Abstract
Our early life nutritional environment can influence several aspects of physiology, including our propensity to become obese. There is now evidence to suggest perinatal diet can also independently influence development of our innate immune system. This review will address three not-necessarily-exclusive mechanisms by which perinatal nutrition can program neuroimmune function long-term: by predisposing the individual to obesity, by altering the gut microbiota, and by inducing epigenetic modifications that alter gene transcription throughout life.
Keywords: hypothalamic-pituitary-adrenal axis, obesity, glucorticoids, gut microbiota, epigenetics
Perinatal dietary influence on immune system development
The immune system of a newborn animal is relatively naïve and influences from the environment are necessary to allow it to become fully functional. Early exposure to pathogens develops an adaptive (Flajnik and Kasahara, 2010) and innate (Galic et al., 2009; Spencer et al., 2011) immunity that will facilitate appropriate responses to additional pathogens throughout life. However, there is now evidence that the early life diet is also crucial in programming long-term immune function.
Specific nutrients in perinatal diet influence immune system development
Specific nutrients within an individual diet can influence immune system development in different ways. Thus, antioxidants, oligosaccharides, polyunsaturated fatty acids (PUFAs), folate, and other vitamins have all been implicated in programming the developing immune system (West et al., 2010). For example, omega-3 PUFAs, those found in fish, fish oils, green plants, and some nuts and seeds (Huffman et al., 2011; Kremmyda et al., 2011), have an anti-inflammatory role. They inhibit cytokine production and may also alter gene expression and stimulate eicosanoid metabolism to control inflammation (Shek et al., 2012). Thus, a diet high in omega-3 PUFAs leads to a suppression of arachidonic acid-derived eicosanoids such as prostaglandin E2 (PGE2). PGE2 exerts pro-inflammatory effects and reduces the production of T helper type 1 (Th1) cytokines, such as interferon (IF) γ and interleukin (IL)2 and enhances production of Th2 cytokines like IL-4 and IL-5. A shift in the Th1/Th2 balance toward a Th2-dominant profile is associated with impaired immune tolerance and allergies (Gottrand, 2008). High maternal intake of fish is thus associated with protection, in the infant, from allergic diseases such as eczema and asthma (Calvani et al., 2006; Romieu et al., 2007; Sausenthaler et al., 2007). Omega-6 PUFAs, those found in vegetable oils (Huffman et al., 2011; Kremmyda et al., 2011), are pro-inflammatory and may contribute to metabolic syndrome and cardiovascular disease (Patterson et al., 2012). A shift in the ratio of omega-3 to omega-6 PUFA intake in the diet to favor omega-6 encourages an allergenic Th2-dominant profile and is thus likely contributing to the recent increase in the incidence of childhood allergies (Shek et al., 2012). Folic acid is another example of a dietary component that influences immune development. Folic acid supplementation during pregnancy reduces the risk of neural tube defects and other congenital malformations (Wilcox et al., 2007), but it also predisposes infants toward allergies and immune dysfunction. For instance, folate supplementation in the first trimester of pregnancy predisposes infants to developing wheeze and respiratory infections in early life (Haberg et al., 2009).
Perinatal diet influences immune system development through the gut microbiota
One mechanism by which specific nutrients in the early life diet may be able to influence the adult immune system is by affecting the development, diversity, and function of the gut microbiota. In humans, the gastrointestinal tract is home to more than 100 trillion bacteria comprised of more than 1000 species (Qin et al., 2010). It also hosts numerous viruses, archaea, parasites, and fungi that together make up the gut microbiota (Ashida et al., 2012). This microbiota exists in a symbiotic relationship with its human hosts and can influence barrier function, trophic effects, metabolism, and the development of the adaptive and innate immune systems (Matamoros et al., 2013).
An adult's gut microbiome can be influenced by long-term changes in environmental factors. Hildebrandt and colleagues have shown 3 months of high fat diet (HFD)-feeding can influence a change in gut microbiota composition toward an increase in Firmicutes and Proteobacteria and a decrease in Bacteroidetes phyla in female mice (Hildebrandt et al., 2009). Although these changes were independent of obesity, other groups have shown a high fat, high sugar diet encourages increased adiposity and this phenotype can be transmitted to initially lean (normal diet) animals via transplantation of the microbiota (Turnbaugh et al., 2008). Most evidence, however, suggests the adult gut flora is very stable and short-term environmental influences in adulthood have limited effect (Wu et al., 2011). An infant, on the other hand, is not born with an established gut microbiome. Rather, the gut is colonized from bacteria in the environment in the first hours to days of life and the microbiome gains diversity and becomes stable and adult-like by around 3 years of age (Mackie et al., 1999; Palmer et al., 2007). In particular, an individual's diet during the early colonization phase can be tremendously important in determining the later composition of the gut microbiota.
Breast milk is a major source of the bacteria that colonize the gut (Martin et al., 2012). Breast-fed infants have higher counts of Bifidobacteria, Lactobacilli, and lower counts of Bacteroides, Clostridium coccoides group, Staphylococcus, and Enterobacteriaceae than formula-fed (Rinne et al., 2005; Fallani et al., 2010). Breast milk is also rich in oligosaccharides, which have a strong pre-biotic effect, promoting bacterial growth (Sela and Mills, 2010). Oligosaccharides found in human milk can improve the diversity of the microbiota, particularly promoting growth and metabolism of Bifidobacteria (Scholz-Ahrens et al., 2007). Human milk oligosaccharides can even improve glucose homeostasis (Laitinen et al., 2009). This interaction between breast milk oligosaccharides and gut bacteria also encourages immune system development and prevention of disease (Innis, 2007). Maternal diet strongly influences the composition of the breast milk and probably, therefore, the types of bacteria available to colonize the infant's gut. For instance, in rats, a maternal diet high in olive oil leads to high oleic-acid levels in the milk. A maternal diet high in PUFA is reflected in high PUFA concentrations in milk. Saturated fats are also transferred to the milk (Priego et al., 2013). When infants are introduced to a more complex diet at weaning there is a marked increase in Bacteroidetes, and a shift toward a more diverse colony (Koenig et al., 2011). The infant's post-weaning diet can therefore also affect the makeup of the gut microbiota. High dietary fat, for example, can cause a shift toward increased representation of Clostridium populeti bacteria and a reduction in Lactobacillus and Bacteroides species (Patrone et al., 2012). In weaned piglets the quantity and type of carbohydrate in the diet can influence the gut microbiota so that a diet high in hulled barley supplemented with beta-glucan encourages a Lactobacilli-dominant gut microbiota (Pieper et al., 2008).
Exactly what this means for the infant is yet unclear as there is no consensus as to what constitutes a “normal” gut microbiome (Matamoros et al., 2013). However, changing the makeup of the bacterial colony can certainly influence the immune system long-term. Infants given Bacteroides fragilis supplements early in life have high salivary secretory immunoglobulin A (IgA) and more basal IFN-γ production. They also have reduced expression of the pathogen-associated molecular pattern receptor, toll-like receptor (TLR)4, mRNA and an attenuated pro-inflammatory response to stimulation with lipopolysaccharide (LPS) than those not given the B. fragilis supplements (Gronlund et al., 2000; Sjogren et al., 2009). These findings suggest the possibility that early colonization with B. fragilis can accelerate the maturation of the IgA system, leading to improved Th1/Th2 balance, a reduced likelihood of allergies developing, and a reduced response to LPS. A Lactobacilli-dominant colonization in infancy also reduces the likelihood the child will develop allergies by age five, while a Staphylococcus aureaus-dominant colonization has the opposite effect (Johansson et al., 2011). This latter bacterium has been linked to asthma and allergic rhinitis in childhood (Bjorksten et al., 2001). Lactobacillis tends to suppress numbers of interleukin (IL)-4, IL-10, and IFN-γ -secreting cells after stimulation with phytohaemagglutinin, whereas early life S. aureaus colonization has the opposite effect. Colonization with Lactobacilli also lowers cytokine responses to stimulation with an allergen (Martino et al., 2008). The Lactobacilli data suggest that this bacterium is able to suppress the immune response.
