Abstract
Adoptive cellular immunotherapy utilizing tumor-reactive T cells has proven to be a promising strategy for cancer treatment. However, we hypothesize that successful treatment strategies will have to appropriately stimulate not only cellular immunity, but also humoral immunity. We previously reported that B cells in tumor-draining lymph nodes (TDLN) may function as antigen-presenting cells. In this study, we identified TDLN B cells as effector cells in an adoptive immunotherapy model. In vivo primed and in vitro activated TDLN B cells alone mediated effective (p<0.05) tumor regression after adoptive transfer into two histologically distinct murine pulmonary metastatic tumor models. Prior lymphodepletion of the host with either chemotherapy or whole-body irradiation augmented the therapeutic efficacy of the adoptively transferred TDLN B cells in the treatment of subcutaneous tumors as well as metastatic pulmonary tumors. Furthermore, B cell plus T cell transfers resulted in substantially more efficient antitumor responses than B cells or T cells alone (p<0.05). Activated TDLN B cells conferred strong humoral responses to tumor. This was evident by the production of IgM, IgG and IgG2b, which bound specifically to tumor cells and led to specific tumor cell lysis in the presence of complement. Collectively, these data indicate that in vivo primed and in vitro activated B cells can be employed as effector cells for cancer therapy. The synergistic antitumor efficacy of co-transferred activated B effector cells and T effector cells represents a novel approach for cancer adoptive immunotherapy.
Keywords: B Cells, T Cells, Antibody, Cytotoxicity, Cancer Immunotherapy
Introduction
T cell-based therapy of advanced cancers has been one of the few cellular strategies that have resulted in clinically significant and durable responses. Rosenberg and co-workers have reported that the adoptive transfer of tumor-infiltrating lymphocytes can result in regression of large burdens of metastatic tumor in melanoma patients (1). Our laboratory has been involved in investigating tumor-primed lymph nodes as a source of lymphoid cells that could be secondarily sensitized by in vitro methods to generate effector T cells for adoptive immunotherapy (2–6). In patients with advanced renal cell cancers, we have documented the ability to obtain durable clinical responses using vaccine-primed lymph node cells in adoptive immunotherapy (7). However, clinical responses have been confined to a subset of patients. Novel approaches are needed to improve the efficacy of adoptive T cell therapies.
Cellular and humoral responses represent two critical arms of immunity. We hypothesize that any successful cancer treatment strategy will, in the final analysis, have to appropriately stimulate both the humoral as well as the cellular immune responses. Unfortunately, the current knowledge with respect to the effective induction of anti-cancer humoral responses lags well behind that of the induction of cellular responses. To date, the predominant investigative focus of adoptive immunotherapy for cancer has been understanding the mechanisms involved in the induction, activation, proliferation, and trafficking of effector T cells.
We have previously shown that approximately 60% of tumor-draining lymph node (TDLN) cells are CD3+ T cells. In vitro activation of TDLN cells with anti-CD3/anti-CD28 mAbs results in the generation of therapeutic effector T cells (>90% CD3+ cells) (8–10). Nevertheless, the immune function of B cells, which comprise > 30% of TDLN cells, and their potential antitumor reactivity have not been well characterized. To test our hypothesis that TDLN B cells may function as antigen-presenting cells (APCs), we have reported that the simultaneous in vitro targeting of CD3 on T cells and CD40 on B cells or DC resulted in the generation of more potent effector cells when adoptively transferred into tumor-bearing mice (5). These results established a role for engaging CD40 on TDLN B cells or DCs as APCs in the generation of effector T cells. Depletion of B cells and/or DC cells from the TDLN cells prior to in vitro activation abrogated the anti-CD3 and anti-CD40 augmented antitumor immunity (5).
More recently, we examined the immune modulating effects of IL-21 when administrated concomitantly with T cell transfer for cancer therapy (6). IL-21 administration promoted both T and B cell antitumor reactivity. The humoral response associated with IL-21 administration was manifested by increased levels of tumor-specific IgG2b in the sera of animals. Use of B cell-deficient mice provided direct evidence that host B cells contributed to T cell plus IL-21-elicited antitumor immunity because the IL-21-augmented therapeutic efficacy of the transferred T cells in the wild-type host was significantly reduced in the B−/− host.
In this present report, we have identified that B cells purified from tumor draining lymph nodes can function as effector cells in the adoptive cellular therapy of established cancers. Furthermore, the combined use of B effector cells and T effector cells in adoptive cellular transfer results in substantially more effective antitumor responses.
Materials and Methods
Mice
Female C57BL/6 (B6) mice from Jackson Laboratories, Bar Harbor, ME were maintained in a pathogen-free environment and used at age 8 weeks or older. Principles of laboratory animal care (NIH publication No. 85-23, revised 1985) were followed. The University of Michigan Laboratory of Animal Medicine approved all the animal protocols.
Murine tumor cells
MCA 205 is a weakly immunogenic 3-methylcholanthrene-induced fibrosarcoma that is syngeneic to B6 mice. The D5 melanoma tumor line is a clone of the B16-BL6 tumor line that is poorly immunogenic and syngeneic to B6 mice. D5G6 is a GM-CSF-secreting D5 melanoma tumor line generated in our laboratory (11). MCA 205 tumors were maintained in vivo by subcutaneous (s.c) transplantation in B6 mice and used within the eighth transplant generation. Tumor cells were isolated from solid tumors and resuspended in phosphate buffered solution (PBS) for administration into mice or in complete medium (CM) for in vitro assays as previously described (9).
Tumor Draining Lymph Nodes (TDLN)
To induce TDLN, 1×106 MCA 205 or D5G6 tumor cells in 0.1 ml PBS were injected subcutaneously (s.c) into the lower flanks of normal syngeneic mice. The draining inguinal lymph nodes were collected 9 days later and processed using mechanical dissociation, then filtered through nylon mesh and washed in HBSS. Multiple inguinal TDLN were pooled from groups of mice for lymphoid cell suspension preparation. CD3+ T cells and CD19+ B cells were purified from TDLN cells by using antibody-coupled Microbeads and MACS separator (Miltenyi Biotec. Inc., Sunnyvale, CA).
Cell activation and expansion
T cells and/or B cells were activated with immobilized anti-CD3 plus anti-CD28 mAbs in CM containing hrIL-2 and/or LPS (Sigma-Aldrich, St. Louis, MO) plus anti-CD40. Anti-CD3 and anti-CD28 (BD Biosciences, San Jose, CA) were immobilized on 24-well or 6-well tissue culture plates (Costar, Cambridge, MA). Immobilization was achieved by placing 1 mL of antibody solution in PBS in the wells of the plates and incubating overnight at 4°C or at room temperature for 5 hours. The coated plates were washed with PBS and then used for TDLN cell activation at a concentration of 2×106 cells/mL. CM was used for T cell activation in the presence of recombinant IL-2 (60 IU/mL) (Novartis, Basel Switzerland). These cells were activated/ expanded at 37°C with 5% CO2 for 4 days. Anti-CD40 (FGK45) mAb ascites (1/300 dilution) were used in a soluble form. The anti-CD40 ascites were produced by using FGK45 hybridoma cells (American Type Culture Collection, Rockville, MD). The use of anti-CD40 ascites at 1/300 dilution was determined by previous titrating tests to be optimal for B cell expansion in combination with LPS (5 µg/mL). We determined the IgG content in anti-CD40 ascites using ELISA, and found that the 1/300 dilution is equivalent to an IgG concentration of 0.2–0.5 µg/mL. Activated and expanded TDLN T and/or B cells were used for adoptive immunotherapy, phenotype and immune function analyses.
Antibody production assessment
Supernatants at the end of cell activation were collected and analyzed for IgM, IgG, and IgG2b production using enzyme linked immunosorbent assay (ELISA) (BD PharMingen San Diego, CA). In addition, we also tested IgG and IgM production in response to tumor antigen stimulation. After activation and expansion, 1×106 TDLN T and B cells were co-cultured with 2.5×105 irradiated (6,000 cGy) tumor stimulator cells in 24-well tissue culture plates at 37°C with 5% CO2 for 24 hours. The supernatant was then collected and analyzed for the production of IgG and IgM in response to tumor by ELISA.
Adoptive T and/or B cell therapy of pulmonary metastatic tumor and s.c tumor
B6 mice were inoculated intravenously (i.v.) by tail vein with 2 × 105 MCA 205 or 1 × 105 D5 tumor cells to establish pulmonary metastases. Three or five days after tumor inoculation, the tumor-bearing mice were treated with tail vein injection of activated T cells and/or B cells purified from MCA 205 TDLN or D5G6 TDLN cells. In separate experiments, tumor-bearing mice were treated with unfractionated TDLN cells activated with anti-CD3/anti-CD28/IL-2 or anti-CD3/anti-CD28/IL-2 + LPS/anti-CD40. Commencing on the day of the cell transfer, intraperitoneal (i.p.) injections of IL-2 (40,000 IU) were administered in 0.5 ml of PBS and continued twice daily for 8 doses. Approximately 14 days after T cell transfer, all mice were sacrificed, and lungs were harvested for enumeration of pulmonary metastatic nodules. S.c tumors were treated in combination with whole body irradiation as described below.
