Abstract
Objective
Subarachnoid hemorrhage (SAH) is associated with inflammation which may mediate poor outcome in SAH. We hypothesize that elevated serum tumor necrosis factor-alpha (TNFα) and interleukin-6 (IL-6) are associated with vasospasm and poor outcome in SAH.
Methods
In 52 consecutive SAH subjects, we compared TNFα and IL-6 levels on post-SAH days 0–1, 2–3, 4–5, 6–8, and 10–14 with respect to vasospasm and to poor outcome at 3- and 6-months. Vasospasm was defined as >50% reduction in vessel caliber on angiography. Poor outcome was defined as modified Rankin score >2.
Results
Elevated TNFα on post-SAH days 2–3 was associated with poor 3-month outcome (p=0.0004). Global elevation of TNFα over time (post-SAH days 0–14) was independently associated with poor 3-month outcome after adjusting for Hunt-and-Hess grade and age (p=0.02). Neither cross-sectional nor IL-6 levels over time were associated with outcome. Neither TNFα nor IL-6 levels were associated with vasospasm.
Conclusions
Elevation in serum TNFα on post-SAH days 2–3 and global elevation of TNFα over time are associated with poor outcome but not with angiographic vasospasm in this small cohort. Future studies are needed to define the role of TNFα in SAH-related brain injury and its potential as a SAH outcome biomarker.
Keywords: subarachnoid hemorrhage, tumor necrosis factor-alpha, inflammation
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (SAH) and subsequent delayed vasospasm continue to leave more than half of its survivors in severe long-term disability while the pathogenesis of vasospasm and SAH-related brain injury remain incompletely understood. There is increasing evidence that SAH is associated with leukocytosis and central nervous system (CNS) release of inflammatory mediators such as cytokines which may contribute to the pathogenesis of both vasospasm and brain injury in SAH [1–5].
Microglia and astrocytes release 3 major cytokines after injury: tumor necrosis factor-alpha (TNFα), interleukin-6 (IL-6), and interleukin 1-beta (IL-1β) [2]. In animal models, both TNFα and IL-1β are increased acutely after cerebral ischemia and have many overlapping functions. Of these 3 cytokines, TNFα and IL-6 elevations have been reliably detected in the cerebrospinal fluid (CSF) and microdialysate [6] from SAH patients and are associated with evidence of vasospasm by transcranial Doppler ultrasound, poor SAH outcome [7], and with delayed ischemic deficit [7–9]. TNFα is a pro-inflammatory cytokine associated with SAH-related oxidative stress, endothelial cell apoptosis [10], and recruitment of inflammatory mediators of vasospasm [11]. TNFα causes release of many downstream cytokines, including IL-6 [12]. Unlike TNFα, IL-6 both causes and inhibits inflammation. The anti-inflammatory effect of IL-6 includes inhibition of TNFα production and upregulation of soluble TNFα receptor which antagonizes TNFα [13]. However, IL-6 is associated with increased endothelial permeability in the CNS and upregulation of molecular mediators of ischemic brain injury such as leukotrienes and prostaglandins, all of which may contribute to brain injury in SAH [14]. In fact, elevated plasma and CSF IL-6 levels in humans are associated with poor clinical outcome after cerebral ischemia [13].
While elevation of TNFα and IL-6 in CSF is associated with SAH and possibly with vasospasm and poor outcome, CSF is not routinely accessible in all SAH patients. In this study, we investigate the association of serum TNFα and IL-6 with cerebral vasospasm and SAH outcome. We hypothesize that, like in CSF, elevation of serum TNFα and IL-6 are associated with both angiographic vasospasm and with SAH outcome.
MATERIALS AND METHODS
52 consecutive SAH patients within 96 hours of spontaneous SAH were prospectively enrolled. Patients with traumatic SAH, pregnancy, end-stage renal or hepatic disease, intracranial malignancies, or infectious meningitis were excluded. All subjects or their next-of-kin provided informed consent for study participation in accordance with institutional review board approved protocols.
