeostasis, as well as the intravenous volume load given with this regimen limits applicability in HF treatment, especially in patients who are already volume-overloaded. Acipimox, a nicotinic acid derivative, has also been tried as a metabolic modulator therapy for HF. Acipimox given acutely in one study decreased plasma FFA levels and hence myocardial FA uptake by ∼80% in both HF patients and controls [28]. However, in contrast to what was hypothesized, the treatment resulted in a decrease in cardiac function. The HF patients had a decrease in cardiac output, stroke volume, and efficiency. In the controls, efficiency increased, but they too experienced a similar decrease in cardiac output and stroke volume (although the difference was not significant, perhaps in part due to fewer [N=8] controls than HF patients [N=18]). In sum, currently there are no easily administered treatments for acutely decreasing FA delivery to the heart for the purpose of improving its function. Moreover, significant, acute decreases in plasma FA levels appear to be harmful to patients with HF.
In contrast, longer-term treatment with partial FA beta-oxidation inhibitors (such as trimetazidine) appears to increase cardiac function. Trimetazidine (which is not approved by the FDA and not available in the United States) increases systolic function [43], wall motion, and response to dobutamine in patients with ischemic cardiomyopathy. Trimetazidine also improved exercise capacity [44], functional class [44], markers of inflammation [45], and most importantly, 2 y outcomes in patients with ischemic cardiomyopathy [46]. There are fewer studies of trimetazidine performed solely in patients with nonischemic HF. However, it appears to have salutary effects in this group as well. In nonischemic patients, it improves cardiac energetics, improves glucose tolerance, and increases ejection fraction [47]. Interestingly, trimetazidine only decreased myocardial FA oxidation to a small degree [47] and no study in humans with HF has actually determined if glucose oxidation increases in response to this treatment. Ranolazine is another drug that may partially inhibit FA oxidation, and/or it may exert its beneficial effects through its actions on sodium channels, or another mechanism. There are no studies in humans, in vivo, to confirm its mechanism(s) of action in humans. Although ranolazine is approved only for the treatment of ischemia, there are some animal studies suggesting that it, too, has beneficial effects on heart failure [48,49].
Drugs that primarily inhibit FA oxidation via inhibiting carnitine palmitoyltransferase (CPT)-1 have also been tried as treatments for HF. Etomoxir is often used to modulate FA oxidation in animal models of HF, but a recent trial in humans demonstrated that it has liver toxicity [50]. Perhexiline, another CPT-1 inhibitor, has also been tried in the treatment of HF but with mixed results. In one study, symptoms improved [51], but in another, neither exercise capacity nor function nor 1 y hospital admission rates changed [52]. Currently, perhexiline is mainly used in Australia and New Zealand. Its use is complicated by the fact that certain individuals are poor metabolizers of the drug, which leads to an increased risk of toxicity. Monitoring of plasma drug levels and Cis-hydroxyperhexiline levels are recommended to help avoid peripheral neuropathy and liver damage.
Conclusions
In contrast to the normal adult heart, the nonischemic failing human heart generally uses relatively more glucose at the expense of FA oxidation. This conclusion, however, may need to be refined as there are some suggestions that not all heart failure is metabolically the same. Co-morbid conditions, degree of ischemia, degree of FA oxidative metabolism machinery dysfunction, and plasma substrate levels, also appear to affect myocardial metabolism. Moreover, whether an increase in glucose metabolism is adaptive or maladaptive likely depends upon the demands placed on the heart and other superimposed conditions. Certainly, glucose is a more oxygen efficient fuel. However, if oxygen is not limiting, the rationale for decreasing FA metabolism is less compelling. A “normal” pattern of ∼60-70% FA use would seem to be a logical goal for the nonischemic failing heart. Indeed, studies of metabolic modulator therapy demonstrate that an acute, marked decrease in myocardial FA metabolism is detrimental. However, chronic, mild FA oxidation inhibition by trimetazidine appears to be well-tolerated and beneficial in HF patients.
Footnotes
Author Disclosure
Dr. Peterson is on the Merck Speaker's Bureau.
Drs. Coggan and Kadkhodayan have no conflicts of interest or financial ties to disclose.