Treatment of s.c tumors with purified B cells in combination with total body irradiation (TBI) and the induction of lymphodelpletion
Established (5-Day) s.c MCA 205 tumor-bearing mice were treated with TBI (5 Gy) followed by adoptive transfer of purified and activated MCA 205 TDLN B cells. Tumor size was then monitored. In other experiments, we utilized a clinically relevant protocol for the induction of non-myeloablative lymphodepletion and executed B cell therapy of cancer under lymphopenic conditions. In these experiments, we used the D5 melanoma tumor which is resistant to cyclophosphamide and fludara chemotherapy. In this model, D5 tumor cells were inoculated on day 0, chemotherapy (1mg cyclophosphamide and 2 mg fludara) was administered i.p. on days 3 and 4; and activated B cells were adoptively transferred i.v. on day 5.
Flow cytometry analysis
Cell surface expression of CD3, CD19, and B220 was analyzed by immunofluorescence assay and flow cytometry using a FACS Calibur flow cytometer (Becton Dickson & Co., Mountain View, CA). Fluorescence profiles were generated by analyzing 10,000 cells and displayed as logarithmically increased fluorescence intensity versus cell numbers. All FITC- or PE-conjugated antibodies were from BD PharMingen. To block binding to the Fc receptors, mouse BD Fc Block™ (purified anti-mouse CD16/CD32, BD PharMingen) was used to incubate the cells before the addition of FITC- or PE-conjugated antibodies. Binding of IgG2b produced by activated B cells to tumor cells was detected using FITC-anti-mouse IgG2b or matched isotype control (both from BD Biosciences) following the incubation of tumor cells with B cell culture supernatants. FACS data analysis was performed using the CellQuest software (BD Biosciences).
Antibody and complement-mediated cytotoxicity
Tumor cell lysis mediated by antibodies produced during TDLN activation was assessed by incubating tumor cells with culture supernatants in test tubes on ice for one hour followed by cell culture in the presence of rabbit complement (CalBiochem, Darmstadt, Germany) in a 37°C water bath for another hour. Viable cells were then counted under a microscope after trypan blue staining to calculate cell lysis. Alternatively, cytotoxicity was analyzed in 96-well flat bottom tissue culture plates and was determined using the Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA) according to the manufacturer’s instructions. OD values were measured via a multi-well spectrophotometer to evaluate tumor cell lysis.
Statistical Analysis
The significance of differences in numbers of metastatic nodules; the size of s.c tumors; the concentration of immunoglobulin, and cell lysis was determined using one-way analysis of variance (Newman- Keuls post hoc test) or unpaired t test. p values of < 0.05 were considered statistically significant between the experiment groups.
Results
1. Tumor-primed and ex vivo activated B cells produce IgG and IgM in response to tumor
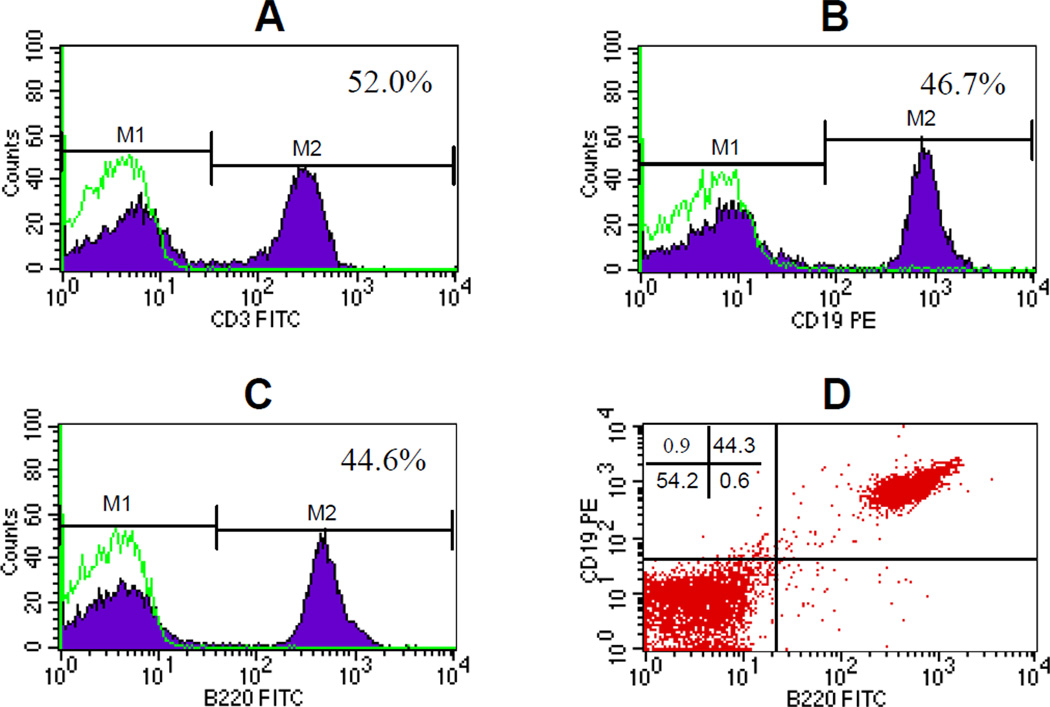
To date, molecular targets chosen for antibody activation to generate antitumor effector cells have been confined on T cells. We reported previously (5) that while tumor-draining lymph node cells are composed mainly of T cells, which are about 55% of the whole TDLN cell population, the rest of the TDLN cells are CD3− cells. In this study, we further characterized these CD3− cells in the TDLN. Flow cytometry analysis (Fig. 1) indicate that in addition to the 50–55% of CD3+ TDLN T cells (Fig 1A), the remainder of the approximately 45% TDLN cells are B cells. This was revealed by their positive staining of CD19 (Fig 1B) or B220 (Fig 1C). PE-anti-CD19 and FITC-anti-B220 double staining revealed that these TDLN B cells express both CD19 and B220 (Fig 1D).
Fig 1.
CD19+ B cells in the TDLN. TDLN cell suspension was prepared from freshly harvested TDLNs and stained with FITC-anti-CD3 (A), PE-anti-CD19 (B), or FITC-anti-B220 (C) respectively, or double-stained with PE-anti-CD19 and FITC-anti-B220 (D). Stained cells were then analyzed by flow cytometry using a FACScan flow microfluorometer as described in the Materials and Methods. Data are representative of five experiments performed.
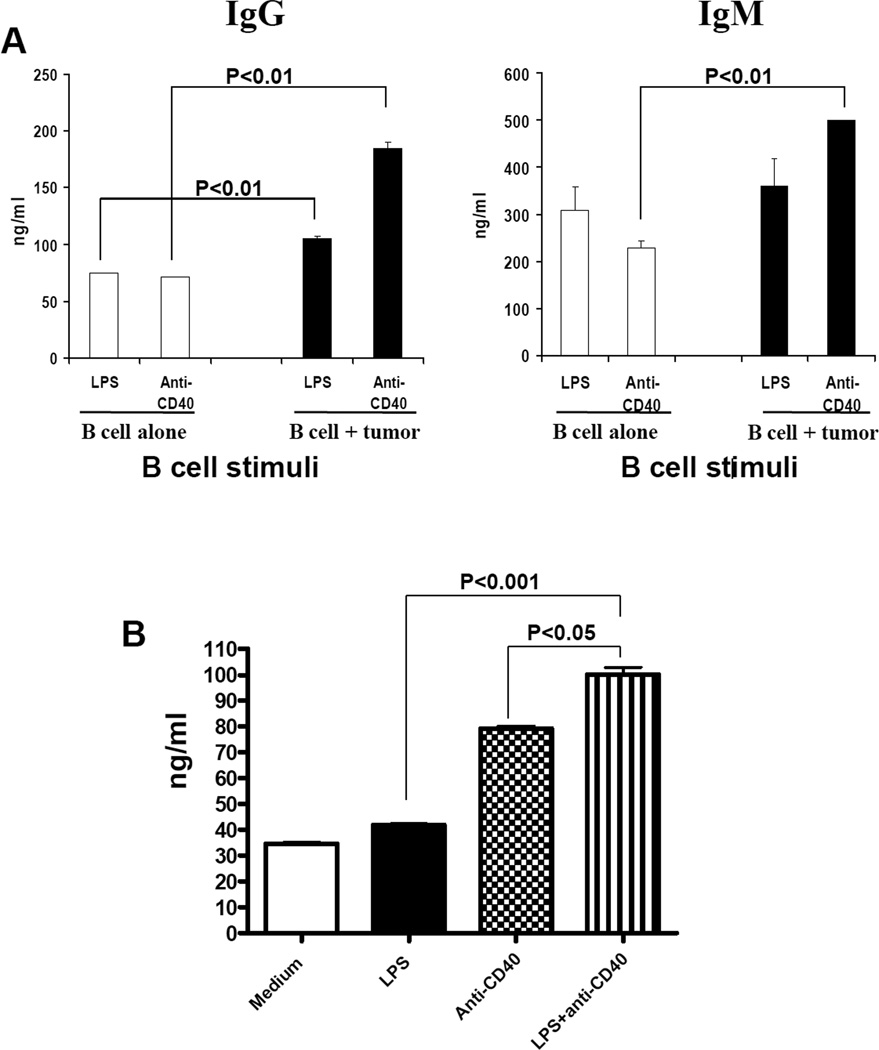
To test our hypothesis that in vivo sensitized TDLN B cells may have the potential to mediate antitumor responses, we purified these B cells from the TDLN cells. Utilizing anti-CD19 coupled magnetic beads, we have consistently obtained an enriched population of B cells from TDLN (>95% CD19+, data not show). Purified TDLN B cells were put into 24-well culture plate at 2×106 cells/ml, 2ml per well in complete medium, and activated with LPS or anti-CD40 respectively for 4 days. Activated B cells were harvested for phenotype and function analyses. In vitro studies with varying concentrations of LPS or anti-CD40 revealed that LPS activation increased IgG and IgM production by increasing B cell proliferation (data not shown). Anti-CD40 increased Ig production by stimulating B cell differentiation (indicated by CD38 modulation, data not shown). These activated B cells when cultured with irradiated tumor cells resulted in significantly greater Ig production compared to the absence of tumor cells (B cell alone) (Fig 2A). These experiments suggested that tumor-primed, and LPS or anti-CD40 activated TDLN B cells produce antibody in response to tumor. Furthermore, the combination of LPS and anti-CD40 resulted in significantly (p<0.05) enhanced Ig production compared to LPS or anti-CD40 activation alone (Fig 2B). We therefore used this LPS and anti-CD40 combination as TDLN B cell stimuli in this study.