Perinatal diet influences immune system development through epigenetic modifications
An interrelated mechanism by which perinatal diet can influence the innate immune system is through changes in the epigenome. Epigenetics refers to stable, heritable, environmentally-induced modifications to gene expression that occur independently of alterations to the DNA sequence (Christensen and Marsit, 2011). These modifications include changes in cytosine methylation, histone modification, and changes in non-coding RNAs such as microRNAs (Milagro et al., 2013). Together these mechanisms are responsible for regulating the degree of expression of a particular gene and the timing of its expression (Zeisel, 2009; McKay and Mathers, 2011). While epigenetic research is still a very young field, there is a large body of evidence accumulating to suggest diet, particularly in early life, can influence this epigenome long-term (Lillycrop et al., 2008). There are now data revealing almost every dietary component, from broccoli to betaine can influence the epigenome. Broccoli, for example contains sulforaphane, which induces histone modifications and has been implicated in preventing cancer (Dashwood and Ho, 2008; Delage and Dashwood, 2008; Nian et al., 2009). Betaine is found in grains and some vegetables and has been found to influence DNA methylation to promote fetal brain development (Sinclair et al., 2007; Mehedint et al., 2010; Zeisel, 2011).
Dietary components may also alter the epigenome to influence immune function, and there is a particular window of vulnerability for this during early development (West et al., 2011). Folate for example is a methyl donor. At least in mice, folate supplementation in pregnancy causes DNA hypermethylation, particularly in key metabolic genes (Waterland et al., 2008). Supplementation with methyl donors such as folate is also associated with altered immune function resulting in increased development of allergic asthma and eczema (Hollingsworth et al., 2008; Haberg et al., 2009). PUFAs also have the potential to cause epigenetic modifications. Crucially, PUFAs may be able to alter nuclear factor κ B (NFκ B)-mediated transcription of pro-inflammatory cytokines to influence the sensitivity of the immune response (Benatti et al., 2004; Waterland, 2006). NFκ B is a transcription factor responsible for regulating the expression of more than 400 genes, including those responsible for pro-inflammatory cytokines, chemokines, and adhesion molecules (Vanden Berghe et al., 2006). NFκ B-mediated transcription may be particularly vulnerable to early life influence and may be a principal mechanism by which epigenetic programming can influence immune system development long-term (Benatti et al., 2004; Vanden Berghe et al., 2006). NFκ B itself is closely regulated by glucocorticoids and these are influenced by hypothalamic-pituitary-adrenal (HPA) axis activation (Sapolsky et al., 2000). Epigenetic programming by early life stress can result in changes in the methylation status of the glucocortiocoid receptor (GR) in the hippocampus and hypothalamus, altering negative feedback onto the hypothalamus, and thus HPA axis sensitivity (Liu et al., 1997; Weaver et al., 2004; Stevens et al., 2010; Begum et al., 2012). Early life diet can also impact HPA axis reactivity long-term (Boullu-Ciocca et al., 2005; Spencer and Tilbrook, 2009; Bulfin et al., 2011), altering the glucocorticoid response to stress. Enhanced circulating glucocorticoid levels in response to stress then feed back to inhibit NFκ B-mediated cytokine production (Figure 1). The HPA axis in particular will be discussed in the next section.
Figure 1.
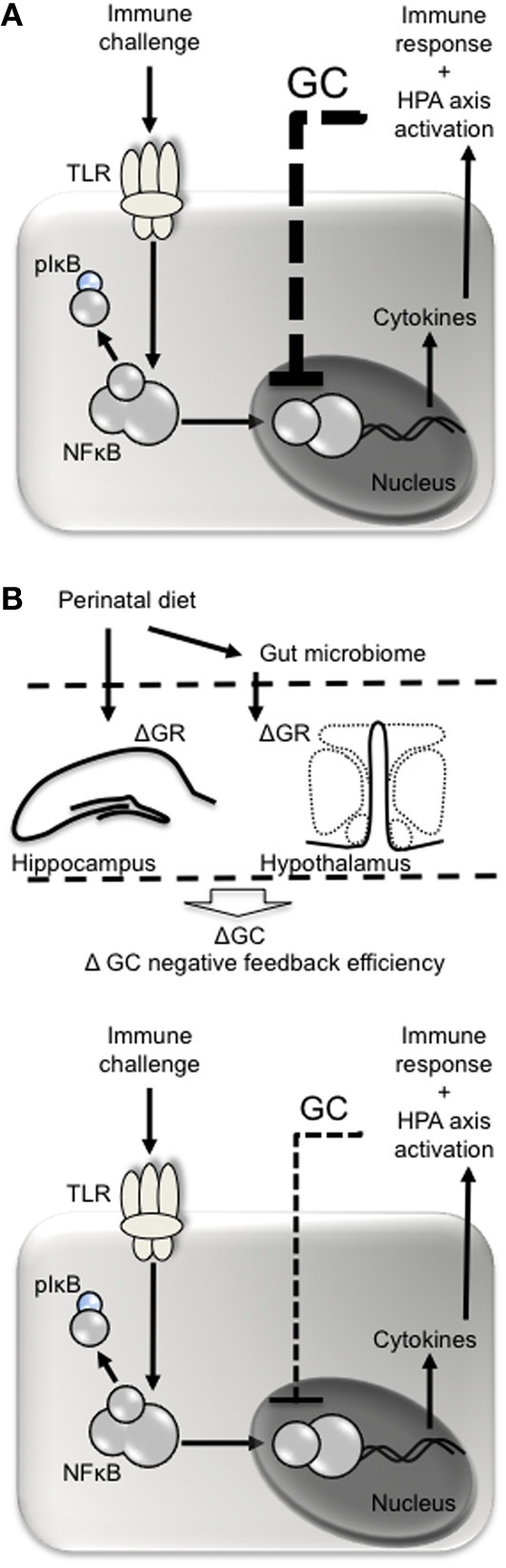
Perinatal diet may influence glucocorticoid negative feedback following immune challenge. (A) Pathogens such as lipopolysaccharide act at toll-like receptors (e.g., TLR4) on immune cells leading to phosphorylation of inhibitory factor (I)κ B, releasing nuclear factor (NF)κ B from its complex and allowing it to be translocated to the nucleus. NKκ B is responsible for the transcription of pro- and anti-inflammatory cytokines, the former of which stimulate the cyclo-oxygenase 2-mediated conversion of aracidonic acid into prostaglandins. Prostaglandins (e.g., PGE2) act at the brain to stimulate fever and sickness behavior and recruit the HPA axis. Once released, glucocorticoids (GC) negatively feed back to inhibit further NFκ B-mediated transcription of cytokines. (B) The perinatal diet may influence glucocorticoid negative feedback by altering expression of glucocorticoid receptors (GR) in the hippocampus and hypothalamus leading to less efficient glucocorticoid-mediated inhibition of NFκ B and an exacerbated immune response.
Perinatal dietary influence on adiposity—links to immune system development
Dietary factors in early life clearly have a crucial influence on immune system development. The second half of this review will focus on how early life nutrition can program a pro-inflammatory basal immune profile by pre-disposing an individual to an obese phenotype.
Obesity is becoming a huge problem worldwide. In developed countries such as Australia and the US, 70–74% of adult males and 56–64% of adult females are now either overweight or obese, with 28% of both classified as obese (BMI > 30). As many as 25–32% of Australian and US children are classified as overweight or obese (Cretikos et al., 2008; Nhanes, 2009-2010; AHS, 2011-2012).
