Abstract
BACKGROUND
Ovarian cancer (OC) is associated with a >75% risk of recurrence after completion of primary therapy. Several clinical trials have explored the role of continued therapy after complete response to primary adjuvant therapy to reduce the risk of recurrence; however, these trials have largely been underpowered, leading to inconclusive results.
METHODS
A systematic search strategy was initiated to identify all clinical trials involving consolidation or maintenance therapy regimens for OC in first complete remission. A meta-analysis was conducted to evaluate toxicity and progression-free (PFS) and overall survival (OS).
RESULTS
There were 37 publications meeting all eligibility criteria, representing 20 consolidation and 9 maintenance therapy trials. Consolidation and maintenance therapies were associated with improved PFS (hazard ratio [HR], 0.79 [P = .003] and HR, 0.82 [P = .02], respectively) and OS (HR, 0.68 [P = .0008] and HR, 0.68 [P = .007], respectively). This relationship remained statistically significant when the analysis was limited to randomized trials and across other sensitivity analyses.
CONCLUSIONS
Although individual studies have not yet convincingly shown a survival advantage with maintenance chemotherapy in OC, this metaanalysis demonstrates that continued chemotherapy after completion of primary therapy for OC improves PFS and OS. Benefits are greatest in patients with advanced stage OC who reach complete clinical or pathologic response after primary therapy.
Keywords: consolidation, maintenance, ovarian cancer, chemotherapy, meta-analysis
Ovarian cancer (OC) has the highest mortality rate of all cancers of the reproductive system, and is the fourth leading cause of cancer-related death among women.1 Women with OC are faced with a 5-year survival rate of 46% for all stages, which drops to 31% for advanced disease.1 Since the 1970s, treatment advances have led to <5 months improvement in overall survival among women with OC.2 The majority of OC patients respond to primary therapy consisting of debulking surgery followed by platinum and taxane-based chemotherapy. However, most women recur, and recurrent OC is invariably fatal. Thus, significant effort has been dedicated to improving the outcome of primary therapy to avoid recurrences, including increased intensity of primary chemotherapy,3,4 addition of new agents to the standard regimen,5 or continuation of primary therapy in the form of either consolidation or maintenance therapy.6,7 In general, consolidation therapy is designed to be a short-term treatment boost after initial therapy to consolidate the initial response to therapy.8 Maintenance therapy is designed to maintain the disease-free period as long as possible, while delivering a reduced dose of chemotherapy over a longer period of time (eg, 6 cycles or more).7 It has been speculated that continued reduction of residual cancer cells by extending the period of chemotherapy administration will diminish the risk of tumor regrowth. The concept of maintenance therapy has its roots in the experience extracted from the treatment of acute lymphoblastic leukemias, where postremission therapy continued to reduce the burden of residual cancer cells, eventually yielding eradication of the disease.9,10 Although this hypothesis has not yet been validated experimentally in solid tumors, the emergence of the cancer stem cell theory11,12 further supports the idea that residual cancer cells with stem cell properties persist after completion of chemotherapy and eventually give rise to recurrent metastases that recapitulate the characteristics of the original tumor. This concept strengthens the rationale for investigating postremission therapy to eradicate residual chemotherapy-resistant tumors. The purpose of this meta-analysis was to pool and analyze the existent data on consolidation and maintenance therapy in OC.
The largest and most compelling randomized trial conducted to test this theory evaluated 12 versus 3 cycles of monthly paclitaxel as maintenance therapy in OC patients in first remission.7 At the time of a planned interim analysis, the Southwest Oncology Group Data and Safety Monitoring Committee noted significant differences in progression-free survival (PFS) and recommended study closure.7 As a result, enrollment was halted, which led to a reduction in the statistical power to detect an overall survival (OS) benefit.13 Another nonrandomized small study using the same regimen as the Southwest Oncology Group trial recorded improved PFS in patients receiving 12 cycles of therapy compared with controls (11 vs 24 months, P = .0062) and an improved median OS (38 months vs not reached; P =.0019).14 On the contrary, a randomized trial of 6 courses of monthly paclitaxel versus no further treatment found no significant improvement in PFS or OS, but this study was also underpowered.15 Those and other studies’ discrepant results led to unclear interpretation of the data for application in clinical practice.16–18 As a result, the choice of whether to pursue continued therapy is generally made based on the physician’s own viewpoint about its value. To address these inconclusive data, a meta-analysis was conducted to assess the impact of consolidation and maintenance therapy on survival and toxicity outcomes among patients with OC in first remission.
MATERIALS AND METHODS
Trial Inclusion Criteria
To be included in the analysis, continued therapy was defined as any postprimary treatment for OC patients after completion of primary therapy. Eligible studies were required to include at least 2 treatment groups, with continued therapy in at least 1 treatment group. Studies were also required to include participants with ovarian or peritoneal cancer, and to report data on PFS or OS. Review articles, case reports, retrospective studies, studies addressing treatment for persistent or recurrent OC or post-front-line maintenance therapy regimen (eg, post-secondor third-line treatment), and those allowing an extended treatment-free interval were excluded. Studies of continued therapy were then categorized into consolidation regimens (treatment period <6 treatment cycles) and maintenance regimens (at least 6 treatment cycles).
Trial Identification
The following search terms were used to identify ovarian/ peritoneal cancer research studies: “ovarian neoplasms”; “ovarian carcinoma”; “ovarian cancer” or “peritoneal neoplasms”; “peritoneal carcinoma”; and “peritoneal cancer.” The following search terms were used to identify studies that investigate maintenance therapies: “consolidation,” “consolidation therapy,” “maintenance therapy,” and “maintenance treatment.” Studies considered for potential study inclusion required at least 1 ovarian/ peritoneal and 1 maintenance term as described above. All terms were expanded to include all subcategories and searched as keywords to attempt to obtain all published research that potentially fit the inclusion criteria. OVID/ Medline 1950 to December 2009, the Society of Gynecologic Oncology abstracts, the Cochrane register of controlled trials, and the American Society of Clinical Oncology abstracts were reviewed.