Fig 2.
Immunoglobulin production of TDLN B cells after activation. 2A. Purified and activated TDLN B cells produce IgG and IgM in response to tumor. B cells purified from MCA 205 TDLNs were activated with LPS or anti-CD40 respectively. Activated TDLN B cells (1×106) were then re-stimulated with irradiated MCA 205 tumor cells for 24 hours. The culture supernatant with tumor re-stimulation (B cell + tumor) or without tumor re-stimulation (B cell alone) were collected and analyzed for the secretion of IgG and IgM by ELISA. 2B. LPS plus anti-CD40 activation significantly enhanced IgM production of TDLN B cells compared with LPS or anti-CD40 activation alone. Purified B cells from TDLN were activated with LPS and/or anti-CD40. Cultured supernatants at the end of cell activation were then collected and analyzed for immunoglobulin production using ELISA.
2. In vivo primed and in vitro activated TDLN B cells mediate significant tumor regression after adoptive transfer
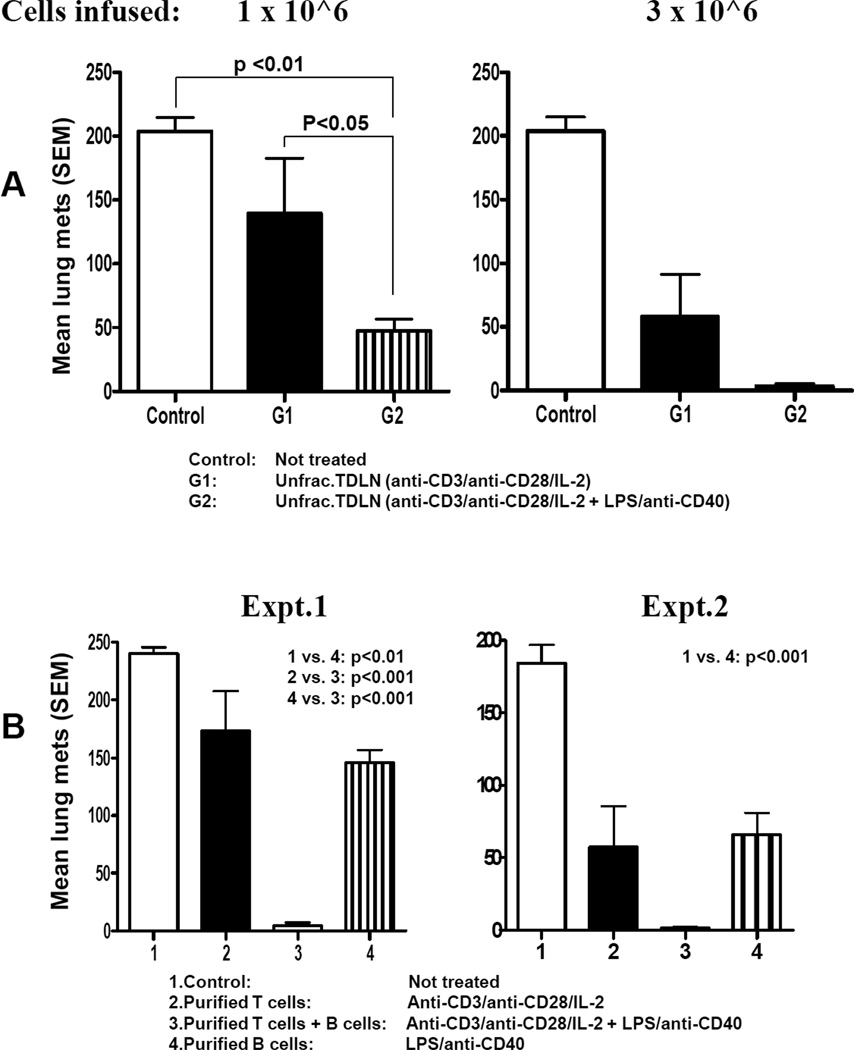
To understand the immunological significance of antibody production by in vivo sensitized and in vitro activated B cells derived from TDLN, we performed adoptive therapy experiments using whole TDLN cells after simultaneous activation of T cells with anti-CD3/ anti-CD28 plus activation of B cells with LPS/anti-CD40. Utilizing the MCA 205 tumor model, we compared the antitumor reactivity of these activated TDLN cells with TDLN cells activated with anti-CD3/anti-CD28 alone in the adoptive immunotherapy of 3-day established pulmonary metastases. As shown in Fig 3A, unfractionated TDLN cells simultaneously activated with anti-CD3/anti-CD28 plus LPS/anti-CD40 to stimulate T cells and B cells (group 2) resulted in significantly enhanced tumor regression in adoptive transfer compared to an equivalent number of cells activated with anti-CD3/anti-CD28 only (T cells alone, group 1). By FACS, TDLN activated with anti-CD3/CD28 alone had approx. 95% T cells compared to ~ 70%:30% T:B cells with anti-CD3/CD28 plus LPS/anti-CD40 activation. These experiments clearly indicated that antitumor efficacy of in vitro activated T cells plus B cells was more potent than using T cells alone.
Fig 3.
Activated TDLN B cells mediated effective tumor regression. 3A. Simultaneously activated T and B cells in unfractionated TDLN cells mediated significantly more efficient tumor regression than T cells alone. B6 mice were inoculated i.v. by tail vein with MCA 205 tumor cells to establish pulmonary metastases. Three days after tumor inoculation, the tumor-bearing mice were treated with tail vein injection of various doses of unfractionated MCA 205 TDLN cells activated with anti-CD3/anti-CD28/IL-2 or anti-CD3/anti-CD28/IL-2 + LPS/anti-CD40 respectively. Approximately 14 days after cell transfer, all mice were randomized and sacrificed, and lungs were harvested for enumeration of pulmonary metastatic nodules. There were 5 mice in each group, and all the animals formed tumor except for G2 when 3 × 106 cells were infused. Data are representative of four experiments performed. 3B. Purified and activated TDLN B cells alone mediated tumor regression and the therapeutic efficacy can be significantly augmented by purified B cells plus T cells. Three-day pulmonary metastases were established as in 3A, but treated with purified and activated B cells alone, T cells alone or T cells plus B cells. Lungs were harvested for enumerations of pulmonary metastatic nodules as in 3A. There were 5 mice in each group, and all the animals formed tumor except for group 3 in both experiments. Data are representative of five experiments performed.
We proceeded to investigate whether purified TDLN B cells alone were capable of mediating tumor regression in adoptive transfer models. We enriched B cells by immunomagnetic beads and secondarily activated them with LPS and anti-CD40. These activated B cells were adoptively transferred into mice with 3-day established lung metastases. The results are illustrated in Fig 3B. Activated B cells alone mediated significant (p<0.01) tumor regression compared with untreated control animals. We also evaluated the antitumor activity of the mixture of purified T and B cells from TDLN that were activated together with anti-CD3/anti-CD28 plus LPS/anti-CD40. When purified T and B cells from TDLN were mixed at a T:B ratio of 1:2 and activated, a 65%:35% T:B cell mixture was obtained. These cells mediated significantly greater reduction of tumor metastases (group 3) upon adoptive transfer compared to equal numbers of purified T cells (group 2) or B cells (group 4) that were activated by anti-CD3/anti-CD28 or LPS/anti-CD40, respectively (Fig. 3B). Collectively, these data indicate that enhanced antitumor efficacy of cell therapy can be achieved by co-transferring T cells plus B cells after in vivo priming and in vitro activation. In vivo primed and in vitro activated B cell may represent a novel effector cell type for cancer immunotherapy.