References
- 1.Yuan CL, Sharma N, Gilge DA, Stanley WC, Li Y, Hatzoglou M, Previs SF. Preserved protein synthesis in the heart in response to acute fasting and chronic food restriction despite reductions in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E216–22. doi: 10.1152/ajpendo.00545.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill M, France J, Summers M, McBride BW, Milligan LP. Simulation of the energy costs associated with protein turnover and Na+,K+-transport in growing lambs. J Nutr. 1989;119:1287–1299. doi: 10.1093/jn/119.9.1287. [DOI] [PubMed] [Google Scholar]
- 3.Opie LH. In: The Heart: Physiology, from Cell to Circulation. Philadelphia PA, editor. 1998. p. 295. [Google Scholar]
- 4.Bing RJ. Cardiac Metabolism. Physiol Rev. 1965;45:171–213. doi: 10.1152/physrev.1965.45.2.171. [DOI] [PubMed] [Google Scholar]
- 5.Peterson LR, Herrero P, McGill J, Schechtman KB, Kisrieva-Ware Z, Lesniak D, Gropler RJ. Fatty acids and insulin modulate myocardial substrate metabolism in humans with type 1 diabetes. Diabetes. 2008;57:32–40. doi: 10.2337/db07-1199. [DOI] [PubMed] [Google Scholar]
- 6.Kaijser L, Lassers BW, Wahlqvist ML, Carlson LA. Myocardial lipid and carbohydrate metabolism in fasting men during prolonged exercise. J Appl Physiol. 1972;32:847–858. doi: 10.1152/jappl.1972.32.6.847. [DOI] [PubMed] [Google Scholar]
- 7.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. 1988;82:2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 10.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 11.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 12.Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, Dence C, Gropler RJ. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging. 2008;1:424–433. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 14.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 16.Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, Gropler RJ. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 17.McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, Demoss AJ, Schechtman KB, Dence CS, Gropler RJ. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol. 2011;18:421–9. doi: 10.1007/s12350-011-9362-3. quiz 432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, Recklein CL, Coggan AR, Demoss AJ, Dence CS, Gropler RJ. Sex and Type 2 Diabetes: Obesity-Independent Effects on LV Substrate Metabolism and Relaxation in Humans. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol. 1970;218:153–159. doi: 10.1152/ajplegacy.1970.218.1.153. [DOI] [PubMed] [Google Scholar]
- 21.Wittels B, Spann JFJ. Defective lipid metabolism in the failing heart. J Clin Invest. 1968;47:1787–1794. doi: 10.1172/JCI105868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantor PF, Robertson MA, Coe JY, Lopaschuk GD. Volume overload hypertrophy of the newborn heart slows the maturation of enzymes involved in the regulation of fatty acid metabolism. J Am Coll Cardiol. 1999;33:1724–1734. doi: 10.1016/s0735-1097(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 23.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 24.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med. 2001;42:55–62. [PubMed] [Google Scholar]
- 25.Funada J, Betts TR, Hodson L, Humphreys SM, Timperley J, Frayn KN, Karpe F. PLOS one. 4:e7533. doi: 10.1371/journal.pone.0007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuunanen H, Engblom E, Naum A, Scheinin M, Nagren K, Airaksinen J, Nuutila P, Iozzo P, Ukkonen H, Knuuti J. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and LV dysfunction. J Card Fail. 2006;12:644–652. doi: 10.1016/j.cardfail.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Shah A, Shannon RP. Insulin resistance in dilated cardiomyopathy. Rev Cardiovasc Med. 2003;4(6):S50–7. [PubMed] [Google Scholar]
- 28.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 29.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L'Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–8. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 30.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 31.de las Fuentes L, Herrero P, Peterson LR, Kelly DP, Gropler RJ, Dávila-Román VG. Myocardial fatty acid metabolism: independent predictor of LV mass in hypertensive heart disease. Hypertension. 2003;41:83–87. doi: 10.1161/01.hyp.0000047668.48494.39. [DOI] [PubMed] [Google Scholar]