Perinatal nutrition can program adult weight and metabolism leading to adipose-dependent changes in immune function
Obesity itself, whether due to metabolic changes programmed in early life or to adult factors, is linked to changes in the inflammatory profile. It is now recognized that obesity is associated with, and may even be precipitated by, a chronic low-grade systemic and local inflammation (Gregor and Hotamisligil, 2011). This metabolic inflammation can contribute to insulin- and leptin-resistance at various levels, including at the hypothalamus (Thaler and Schwartz, 2010).
Dietary factors such as PUFAs and glucose, as well as changes in the gut microbiota, are able to trigger a chronic low-grade inflammatory profile initially in white adipose tissue (WAT). This change is characterized by macrophage infiltration into WAT, apoptosis and necrosis of adipocytes, and reduced vascularity (Shu et al., 2012). These changes result in an abnormal preponderance of adipose-tissue macrophages, and these can make up almost 40% of the cells in obese adipose tissue (Weisberg et al., 2003; Xu et al., 2003). Adipose tissue macrophages, and potentially an increase in pattern recognition receptors on adipocytes themselves, lead to local inflammation with a predominance of pro-inflammatory over anti-inflammatory cytokines released (Shu et al., 2012). Hotamisligil and colleagues showed early on there is a substantial increase in expression of the pro-inflammatory cytokine tumor necrosis factor (TNF)α in several rodent models of obesity and that neutralizing TNFα could improve insulin-sensitivity in these animals (Hotamisligil et al., 1993, 1995; Uysal et al., 1997). The pro-inflammatory profile in the adipose leads to cytokine, adipokine, and fatty acid release into circulation, which have downstream effects on liver, muscle, and brain, and ultimately contribute to insulin-resistance (Shu et al., 2012).
As a result of these changes in the inflammatory profile, obese subjects have compromised immune function and are more likely to die from an acute infection than those of normal weight (Falagas and Kompoti, 2006). For instance, excessive body weight gain immediately postnatally predisposes infants to atopy and wheezing disorders (Pike et al., 2010). Obese patients in general are also twice as likely to die in intensive care due to infection-related complications as normal weight patients (Falagas and Kompoti, 2006).
It has been clear for some time that early life nutrition is able to program growth and can influence development of the central pathways subserving feeding and metabolism (Spencer, 2012). Babies born to overweight or obese mothers are significantly more likely to become overweight or obese themselves (Dabelea et al., 2000; Ruager-Martin et al., 2010), and babies born to mothers who ate a high fat, junk food diet while pregnant have higher levels of body fat when they are born, irrespective of whether or not the mothers were obese during pregnancy (Albuquerque et al., 2006; Srinivasan et al., 2006; Ashino et al., 2012). Associated with this excess body fat are indices of metabolic syndrome such as hyperinsulinemia and insulin resistance (Dabelea et al., 2000; Boney et al., 2005; Sewell et al., 2006; Catalano et al., 2009).
Paradoxically, babies that were undernourished in utero are also more likely to develop obesity and associated metabolic disorders (Spencer, 2012). In the first instance, in utero factors that cause the baby to be born small may also alter its metabolic pathways to encourage energy storage when food is available (Vickers et al., 2000, 2003; Bellinger et al., 2004; Bellinger and Langley-Evans, 2005). Secondly, preferred practise with small for gestational age babies is a program of intensive feeding to encourage appropriate brain and lung development (Lubchenco et al., 1972a,b; Brandt et al., 2003) and this catch up growth in the postnatal period also predisposes an individual to obesity (Ong et al., 2000, 2006; Brandt et al., 2003; Desai et al., 2005).
The importance of these findings is reflected in statistics showing overweight children are significantly more likely to be overweight adults than those of normal weight. As mentioned, excessive weight gain in the first week of life increases the long-term risk of obesity (Stettler et al., 2005). Furthermore, compared with children with a BMI below the 50th percentile, children between the 50 and 74th percentiles of BMI are approximately five times more likely to become overweight adults (Baird et al., 2005; Field et al., 2005; Druet et al., 2012).
Perinatal nutrition can program changes in immune function that are independent of adiposity
We can conclude from these studies there is an obvious connection between early life events programming an increased propensity to obesity and obesity itself resulting in a basal pro-inflammatory profile and susceptibility to infection. However, it is also apparent being overweight in early life can have independent and compounding effects on the inflammatory profile in adulthood.
Interesting evidence for the long-term effects of early life diet on the adult immune system comes from individuals who were undernourished in utero or as infants and did not develop obesity. Thus, a study of three rural villages in Gambia revealed subjects were significantly more likely to die of infectious disease in adulthood if they had been born during the nutritionally debilitating “hungry” season of July–December than during January–June when food was plentiful (Moore et al., 1999). A calorie restricted perinatal diet has also been shown to influence macrophage activation in adulthood so that adult rats undernourished during lactation had fewer alveolar macrophages and these released less nitric oxide in response to a fluoxetine challenge (Ferreira et al., 2009). Similarly, adult rats undernourished during lactation showed no change in immune parameters after an immune challenge either under control conditions or after being subjected to footshock, while control rats (normal diet during lactation) had elevated leukocyte counts and antibody titers (Barreto-Medeiros et al., 2007). These data suggest neonatal malnutrition can lead to a less reactive or less efficient immune response.
There is also some evidence that animals made obese as a result of perinatal diet can have changes in neuroimmune function in later life that are independent of the obesity per se. Several groups have now shown rats suckled in small litters, where they have greater access to the dam's milk, gain weight faster and maintain a higher body weight into adulthood (Plagemann et al., 1999; Schmidt et al., 2001; Morris et al., 2005; Rodel et al., 2008). We have shown these overweight rats, both males and females, have a significantly exacerbated neuroimmune response to LPS. This response is categorized by exacerbated NFκ B activation in the overweight rats, more circulating pro-inflammatory cytokines, and bigger fevers (Clarke et al., 2012).
Importantly, there are some fundamental differences between the changes in neuroimmune function in rats made overweight due to early life overfeeding and those in rats made overweight due to HFD-feeding in adulthood. Firstly, neonatally overfed rats do not have a profile of basal inflammation. There are no differences in basal circulating pro-inflammatory cytokine concentrations between those suckled in small litters (overweight) and those suckled in control litters (Clarke et al., 2012). As discussed above, several studies have shown human (Hak et al., 1999; Yudkin et al., 1999) and rodent (Hotamisligil et al., 1993) obese subjects have higher levels of circulating pro-inflammatory cytokines under unstimulated conditions, reflecting a pro-inflammatory profile. This difference may be a result of the degree of obesity, dietary composition, and/or that the perinatal overfeeding is able to prime the system to display an over-active response to immune challenge without affecting the basal inflammatory profile (Pohl et al., 2009).
The second key difference between immune dysfunction as a result of perinatal obesity and that of diet-induced obesity in adulthood is that perinatal obesity leads to an exacerbated immune response to a TLR4-mediated challenge, but not to a TLR3-mediated one (Clarke et al., 2012). The TLR family contains as many as 13 mammalian TLR, most of which respond to specific pathogen-associated molecular patterns. In the case of Gram negative bacteria, the pyrogenic moiety, LPS, interacts with cluster of differentiation (CD)14 on the cell membrane, allowing MD2 to associate with TLR4. This interaction activates a myeloid differentiation primary response gene (MyD88)-dependent pathway, culminating in the phosphorylation of NFκ B-interacting inhibitory factor (I) κ B, which releases NFκ B from its complex. NFκ B is then translocated to the nucleus of the cell where it stimulates the transcription of pro- and anti-inflammatory cytokines (Cartmell et al., 2003; Conti et al., 2004; Galic et al., 2009). Pro-inflammatory cytokines act at the brain to stimulate cyclo-oxygenase 2-mediated conversion of arachadonic acid into prostaglandins. These then act in the ventromedial preoptic area of the hypothalamus to disinhibit neuronal pathways that normally stimulate heat conservation, ultimately resulting in a regulated increase in body temperature; fever (Figure 1A) (Blatteis et al., 2000; Morrison et al., 2008). In the case of a virus, the viral double-stranded RNA interacts with TLR3 to stimulate the immune cascade via an interferon regulatory factor 3-dependent pathway. Polyinosinic:polycytidylic acid (PolyI:C) is a synthetic double-stranded RNA that mimics a virally-induced immune response and fever by activating TLR3. In neonatally overfed rats the response to LPS is exacerbated, while the response to PolyI:C remains normal (Clarke et al., 2012).