Data Collection
Each publication underwent data abstraction by 2 independent reviewers and was matched for accuracy. A third independent reviewer served as a tiebreaker for all discrepancies, followed by a third review for accuracy. For studies reported in >1 publication, data abstracted were limited to the most recent for each variable. When data were missing from the primary publication, the authors were contacted to attempt to obtain complete data.
Study quality was assessed using the Cho and Bero scale,19 which grades each study based on 24 factors (eg, study design, randomization, statistical power). The resulting quality score ranges from 0.0 to 1.0, with 1.0 reflecting highest study quality.
Data Analysis
Hazard ratios (HR), 95% confidence intervals (CIs), and variances were calculated using the Excel software described by Tierney and colleagues20 when not explicitly stated in the original articles. Odds ratios (ORs) were calculated for toxicities. Data were analyzed using the software package Comprehensive Meta-Analysis V2.2.050 (Biostat, Englewood, NJ). In the pooled analysis, chemotherapy was considered the experimental treatment if not explicitly stated. Whenever possible, survival data were limited to patients who had experienced complete response (CR) after primary therapy. Median values were obtained from the survival curves when not reported in the article.
Tests of homogeneity (Q statistic) were performed to assess the extent of variability among included studies that may be because of more than sampling error.21 The planned endpoints included OS, PFS, and toxicity. The influence of publication bias was determined by assessing the fail-safe N (the number of unpublished, nonsignificant studies needed to bring the pooled P value to >.05).22 In addition, a trim and fill analysis23 was conducted.
Sensitivity analyses were performed by subgroups to further test the stability of the survival analyses, including only randomized trials, type of treatment regimen, stage of disease at diagnosis, and extent of disease after primary therapy.
RESULTS
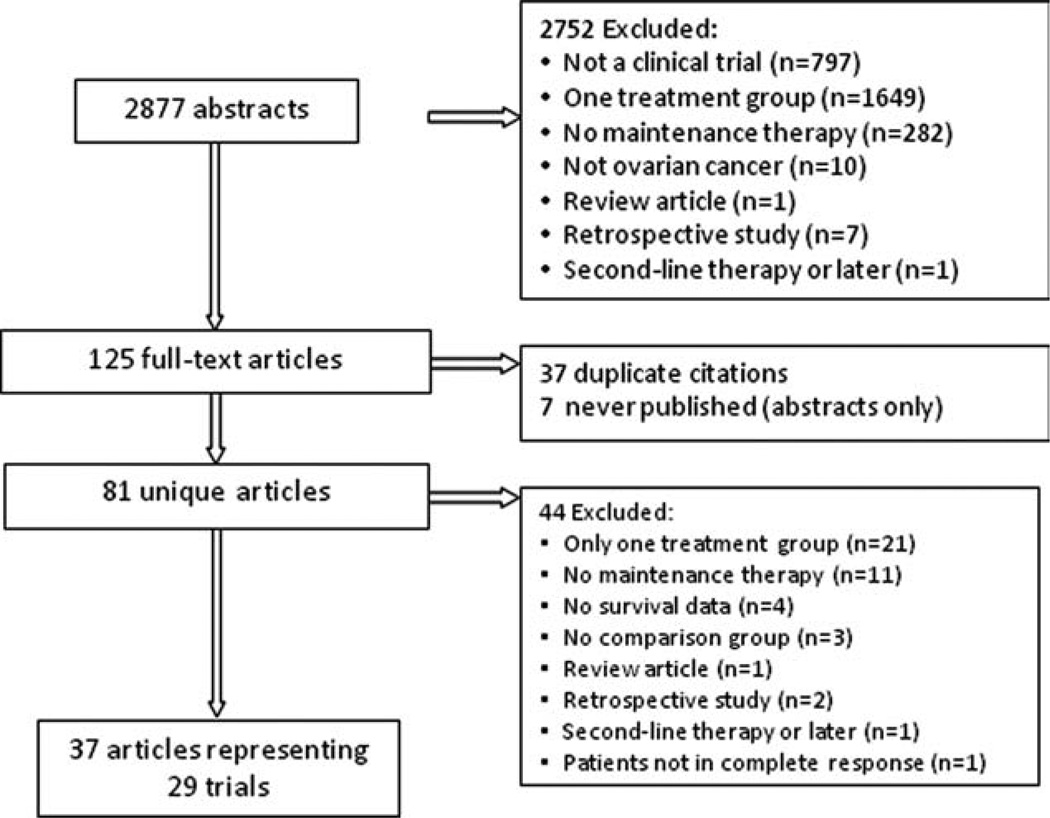
Figure 1 demonstrates the process of selection and the results of the search strategy. The initial search strategy found 2877 publications fulfilling the wide search criteria. Of these, the primary reasons for exclusion included reporting of results of only 1 treatment group (n = 1649) and being other than a clinical trial (n = 797). For abstracts that were not initially excluded (n = 125), the full text articles were obtained and reviewed. A total of 37 articles representing 29 trials met eligibility criteria and underwent data abstraction (Table 1). These trials included 20 consolidation and 9 maintenance trials (1 trial was a 3-arm study, including 2 trials of consolidation and maintenance therapies, respectively, vs control). Eleven (55%) consolidation trials and 7 (78%) maintenance trials were randomized. Twelve (60%) of the consolidation trials and 5 (56%) of the maintenance trials enrolled only patients achieving CR or pathologic CR after primary therapy. Ten (20%) consolidation trials and 4 (44%) maintenance trials were limited to patients with advanced OC (stages III-IV). Although the majority of trials permitted enrollment of patients with stage I–II disease, early stage disease represented a minority of enrolled patients. Only 3 consolidation trials included a treatment group that included 40% to 50% of patients with early stage disease, 2 of which disproportionately enrolled patients with early stage OC to the control regimen. The Q statistic was statistically significant, demonstrating heterogeneity across all trials (Q value = 46.42, P = .006), consolidation trials (Q = 32.69, P = .008), and maintenance trials (Q = 7.43, P = .02). Therefore, a random effects model was used for all analyses. The median OS data were reported descriptively for each study separately, and were available for 14 consolidation trials and for 9 maintenance trials. It is interesting to note that the average gain in survival for the experimental as compared with the control treatment was 16.5 months for consolidation therapy and 8.3 months for maintenance therapy.