- 32.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 33.Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–299. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 34.Peterson LR, Soto PF, Herrero P, Schechtman KB, Dence C, Gropler RJ. Sex differences in myocardial oxygen and glucose metabolism. J Nucl Cardiol. 2007;14:573–581. doi: 10.1016/j.nuclcard.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallhaus TR, Taylor M, DeGrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 36.Eichhorn EJ, Heesch CM, Barnett JH, Alvarez LG, Fass SM, Grayburn PA, Hatfield BA, Marcoux LG, Malloy CR. Effect of metoprolol on myocardial function and energetics in patients with nonischemic dilated cardiomyopathy: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1994;24:1310–1320. doi: 10.1016/0735-1097(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 37.Beanlands RS, Nahmias C, Gordon E, Coates G, deKemp R, Firnau G, Fallen E. The effects of beta(1)-blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with LV dysfunction: A double-blind, placebo-controlled, positron-emission tomography study. Circulation. 2000;102:2070–2075. doi: 10.1161/01.cir.102.17.2070. [DOI] [PubMed] [Google Scholar]
- 38.Panchal AR, Stanley WC, Kerner J, Sabbah HN. Beta-receptor blockade decreases carnitine palmitoyl transferase 1 activity in dogs with heart failure. J Card Fail. 1998;4:121–126. doi: 10.1016/s1071-9164(98)90252-4. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi S, Takeishi Y, Minamihaba O, Arimoto T, Hirono O, Takahashi H, Miyamoto T, Nitobe J, Nozaki N, Tachibana H, Watanabe T, Fukui A, Kubota I. Angiotensin-converting enzyme inhibition improves cardiac fatty acid metabolism in patients with congestive heart failure. Nucl Med Commun. 2003;24:901–906. doi: 10.1097/01.mnm.0000084579.51410.69. [DOI] [PubMed] [Google Scholar]
- 40.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 41.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Cottin Y, Lhuillier I, Gilson L, Zeller M, Bonnet C, Toulouse C, Louis P, Rochette L, Girard C, Wolf JE. Glucose insulin potassium infusion improves systolic function in patients with chronic ischemic cardiomyopathy. Eur J Heart Fail. 2002;4:181–184. doi: 10.1016/s1388-9842(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 43.Vitale C, Wajngaten M, Sposato B, Gebara O, Rossini P, Fini M, Volterrani M, Rosano GM. Trimetazidine improves LV function and quality of life in elderly patients with coronary artery disease. Eur Heart J. 2004;25:1814–1821. doi: 10.1016/j.ehj.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 44.Fragasso G, Perseghin G, De Cobelli F, Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, Del Maschio A, Margonato A. Effects of metabolic modulation by trimetazidine on LV function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J. 2006;27:942–948. doi: 10.1093/eurheartj/ehi816. [DOI] [PubMed] [Google Scholar]
- 45.Di Napoli P, Taccardi AA, Barsotti A. Long term cardioprotective action of trimetazidine and potential effect on the inflammatory process in patients with ischaemic dilated cardiomyopathy. Heart. 2005;91:161–165. doi: 10.1136/hrt.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Kady T, El-Sabban K, Gabaly M, Sabry A, Abdel-Hady S. Effects of trimetazidine on myocardial perfusion and the contractile response of chronically dysfunctional myocardium in ischemic cardiomyopathy: a 24-month study. Am J Cardiovasc Drugs. 2005;5:271–278. doi: 10.2165/00129784-200505040-00006. [DOI] [PubMed] [Google Scholar]
- 47.Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–1258. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- 48.Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, Wolff A, Sabbah HN. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- 49.Sabbah HN, Chandler MP, Mishima T, Suzuki G, Chaudhry P, Nass O, Biesiadecki BJ, Blackburn B, Wolff A, Stanley WC. Ranolazine, a partial fatty acid oxidation (pFOX) inhibitor, improves LV function in dogs with chronic heart failure. J Card Fail. 2002;8:416–422. doi: 10.1054/jcaf.2002.129232. [DOI] [PubMed] [Google Scholar]
- 50.Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 2007;113:205–212. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 51.Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- 52.Bansal M, Chan J, Leano R, Pillans P, Horowitz J, Marwick TH. Effects of perhexiline on myocardial deformation in patients with ischaemic LV dysfunction. Int J Cardiol. 2010;139:107–112. doi: 10.1016/j.ijcard.2009.08.007. [DOI] [PubMed] [Google Scholar]