3. Radiation and chemotherapy significantly enhance the therapeutic efficacy of adoptively transferred B cells
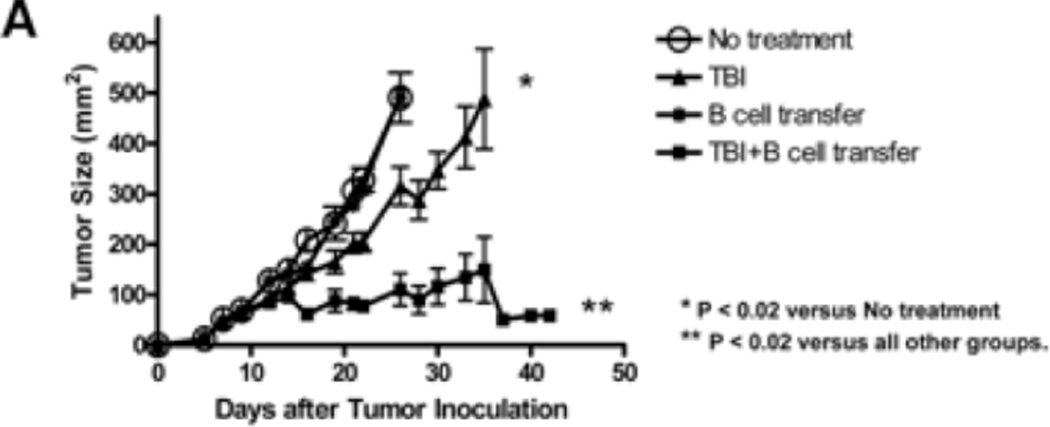
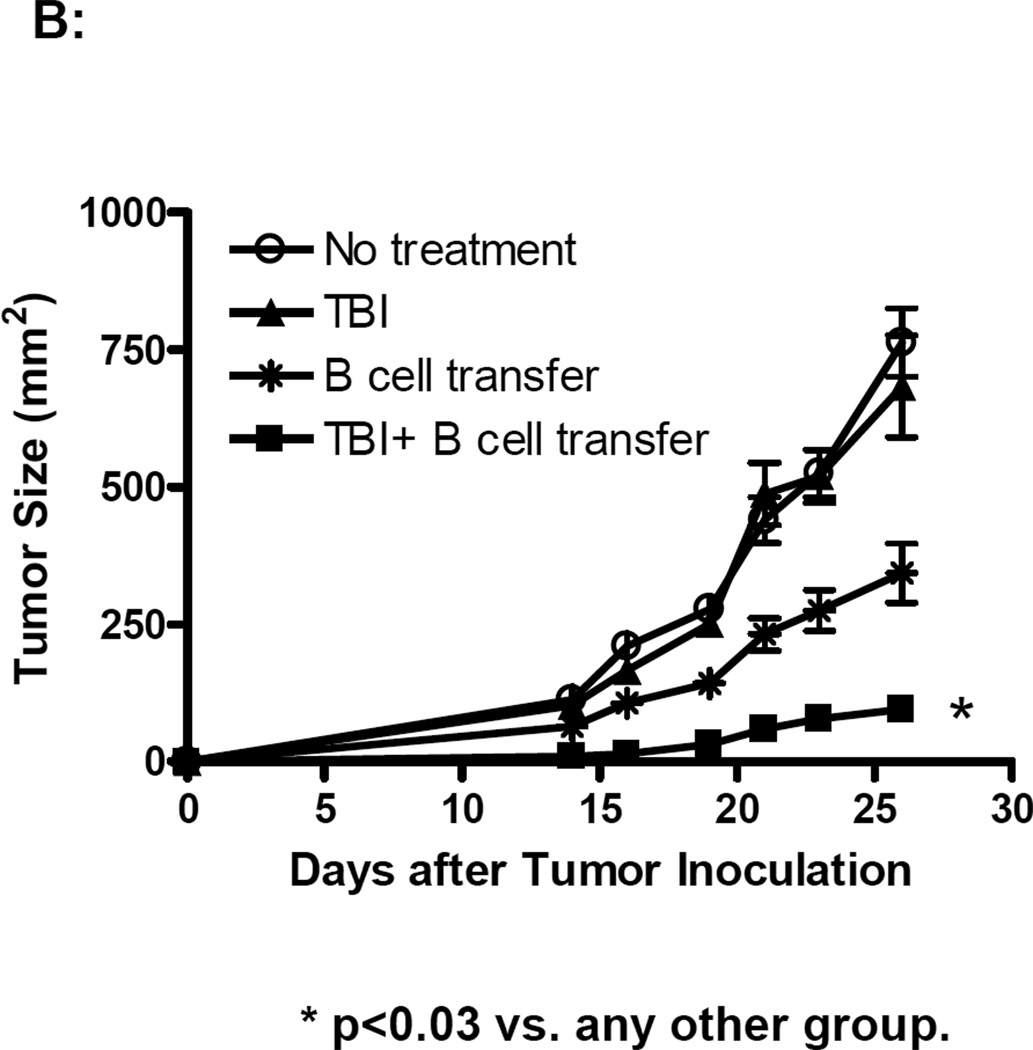
S.c tumor models are generally more resistant to cellular therapy than pulmonary metastatic tumor models. We proceeded by examining whether adoptively transferred effector B cells could mediate effective antitumor effects in a s.c tumor model. In Fig. 4A, established (5-day) s.c MCA 205 tumor-bearing mice were treated with TBI (5 Gy) followed by the adoptive transfer of purified and activated MCA 205 TDLN B cells. While TBI alone slightly suppressed tumor growth, B cell transfer alone showed no therapeutic efficacy in the s.c tumor model. However, B cell transfer following TBI synergistically and significantly inhibited tumor growth. In a similar way as described for the treatment of s.c MCA 205 tumors in Fig. 4A, we treated s.c. D5 tumors with TBI (5 Gy) and/or B cells (5 × 106). The results are summarized in Fig. 4B. Compared with the control (No treatment), TBI alone showed no therapeutic efficacy, while B cell transfer inhibited the s.c. D5 tumor growth to a modest extent. Importantly, B cell transfer following TBI resulted in significant inhibition of tumor growth (p<0.03 compared with any other group).
Fig 4.
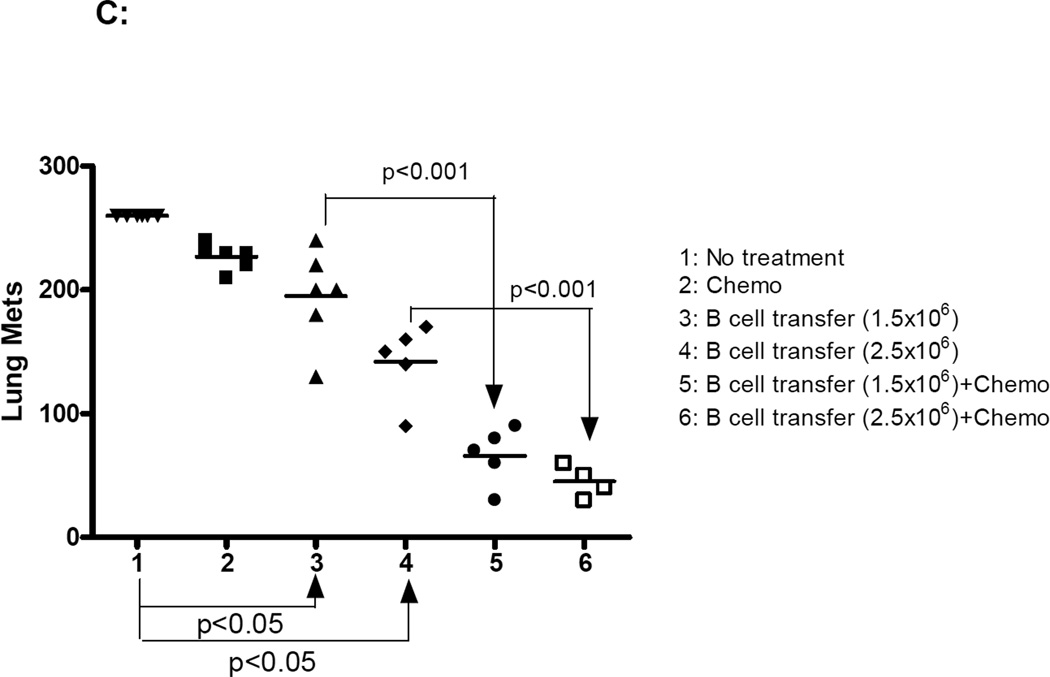
TBI or chemotherapy significantly augmented the therapeutic efficacy of adoptively transferred B cells. 4A. TBI significantly augmented the therapeutic efficacy of adoptively transferred B cells in the s.c MCA 205 tumor model. Established (5-Day) s.c MCA 205 tumor-bearing mice were treated with TBI (5 Gy) followed by adoptive transfer of purified and activated MCA 205 TDLN B cells. Animals treated with TBI or B cells alone served as controls. Tumor size was then monitored. There were 6 mice in each group, and all the animals formed tumors. Data are representative of two independently conducted experiments. 4B. TBI significantly augmented the therapeutic efficacy of adoptively transferred B cells in the s.c D5 tumor model. Established s.c D5 tumor-bearing mice were treated with TBI and/or adoptive transfer of purified and activated D5G6 TDLN B cells as in 4A. There were 6 mice in each group, and all the animals formed tumor. Data are representative of two experiments performed. 4C. The poorly immunogenic tumor, D5 melanoma, was used in this experiment. Five-day pulmonary metastases were established as in Fig. 3. In relevance to inoculation of D5 tumor cells on day 0, chemotherapy (1mg cyclophosphamide and 2 mg fludara) was administered i.p. on days 3 and 4 to induce lymphodelpletion. Various doses of purified and activated D5G6 TDLN B cells were given i.v. on day 5. Lungs were harvested for enumerations of pulmonary metastatic nodules as in Fig. 3. There were 6 mice in each group, and all the animals formed tumor.