Figure 1.
Results of search strategy are shown.
Table 1.
Eligible Studies
| Study | Disease After Primary Therapy |
Treatment Regimens | No. | Median PFS | Median OS |
|---|---|---|---|---|---|
| Consolidation | |||||
| Alberts 20066 | No pathologic evidence of disease (pCR) |
lnterferon-a every week, 6 weeks | 35 | 47 months | NR at 147 months |
| Observation | 35 | 94 months | 87 months | ||
| Barakat 199827 | pCR | Intraperitoneal cisplatin plus etopo side every 28 days, 3 cycles |
36 | NR, 61 % at 36 months | NA |
| Observation | 46 | 28.5 months | NA | ||
| Ben-Baruch 199428 Menczer 198929 |
pCR or clinical CR | Intraperitoneal cisplatin every 28 days, 3 cycles |
19 | 36 months | 60 months |
| Radiotherapy | 18 | 23 months | 39 months | ||
| Bolis 200630 | pCR or CR prior to second Look |
Epidoxorubicin, every 21 days, 4 Cycles |
64 | NA | 57.8% alive at 5 years |
| No treatment | 74 | NA | 54.5% alive at 5 years | ||
| Bruzzone 199031 | CR or SD | Continuation of initial front-line ther apy regimen, 3 courses |
21 | NR, 71.5% NED at 22 months | 48 months |
| Radiotherapy | 20 | 16 months | 24 months | ||
| De Placido 20048 | CR or PR | Topotecan, Days 1–5 every 3 weeks, 4Cycles |
137 | 18.2 months | NA |
| Observation | 136 | 28.4 months | NA | ||
| Goldberg 200132 | CR | Abdominal irradiation | 60 | NA | 30 months |
| No treatment | 79 | NA | 41 months | ||
| Goldhirsch 198833 | pCR | Whole abdominal radiotherapy | 24 | NR, 83% NED at 3 years | NA |
| Any other treatment | 21 | 29.2 months | NA | ||
| Gori 200534 | CR | Intraperitoneal cisplatin plus Hyperthermia |
29 | NA, 3.1% NED at 5 years | 64.4 months |
| No treatment | 19 | NA, 3.7% NED at 5 years | 46.4 months | ||
| Lambert 199335 | Residual disease <2 cm | Carboplatin, 5 cycles | 59 | NR, 76% NED at 5 years | 46 months |
| Whole abdominal radiotherapy | 58 | 49 months | |||
| Lee 200636 | CR | Paclitaxel plus cisplatin, 3 cycles | 42 | 25 months | 74.3 months |
| Observation | 39 | 26 months | 78 months | ||
| Menczer 199237 | CR | Intraperitoneal cisplatin every 28 days, 3 cycles |
25 | NA | 69.3 months |
| No additional treatment | 12 | NA | 45.7 months | ||
| Nicholson 199838 | pCR (experimental); pCR or CR (controls) |
Intraperitoneal radioimmunotherapy, HMFG1 labeled with yttrium-90 |
25 | NA | 94 months |
| No radioimmunotherapy | 20 | NA | 60 months | ||
| Nicoletto 200439 | pCR | 5-FU (for 5 days), followed by cispla tin (Day 6 or 7), every 28 days, 3 Cycles |
61 | 68 months | 87 months |
| No treatment | 61 | 73 months | 89 months | ||
| Oei 200740; Verheijen 200641 | No macroscopic disease at second look |
Intraperitoneal radioimmunotherapy, HMFG1 labeled with yttrium-90 |
224 | Mean, 64 months | NR, 68.7% alive at 3.5 years |
| Standard treatment | 223 | Mean, 67 months | NR, 72.6% alive at 3.5 years | ||
| Papadimitriou 200842 | CR | Cyclophosphamide plus melphalan | 37 | 7.1 years | 96 months |
| Observation | 43 | 1.5 years | 73.2 months | ||
| Piccart 200343 | pCR | Intraperitoneal cisplatin every 3 weeks, 4 cycles |
76 | 64 months | 117 months |
| Observation | 76 | 40 months | 87 months | ||
| Pickel 199944; Pickel 199145 | NED | Whole abdominal radiotherapy | 32 | 76 months | NR at 104 months |
| No radiotherapy | 32 | 26 months | 40 months | ||
| Sorbe 200346’47 (consolidation vs control) |
pCR | Abdominal radiotherapy | 32 | 116 months | 120 months |
| No treatment | 31 | 32 months | 70.6 months | ||
| Varia 200348 | pCR | Intraperitoneal radioactive Phosphorus |
104 | 43.3 months | 84 months |
| No treatment | 98 | 32.9 months | 73 months | ||
| Maintenance | |||||
| Berek 200949; Berek 200450 Berek 200851 |
NED | Oregovomab every 4 weeks, 3 cycles, followed by every 12 weeks, up to 2 years |
73 | 13.3 months | 57.5 months |
| Placebo | 72 | 10.3 months | 48.6 months | ||
| Hall 200452; Hall, unpublished data |
NED | lnterferon-a-2a, 3 days every weeks | 89 | 14.4 months | 49 months |
| Observation | 95 | 15.9 months | 38 months | ||
| Hirte 200653 | NED, CR, and PR (residual dis ease <2 cm) |
Tanomastat | 122 | 10.4 months | 13.9 months |
| Placebo | 121 | 9.2 months | 11.8 months | ||
| Lawton 199054 | <2 cm residual disease | Chlorambucil every days, 14 days every 28 days, 12 cycles |
25 | 20.6 months | |
| Total abdominal and pelvic radiotherapy |
25 | 15.5 months | |||
| Markman 20037; Markman 200913 | CR | Paclitaxel every 28 days, 12 cycles | 150 | 22 months | 53 months |
| Paclitaxel every 28 days, 3 cycles | 146 | 14 months | 48 months | ||
| Micha 200614; Micha 200955; Micha 200556 |
CR | Paclitaxel every 21 days, 3 cycles | 13 | 11 months | 38 months |
| Paclitaxel every 21 days, 12 cycles | 13 | 24 months | NR | ||
| Pecorelli 200915 | pCR, CR | Paclitaxel every 21 days, 6 cycles | 101 | 34 months | 77 months |
| Observation | 99 | 30 months | 84 months | ||
| Sorbe 200346’47 (maintenance vs control) |
pCR | Carboplatin plus doxorubicin or epirubicin, 6 cycles |
35 | 37 months | 78 months |
| No treatment | 31 | 32 months | 70.6 months | ||
| Umesaki 199957 | pCRorCA 125 <8 U/mL | Cisplatin every day for 5 days, every 3–4 months, 3–5 years |
15 | NA | 79.3 months |
| No treatment or <1 year of study treatment |
10 | NA | 67.4 months | ||
PFS indicates progression-free survival; OS, overall survival; pCR, pathologic complete response; NR, not reached; NA, not available; CR, complete response; SD, stable disease; NED, no evidence of disease; PR, partial response; HMFG1, human milk fat globulin 1; 5-FU, 5-fluorouracil.