As with TLR4, there is increased TLR3 expression in neonatally overfed rat adipose tissue (Clarke et al., 2012). However, unlike in humans with adult-onset obesity (MMWR, 2009; Fuhrman et al., 2011) and adult HFD-fed rodents (Smith et al., 2007), the immune response to a TLR3 ligand is not altered in rats made obese due to neonatal overfeeding (Clarke et al., 2012). A possible explanation for differential effects on TLR4 and TLR3 signaling is in the receptor location, with TLR4 being membrane-bound and TLR3 internalized (Kumar et al., 2009; Konner and Bruning, 2011). Thus, although obesity in general may increase TLR3 expression, perinatally-induced obesity may not cause corresponding changes in transport of the ligand into the cell. For the patient, this may mean early-life programming of obesity may be associated with some form of protection against a viral infection in comparison with adult-onset obesity.
Perinatal overfeeding is, unlike adult onset obesity, also able to exacerbate immune responses independently of sickness behavior. Generally, an immune response elicits a variety of sickness behaviors in addition to the pro-inflammatory and febrile changes. These include anorexia, lethargy, depression, reduced activity, loss of libido (Dantzer and Kelley, 2007). Although there is a typical expression of sickness behavior with LPS in perinatally overfed rats, this is not exacerbated in these animals as the pro-inflammatory and febrile responses are (Clarke et al., 2012). In contrast, adult-onset obesity is strongly associated with an increase in sickness behavior relative to lean adults (Lawrence et al., 2012). Several aspects of sickness behavior are likely to be mediated centrally. For instance, leptin is a significant modulator of the anorexia associated with infection (Luheshi et al., 1999), and treatment with leptin anti-serum can reverse LPS-induced anorexia (Sachot et al., 2004; Harden et al., 2006). Leptin responses to LPS are similar in neonatally overfed and control rats despite pronounced differences in other cytokines, potentially facilitating similar sickness responses (Clarke et al., 2012).
It is yet unclear what this absence of an exacerbated sickness response after perinatal overfeeding would mean for a human subject. On one hand the subject is likely to be resilient to the feeling of sickness associated with an immune challenge, despite having exacerbated pro-inflammatory and febrile response, allowing them to continue life as normal when sick. On the other hand, sickness behavior is very important in promoting withdrawal so the body's resources are fully available to effectively combat the infection (Carlton et al., 2012).
The hypothalamic-pituitary-adrenal axis
As a possible key explanation for how the early life nutritional environment apparently programs adult immune function independently of obesity is in epigenetic changes to key aspects of the HPA axis. The HPA axis plays a significant modulatory role in the immune response with glucocorticoids acting to inhibit NFκ B activation and downstream transcription of pro- and anti-inflammatory cytokines (Figure 1) (Spencer et al., 2011). The HPA axis is also exceptionally sensitive to the early life environment. It has previously been established early life changes in HPA axis function are associated with changes in responses to LPS in adulthood. For instance, early life exposure to an immune challenge can permanently alter HPA axis function (Shanks et al., 1995, 2000; Hodgson et al., 2001). Early life immune challenge leads to an exacerbated HPA axis response to LPS in later life and blocking this increase with RU486 can restore a normal febrile response and cytokine profile (Ellis et al., 2005; Mouihate et al., 2010). At least some of the changes in HPA axis function that derive from early life events are linked to epigenetic modifications. For instance, rats that received high levels of care from their dams as pups (high levels of licking and grooming) have hypomethylation of the GR in the hippocampus and this is associated with increased hippocampal GR mRNA and a more efficient glucocorticoid negative feedback response to stress compared with rats that were given less attention as pups (Liu et al., 1997; Weaver et al., 2004). The hippocampal GR system plays a crucial role in glucocorticoid negative feedback regulation of the HPA axis, with glucocorticoids acting on GR at the hippocampus to inhibit PVN activation (De Kloet et al., 2009). As such, epigenetic modification of hippocampal GR may have significant effects on HPA axis function (Liu et al., 1997; Weaver et al., 2004; Mueller and Bale, 2006). Undoubtedly, glucocorticoid negative feedback at the hypothalamus itself is also important and can be altered by changes to the epigenome. For instance, maternal undernutrition is linked to increased histone acetylation and hypomethylation of the GR in the hypothalamus of the offspring, with a substantial increase in GR expression in this region. These modifications are closely linked with enhanced weight gain, and subsequent obesity, in these offspring (Stevens et al., 2010; Begum et al., 2012).
Early life diet is certainly capable of altering how the HPA axis functions. Females that become overweight as a result of early life overfeeding have enhanced PVN and corticosterone responses to acute stress (Spencer and Tilbrook, 2009). Conversely, males made lean by early underfeeding have more efficient HPA axis responses to stress, with reduced PVN neuronal activation and corticosterone responses that return to baseline more quickly (Bulfin et al., 2011). Neonatally overfed rats also have increased expression of the GR and increased glucocorticoid signaling in adipose tissue as adults (Boullu-Ciocca et al., 2005). In neonatally overfed rats, adult LPS leads to a significantly enhanced PVN response to stress and a corticosterone response that is significantly less efficient. Plasma corticosterone reaches a peak 30 min after LPS in control animals before returning to baseline, but in neonatally overfed rats the corticosterone levels still appears to be increasing after 90 min (Clarke et al., 2012). Together these data indicate early life overfeeding may lead to impaired development of central and peripheral HPA axis and glucocorticoid regulation resulting in altered HPA axis function in later life. These changes are likely to be responsible for a delay in the glucocorticoid response to an immune challenge, which would culminate in exacerbated PVN/HPA axis activation and a less effective glucocorticoid-mediated suppression of cytokine release, and fever (Figure 1).
Sexual dimorphism in perinatal nutritional programming of immune function
Obesity can be manifested very differently in males and females. For instance, Australian and US statistics show a greater proportion of males than females are overweight or obese (Cretikos et al., 2008; Nhanes, 2009-2010; AHS, 2011-2012). Males are also more likely to accumulate visceral fat, a distribution that is more closely associated with complications such as heart disease (Bjorntorp, 1996). HPA axis responses to psychological stress also differ as a function of adiposity between males and females. Thus, perceived stress has been associated with greater increases in BMI in women, but not in men (Fowler-Brown et al., 2009), and female rats overfed as neonates have exacerbated HPA axis responses to restraint, while males do not (Spencer and Tilbrook, 2009). On the other hand, there is little evidence to suggest there are substantial sex differences in the neuroimmune response to an immune challenge in terms of how it is programmed by the perinatal environment. Most studies examining the effects of changes in gut flora on immune function have either only included males, or have found no effect of sex on immune-related outcomes (e.g., Calvani et al., 2006; Haberg et al., 2009; Patterson et al., 2012; Shek et al., 2012). There are some sex differences in the effects of gut flora on central nervous system circuitry. For example, hippocampal serotonin concentrations are elevated in male germ-free mice, but not females, compared with control mice with typical gut flora colonization (Clarke et al., 2013). However, immunological and neuroendocrine effects of changes to gut flora appear to be similar between males and females (Clarke et al., 2013). There has also been limited study on sex differences in epigenetic changes imposed by the early life environment. In humans, women have reduced global DNA methylation in peripheral blood compared with men, implying there may be differences in vulnerability to a challenge that influences methylation status (Zhang et al., 2011). However, methylation status of inflammatory markers such as IL-6 does not appear to be affected by sex (Zhang et al., 2012). Although there are sexually dimorphic effects of neonatal overfeeding on HPA axis responses to psychological stress (Spencer and Tilbrook, 2009), these do not seem to be apparent in the response to an immune challenge. As such, we have seen adult immune responses to LPS are exacerbated in both males and females made overweight due to neonatal overfeeding (Clarke et al., 2012). Thus, further work is necessary to clarify the differences, if there are any, between males and females in perinatal programming of neuroimmune function.