TBI of 5 Gy induces lymphodepletion (12). The results revealed in Fig. 4A and 4B suggest that adoptively transferred TDLN B cells suppress tumor growth more effectively in the lymphopenic host. To further evaluate the function of adoptively transferred effector B cells in the lymphopenic host, we utilized a clinically relevant protocol for the induction of non-myeloablative lymphodepletion and executed B cell therapy of cancer under these lymphopenic conditions. In this experiment, we used the D5 melanoma tumor because it is resistant to cyclophosphamide and fludara chemotherapy. TDLN were induced by inoculating B6 mice with a GM-CSF-secreting D5 tumor line in the flank (11). D5G6 TDLN B cells were enriched for subsequent activation with LPS and anti-CD40. These cells were transferred into chemotherapy-conditioned lymphopenic hosts for the treatment of established 5-day D5 melanoma lung metastases. Chemotherapy mediated a profound, but transient, reduction in total white blood cell counts in the peripheral blood (data not shown). As demonstrated in Fig 4C, chemotherapy alone had no significant therapeutic effect on D5 tumor growth. In vivo sensitized and in vitro activated B cells mediated D5 tumor regression (p<0.05) in a dose dependent manner. These results verified the therapeutic efficacy of TDLN B cells in a second tumor model tested in this study. More importantly, for both doses of B cells infused, there was significantly (p<0.001) greater therapeutic effect in the lymphodepleted hosts. These experiments indicate that induction of lymphopenia prior to adoptive transfer of TDLN B cells could significantly augment the therapeutic efficacy of these cells.
4. Therapeutic efficacy of TDLN B cells is associated with the antibody production and tumor specific cytotoxicity
To define the possible mechanisms underlying the antitumor effects of TDLN B cells, we compared the antibody production and examined the presence of cytotoxic antibody subclasses, e.g. IgG2b, after culture of the purified B cells used for adoptive immunotherapy.
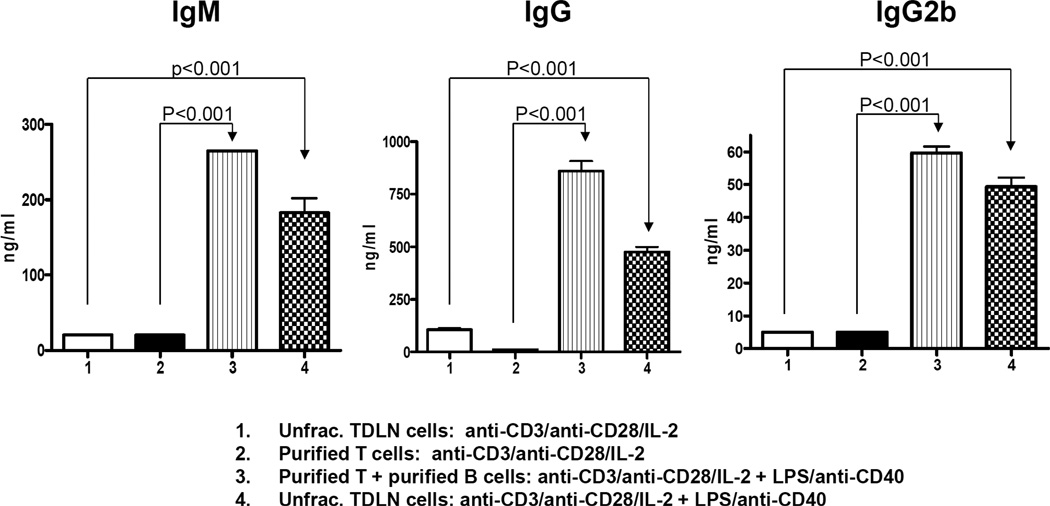
At the end of cell activation and expansion with anti-CD3/anti-CD28 and/or LPS/anti-CD40, cell culture supernatants were collected for immunoglobulin detection (Fig. 5). Significantly increased IgM, IgG and IgG2b production (p<0.001) in groups 3 and 4 correlated with the significantly increased therapeutic efficacy observed using these cells (T+B at 65–70%:30–35%) in adoptive immunotherapy of established pulmonary metastases (Fig. 3A and 3B).
Fig 5.
Significantly increased IgM, IgG and IgG2b production in the culture of T + B cells. Supernatant collected at the end of T and/or B cell activation with anti-CD3/anti-CD28/IL-2 and/or LPS/anti-CD40 were tested for IgM, IgG and IgG2b using ELISA. Data are representative of four independently performed experiments.
In Fig. 4A and 4B, we tested the therapeutic efficacy of purified B cells to treat established (5-day) s.c. tumors in combination with TBI (Fig. 4A) or to treat established D5 pulmonary metastatic cancer in a lymphodepleted host (Fig. 4B). Antibody production was also assessed in the culture supernatant of these purified B cells used for adoptive transfer in these experiments. Table 1 shows that these purified TDLN B cells consistently produce IgM, IgG, and IgG2b after activation with LPS/anti-CD40 before infusion.
Table 1.
Secretion of antibodies by activated B cells enriched from TDLNs (ng/ml, mean ± SEM)
| D5G6 TDLN B cells | MCA205 TDLN B cells | |||
|---|---|---|---|---|
| Expt. 1 | Expt. 2 | Expt. 1 | Expt. 2 | |
| IgM | 186±50 | 158±7 | 122±14 | 410±153 |
| IgG | 931±382 | 375±12 | 685±263 | 838±333 |
| IgG2b | 86±29 | 47±4 | 73±26 | 62±18 |
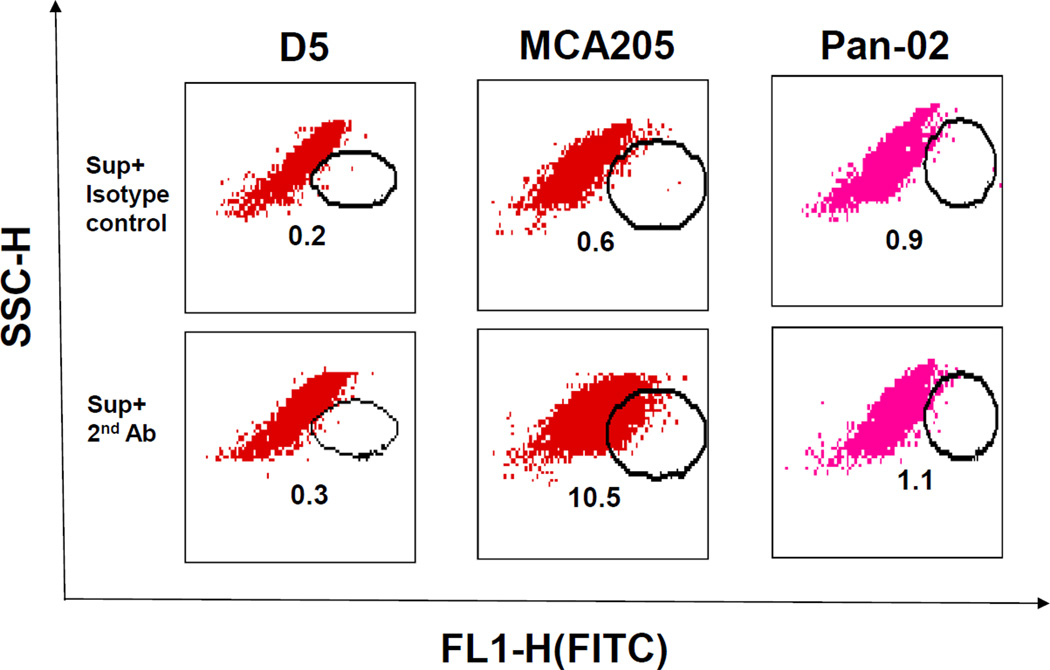
IgG2b was reported to mediate hyperacute rejection in rat cardiac xenografts in a complement dependent manner (13). We tested the binding of IgG2b to tumor cells by flow cytometry in the following experiments. Fig. 6 shows that MCA 205 TDLN B cell-produced IgG2b, as shown in Table 1, binds to MCA 205 tumor cells (10.5% vs. 0.6% control) in a tumor specific fashion and did not bind to D5 or Pan-02 tumor cells.
Fig 6.
TDLN B cell-produced IgG2b binds specifically to tumor cells. D5, MCA205, and Pan-02 tumor cells were incubated with the culture supernatant (Sup) of MCA 205 TDLN B cells. Bound IgG2b onto the tumor cells was then detected by secondary antibody FITC-anti-mouse IgG2b. FITC-coupled secondary antibody isotype-matching control was also used. Numbers within each immunofluorescence dot plots indicate the percentage of viable cells stained positive with the FITC-coupled antibodies. Data are representative of three independently performed experiments.