PFS
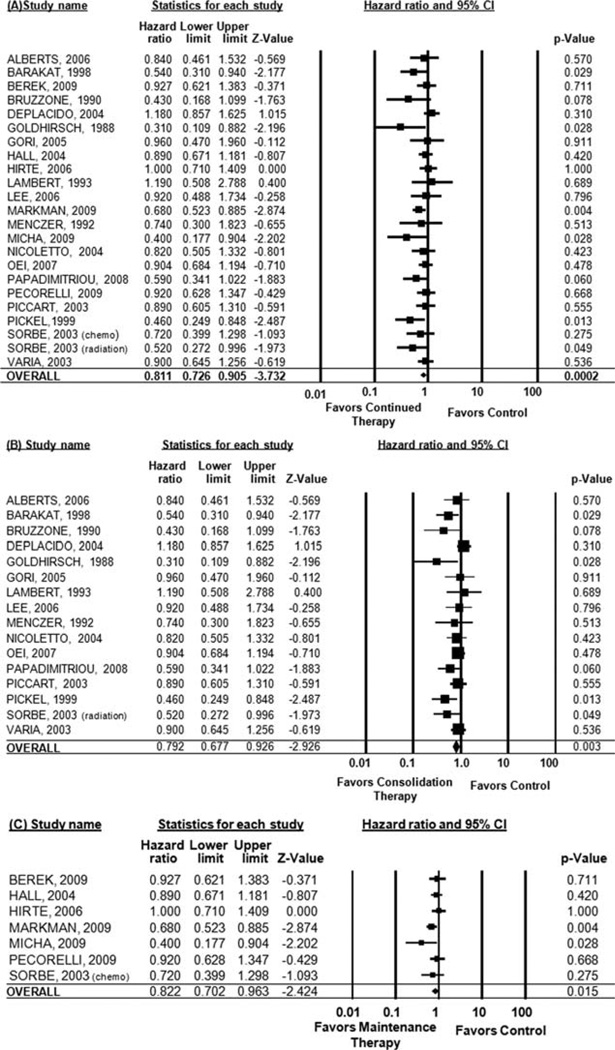
Data on PFS were not provided in the Bolis, Goldberg, Lawton, Nicholson, and Umesaki studies, and only progression rates were available for the Ben-Baruch study, so a hazard ratio could not be estimated. Therefore, these studies were excluded from the PFS analysis. When considering all studies, continued therapy was significantly associated with improved PFS (HR, 0.81; 95% CI, 0.73–0.91 [P = .0002]). For consolidation therapy regimens (<6 cycles of continued therapy), there was also a significant improvement in PFS (HR, 0.79; 95% CI, 0.68–0.93 [P = .003]). When limited to maintenance therapy regimens, there remained a significant improvement in PFS (HR, 0.82; 95% CI, 0.70–0.96 [P = .02]) (Fig. 2).
Figure 2.
Pooled progression-free survival is shown for (A) all studies, (B) consolidation therapy studies, and (C) maintenance therapy studies. CI indicates confidence interval.
OS
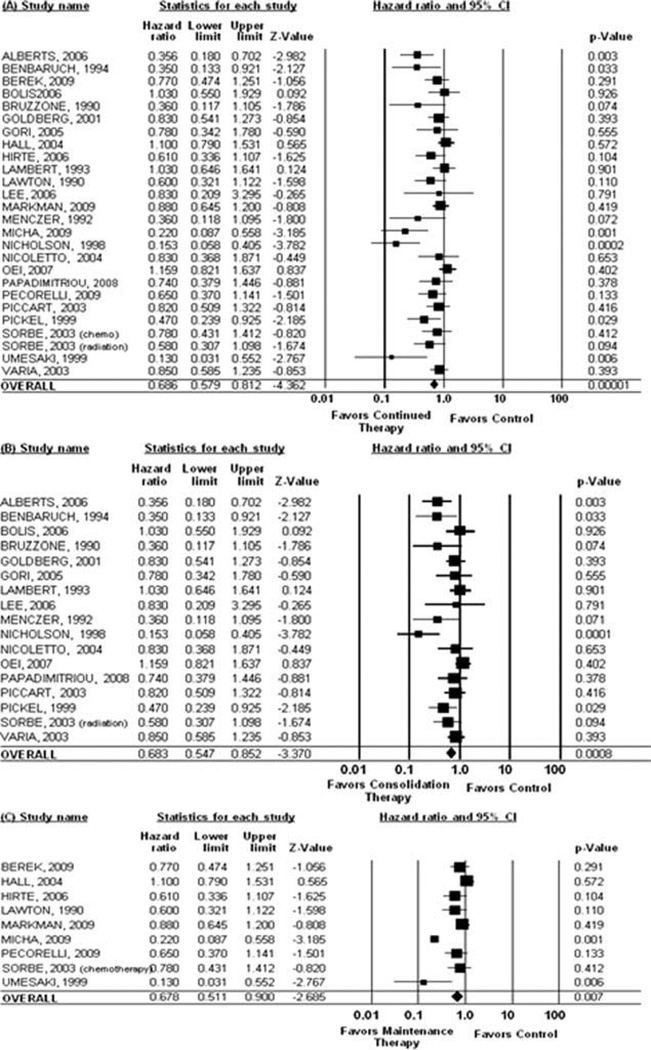
Data on OS were not reported in the Barakat, DePlacido, and Goldhirsch studies, which were therefore excluded from the OS analysis. When considering all studies, continued treatment was significantly associated with improved OS (HR, 0.69; 95% CI, 0.58–0.81 [P = .00001]). When considering only consolidation regimens, treatment was significantly associated with improved OS (HR, 0.68; 95% CI, 0.55–0.85 [P = .0008]). When considering only maintenance therapy, treatment remained significantly associated with improved OS (HR, 0.68; 95% CI, 0.51–0.90 [P = .007]) (Fig. 3).