All subjects underwent conventional cerebral angiography on admission and again between post-SAH days 6–8 according to standardized clinical protocol. Angiographic cerebral vasospasm was defined as reduction in cerebral arterial diameter by > 50% in any vessel on cerebral angiography on post-SAH day 6–8. Long-term functional outcome at 3 and 6-months were assessed using modified Rankin score (mRS) via telephone interview. A standardized questionnaire was used to perform mRS scoring to reduce inter-rater variability. Poor outcome was defined as mRS>2.
We collected important clinical and demographic information including age, gender, ethnicity, SAH clinical severity grade (Hunt and Hess Grade), SAH radiographic severity grade (Fisher group), the incidence of hydrocephalus, the modality of aneurysm treatment, and the incidence of delayed cerebral infarction. Delayed cerebral infarction is defined as any new area of hypodensity on brain computed tomography developing after post-SAH day 3 and is not attributable to another etiology such as surgical retraction injury or edema along the external ventricular drain track.
Blood samples from enrolled subjects were collected on post-SAH days 0–1, 2–3, 4–5, 6–8, and 10–14. All samples were immediately centrifuged at 3900 RPM for 15 minutes, aliquotted, and stored frozen at −80C until analysis. Serum TNFα was measured by ELISA (R&D Systems), and IL-6 was measured using chemluminescent immunoassay (Beckman-Couter) in the CLIA-certified Harvard Catalyst Central Laboratory.
Continuous variables were compared using a 2-tailed student t-test or Wilcoxon Rank Sum test depending on data distribution. For each biomarker, we performed cross-sectional, between-group comparisons at each of the 5 time-points of sample collection, and used Bonferroni correction for multiple comparisons (threshold of p<0.01 for significance). We used longitudinal regression with random effects to study the association between global TNFα and IL-6 levels over time (measured repeatedly during post-SAH days 0–14) and SAH outcome and vasospasm. In the longitudinal regression models, we adjusted for two pre-specified important confounders for vasospasm (Hunt and Hess grade and Fisher group) and for SAH outcome (Hunt and Hess grade and age). All statistical analyses were performed using JMP 8.0 and SAS 9.2.
RESULTS
The study population consisted of 52 subjects with mean age of 53.6 years (Table 1). Fifty percent of subjects presented with Hunt and Hess (HH) grade 3 or higher. All except 2 subjects in this cohort were independent in activities of daily living prior to SAH. Of the two subjects who were not entirely independent, one requires help because of recent hospitalization for deep venous thrombosis 1 month prior to SAH, and the other subject has mild cognitive deficit that intermittently requires supervision, but was otherwise independent. The majority of subjects (71%) had Fisher group 3 SAH. Twenty subjects (38%) developed vasospasm, and 14 subjects (27%) had poor outcome (mRS>2) at 3-months. Fifty percent of all patients were treated with craniotomy and aneurysm clipping, and 29% treated with endovascular coil embolization. Patients whose aneurysms were not amendable to either therapy, or patients who had angionegative SAH, were medically treated. At 3-month follow up, two out of 52 SAH patients had died. At 6-month, one more patient had died. The total mortality of this cohort is 3.8% at 3 month post SAH and 5.7% at 6-months post SAH.
Table 1.