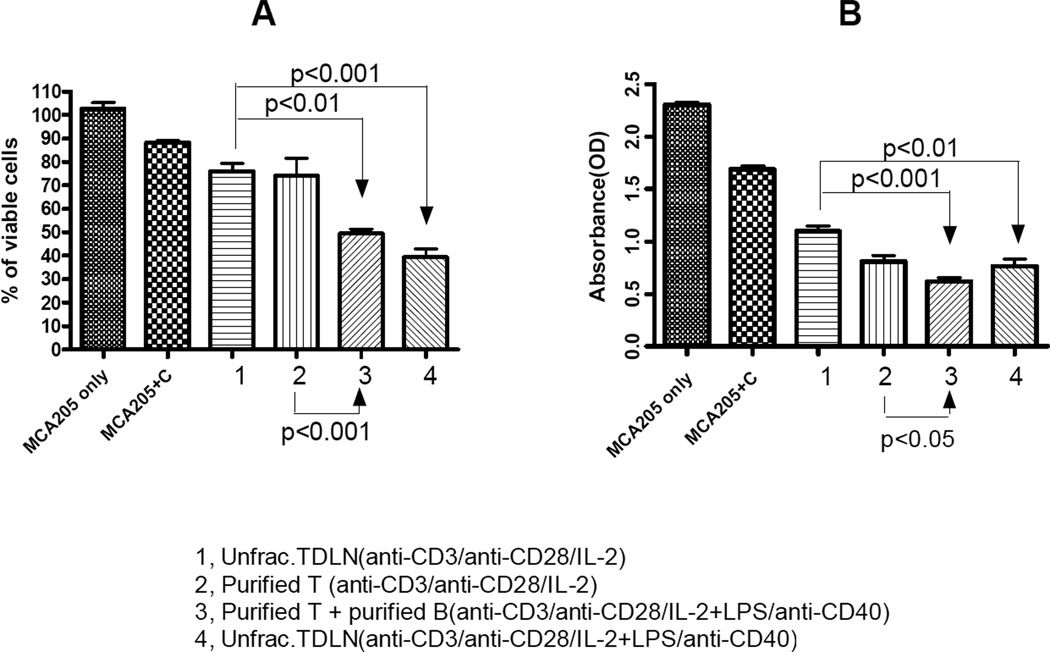
We then tested antibody and complement-mediated cytotoxicity. As indicated in Fig. 7A, antibody from supernatants where B cell activations were performed (groups 3 and 4) demonstrated significantly more efficient tumor cell killing compared with supernatants derived from cell cultures where only T cell activations were performed (groups 1 and 2). This enhanced tumor cell killing correlated with the significantly increased Ab production (Fig. 5) and therapeutic efficacy observed using these cells (T+B at 65–70%:30–35%, Fig. 3A, 3B) in adoptive immunotherapy of established pulmonary metastatic cancers. Alternatively, antibody-mediated cytotoxicity was analyzed by a spectrophotometric assay. In this assay, a special substrate 4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1, 3- benzene disulfonate (WST-1) was added to tumor cell cultures at the end of antibody (cell culture supernatant 1–4) and complement incubation. The assay is based on the cleavage of WST-1 to formazan dye by cellular mitochondrial dehydrogenases. Since cleavage of WST-1 to formazan dye occurs only in viable cells, the amount of dye produced, measured in OD values, directly correlates with the number of viable cells present in the culture. As shown in Fig. 7B, supernatants from cultures where B cells were activated (groups 3 and 4) demonstrated significantly increased tumor cell killing compared to cultures where only T cells were activated (groups 1 and 2). This was indicated by the significantly (p<0.05) reduced OD values in these groups. These experiments verified the antibody plus complement-mediated tumor cell lysis data measured by visual viable cell counting under the microscope (Fig. 7A).
Fig 7.
Antibody and complement-mediated tumor cell lysis. 7A: IgG2b-enriched cell culture supernatant induces tumor cell cytotoxicity. Viable MCA 205 tumor cells (0.5 × 106) were put in 4 mL test tubes in 450 µl CM plus 50µl cell culture supernatant from four cell groups respectively as indicated. Cells were incubated on ice for 1 hour. After centrifugation, culture supernatants were discarded and 450 µl fresh CM plus 50µl rabbit complement was added to each tube followed by incubation in a 37°C water bath for another one hour. Control groups included MCA 205 cells similarly cultured without cell culture supernatants or complement (MCA 205 only), or with complement alone (MCA 205 + C). Viable cells were then counted under the microscope after trypan blue staining. Percentage (%) of viable cells was calculated by dividing the remaining viable cells with 0.5 × 106. Data are representative of two independent experiments conducted. 7B: Antibody and complement-mediated cytotoxicity was analyzed in 96-well culture plates and determined using the Quick Cell Proliferation Assay Kit. This assay was performed according to the manufacturer’s instructions. MCA 205 tumor cells were incubated with or without antibody-enriched cell culture supernatants and complement as in Fig. 7A. OD values were then measured via a multi-well spectrophotometer to evaluate cell lysis. Data are representative of three independent experiments conducted.
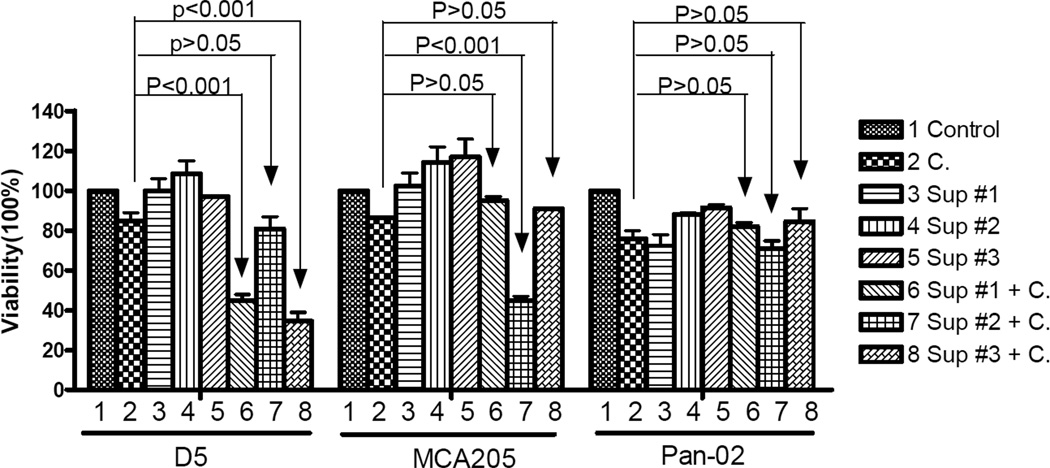
The specificity of tumor cell lysis by TDLN B cell-produced antibody and complement was also examined. As indicated in Fig. 8, D5-TDLN B cell culture supernatants (groups 6 and 8) plus complement mediated significant (p<0.001) D5 tumor cell killing, while MCA 205-TDLN B cell culture supernatant (group 7) showed no D5 cell lysis in the presence of complement compared with the use of complement alone (p>0.05). As indicated previously, IgM, IgG and IgG2b production by these activated TDLN B cells was shown by Table 1. By comparison, MCA 205-TDLN B cell culture supernatant plus complement (group 7) mediated significant (p<0.001) MCA 205 tumor cell killing, while D5-TDLN B cell culture supernatants (groups 6 and 8) did not lyse MCA 205 cells in the presence of complement and was similar to the use of complement alone (p>0.05). Use of Pan-02 tumor cells further confirmed that this tumor killing was immunologically specific.
Fig 8.
Tumor specific cytotoxicity mediated by antibodies and complement. Viable D5, MCA 205, or Pan-2 tumor cells (0.5 × 106) were incubated with culture supernatants of D5G6 TDLN B cells from two separate experiments (Sup#1 and Sup#3) or with culture supernatant of MCA 205 TDLN B cells (Sup #2) in 4 mL test tubes as indicated. After that, rabbit complement was added for incubation for one hour. Control groups included tumor cells cultured with complete medium (Control), complement only (C), or antibodies without complement. Viable cells were then counted under the microscope after trypan blue staining. Percentage (%) of viable cells was calculated by dividing the remaining viable cells with 0.5 × 106.
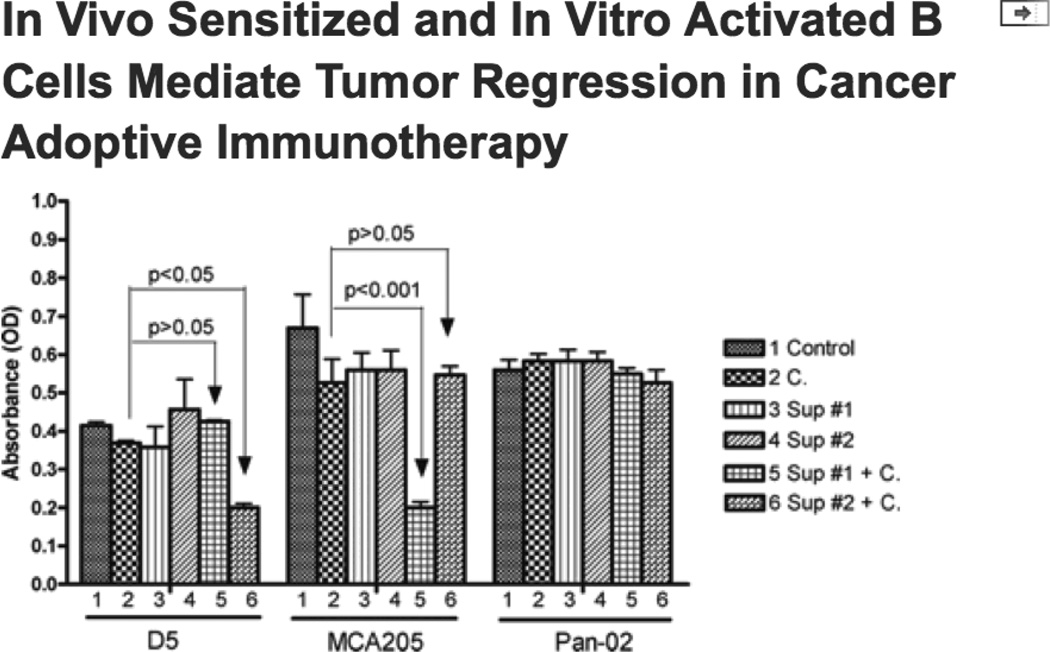
Alternatively, antibody-mediated tumor-specific cytotoxicity was analyzed in the WST-1 assay described above. As shown in Fig. 9, D5-TDLN B cell culture supernatant plus complement (group 6) significantly killed D5 tumor (p<0.05), but not MCA 205 tumor (p>0.05). By comparison, MCA 205-TDLN B cell culture supernatant plus complement (group 5) significantly killed MCA 205 tumor (p<0.001), but not D5 tumor (p>0.05). In addition, the use of Pan-02 tumor cells confirmed that this tumor killing was indeed immunologically specific.