Figure 3.
Pooled overall survival is shown for (A) all studies, (B) consolidation therapy studies, and (C) maintenance therapy studies. CI indicates confidence interval.
Toxicity
The majority of studies (85.7%, n = 24) reported toxicity data on at least 1 of the treatment groups. However, more than half of the studies could not be included in the pooled analysis because of a lack of reporting any toxicity data (n = 5) or because of reporting toxicity in the experimental treatment group but not in the control group (n = 12). Two additional studies reported toxicities by treatment group, but did not report any of the same toxicities for comparison purposes. The 10 (35.7%) remaining studies reporting at least 1 type of toxicity for the experimental and control groups were included. Hematologic toxicities were reported inconsistently (eg, 3 studies reported thrombocytopenia, 2 reported leukopenia, and 3 reported anemia or thrombocytopenia, whereas 3 others reported “hematologic” toxicities without specification). Cardiotoxicity (reported in 3 trials), infection (2 trials), and a series of trials reporting unique toxicities could not be pooled for analysis. Nausea/vomiting (OR, 1.76; 95% CI, 0.86–3.60 [P = .12]; 6 studies), pain (OR, 1.06; 95% CI, 0.58–1.96 [P = .89]; 5 studies), and fatigue (OR, 1.79; 95% CI, 0.96–3.35 [P = .07]; 4 studies) were not significantly higher among those treated with continued therapy. However, neurotoxicity was significantly higher among patients treated with continued therapy (OR, 2.25; 95% CI, 1.25–4.07 [P = .007]; 6 studies).
Publication Bias and Study Quality
Table 2 shows the result of the fail-safe N analysis. The very large number of insignificant studies required to remove the observed significant effect indicated that publication bias, even if it existed, would not have changed the conclusion of the study. The trim and fill analysis and associated funnel plot suggested that 10 trials may have been suppressed in the literature; however, these were limited to consolidation trials. In the range of 0 to 1.0, with zero representing worst possible and 1.0 representing ideal study quality, the average quality of consolidation trials was 0.68 (range, 0.47–0.94) and of maintenance trials was 0.70 (range, 0.37–0.92).
Table 2.
Results of Fail-Safe N Analyses
| No. of Missing Studies Needed to Bring P Value >.05 |
All Continued Therapy |
Consolidation Therapy |
Maintenance Therapy |
|---|---|---|---|
| Progression-free survival | 149 | 46 | 9 |
| Overall survival | 197 | 78 | 34 |
Sensitivity Analyses of PFS
Six of the 20 consolidation trials evaluated radiation therapy, 5 of which reported PFS data. These trials demonstrated improvement in PFS (HR for radiation therapy, 0.68; 95% CI, 0.49–0.94 [P = .02]). Chemotherapybased consolidation regimens (n = 11 trials) were not associated with improved PFS (HR, 0.85; 95% CI, 0.71–1.01 [P = .07]). None of the maintenance trials evaluated radiation therapy.
When limited to randomized trials, both consolidation therapy (HR, 0.82; 95% CI, 0.69–0.98 [P = .03]; 11 trials) and maintenance therapy (HR, 0.84; 95% CI, 0.73–0.97 [P = .01]; 6 trials) were significantly associated with improved PFS. When limited to trials that enrolled >90% of patients with stage III-IV disease, consolidation therapy significantly improved PFS (HR, 0.70; 95% CI, 0.55–0.87 [P = .002]; 9 trials), but when limited to studies that included >10% of patients with early stage disease, consolidation therapy was not associated with improved PFS (HR, 0.91; 95% CI, 0.76–1.10 [P = .34]; 7 trials). Similarly, when limited to those with »90% of patients with stage III-IV disease, maintenance therapy was associated with improved PFS (HR, 0.78; 95% CI, 0.61–0.99 [P = .04]; 5 trials), but when >10% of the study population had early stage disease, this relationship was not significant (HR for PFS, 0.90; 95% CI, 0.72–1.13 [P = .37]; 2 studies). When limited to patients in CR or pathologic CR, PFS was significantly improved with both consolidation (HR, 0.78; 95% CI, 0.66–0.92 [P = .004]; 8 trials) and maintenance therapy (HR, 0.76; 95% CI, 0.63–0.93 [P = .009]; 5 trials). When patients with residual disease after primary therapy were included in the analysis, these trials did not demonstrate improved PFS (consolidation HR, 0.85; 95% CI, 0.65–1.12 [P = .25]; 7 trials; maintenance HR, 0.96; 95% CI, 0.75–1.24 [P = .77]; 2 trials).
Sensitivity Analyses of OS
When evaluating subgroups of trials by consolidation regimen (chemotherapy or radiation therapy), consolidation chemotherapy was associated with a significant survival advantage (OS HR for chemotherapy regimens, 0.69; 95% CI, 0.52–0.69 [P = .006]; 10 studies). Radiation therapy was associated with improved OS (HR for radiation therapy, 0.66; 95% CI, 0.45–0.66 [P = .04]; 6 studies). No maintenance trials involved radiation therapy as the experimental treatment regimen. When limited to randomized trials, both consolidation (HR for OS, 0.77; 95% CI, 0.62–0.96 [P = .02]; 12 trials) and maintenance therapy (HR for OS, 0.84; 95% CI, 0.84–0.99 [P = .04]; 7 trials) were associated with a significant survival advantage. When limited to studies with >90% of participants with stage III–IV disease, consolidation therapy was significantly associated with OS (HR, 0.69; 95% CI, 0.55–0.86 [P = .001]; 9 trials). When limited to trials that enrolled >10% of participants with early stage OC, consolidation therapy no longer demonstrated a significant OS advantage (HR, 0.66; 95% CI, 0.44–1.00 [P ¼ .05]; 8 trials). When limited to studies with >90% of participants having stage III-IV disease, there also remained a significant survival advantage (HR, 0.61; 95% CI, 0.43–0.86 [P = .004]; 7 trials). When limited to trials of >10% of patients with early stage disease, maintenance therapy was no longer associated with improved OS (HR, 0.89; P = .65); however, there were only 2 trials (384 patients) reporting OS that met this criterion, which may limit the ability to definitively evaluate this relationship.