Summary and future perspectives
Clearly, early life diet is essential for programming many aspects of adult physiology, including immune function and later susceptibility to disease. The gut microbiome and changes to the epigenetic profile may be particular mechanisms by which early life diet can alter immune function. In conjunction with these mechanisms, early life diet can predispose a subject to obesity, which has its own consequences for long-term immune function. Obesity that occurs as a result of early life diet may have independent implications for the immune system. Recent studies even imply that if one must become obese, there appear to be certain health advantages to doing it early on. At least, the basal pro-inflammatory profile, responses to a viral infection, and sickness behaviors seem to be unaffected in animals made obese by early life overfeeding, although febrile and cytokine responses to LPS are highly exacerbated. What this means for obese humans and for designing appropriate early life diets remains to be seen, but the implications for our immune systems are significant and clearly more work is needed in this field. Future research is needed to determine (1) how the early life gut microbiome can influence immune system development and if we can alter this with diet, (2) how early life influences, including diet, can cause epigenetic modifications to alter immune system development and if these can be reversed, and (3) how perinatal diet influences immune function independently of adult adiposity and if there is potential for early life interventions to reverse or ameliorate these effects.
Acknowledgments and funding sources
This work was supported by a Discovery Project Grant from the Australian Research Council (ARC) to Sarah J. Spencer (DP109339), and Project Grant from the National Health and Medical Research Council (NHMRC) to Dr Zane Andrews and Sarah J. Spencer (APP1011274). Sarah J. Spencer is an ARC Future Fellow (FT110100084) and an RMIT University VC Senior Research Fellow.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- AHS , (2011-2012). Australian Health Survey: First Results, Canberra: Australian Bureau of Statistics [Google Scholar]
- Albuquerque K. T., Sardinha F. L., Telles M. M., Watanabe R. L., Nascimento C. M., Tavares Do Carmo M. G., et al. (2006). Intake of trans fatty acid-rich hydrogenated fat during pregnancy and lactation inhibits the hypophagic effect of central insulin in the adult offspring. Nutrition 22, 820–829 10.1016/j.nut.2006.04.009 [DOI] [PubMed] [Google Scholar]
- Ashida H., Ogawa M., Kim M., Mimuro H., Sasakawa C. (2012). Bacteria and host interactions in the gut epithelial barrier. Nat. Chem. Biol. 8, 36–45 10.1038/nchembio.741 [DOI] [PubMed] [Google Scholar]
- Ashino N. G., Saito K. N., Souza F. D., Nakutz F. S., Roman E. A., Velloso L. A., et al. (2012). Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J. Nutr. Biochem. 23, 341–348 10.1016/j.jnutbio.2010.12.011 [DOI] [PubMed] [Google Scholar]
- Baird J., Fisher D., Lucas P., Kleijnen J., Roberts H., Law C. (2005). Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 331, 929 10.1136/bmj.38586.411273.E0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto-Medeiros J., Queiros-Santos A., Cabral-Filho J. E., Ferreira E. S. W. T., Leandro C. G., Deiro T. C., et al. (2007). Stress/aggressiveness-induced immune changes are altered in adult rats submitted to neonatal malnutrition. Neuroimmunomodulation 14, 229–334 10.1159/000112047 [DOI] [PubMed] [Google Scholar]
- Begum G., Stevens A., Smith E. B., Connor K., Challis J. R., Bloomfield F., et al. (2012). Epigenetic changes in fetal hypothalamic energy regulating pathways are associated with maternal undernutrition and twinning. FASEB J. 26, 1694–1703 10.1096/fj.11-198762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger L., Langley-Evans S. C. (2005). Fetal programming of appetite by exposure to a maternal low-protein diet in the rat. Clin. Sci. (Lond). 109, 413–420 10.1042/CS20050127 [DOI] [PubMed] [Google Scholar]
- Bellinger L., Lilley C., Langley-Evans S. C. (2004). Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br. J. Nutr. 92, 513–520 10.1079/BJN20041224 [DOI] [PubMed] [Google Scholar]
- Benatti P., Peluso G., Nicolai R., Calvani M. (2004). Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 23, 281–302 10.1080/07315724.2004.10719371 [DOI] [PubMed] [Google Scholar]
- Bjorksten B., Sepp E., Julge K., Voor T., Mikelsaar M. (2001). Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108, 516–520 10.1067/mai.2001.118130 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. (1996). The regulation of adipose tissue distribution in humans. Int. J. Obes. Relat. Metab. Disord. 20, 291–302 [PubMed] [Google Scholar]
- Blatteis C. M., Sehic E., Li S. (2000). Pyrogen sensing and signaling: old views and new concepts. Clin. Infect. Dis. 31Suppl. 5, S168–S177 [DOI] [PubMed] [Google Scholar]
- Boney C. M., Verma A., Tucker R., Vohr B. R. (2005). Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115, e290–e296 10.1542/peds.2004-1808 [DOI] [PubMed] [Google Scholar]
- Boullu-Ciocca S., Dutour A., Guillaume V., Achard V., Oliver C., Grino M. (2005). Postnatal diet-induced obesity in rats upregulates systemic and adipose tissue glucocorticoid metabolism during development and in adulthood: its relationship with the metabolic syndrome. Diabetes 54, 197–203 10.2337/diabetes.54.1.197 [DOI] [PubMed] [Google Scholar]
- Brandt I., Sticker E. J., Lentze M. J. (2003). Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood. J. Pediatr. 142, 463–468 10.1067/mpd.2003.149 [DOI] [PubMed] [Google Scholar]
- Bulfin L. J., Clarke M. A., Buller K. M., Spencer S. J. (2011). Anxiety and hypothalamic-pituitary-adrenal axis responses to psychological stress are attenuated in male rats made lean by large litter rearing. Psychoneuroendocrinology 36, 1080–1091 10.1016/j.psyneuen.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Calvani M., Alessandri C., Sopo S. M., Panetta V., Pingitore G., Tripodi S., et al. (2006). Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr. Allergy Immunol. 17, 94–102 10.1111/j.1399-3038.2005.00367.x [DOI] [PubMed] [Google Scholar]
- Carlton E. D., Demas G. E., French S. S. (2012). Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm. Behav. 62, 272–279 10.1016/j.yhbeh.2012.04.010 [DOI] [PubMed] [Google Scholar]
- Cartmell T., Ball C., Bristow A. F., Mitchell D., Poole S. (2003). Endogenous interleukin-10 is required for the defervescence of fever evoked by local lipopolysaccharide-induced and Staphylococcus aureus-induced inflammation in rats. J. Physiol. 549, 653–664 10.1113/jphysiol.2002.037291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano P. M., Presley L., Minium J., Hauguel-De Mouzon S. (2009). Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32, 1076–1080 10.2337/dc08-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. C., Marsit C. J. (2011). Epigenomics in environmental health. Front. Genet. 2:84 10.3389/fgene.2011.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R. D., Shanahan F., et al. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673 10.1038/mp.2012.77 [DOI] [PubMed] [Google Scholar]
- Clarke M. A., Stefanidis A., Spencer S. J. (2012). Postnatal overfeeding leads to obesity and exacerbated febrile responses to lipopolysaccharide throughout life. J. Neuroendocrinol. 24, 511–524 [DOI] [PubMed] [Google Scholar]
- Conti B., Tabarean I., Andrei C., Bartfai T. (2004). Cytokines and fever. Front. Biosci. 9, 1433–1449 10.2741/1341 [DOI] [PubMed] [Google Scholar]
- Cretikos M. A., Valenti L., Britt H. C., Baur L. A. (2008). General practice management of overweight and obesity in children and adolescents in Australia. Med. Care 46, 1163–1169 10.1097/MLR.0b013e318179259a [DOI] [PubMed] [Google Scholar]