Fig 9.
Tumor specific cytotoxicity analyzed in 96-well culture plates and determined using the Quick Cell Proliferation Assay Kit. This assay was performed similarly as in Fig. 7B. D5 and MCA205 tumor cells were incubated with antibody-enriched cell culture supernatant of MCA 205 TDLN B cells (Sup#1) or D5G6 TDLN B cells (Sup#2) and complement. OD values were then measured via a multi-well spectrophotometer to evaluate cell lysis. Pan-02 tumor cells; tumor cells incubated with complete medium (Control), or complement only (C), or with antibody but no complement served as controls. Data were repeated in a second experiment.
Discussion
The experimental results we described in this study using two histologically distinct weakly immunogenic and non-immunogenic tumors provide direct evidence that in vivo primed and in vitro activated B cells isolated from TDLN can serve as a cellular reagent to mediate regression of established pulmonary metastases and subcutaneous tumors upon adoptive transfer. Mechanistically, we observed significantly enhanced tumor-associated antibody responses. Cytotoxic antibody subclass IgG2b produced by these in vivo sensitized and in vitro activated B cells bound specifically to tumor cells, and mediated specific tumor cell lysis in the presence of complement. The application of activated B cells in this setting represents a novel approach which may significantly augment the efficacy of current T cell therapies. The only other report that we are aware of where activated B cells mediated tumor regression was by Harada et al. (14) In that report, normal B cells were bound to CD3 and mediated tumor regression presumably by binding to T cells. The mechanism by which activated normal B cells mediated tumor responses was not examined in that study.
Several recent studies have shown that T cells and B cells collaborate to elicit immune responses. Down-regulated B cell function decreased T cell immunity in two autoimmune disease models (15, 16). B cells are required for the generation of protective effector and memory CD4 cells in response to infection (17). Both T cells and B cells are required to mediate significant immune responses in prion diseases (18). Recent studies suggested that B cells can function as antigen-presenting cells to T cells in multiple sclerosis (19) and in hemolytic diseases (20). New experimental evidence has also been documented that antibody plays an important role in renal transplantation through circulating IgG-mediated hydrolysis of coagulation factors (21).
The role played by B cells in the host immune response to cancer is complex and controversial. Depending upon their state of activation, B cells have had divergent roles on T cell differentiation and effector function. In tumor models, resting B cells have been reported to suppress T cell mediated antitumor immunity (22–25). The mechanisms of the suppression by resting B cells on the antitumor immune response is poorly understood (23, 25). On the other hand, activation of B cells with antigen or Ig cross-linking, but not LPS activation, has been reported to upregulate the chemokine CCL4 and attract CD4+CD25+ Treg cells (26). As opposed to resting B cells, several reports have indicated the efficacy of activated B cells in cellular immunotherapy of malignancies. Some of these reports have been focused on how activated B cells can be used as effective antigen presenting cells for T cell sensitization (27–31). Anti-CD40 activated human B cells were found to mediate tumor cell killing via a TRAIL/Apo-2L-dependent mechanism (32). CD4 T cell therapy of cancer have not worked effectively in B−/− mice (33), and T cells by themselves were not enough in suppressing tumor growth in cancer patients (34). Kawakami et al. reported that the administration of a DNA-based vaccine resulted in antitumor effects of established murine tumors that were mediated by both T and B cells (35). In this study, we have identified that B cells from tumor-primed lymph nodes possess antitumor reactivity and can be secondarily activated to mediate tumor regression in adoptive transfer models. Other than antibody production and their specific binding and cytotoxicity towards tumor cells as we described herein, other mechanism by which the transferred TDLN B cells mediated tumor destruction is largely unknown. Evaluation of the interactions of tumor-primed T and B cells during in vitro activation, and in vivo after adoptive transfer warrants further investigation.
In our previously reported adoptive immunotherapy studies utilizing T cells, we have described the role played by IFNγ in antitumor responses both in animal studies (5, 6, 9, 10, 36) and in clinical trials (7). In this study, IFNγ production by activated T cells, B cells, and T cells plus B cells derived from the TDLN was also evaluated. While purified and activated T cells did not produce antibody (Fig. 5), they produced large amounts of IFNγ (data not show). In contrast, whereas purified and activated B cells produced large amounts of antibodies, they did not produce IFNγ (data not shown). Of note, activated T cells plus B cells released both antibody (Fig, 5) and IFNγ(data not shown) at high levels. These data demonstrated an association between T cell-mediated therapeutic efficacy with IFNγ production as we reported before, and a correlation between B cell-mediated therapeutic efficacy with the observed antibody production. These data are supportive of our hypothesis that the enhanced therapeutic efficacy mediated by T cells combined with B cells in this study is due to concurrent cellular and humoral antitumor responses.
We found that radiation and chemotherapy significantly enhanced the therapeutic efficacy of adoptively transferred B cells in the s.c and pulmonary metastatic tumor models, respectively. TBI leads to lymphodepletion (12). Our subsequent use of a clinically relevant chemotherapy protocol (cyclophosphamide and fludara) (1) for the induction of non-myeloablative lymphodepletion further verified that B cell therapy of cancer could be significantly enhanced in the lymphopenic host. A clinical trial of adoptive transfer of autologous T cells in patients with advanced melanoma pretreated with this non-myeloablative but lymphodepleting chemotherapy regimen has been reported with promising clinical responses (1). The intent of fludarabine and cyclophophamide treatment is to induce lymphopenia and potentially eliminate cellular cytokine “sinks” for homeostatic cytokines such as IL-7, IL-15 and IL-21 that can activate and expand tumor reactive T cells as well as result in the depletion of Treg cells (37–39). Mechanisms involved in the enhanced tumor reactivity of adoptively transferred B cells in the lymphodepleted host have not been elucidated to date. These studies may provide more experiment evidence to support the use of B effector cells as a novel cellular reagent for cancer immunotherapy.
In a previous study (40), we tested the target recognition efficiency of TDLN-isolated T cells without in vitro activation. We found that freshly harvested, non-activated TDLN T cells had very limited immunity (e.g. IFNγ production) in response to tumor cell stimulation. In this study, we also performed experiments by culturing the TDLN-isolated B cells without LPS/anti-CD40 activation. These B cells did not grow, and were almost all dead by the end of 4 days in culture. B cells isolated form the spleens obtained from the tumor-bearing mice as well as normal mice did not grow either without LPS/anti-CD40 activation (data not shown). The very limited B cell number (due to the lack of cell expansion) did not allow us to perform adoptive transfer experiments to compare their antitumor efficacy with activated B cells. Also, significant cell death in the absence of B cell activation did not allow us to collect culture supernatants for IgG and/or IgM detection or for binding assays. These series of experiments underscore the essential requriment of in vitro activation for in vivo sensitized B cells to mediate tumor regression in cancer adoptive immunotherapy. The use of LPS plus anti-CD40 as TDLN B cell stimuli in this report may represent a combined stimulation of innate immunity (by LPS) and adaptive immunity (by anti-CD40 to mimic the CD40L signaling from T helper cells). Evaluation of additional B cell activation pathways, such as the engagement of the B cell antigen receptor (BCR) by using available tumor specific antigen or by using anti-Ig (e.g. anti-IgM or anti-IgD) in the forms of F(ab’)2 may lead to the better understanding of requirements and the optimization of methods for pre-effector B cell secondary activation in vitro. Furthermore, successful T cell therapy required the infiltration of infused T cells within the tumor mass (41). Studies on the infiltration and accumulation of infused B cells in the subcutaneous tumors and the assessment of proliferation and survival of the infused B cells would increase our understanding of the events behind the observed therapeutic efficacy of adoptively transferred TDLN B cells.
In summary, we have identified that tumor-primed B cells that are secondarily activated in vitro can mediate regression of established tumors upon adoptive transfer. This represents a novel effector cell population that can enhance the therapeutic efficacy of adoptive T cell therapies. Further investigations are required to determine the mechanisms by which activated B cells mediate in vivo tumor regression and how they interact with effector T cells. We have demonstrated that it is quite feasible to generate both T and B effector cells together by in vivo sensitization and in vitro methods that can have direct clinical applications.