Patient Demographics
| All SAH (n = 52) |
VSP + (n = 20) |
VSP – (n = 32) |
3 month mRS <= 2 (n = 37) |
3 month mRS > 2 (n = 14 ) |
6 month mRS <= 2 (n = 39) |
6 month mRS > 2 (n = 9 ) |
||
|---|---|---|---|---|---|---|---|---|
| Mean Age | 53.6 [49 – 58] |
50.1 [42 – 58] |
55.8 [50 – 62] |
50.3 [46 – 55] |
64.4 [55 – 74] |
50.7 [46 – 55] |
69.1 [55 – 83] |
|
| Gender | ||||||||
| (% Female) | 58% | 65% | 53% | 54% | 71% | 56% | 78% | |
| HH Grade | ||||||||
| 1 | 6 (12%) | 2 (10%) | 4 (13%) | 6 (16%) | 0 | 5 (13%) | 0 | |
| 2 | 20 (38%) | 7 (35%) | 13 (41%) | 16 (43%) | 4 (29%) | 18 (46%) | 1 (11%) | |
| 3 | 13 (25%) | 5 (25%) | 8 (25%) | 9 (24%) | 3 (21%) | 8 (21%) | 3 (33%) | |
| 4 | 9 (17%) | 3 (15%) | 6 (19%) | 5 (14%) | 4 (29%) | 7 (18% | 2 (22%) | |
| 5 | 4 (8%) | 2 (15%) | 1 (3%) | 1 (3%) | 3 (21%) | 1 (3%) | 3 (33%) | |
| Fisher Grade | ||||||||
| 1 | 2 (4%) | 1 (5%) | 1 (3%) | 1 (3%) | 1 (7%) | 1 (3%) | 0 | |
| 2 | 11 (21%) | 2 (10%) | 9 (28%) | 10 (27%) | 1 (7%) | 10 (26%) | 1 (11%) | |
| 3 | 37 (71%) | 17 (85%) | 20 (63%) | 25 (68%) | 11 (79%) | 27 (69%) | 7 (78%) | |
| 4 | 2 (4%) | 0 | 2 (6%) | 1 (3%) | 1 (7%) | 1 (3%) | 1(11%) | |
| Hydrocephalus | 34 (65%) | 18 (90%) | 16 (50%) | 22 (59%) | 11 (79%) | 26 (67%) | 7 (78%) | |
| Treatment | ||||||||
| Clip | 26 (50%) | 12 (60%) | 14 (44%) | 21 (57%) | 5 (36%) | 22 (56%) | 3 (33%) | |
| Coil | 15 (29%) | 6 (30%) | 9 (28%) | 7 (19%) | 7 (50%) | 9 (23%) | 5 (56%) | |
| Other | 11 (21%) | 2 (10%) | 9 (28%) | 9 (24%) | 2 (14%) | 8 (21%) | 1 (11%) | |
|
Delayed Cerebral Infarction |
5 (10%) | 1 (3%) | 4 (20%) | 3 (8%) | 2 (14%) | 2 (5%) | 2 (22%) | |
| Ethnicity | ||||||||
| African American | 6 (12%) | 0 | 6 (19%) | 2 (5%) | 4 (29%) | 3 (8%) | 2 (22%) | |
| Asian | 2 (4%) | 1 (5%) | 1 (3%) | 1 (3%) | 0 | 1 (3%) | 0 | |
| Caucasian | 35 (67%) | 15 (75%) | 20 (63%) | 27 (73%) | 8 (57%) | 28 (72%) | 5 (56%) | |
| Hispanic | 9 (17%) | 4 (20%) | 5 (16%) | 7 (19%) | 2 (14%) | 7 (18%) | 2 (22%) | |
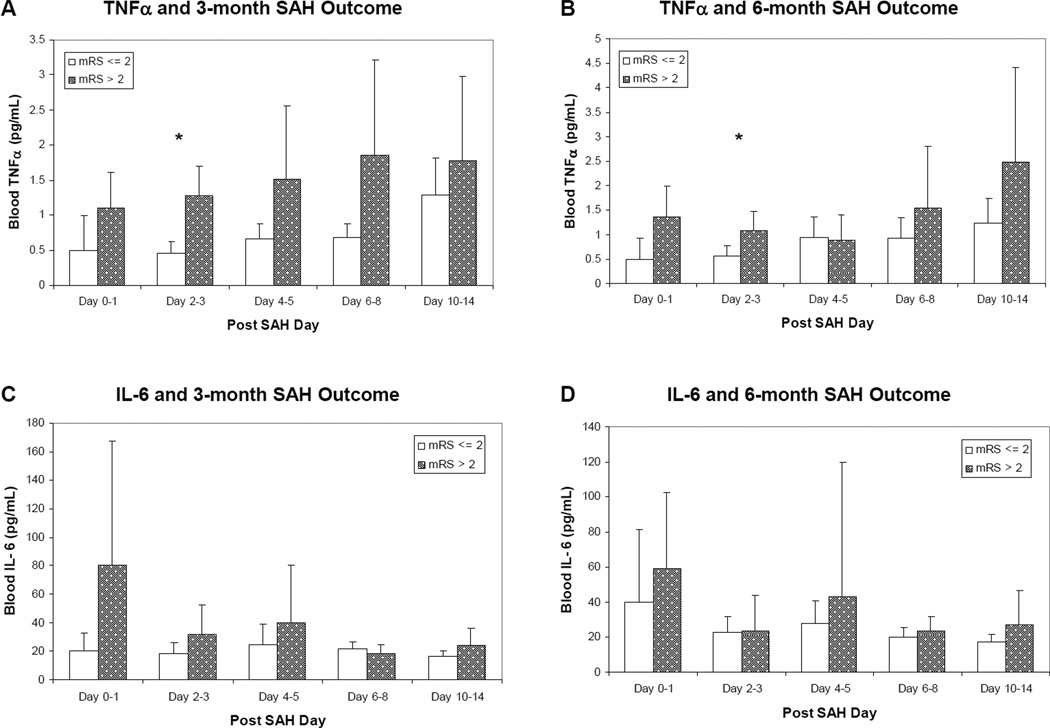
Cross-sectional elevation of serum TNFα levels on post-SAH days 2–3 was associated with poor 3-month outcome (p=0.0004) and showed trend towards association with poor 6-month outcome (p=0.017) (Figure 1 a – b). Cross-sectional elevation of TNFα levels on post SAH days 4–5 (p=0.03) and 6–8 (p=0.04) also showed trend towards association with poor 3-month outcome. Global TNFα elevation over time (measured repeatedly during post SAH days 0–14) is associated with poor 3-month SAH outcome (p=0.02) after adjusting for Hunt and Hess grade and age using longitudinal regression. Cross-sectional TNFα levels and its global effects over time showed no association with angiographic vasospasm in this small cohort.