DISCUSSION
Studies published to date evaluating different strategies of maintenance or consolidation therapy for OC patients in remission at the end of primary therapy have failed to detect a survival benefit. This has been largely attributed to lack of adequate statistical power for these trials. Indeed, the average sample size among the studies identified and included in this meta-analysis was 114 patients, corresponding to 57 per treatment group. Power calculations for the Southwest Oncology Group trial predicted that 225 patients per arm were needed to detect a significant OS improvement caused by maintenance therapy.7 However, this trial was stopped early, so that only 150 and 146 patients were enrolled to the maintenance and control arms, respectively. This early stopping and allowed crossover of patients randomized to the control arm to maintenance strategy led to severe underpowering of the trial for detection of an OS benefit.13
Thus, there has been no single maintenance trial conducted to date that has reached a sample size with enough power to detect survival benefits. A meta-analysis is an appropriate strategy to increase the power to detect differences by pooling the results across studies that may be underpowered individually.24 By pooling the results of these trials to include 2205 patients enrolled in consolidation therapy trials, and 1240 patients enrolled in maintenance therapy trials, this meta-analysis has increased the power to detect survival benefits. This meta-analysis demonstrates a statistically significant improvement in PFS and OS for both consolidation and maintenance therapy, providing a strong rationale for continued investigation of this strategy in OC. Importantly, survival analyses remained statistically significant when limited to randomized trials (consolidation OS HR, 0.77; 95% CI, 0.62–0.96 [P = .02]; maintenance therapy OS HR, 0.84; 95% CI, 0.84–0.99 [P = .04]).
Prospective studies addressing this question are ongoing. The Gynecologic Oncology Group has activated a randomized phase 3 trial testing 12 months of maintenance paclitaxel versus CT-2103 (paclitaxel polyglutamate) versus placebo control for patients with advanced OC in clinical CR at the end of primary therapy (Gynecologic Oncology Group protocol 212). This study is powered to demonstrate a 25% improvement in OS, and anticipates enrolling 1130 patients. Other trials that are currently active or are awaiting analysis include studies testing immunotherapy (eg, abagovomab), antiangiogenic treatments (bevacizumab, sorafenib), or other biological pathways inhibitors (eg, hedgehog inhibitors, poly adenosine diphosphate-ribose polymerase inhibitors). An early yet unpublished report of maintenance antiangiogenic therapy with bevacizumab suggests that this strategy is associated with PFS benefit.25
This meta-analysis shows that the impact of maintenance therapy on survival is greater for trials including a greater proportion of patients with advanced stage OC and for treatment trials as postremission intervention. This is consistent with what is known about higher cure rates for patients with early stage OC, where presumably additional cycles of therapy (6 vs 3) yields no added benefit.26 It is therefore not surprising that continued therapy after a CR does not provide increased benefit in this setting. Likewise, the benefits of systemic chemotherapy compared with radiation therapy can be attributed to its ability to target residual cancer cells regardless of their location.
A weakness of our analysis continues to be the reliance on limited reporting of toxicity data. Neurotoxicity remains a concern for continued therapy, and is largely dependent on the nature of primary therapy and continuation treatment. This is significant issue for patients reaching a meaningful CR when further treatment-related toxicity should be minimized. It is important that future trials of maintenance therapy incorporate measures of patient quality of life prospectively and report adverse events to offer a clear measure of benefits and costs associated with continued therapy.
In summary, by pooling results of many studies that have investigated maintenance and consolidation treatments, we show that postremission therapy improves PFS and OS in women with OC. Our findings that continued therapy is associated with a 32% improvement in OS provide a strong rationale for continuing to address this concept in prospective, adequately powered trials. However, the burden of continuation therapy will have to be weighed against the anticipated clinical benefits. This burden may relate to treatment-related toxicity or to financial costs associated with prolonged therapy. Anticipating that the role of maintenance therapy will be ultimately proven in the setting of a randomized clinical trial, the next critical issue will be to identify the type of maintenance intervention able to prevent cancer recurrence, while avoiding toxicity and preserving quality of life.