- Dabelea D., Hanson R. L., Lindsay R. S., Pettitt D. J., Imperatore G., Gabir M. M., et al. (2000). Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49, 2208–2211 10.2337/diabetes.49.12.2208 [DOI] [PubMed] [Google Scholar]
- Dantzer R., Kelley K. W. (2007). Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 21, 153–160 10.1016/j.bbi.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood R. H., Ho E. (2008). Dietary agents as histone deacetylase inhibitors: sulforaphane and structurally related isothiocyanates. Nutr. Rev. 66Suppl. 1, S36–S38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E. R., Fitzsimons C. P., Datson N. A., Meijer O. C., Vreugdenhil E. (2009). Glucocorticoid signaling and stress-related limbic susceptibility pathway: about receptors, transcription machinery and microRNA. Brain Res. 1293, 129–141 10.1016/j.brainres.2009.03.039 [DOI] [PubMed] [Google Scholar]
- Delage B., Dashwood R. H. (2008). Dietary manipulation of histone structure and function. Annu. Rev. Nutr. 28, 347–366 10.1146/annurev.nutr.28.061807.155354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M., Gayle D., Babu J., Ross M. G. (2005). Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R91–R96 [DOI] [PubMed] [Google Scholar]
- Druet C., Stettler N., Sharp S., Simmons R. K., Cooper C., Smith G. D., et al. (2012). Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr. Perinat. Epidemiol. 26, 19–26 10.1111/j.1365-3016.2011.01213.x [DOI] [PubMed] [Google Scholar]
- Ellis S., Mouihate A., Pittman Q. J. (2005). Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 19, 1519–1521 [DOI] [PubMed] [Google Scholar]
- Falagas M. E., Kompoti M. (2006). Obesity and infection. Lancet Infect. Dis. 6, 438–446 10.1016/S1473-309970523-0 [DOI] [PubMed] [Google Scholar]
- Fallani M., Young D., Scott J., Norin E., Amarri S., Adam R., et al. (2010). Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 51, 77–84 10.1097/MPG.0b013e3181d1b11e [DOI] [PubMed] [Google Scholar]
- Ferreira E. S. W. T., Galvao B. A., Ferraz-Pereira K. N., De-Castro C. B., Manhaes-De-Castro R. (2009). Perinatal malnutrition programs sustained alterations in nitric oxide released by activated macrophages in response to fluoxetine in adult rats. Neuroimmunomodulation 16, 219–227 10.1159/000212382 [DOI] [PubMed] [Google Scholar]
- Field A. E., Cook N. R., Gillman M. W. (2005). Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes. Res. 13, 163–169 10.1038/oby.2005.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik M. F., Kasahara M. (2010). Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 11, 47–59 10.1038/nrg2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler-Brown A. G., Bennett G. G., Goodman M. S., Wee C. C., Corbie-Smith G. M., James S. A. (2009). Psychosocial stress and 13-year bmi change among blacks: the pitt county study. Obesity (Silver Spring). 10.1038/oby.2009.130 [DOI] [PubMed] [Google Scholar]
- Fuhrman C., Bonmarin I., Bitar D., Cardoso T., Duport N., Herida M., et al. (2011). Adult intensive-care patients with 2009 pandemic influenza A(H1N1) infection. Epidemiol. Infect. 139, 1202–1209 10.1017/S0950268810002414 [DOI] [PubMed] [Google Scholar]
- Galic M. A., Spencer S. J., Mouihate A., Pittman Q. J. (2009). Postnatal programming of the innate immune response. Integr. Comp. Biol. 49, 237–245 10.1093/icb/icp025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottrand F. (2008). Long-chain polyunsaturated fatty acids influence the immune system of infants. J. Nutr. 138, 1807S–1812S [DOI] [PubMed] [Google Scholar]
- Gregor M. F., Hotamisligil G. S. (2011). Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- Gronlund M. M., Arvilommi H., Kero P., Lehtonen O. P., Isolauri E. (2000). Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0-6 months. Arch. Dis. Child Fetal Neonatal Ed. 83, F186–F192 10.1136/fn.83.3.F186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberg S. E., London S. J., Stigum H., Nafstad P., Nystad W. (2009). Folic acid supplements in pregnancy and early childhood respiratory health. Arch. Dis. Child 94, 180–184 10.1136/adc.2008.142448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hak A. E., Stehouwer C. D., Bots M. L., Polderman K. H., Schalkwijk C. G., Westendorp I. C., et al. (1999). Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler. Thromb. Vasc. Biol. 19, 1986–1991 10.1161/01.ATV.19.8.1986 [DOI] [PubMed] [Google Scholar]
- Harden L. M., Du Plessis I., Poole S., Laburn H. P. (2006). Interleukin-6 and leptin mediate lipopolysaccharide-induced fever and sickness behavior. Physiol. Behav. 89, 146–155 10.1016/j.physbeh.2006.05.016 [DOI] [PubMed] [Google Scholar]
- Hildebrandt M. A., Hoffmann C., Sherrill-Mix S. A., Keilbaugh S. A., Hamady M., Chen Y. Y., et al. (2009). High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137, 1716–1724 e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson D. M., Knott B., Walker F. R. (2001). Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr. Res. 50, 750–755 10.1203/00006450-200112000-00020 [DOI] [PubMed] [Google Scholar]
- Hollingsworth J. W., Maruoka S., Boon K., Garantziotis S., Li Z., Tomfohr J., et al. (2008). In utero supplementation with methyl donors enhances allergic airway disease in mice. J. Clin. Invest. 118, 3462–3469 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. (1995). Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 10.1172/JCI117936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993). Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- Huffman S. L., Harika R. K., Eilander A., Osendarp S. J. (2011). Essential fats: how do they affect growth and development of infants and young children in developing countries? A literature review. Matern. Child Nutr. 7Suppl. 3, 44–65 10.1111/j.1740-8709.2011.00356.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis S. M. (2007). Human milk: maternal dietary lipids and infant development. Proc. Nutr. Soc. 66, 397–404 10.1017/S0029665107005666 [DOI] [PubMed] [Google Scholar]
- Johansson M. A., Sjogren Y. M., Persson J. O., Nilsson C., Sverremark-Ekstrom E. (2011). Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS ONE 6:e23031 10.1371/journal.pone.0023031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J. E., Spor A., Scalfone N., Fricker A. D., Stombaugh J., Knight R., et al. (2011). Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1), 4578–4585 10.1073/pnas.1000081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner A. C., Bruning J. C. (2011). Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol. Metab. 22, 16–23 10.1016/j.tem.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Kremmyda L. S., Vlachava M., Noakes P. S., Diaper N. D., Miles E. A., Calder P. C. (2011). Atopy risk in infants and children in relation to early exposure to fish, oily fish, or long-chain omega-3 fatty acids: a systematic review. Clin. Rev. Allergy Immunol. 41, 36–66 10.1007/s12016-009-8186-2 [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. (2009). Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388, 621–625 10.1016/j.bbrc.2009.08.062 [DOI] [PubMed] [Google Scholar]
- Laitinen K., Poussa T., Isolauri E. (2009). Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br. J. Nutr. 101, 1679–1687 10.1017/S0007114508111461 [DOI] [PubMed] [Google Scholar]
- Lawrence C. B., Brough D., Knight E. M. (2012). Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Dis. Model. Mech. 5, 649–659 10.1242/dmm.009068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Phillips E. S., Torrens C., Hanson M. A., Jackson A. A., Burdge G. C. (2008). Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br. J. Nutr. 100, 278–282 10.1017/S0007114507894438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., et al. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659–1662 10.1126/science.277.5332.1659 [DOI] [PubMed] [Google Scholar]