Figure 1. Blood TNFα and IL-6 Levels and SAH Outcome.
(A) Blood TNFα and 3-month SAH outcome (*: p < 0.01). (B) Blood TNFα and 6-month SAH outcome (*: p < 0.01). (C) Blood IL-6 and 3-month SAH outcome. (D) Blood IL-6 and 6-month SAH outcome.
Neither cross-sectional nor global IL-6 levels over time were associated with 3 and 6-month SAH outcome (Figure 1 c – d) or with vasospasm.
DISCUSSION
TNFα and IL-6 are known to be elevated in CSF after SAH, and cross-sectional elevations of these cytokines are associated with vasospasm and possibly poor outcome in SAH [7]. In this study, we examined serum levels of these cytokines in SAH, their time course, and their relationships with vasospasm and SAH clinical outcome. We found that cross-sectional elevation of serum TNFα on post SAH day 2–3 is significantly associated with poor 3-month SAH outcome and shows a trend towards similar effects at 6 months post SAH. Furthermore, global elevation of TNFα levels over time (post-SAH days 0–14) is significantly associated with poor SAH outcome at 3 months after adjusting for SAH clinical severity and age. To our knowledge, this is the first report of association between serum TNFα elevation and poor long-term outcome in an SAH cohort. Our results are consistent with prior CSF studies and suggest that the association between TNFα and poor outcome in SAH is consistent across blood and CSF compartments. The design of this study does not address whether serum TNFα mediates secondary brain injury in SAH and leads to poor outcome, or is elevated as a result of secondary brain injury in SAH, or may even reflect a forme fruste attempt at neural repair following SAH [15].
In this small cohort, we did not observe a significant association between elevated serum TNFα over time and angiographic vasospasm, though we did observe a significant association with poor SAH clinical outcome. This differential association may be partly due to a small sample size which limits our power to detect a small association between TNFα and angiographic vasospasm, or it may support the growing literature suggesting there are separate pathophysiologic pathways leading to angiographic vasospasm and delayed neurologic deterioration in SAH [8, 16]. Unlike TNFα, neither cross-sectional nor global serum IL-6 levels over time showed any association with SAH outcome. These findings suggest that the association between high serum TNFα and poor SAH outcome may not be the result of a non-specific generalized surge of cytokines due to acute phase reaction in SAH patients with higher disease severity. Rather, these results suggest there may be cytokine-specific mechanisms leading to poor outcome in SAH.