Acknowledgments
CONFLICT OF INTEREST DISCLOSURES
Supported by a Project Development Team within the Indiana Clinical and Translational Science Institute National Institutes of Health/National Center for Research Resources RR025761. Its contents are the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Huang L, Cronin KA, Johnson KA, et al. Improved survival time: what can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer. 2008;112:2289–2300. doi: 10.1002/cncr.23425. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen A, Bertelsen K, Andersen JE, et al. Dose-effect study of carboplatin in ovarian cancer. J Clin Oncol. 1997;15:193–198. doi: 10.1200/JCO.1997.15.1.193. [DOI] [PubMed] [Google Scholar]
- 4.Gore M, Mainwaring P, A’Hern R, et al. Randomized trial of dose-intensity with single-agent carboplatin in patients with epithelial ovarian cancer. London Gynaecological Oncology Group. J Clin Oncol. 1998;16:2426–2434. doi: 10.1200/JCO.1998.16.7.2426. [DOI] [PubMed] [Google Scholar]
- 5.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advancedstage ovarian cancer. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberts DS, Hannigan EV, Liu PY, et al. Randomized trial of adjuvant intraperitoneal alpha-interferon in stage III ovarian cancer patients who have no evidence of disease after primary surgery and chemotherapy. Gynecol Oncol. 2006;100:133–138. doi: 10.1016/j.ygyno.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 7.Markman M, Liu PY, Wilczynski S, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy. J Clin Oncol. 2003;21:2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 8.De Placido S, Scambia G, Di Vagno G, et al. Topotecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer. J Clin Oncol. 2004;22:2635–2642. doi: 10.1200/JCO.2004.09.088. [DOI] [PubMed] [Google Scholar]
- 9.Cassileth PA, Begg CB, Bennett JM, et al. A randomized study of the efficacy of consolidation therapy in adult acute nonlymphocytic leukemia. Blood. 1984;63:843–847. [PubMed] [Google Scholar]
- 10.Sauter C, Berchtold W, Fopp M, et al. Acute myelogenous leukaemia: maintenance chemotherapy after early consolidation treatment does not prolong survival. Lancet. 1984;1:379–382. doi: 10.1016/s0140-6736(84)90424-0. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markman M, Liu PY, Moon J, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel. Gynecol Oncol. 2009;114:195–198. doi: 10.1016/j.ygyno.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micha JP, Goldstein BH, Graham C, et al. Improved survival with single-agent paclitaxel consolidation/maintenance therapy in advanced ovarian carcinoma. Oncology. 2006;71:49–53. doi: 10.1159/000100987. [DOI] [PubMed] [Google Scholar]
- 15.Pecorelli S, Favalli G, Gadducci A, et al. Phase III trial of observation versus 6 courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after 6 courses of paclitaxel/platinum-based chemotherapy. J Clin Oncol. 2009;27:4642–4648. doi: 10.1200/JCO.2009.21.9691. [DOI] [PubMed] [Google Scholar]
- 16.Herzog TJ, Coleman RL, Markman M, Cella D, Thigpen JT. The role of maintenance therapy and novel taxanes in ovarian cancer. Gynecol Oncol. 2006;102:218–225. doi: 10.1016/j.ygyno.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Foster T, Brown TM, Chang J, et al. A review of the current evidence for maintenance therapy in ovarian cancer. Gynecol Oncol. 2009;115:290–301. doi: 10.1016/j.ygyno.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 18.McGuire WP. Maintenance therapy for ovarian cancer: of Helsinki and Hippocrates. J Clin Oncol. 2009;27:4633–4634. doi: 10.1200/JCO.2009.23.6653. [DOI] [PubMed] [Google Scholar]
- 19.Cho MK, Bero LA. Instruments for assessing the quality of drug studies published in the medical literature. JAMA. 1994;272:101–104. [PubMed] [Google Scholar]
- 20.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipsey MW, Wilson DB. Practical Meta-Analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 22.Persaud R. Misleading meta-analysis. “Fail safe N” is a useful mathematical measure of the stability of results. BMJ. 1996;312:125. doi: 10.1136/bmj.312.7023.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. Trim and fill: a simple funnel-plotbased method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 24.Cohn LD, Becker BJ. How meta-analysis increases statistical power. Psychol Methods. 2003;8:243–253. doi: 10.1037/1082-989X.8.3.243. [DOI] [PubMed] [Google Scholar]
- 25.Burger RA, Brady MF, Bookman MA, et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC) or Fallopian tube cancer (FTC): A Gynecologic Oncology Group study. J Clin Oncol. 2010;28 doi: 10.1016/j.ygyno.2013.07.100. Abstract LBA 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell J, Brady MF, Young RC, et al. Randomized phase III trial of 3 versus 6 cycles of adjuvant carboplatin and pacli-taxel in early stage epithelial ovarian carcinoma. Gynecol Oncol. 2006;102:432–439. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Barakat RR, Almadrones L, Venkatraman ES, et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with stage II-IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol. 1998;69:17–22. doi: 10.1006/gyno.1998.4973. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Baruch G, Menczer J, Feldman B, Rizel S, Brenner H. Intraperitoneal cisplatin chemotherapy versus abdominopelvic irradiation in ovarian carcinoma patients after second look laparotomy. Eur J Gynaecol Oncol. 1994;15:272–276. [PubMed] [Google Scholar]
- 29.Menczer J, Ben-Baruch G, Modan M, Brenner H. Intraperitoneal cisplatin chemotherapy versus abdominopelvic irradiation in ovarian carcinoma patients after second-look laparotomy. Cancer. 1989;63:1509–1513. doi: 10.1002/1097-0142(19890415)63:8<1509::aid-cncr2820630809>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Bolis G, Danese S, Tateo S, et al. Epidoxorubicin versus no treatment as consolidation therapy in advanced ovarian cancer. Int J Gynecol Cancer. 2006;16(suppl 1):74–78. doi: 10.1111/j.1525-1438.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 31.Bruzzone M, Repetto L, Chiara S, et al. Chemotherapy versus radiotherapy in the management of ovarian cancer patients with pathological complete response or minimal residual disease at second look. Gynecol Oncol. 1990;38:392–395. doi: 10.1016/0090-8258(90)90080-5. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg H, Stein ME, Steiner M, Sprecher E, Beck D, Kuten A. Consolidation radiation therapy following cytore-ductive surgery, chemotherapy and second-look laparotomy for epithelial ovarian carcinoma. Tumori. 2001;87:248–251. doi: 10.1177/030089160108700407. [DOI] [PubMed] [Google Scholar]