Abbreviations used in this paper
- TDLN
tumor-draining lymph node
- CM
complete medium
- s.c.
subcutaneous
- i.v.
intravenously
- i.p.
intraperitoneal
- APC
antigen-presenting cells
Footnotes
This work was supported in part by NIH grant CA82529 and the Gillson Longenbaugh Foundation.
References
- 1.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward BA, Shu S, Chou T, Perry-Lalley D, Chang AE. Cellular basis of immunologic interactions in adoptive T cell therapy of established metastases from a syngeneic murine sarcoma. J Immunol. 1988;141:1047–1053. [PubMed] [Google Scholar]
- 3.Chang AE, Perry-Lalley DM, Shu S. Distinct immunologic specificity of tumor regression mediated by effector cells isolated from immunized and tumor-bearing mice. Cellular immunology. 1989;120:419–429. doi: 10.1016/0008-8749(89)90209-8. [DOI] [PubMed] [Google Scholar]
- 4.Geiger JD, Wagner PD, Cameron MJ, Shu S, Chang AE. Generation of T-cells reactive to the poorly immunogenic B16-BL6 melanoma with efficacy in the treatment of spontaneous metastases. J Immunother Emphasis Tumor Immunol. 1993;13:153–165. doi: 10.1097/00002371-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Grover AC, Donald EJ, Carr A, Yu J, Whitfield J, Nelson M, Takeshita N, Chang AE. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. J. Immunol. 2005;175:1424–1432. doi: 10.4049/jimmunol.175.3.1424. [DOI] [PubMed] [Google Scholar]
- 6.Iuchi T, Teitz-Tennenbuam S, Huang J, Redman BG, Hughes SJ, Li M, Jiang G, Chang AE, Li Q. IL-21 augments the efficacy of T cell therapy by eliciting concurrent cellular and humoral responses. Cancer Res. 2008;68:4431–4441. doi: 10.1158/0008-5472.CAN-07-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang AE, Li Q, Jiang G, Sayre DM, Braun TM, Redman BG. Phase II trial of autologous tumor vaccination, anti-CD3-activated vaccine-primed lymphocytes, and interleukin-2 in stage IV renal cell cancer. J Clin Oncol. 2003;21:884–890. doi: 10.1200/JCO.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Yu B, Grover AC, Zeng X, Chang AE. Therapeutic effects of tumor reactive CD4+ cells generated from tumor-primed lymph nodes using anti-CD3/anti-CD28 monoclonal antibodies. J Immunother (1997) 2002;25:304–313. doi: 10.1097/00002371-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer research. 2003;63:2546–2552. [PubMed] [Google Scholar]
- 10.Li Q, Carr AL, Donald EJ, Skitzki JJ, Okuyama R, Stoolman LM, Chang AE. Synergistic effects of IL-12 and IL-18 in skewing tumor-reactive T-cell responses towards a type 1 pattern. Cancer Res. 2005;65:1063–1070. [PubMed] [Google Scholar]
- 11.Arca MJ, Krauss JC, Aruga A, Cameron MJ, Shu S, Chang AE. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer Gene Ther. 1996;3:39–47. [PubMed] [Google Scholar]
- 12.Gattinoni L, Finkelstein SE, Klebanoff CC, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzensinski C, Heimann DM, Surh CD, Rosenburg SA, Restifo NP. Removal of hemeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. The journal of Experimental Medicine. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding JW, Zhou T, Zeng H, Ma L, Verbeek JS, Yin D, Shen J, Chong AS. Hyperacute rejection by anti-Gal IgG1, IgG2a, and IgG2b is dependent on complement and Fc-gamma receptors. J Immunol. 2008;180:261–268. doi: 10.4049/jimmunol.180.1.261. [DOI] [PubMed] [Google Scholar]
- 14.Harada M, Okamoto T, Kurosawa S, Shinomiya Y, Ito O, Takenoyama M, Terao H, Matsuzaki G, Kimura G, Nomoto K. The antitumor activity induced by the in vivo administration of activated B cells bound to anti-CD3 monoclonal antibody. Cellular immunology. 1995;161:132–137. doi: 10.1006/cimm.1995.1017. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chen F, Putt M, Koo YK, Madaio M, Cambier JC, Cohen PL, Eisenberg RA. B cell depletion with anti-CD79 mAbs ameliorates autoimmune disease in MRL/lpr mice. J. Immunol. 2008;181:2961–2972. doi: 10.4049/jimmunol.181.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liossis SC, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cell. Clinical Immunology. 2008;127:280–285. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to pneumocystis lung infection. J. Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 18.Sacquin A, Bergot AS, Aucouturier P, Rosset MB. Contribution of antibody and T cell-specific responses to the progression of 139A-scrapie in C57BL/6 mice immunized with prion protein peptides. The Journal of Immunology. 2008;181:768–775. doi: 10.4049/jimmunol.181.1.768. [DOI] [PubMed] [Google Scholar]
- 19.Harp CT, Lovett-Racke AE, Racke MK, Frohman EM, Monson NL. Impact of myelin-specific antigen presenting B cells on T cell activation in multiple sclerosis. Clinical Immunology. 2008;128:382–391. doi: 10.1016/j.clim.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Brinc D, Le-Tien H, Crow AR, Siragam V, Freedman J, Lazarus AH. Transfusion of IgG-Opsonized foreign red blood cells mediates reduction of antigen-specific B cell priming in a murine model. Journal of Immunology. 2008;181:948–953. doi: 10.4049/jimmunol.181.2.948. [DOI] [PubMed] [Google Scholar]
- 21.Wootla B, Nicoletti A, Patey N, Dimitrov JD, Legendre C, Christophe OD, Friboulet A, Kaveri SV, Desmazes S, Thaunat O. Hydrolysis of coagulation factors by circulating IgG is associated with a reduced risk for chronic allograft nephropathy in renal transplanted patients. J. Immunol. 2008;180:8455–8460. doi: 10.4049/jimmunol.180.12.8455. [DOI] [PubMed] [Google Scholar]
- 22.Perricone MA, Smith KA, Claussen KA, Plog MS, Hempel DM, Roberts BL, St George JA, Kaplan JM. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother (1997) 2004;27:273–281. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH, Rosenblatt JD. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. International journal of cancer. 2005;117:574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 24.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer research. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 25.Watt V, Ronchese F, Ritchie D. Resting B cells suppress tumor immunity via an MHC class-II dependent mechanism. J Immunother (1997) 2007;30:323–332. doi: 10.1097/CJI.0b013e31802bd9c8. [DOI] [PubMed] [Google Scholar]
- 26.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nature immunology. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 27.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. The Journal of clinical investigation. 1997;100:2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, Xia Z, Zeng WY, Wucherpfennig KW, Nadler LM, Schultze JL. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 29.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer research. 2003;63:2836–2843. [PubMed] [Google Scholar]
- 30.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–2054. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 31.Chung Y, Kim BS, Kim YJ, Ko HJ, Ko SY, Kim DH, Kang CY. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer research. 2006;66:6843–6850. doi: 10.1158/0008-5472.CAN-06-0889. [DOI] [PubMed] [Google Scholar]
- 32.Kemp TJ, Moore JM, Griffith TS. Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation. J Immunol. 2004;173:892–899. doi: 10.4049/jimmunol.173.2.892. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Noh HS, Chen J, Kim JH, Falo LD, Jr., You Z. Potent tumor-specific protection ignited by adoptively transferred CD4+ T cells. J. Immunol. 2008;181:4363–4370. doi: 10.4049/jimmunol.181.6.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manjili MH, Kmieciak M, Keeler J. Comment on “Tumor progression can occur despite the induction of very high levers of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J. Immunol. 2006;176:4511. doi: 10.4049/jimmunol.176.8.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami K, Terabe M, Kawakami M, Berzofsky JA, Puri RK. Characterization of a novel human tumor antigen interleukin-13 receptor alpha2 chain. Cancer research. 2006;66:4434–4442. doi: 10.1158/0008-5472.CAN-05-1265. [DOI] [PubMed] [Google Scholar]
- 36.Aruga A, Aruga E, Tanigawa K, Bishop DK, Sondak VK, Chang AE. Type 1 versus type 2 cytokine release by Vbeta T cell subpopulations determines in vivo antitumor reactivity: IL-10 mediates a suppressive role. J Immunol. 1997;159:664–673. [PubMed] [Google Scholar]
- 37.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer research. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 38.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. The Journal of experimental medicine. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nature reviews. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito F, Carr A, Svensson H, Yu J, Chang AE, Li Q. Antitumor Reactivity of Anti-CD3/Anti-CD28 Bead-Activated Lymphoid Cells: Implications for Cell Therapy in a Murine Model. j. Immunotherapy. 2003;26:222–233. doi: 10.1097/00002371-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Skitzki J, Craig RA, Okuyama R, Knibbs RN, McDonagh K, Chang AE, Stoolman LM. Donor cell cycling, trafficking, and accumulation during adoptive immunotherapy for murine lung metastases. Cancer research. 2004;64:2183–2191. doi: 10.1158/0008-5472.can-03-2799. [DOI] [PubMed] [Google Scholar]