This study adds TNFα to the growing list of circulating inflammatory markers associated with poor SAH outcome in humans [1]. Elevated circulating TNFα is in fact associated with worse secondary brain injury in other conditions such as ischemic stroke and intracerebral hemorrhage [17]. At a cellular level, TNFα may participate in brain injury through numerous pleiotropic effects such as activation of the mitogen-activated protein kinases and promotion of downstream inflammatory cascade by increasing the synthesis of adhesion molecules and other cytokines [13]. However, the sources of circulating TNFα in SAH brain injury and how circulating TNFα can affect SAH brain injury remains to be established. Future studies of simultaneous measurements of CSF and serum TNFα over time in acute SAH may help establish the dynamics of this inflammatory cytokine across these two compartments. Furthermore, targeted in vivo studies are needed to further establish the role of circulating TNFα on SAH secondary brain injury.
This study has several caveats. We used angiographic definition for vasospasm rather than clinical symptomatic vasospasm because the high proportion of high clinical grade SAH subjects make the detection of symptomatic vasospasm unreliable in this cohort, and aggressive treatment protocol for angiographic vasospasm may also influence the incidence of secondary development of symptomatic vasospasm. Though presence of vasospasm on angiography can be more objectively measured, angiography has limited ability to detect clinically significant vasospasm, particularly in small-caliber blood vessels. The small sample size limits our ability to examine the main results in different SAH patient subgroups as well as our power to detect smaller effects. Given that elevation of CSF IL-6 is known to be associated with poor outcome in SAH, we must consider the possibility that our study was under-powered to detect small associations between serum IL-6 and SAH outcome. Alternatively, it is also possible that IL-6 elevation in CSF is a nonspecific surrogate for generalized ICP elevation which is known to lead to poor SAH outcome [18]. Because no subject in our cohort had sustain prolonged period of elevated ICP, this may be why we had not observed an association between IL-6 and SAH outcome. To avoid multiple comparisons in a small cohort, we only examined two targeted cytokines in this study. We are therefore unable to comment on the interactions and effects of TNFα on other cytokines and on other mediators of inflammation such as leukocytes and adhesion molecules. A larger SAH cohort will be necessary to study the association of SAH outcome with additional important cytokines such as IL-1β and IL-8. Finally, pro-inflammatory cytokines are not always associated with deleterious effects at all times in a disease process. In fact, inflammation may be necessary for brain injury recovery in the subacute phase of SAH [19]. This study only examined cytokines during the acute phase of SAH and our conclusion on the association between these pro-inflammatory cytokines and outcome cannot be generalized to the subacute and chronic phases of SAH.
Despite the small sample size, consistent association between early TNFα elevation and poor long-term SAH outcome suggests that blood TNFα may be a potential clinical biomarker for SAH outcome. This needs to be validated in a larger prospective cohort, and results from this study may contribute to the design and sample size calculation of such future studies.
ACKNOWLEDGEMENT
The authors thank Drs. F.A. Sorond and G.V. Henderson (Dept. of Neurology, Brigham and Women’s Hospital) for subject recruitment, Dr. A.H. Ropper (Dept. of Neurology, Brigham and Women’s Hospital) for advice and funding support, Dr. Elizabeth Secor for statistical consultation, Dr. J.W. Lee (Dept. of Neurology, Brigham and Women’s Hospital) for editorial advice, and the Harvard Clinical Translational Science Center (Harvard Catalyst) for supporting biomarker analysis.