- 33.Goldhirsch A, Greiner R, Dreher E, et al. Treatment of advanced ovarian cancer with surgery, chemotherapy, and consolidation of response by whole-abdominal radiotherapy. Cancer. 1988;62:40–47. doi: 10.1002/1097-0142(19880701)62:1<40::aid-cncr2820620110>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Gori J, Castano R, Toziano M, et al. Intraperitoneal hyperthermic chemotherapy in ovarian cancer. Int J Gynecol Cancer. 2005;15:233–239. doi: 10.1111/j.1525-1438.2005.15209.x. [DOI] [PubMed] [Google Scholar]
- 35.Lambert HE, Rustin GJ, Gregory WM, Nelstrop AE. A randomized trial comparing single-agent carboplatin with carboplatin followed by radiotherapy for advanced ovarian cancer. J Clin Oncol. 1993;11:440–448. doi: 10.1200/JCO.1993.11.3.440. [DOI] [PubMed] [Google Scholar]
- 36.Lee SJ, Lee JW, Min JA, et al. A pilot study of 3-cycle consolidation chemotherapy with paclitaxel and platinum in epithelial ovarian cancer patients with clinical complete response after paclitaxel and platinum chemotherapy. Int J Gynecol Cancer. 2006;16:95–100. doi: 10.1111/j.1525-1438.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 37.Menczer J, Ben-Baruch G, Rizel S, Brenner H. Intraperitoneal chemotherapy versus no treatment in patients with ovarian carcinoma who are in complete clinical remission. Cancer. 1992;70:1956–1959. doi: 10.1002/1097-0142(19921001)70:7<1956::aid-cncr2820700724>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson S, Gooden CS, Hird V, et al. Radioimmunotherapy after chemotherapy compared to chemotherapy alone in the treatment of advanced ovarian cancer: a matched analysis. Oncol Rep. 1998;5:223–226. doi: 10.3892/or.5.1.223. [DOI] [PubMed] [Google Scholar]
- 39.Nicoletto MO, Tumolo S, Falci C, et al. A randomized study of epithelial ovarian cancer: is chemotherapy useful after complete remission? Int J Med Sci. 2004;1:116–125. doi: 10.7150/ijms.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oei AL, Verheijen RH, Seiden MV, et al. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer. 2007;120:2710–2714. doi: 10.1002/ijc.22663. [DOI] [PubMed] [Google Scholar]
- 41.Verheijen RH, Massuger LF, Benigno BB, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24:571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 42.Papadimitriou C, Dafni U, Anagnostopoulos A, et al. Highdose melphalan and autologous stem cell transplantation as consolidation treatment in patients with chemosensitive ovarian cancer. Bone Marrow Transplant. 2008;41:547–554. doi: 10.1038/sj.bmt.1705925. [DOI] [PubMed] [Google Scholar]
- 43.Piccart MJ, Floquet A, Scarfone G, et al. Intraperitoneal cisplatin versus no further treatment: 8-year results of EORTC 55875. Int J Gynecol Cancer. 2003;13(suppl 2):196–203. doi: 10.1111/j.1525-1438.2003.13360.x. [DOI] [PubMed] [Google Scholar]
- 44.Pickel H, Lahousen M, Petru E, et al. Consolidation radiotherapy after carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol Oncol. 1999;72:215–219. doi: 10.1006/gyno.1998.5184. [DOI] [PubMed] [Google Scholar]
- 45.Pickel H, Petru E, Lahousen M, et al. Consolidation radiotherapy following carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Am J Clin Oncol. 1991;14:184–187. doi: 10.1097/00000421-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Sorbe B. Consolidation treatment of advanced ovarian carcinoma with radiotherapy after induction chemotherapy. Int J Gynecol Cancer. 2003;13(suppl 2):192–195. doi: 10.1111/j.1525-1438.2003.13359.x. [DOI] [PubMed] [Google Scholar]
- 47.Sorbe B. Consolidation treatment of advanced (FIGO stage III) ovarian carcinoma in complete surgical remission after induction chemotherapy: a randomized, controlled, clinical trial. Int J Gynecol Cancer. 2003;13:278–286. doi: 10.1046/j.1525-1438.2003.13193.x. [DOI] [PubMed] [Google Scholar]
- 48.Varia MA, Stehman FB, Bundy BN, et al. Intraperitoneal radioactive phosphorus versus observation after negative second-look laparotomy for stage III ovarian carcinoma. J Clin Oncol. 2003;21:2849–2855. doi: 10.1200/JCO.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Berek J, Taylor P, McGuire W, et al. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27:418–425. doi: 10.1200/JCO.2008.17.8400. [DOI] [PubMed] [Google Scholar]
- 50.Berek JS, Taylor PT, Gordon A, et al. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol. 2004;22:3507–3516. doi: 10.1200/JCO.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Berek JS, Taylor PT, Nicodemus CF. CA125 velocity at relapse is a highly significant predictor of survival post relapse. J Immunother. 2008;31:207–214. doi: 10.1097/CJI.0b013e31816060ce. [DOI] [PubMed] [Google Scholar]
- 52.Hall GD, Brown JM, Coleman RE, et al. Maintenance treatment with interferon for advanced ovarian cancer: results of the Northern and Yorkshire gynaecology group randomised phase III study. Br J Cancer. 2004;91:621–626. doi: 10.1038/sj.bjc.6602037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirte H, Vergote IB, Jeffrey JR, et al. A phase III randomized trial of BAY 12-9566 (tanomastat) as maintenance therapy in patients with advanced ovarian cancer responsive to primary surgery and paclitaxel/platinum containing chemotherapy. Gynecol Oncol. 2006;102:300–308. doi: 10.1016/j.ygyno.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 54.Lawton F, Luesley D, Blackledge G, et al. A randomized trial comparing whole abdominal radiotherapy with chemotherapy following cisplatinum cytoreduction in epithelial ovarian cancer. Clin Oncol (R Coll Radiol) 1990;2:4–9. doi: 10.1016/s0936-6555(05)80210-9. [DOI] [PubMed] [Google Scholar]
- 55.Micha JP, Goldstein BH, Rettenmaier MA, et al. Clinical utility of CA-125 for maintenance therapy in the treatment of advanced stage ovarian carcinoma. Int J Gynecol Cancer. 2009;19:239–241. doi: 10.1111/IGC.0b013e31819c55c9. [DOI] [PubMed] [Google Scholar]
- 56.Micha JP, Goldstein BH, Mattison JA, et al. Experience with single-agent paclitaxel consolidation following primary chemotherapy with carboplatin, paclitaxel, and gemcitabine in advanced ovarian cancer. Gynecol Oncol. 2005;96:132–135. doi: 10.1016/j.ygyno.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Umesaki N, Tanaka T, Muso H, et al. Intermittent cisplatin therapy for stage-III ovarian cancer patients following clinical remission. Gynecol Obstet Invest. 1999;47:139–143. doi: 10.1159/000010078. [DOI] [PubMed] [Google Scholar]