- Lubchenco L. O., Delivoria-Papadopoulos M., Butterfield L. J., Metcalf D., Hix I. E., Jr., et al. (1972a). Long-term follow-up studies of prematurely born infants. I. Relationship of handicaps to nursery routines. J. Pediatr. 80, 501–508 [DOI] [PubMed] [Google Scholar]
- Lubchenco L. O., Delivoria-Papadopoulos M., Searls D. (1972b). Long-term follow-up studies of prematurely born infants. II. Influence of birth weight and gestational age on sequelae. J. Pediatr. 80, 509–512 [DOI] [PubMed] [Google Scholar]
- Luheshi G. N., Gardner J. D., Rushforth D. A., Loudon A. S., Rothwell N. J. (1999). Leptin actions on food intake and body temperature are mediated by IL-1. Proc. Natl. Acad. Sci. U.S.A. 96, 7047–7052 10.1073/pnas.96.12.7047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., Sghir A., Gaskins H. R. (1999). Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69, 1035S–1045S [DOI] [PubMed] [Google Scholar]
- Martin V., Maldonado-Barragan A., Moles L., Rodriguez-Banos M., Campo R. D., Fernandez L., et al. (2012). Sharing of bacterial strains between breast milk and infant feces. J. Hum. Lact. 28, 36–44 10.1177/0890334411424729 [DOI] [PubMed] [Google Scholar]
- Martino D. J., Currie H., Taylor A., Conway P., Prescott S. L. (2008). Relationship between early intestinal colonization, mucosal immunoglobulin a production and systemic immune development. Clin. Exp. Allergy 38, 69–78 [DOI] [PubMed] [Google Scholar]
- Matamoros S., Gras-Leguen C., Le Vacon F., Potel G., De La Cochetiere M. F. (2013). Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 21, 167–173 10.1016/j.tim.2012.12.001 [DOI] [PubMed] [Google Scholar]
- McKay J. A., Mathers J. C. (2011). Diet induced epigenetic changes and their implications for health. Acta Physiol. (Oxf) 202, 103–118 10.1111/j.1748-1716.2011.02278.x [DOI] [PubMed] [Google Scholar]
- Mehedint M. G., Craciunescu C. N., Zeisel S. H. (2010). Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc. Natl. Acad. Sci. U.S.A. 107, 12834–12839 10.1073/pnas.0914328107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagro F. I., Mansego M. L., De Miguel C., Martinez J. A. (2013). Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol. Aspects Med. 34, 782–812 10.1016/j.mam.2012.06.010 [DOI] [PubMed] [Google Scholar]
- MMWR. (2009). Intensive-care patients with severe novel influenza A (H1N1) virus infection - Michigan, June 2009. MMWR Morb. Mortal. Wkly. Rep. 58, 749–752 [PubMed] [Google Scholar]
- Moore S. E., Cole T. J., Collinson A. C., Poskitt E. M., McGregor I. A., Prentice A. M. (1999). Prenatal or early postnatal events predict infectious deaths in young adulthood in rural Africa. Int. J. Epidemiol. 28, 1088–1095 10.1093/ije/28.6.1088 [DOI] [PubMed] [Google Scholar]
- Morris M. J., Velkoska E., Cole T. J. (2005). Central and peripheral contributions to obesity-associated hypertension: impact of early overnourishment. Exp. Physiol. 90, 697–702 10.1113/expphysiol.2005.030783 [DOI] [PubMed] [Google Scholar]
- Morrison S. F., Nakamura K., Madden C. J. (2008). Central control of thermogenesis in mammals. Exp. Physiol. 93, 773–797 10.1113/expphysiol.2007.041848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A., Galic M. A., Ellis S. L., Spencer S. J., Tsutsui S., Pittman Q. J. (2010). Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J. Neurosci. 30, 7975–7983 10.1523/JNEUROSCI.6078-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller B. R., Bale T. L. (2006). Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol. Behav. 88, 605–614 10.1016/j.physbeh.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Nhanes. (2009-2010). National Health and Nutrition Examination Survey, Hyattsville, MD: Centres for Disease Control, National Center for Health Statistics [Google Scholar]
- Nian H., Delage B., Ho E., Dashwood R. H. (2009). Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ. Mol. Mutagen. 50, 213–221 10.1002/em.20454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K. K., Ahmed M. L., Emmett P. M., Preece M. A., Dunger D. B. (2000). Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320, 967–971 10.1136/bmj.320.7240.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K. K., Emmett P. M., Noble S., Ness A., Dunger D. B. (2006). Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics 117, e503–e508 10.1542/peds.2005-1668 [DOI] [PubMed] [Google Scholar]
- Palmer C., Bik E. M., Digiulio D. B., Relman D. A., Brown P. O. (2007). Development of the human infant intestinal microbiota. PLoS Biol. 5:e177 10.1371/journal.pbio.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrone V., Ferrari S., Lizier M., Lucchini F., Minuti A., Tondelli B., et al. (2012). Short-term modifications in the distal gut microbiota of weaning mice induced by a high-fat diet. Microbiology 158, 983–992 10.1099/mic.0.054247-0 [DOI] [PubMed] [Google Scholar]
- Patterson E., Wall R., Fitzgerald G. F., Ross R. P., Stanton C. (2012). Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 539426 10.1155/2012/539426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper R., Jha R., Rossnagel B., Van Kessel A. G., Souffrant W. B., Leterme P. (2008). Effect of barley and oat cultivars with different carbohydrate compositions on the intestinal bacterial communities in weaned piglets. FEMS Microbiol. Ecol. 66, 556–566 10.1111/j.1574-6941.2008.00605.x [DOI] [PubMed] [Google Scholar]
- Pike K. C., Crozier S. R., Lucas J. S., Inskip H. M., Robinson S., Roberts G., et al. (2010). Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax 65, 1099–1106 10.1136/thx.2010.134742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann A., Harder T., Rake A., Voits M., Fink H., Rohde W., et al. (1999). Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 836, 146–155 10.1016/S0006-899301662-5 [DOI] [PubMed] [Google Scholar]
- Pohl J., Woodside B., Luheshi G. N. (2009). Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology 150, 4901–4910 10.1210/en.2009-0526 [DOI] [PubMed] [Google Scholar]
- Priego T., Sanchez J., Garcia A. P., Palou A., Pico C. (2013). Maternal dietary fat affects milk fatty acid profile and impacts on weight gain and thermogenic capacity of suckling rats. Lipids 48, 481–495 10.1007/s11745-013-3764-3768 [DOI] [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K. S., Manichanh C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne M., Kalliomaki M., Arvilommi H., Salminen S., Isolauri E. (2005). Effect of probiotics and breastfeeding on the bifidobacterium and lactobacillus/enterococcus microbiota and humoral immune responses. J. Pediatr. 147, 186–191 10.1016/j.jpeds.2005.03.053 [DOI] [PubMed] [Google Scholar]
- Rodel H. G., Prager G., Stefanski V., Von Holst D., Hudson R. (2008). Separating maternal and litter-size effects on early postnatal growth in two species of altricial small mammals. Physiol. Behav. 93, 826–834 10.1016/j.physbeh.2007.11.047 [DOI] [PubMed] [Google Scholar]
- Romieu I., Torrent M., Garcia-Esteban R., Ferrer C., Ribas-Fito N., Anto J. M., et al. (2007). Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy. 37, 518–525 10.1111/j.1365-2222.2007.02685.x [DOI] [PubMed] [Google Scholar]
- Ruager-Martin R., Hyde M. J., Modi N. (2010). Maternal obesity and infant outcomes. Early Hum. Dev. 86, 715–722 10.1016/j.earlhumdev.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Sachot C., Poole S., Luheshi G. N. (2004). Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. J. Physiol. 561, 263–272 10.1113/jphysiol.2004.074351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U. (2000). How do glucocorticoids influence stress responses. Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Sausenthaler S., Koletzko S., Schaaf B., Lehmann I., Borte M., Herbarth O., et al. (2007). Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am. J. Clin. Nutr. 85, 530–537 [DOI] [PubMed] [Google Scholar]