Sources of Support: This study is sponsored by American Heart Association (10CRP2610341, Chou), Harvard Clinical Translational Research Center (5KL2RR025757, Chou), The American Federation for Medical Research (AFMR Scholar Award, Chou), and the United States National Institute of Health – National Institute of Neurological Disorders and Stroke (K23-NS073806, Chou; R21-NS52498, Ning; R01-NS48422, Ning; R37-NS37074, Lo; P01-NS55104), and a departmental research discretionary fund (Chou).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Chou, Atherton, Ning, Feske, Du, De Jager, Lo and Ms. Konigsberg have no financial relationships to report.
REFERENCES
- 1.Dumont AS, Dumont RJ, Chow MM, et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery. 2003;53:123–133. doi: 10.1227/01.neu.0000068863.37133.9e. discussion 133-125. [DOI] [PubMed] [Google Scholar]
- 2.McKeating EG, Andrews PJ. Cytokines and adhesion molecules in acute brain injury. Br J Anaesth. 1998;80:77–84. doi: 10.1093/bja/80.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Guo ZD, Sun XC, Zhang JH. Mechanisms of early brain injury after SAH: matrix metalloproteinase 9. Acta Neurochir Suppl. 2011;110:63–65. doi: 10.1007/978-3-7091-0353-1_11. [DOI] [PubMed] [Google Scholar]
- 4.Provencio JJ, Vora N. Subarachnoid hemorrhage and inflammation: bench to bedside and back. Semin Neurol. 2005;25:435–444. doi: 10.1055/s-2005-923537. [DOI] [PubMed] [Google Scholar]
- 5.Provencio JJ, Fu X, Siu A, et al. CSF Neutrophils Are Implicated in the Development of Vasospasm in Subarachnoid Hemorrhage. Neurocrit Care. 2009;12:244–251. doi: 10.1007/s12028-009-9308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanafy KA, Grobelny B, Fernandez L, et al. Brain interstitial fluid TNF-alpha after subarachnoid hemorrhage. J Neurol Sci. 2010;291:69–73. doi: 10.1016/j.jns.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassbender K, Hodapp B, Rossol S, et al. Inflammatory cytokines in subarachnoid haemorrhage: association with abnormal blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 2001;70:534–537. doi: 10.1136/jnnp.70.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zemke D, Farooq MU, Mohammed Yahia A, et al. Delayed ischemia after subarachnoid hemorrhage: result of vasospasm alone or a broader vasculopathy? Vasc Med. 2007;12:243–249. doi: 10.1177/1358863X07081316. [DOI] [PubMed] [Google Scholar]
- 9.Osuka K, Suzuki Y, Tanazawa T, et al. Interleukin-6 and development of vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien) 1998;140:943–951. doi: 10.1007/s007010050197. [DOI] [PubMed] [Google Scholar]
- 10.Kimura H, Gules I, Meguro T, et al. Cytotoxicity of cytokines in cerebral microvascular endothelial cell. Brain Res. 2003;990:148–156. doi: 10.1016/s0006-8993(03)03450-4. [DOI] [PubMed] [Google Scholar]
- 11.Vecchione C, Frati A, Di Pardo A, et al. Tumor necrosis factor-alpha mediates hemolysis-induced vasoconstriction and the cerebral vasospasm evoked by subarachnoid hemorrhage. Hypertension. 2009;54:150–156. doi: 10.1161/HYPERTENSIONAHA.108.128124. [DOI] [PubMed] [Google Scholar]
- 12.Aloisi F, Care A, Borsellino G, et al. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 13.Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia: experimental bases and therapeutic perspectives. Arterioscler Thromb Vasc Biol. 1998;18:503–513. doi: 10.1161/01.atv.18.4.503. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 15.Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, et al. The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol. 2001;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- 17.Castillo J, Davalos A, Alvarez-Sabin J, et al. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58:624–629. doi: 10.1212/wnl.58.4.624. [DOI] [PubMed] [Google Scholar]
- 18.Graetz D, Nagel A, Schlenk F, et al. High ICP as trigger of proinflammatory IL-6 cytokine activation in aneurysmal subarachnoid hemorrhage. Neurol Res. 2009;32:728–735. doi: 10.1179/016164109X12464612122650. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]