- Schmidt I., Fritz A., Scholch C., Schneider D., Simon E., Plagemann A. (2001). The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int. J. Obes. Relat. Metab. Disord. 25, 1168–1174 10.1038/sj.ijo.0801669 [DOI] [PubMed] [Google Scholar]
- Scholz-Ahrens K. E., Ade P., Marten B., Weber P., Timm W., Acil Y., et al. (2007). Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 137, 838S–846S [DOI] [PubMed] [Google Scholar]
- Sela D. A., Mills D. A. (2010). Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 18, 298–307 10.1016/j.tim.2010.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell M. F., Huston-Presley L., Super D. M., Catalano P. (2006). Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am. J. Obstet. Gynecol. 195, 1100–1103 10.1016/j.ajog.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Shanks N., Larocque S., Meaney M. J. (1995). Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J. Neurosci. 15, 376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N., Windle R. J., Perks P. A., Harbuz M. S., Jessop D. S., Ingram C. D., et al. (2000). Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc. Natl. Acad. Sci. U.S.A 97, 5645–5650 10.1073/pnas.090571897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek L. P., Chong M. F., Lim J. Y., Soh S. E., Chong Y. S. (2012). Role of dietary long-chain polyunsaturated fatty acids in infant allergies and respiratory diseases. Clin. Dev. Immunol. 2012, 730568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu C. J., Benoist C., Mathis D. (2012). The immune system's involvement in obesity-driven type 2 diabetes. Semin. Immunol. 24, 436–442 10.1016/j.smim.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair K. D., Allegrucci C., Singh R., Gardner D. S., Sebastian S., Bispham J., et al. (2007). DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. U.S.A. 104, 19351–19356 10.1073/pnas.0707258104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren Y. M., Tomicic S., Lundberg A., Bottcher M. F., Bjorksten B., Sverremark-Ekstrom E., et al. (2009). Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin. Exp. Allergy 39, 1842–1851 10.1111/j.1365-2222.2009.03326.x [DOI] [PubMed] [Google Scholar]
- Smith A. G., Sheridan P. A., Harp J. B., Beck M. A. (2007). Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J. Nutr. 137, 1236–1243 [DOI] [PubMed] [Google Scholar]
- Spencer S. J. (2012). Early life programming of obesity: the impact of the perinatal environment on the development of obesity and metabolic dysfunction in the offspring. Curr. Diabetes Rev. 8, 55–68 10.2174/157339912798829214 [DOI] [PubMed] [Google Scholar]
- Spencer S. J., Galic M. A., Pittman Q. J. (2011). Neonatal programming of innate immune function. Am. J. Physiol. Endocrinol. Metab. 300, E11–E18 10.1152/ajpendo.00516.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. J., Tilbrook A. (2009). Neonatal overfeeding alters adult anxiety and stress responsiveness. Psychoneuroendocrinology 34, 1133–1143 10.1016/j.psyneuen.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., Katewa S. D., Palaniyappan A., Pandya J. D., Patel M. S. (2006). Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am. J. Physiol. Endocrinol. Metab. 291, E792–E799 10.1152/ajpendo.00078.2006 [DOI] [PubMed] [Google Scholar]
- Stettler N., Stallings V. A., Troxel A. B., Zhao J., Schinnar R., Nelson S. E., et al. (2005). Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation 111, 1897–1903 10.1161/01.CIR.0000161797.67671.A7 [DOI] [PubMed] [Google Scholar]
- Stevens A., Begum G., Cook A., Connor K., Rumball C., Oliver M., et al. (2010). Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology 151, 3652–3664 10.1210/en.2010-0094 [DOI] [PubMed] [Google Scholar]
- Thaler J. P., Schwartz M. W. (2010). Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151, 4109–4115 10.1210/en.2010-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P. J., Backhed F., Fulton L., Gordon J. I. (2008). Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 10.1016/j.chom.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal K. T., Wiesbrock S. M., Marino M. W., Hotamisligil G. S. (1997). Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389, 610–614 10.1038/39335 [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W., Ndlovu M. N., Hoya-Arias R., Dijsselbloem N., Gerlo S., Haegeman G. (2006). Keeping up NF-kappaB appearances: epigenetic control of immunity or inflammation-triggered epigenetics. Biochem. Pharmacol. 72, 1114–1131 10.1016/j.bcp.2006.07.012 [DOI] [PubMed] [Google Scholar]
- Vickers M. H., Breier B. H., Cutfield W. S., Hofman P. L., Gluckman P. D. (2000). Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 279, E83–E87 [DOI] [PubMed] [Google Scholar]
- Vickers M. H., Breier B. H., McCarthy D., Gluckman P. D. (2003). Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R271–R273 [DOI] [PubMed] [Google Scholar]
- Waterland R. A. (2006). Epigenetic mechanisms and gastrointestinal development. J. Pediatr. 149, S137–S142 10.1016/j.jpeds.2006.06.064 [DOI] [PubMed] [Google Scholar]
- Waterland R. A., Travisano M., Tahiliani K. G., Rached M. T., Mirza S. (2008). Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes. (Lond). 32, 1373–1379 10.1038/ijo.2008.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I. C., Cervoni N., Champagne F. A., D'alessio A. C., Sharma S., Seckl J. R., et al. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003). Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. E., D'vaz N., Prescott S. L. (2011). Dietary immunomodulatory factors in the development of immune tolerance. Curr. Allergy Asthma Rep. 11, 325–333 10.1007/s11882-011-0200-0 [DOI] [PubMed] [Google Scholar]
- West C. E., Videky D. J., Prescott S. L. (2010). Role of diet in the development of immune tolerance in the context of allergic disease. Curr. Opin. Pediatr. 22, 635–641 [DOI] [PubMed] [Google Scholar]
- Wilcox A. J., Lie R. T., Solvoll K., Taylor J., McConnaughey D. R., Abyholm F., et al. (2007). Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ 334, 464 10.1136/bmj.39079.618287.0B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. D., Chen J., Hoffmann C., Bittinger K., Chen Y. Y., Keilbaugh S. A., et al. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., et al. (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. S., Stehouwer C. D., Emeis J. J., Coppack S. W. (1999). C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue. Arterioscler. Thromb. Vasc. Biol. 19, 972–978 [DOI] [PubMed] [Google Scholar]
- Zeisel S. H. (2009). Epigenetic mechanisms for nutrition determinants of later health outcomes. Am. J. Clin. Nutr. 89, 1488S–1493S 10.3945/ajcn.2009.27113B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel S. H. (2011). Nutritional genomics: defining the dietary requirement and effects of choline. J. Nutr. 141, 531–534 10.3945/jn.110.130369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. F., Cardarelli R., Carroll J., Fulda K. G., Kaur M., Gonzalez K., et al. (2011). Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics 6, 623–629 10.4161/epi.6.5.15335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. F., Santella R. M., Wolff M., Kappil M. A., Markowitz S. B., Morabia A. (2012). White blood cell global methylation and IL-6 promoter methylation in association with diet and lifestyle risk factors in a cancer-free population. Epigenetics 7, 606–614 10.4161/epi.20236 [DOI] [PMC free article] [PubMed] [Google Scholar]


