Abstract
Objective
A mixed methods study exploring the UK general public's willingness to donate human biosamples (HBSs) for biomedical research.
Setting
Cross-sectional focus groups followed by an online survey.
Participants
Twelve focus groups (81 participants) selectively sampled to reflect a range of demographic groups; 1110 survey responders recruited through a stratified sampling method with quotas set on sex, age, geographical location, socioeconomic group and ethnicity.
Main outcome measures
(1) Identify participants’ willingness to donate HBSs for biomedical research, (2) explore acceptability towards donating different types of HBSs in various settings and (3) explore preferences regarding use and access to HBSs.
Results
87% of survey participants thought donation of HBSs was important and 75% wanted to be asked to donate in general. Responders who self-reported having some or good knowledge of the medical research process were significantly more likely to want to donate (p<0.001). Reasons why focus group participants saw donation as important included: it was a good way of reciprocating for the medical treatment received; it was an important way of developing drugs and treatments; residual tissue would otherwise go to waste and they or their family members might benefit. The most controversial types of HBSs to donate included: brain post mortem (29% would donate), eyes post mortem (35%), embryos (44%), spare eggs (48%) and sperm (58%). Regarding the use of samples, there were concerns over animal research (34%), research conducted outside the UK (35%), and research conducted by pharmaceutical companies (56%), although education and discussion were found to alleviate such concerns.
Conclusions
There is a high level of public support and willingness to donate HBSs for biomedical research. Underlying concerns exist regarding the use of certain types of HBSs and conditions under which they are used. Improved education and more controlled forms of consent for sensitive samples may mitigate such concerns.
Keywords: PUBLIC HEALTH, GENETICS, PATHOLOGY
Article summary.
Article focus
To explore the UK public's willingness to donate: residual biosamples following a medical procedure, biosamples donated as ‘healthy volunteers’, additional biosamples during surgery and biosamples post mortem for medical research.
The acceptability towards donating different types of biosamples in various settings.
Preferences regarding the use of and access to biosamples.
Key messages
There is a high level of public support for biomedical research and willingness to donate samples for this purpose.
Those responders who self-reported having some or good knowledge of the medical research process were significantly more likely to want to be asked to donate, supporting the need for public education to improve understanding of the research process and the contribution human biological samples (HBSs) make to this.
Concerns exist regarding the use of certain types of samples, the conditions under which they are used and data security; greater transparency and discussion of the safeguards that exist in research are likely to alleviate some of these concerns. More focused communication may also help address the issue that certain subgroups are under-represented and that certain kinds of tissue are infrequently donated.
Strengths and limitations of this study
This study contributes further to our understanding of the UK public's views regarding the types of HBSs acceptable to donate, under what circumstances and for what research purposes. This study highlights the importance of involving the public in a more transparent dialogue about the use of biosamples to encourage greater public involvement and support for this area.
This study presented participants with a series of hypothetical questions about their willingness to donate biosamples for medical research. Therefore, the findings may not necessarily correlate with actual behaviour.
Introduction
A gradual shift in the approach to biomedical research has accelerated the use of human biological samples (HBSs) and the establishment of biobanks with associated skills and infrastructure (‘biobanking’) to acquire, preserve and distribute this increasingly valuable resource. Biobanks are important custodians of HBS collections, usually with access to the donors’ deidentified phenotypic and clinical data.1 Samples can comprise human materials of all kinds, including organs, tissues and biofluids, such as blood, and genetic materials, such as DNA. They may be obtained from a variety of donated sources: from healthy volunteers or as residual tissue surplus to diagnostic requirements following a medical procedure, or alternatively retrieved post mortem. Sample collections may be population based or disease specific, originating from a wide range of people with differing demographics, health, behaviours and lifestyles. Moreover, they may be used by a variety of research organisations, including public and private enterprises.
The public's willingness to donate HBSs is essential to ensure the continued provision of samples for research2; hence, numerous studies have been conducted to examine this issue.3–7 These studies have shown that the public is generally positive towards research using donated HBSs4–6 and the majority is in principle willing to donate.3 7 Less well known are the public's views regarding the types of HBSs acceptable to donate, under what circumstances and for what research purposes, although some research does exist in these areas. For example, research has shown that the public are generally willing to donate diseased tissue or ‘waste material’ (such as cancerous tissue or placental tissue) for biomedical research; however, donation of eyes, brains, lungs and bone is far more contentious.7–9 Regarding access to tissues, research by publicly funded academic researchers has been shown to cause few concerns, in comparison to research conducted by commercial entities.10–12 These issues are important to address to provide an insight into the key drivers that motivate people to donate or prevent people from donating. Knowledge of these can also help inform biobanking governance and ensure consent procedures and patient information addressing any concerns which the public may have. This is important to help the public understand the need for, and the use of, HBSs in biomedical research as well as to increase transparency and engender trust with the public. This study was conducted to broaden our understanding in these areas. Moreover, the findings are intended to inform a biobanking policy for the Strategic Tissue Repository Alliance Through Unified Methods (STRATUM), a UK Government Technology Strategy Board and Industry-funded project seeking to address the problem that there are insufficient numbers of HBSs and associated clinical data of adequate quality to fully support biomedical research in the UK. This research will also help inform the design of new consent templates and deliver guidance and strategies around the consent process for biobanks and researchers.
The aims of this study were to (1) identify participants’ willingness to donate HBSs for biomedical research, (2) explore the acceptability towards donating different types of HBSs in various settings and (3) explore preferences regarding use and access to HBSs. Public views and preferences regarding consent procedures were also investigated and are described in the sister paper related to this study.13
Methods
This was a mixed methods study comprising qualitative focus groups and a quantitative online survey. Focus groups were chosen as this method helps people explore and illuminate their views through debate within the group. They can also help facilitate the expression of ideas that might be left underdeveloped in an interview.14 Focus groups have been used successfully to study the attitudes of the general public in relation to biobanking in previous research.15 16 A more detailed presentation of the methods can be found in the paper related to this study.13
Focus groups
Twelve focus groups (including one pilot group) were conducted between May and July 2012 in six different geographical locations across the UK. Participants were recruited face to face in the street by the market research company, The Focus Group. Participants were purposively sampled; each group was chosen to reflect a particular demographic (age, socioeconomic group (SEG), ethnicity, ‘patients’ who were affected by a condition or had had an operation in the past 2 years) in order to gather a wide spectrum of views and enable comparisons across groups. Prior to the day, focus group participants were given an information sheet about the use of biosamples in research so that they would have some background knowledge about the subject matter and to get them thinking about the key issues (see online supplementary appendix I). Focus groups were held in ‘neutral’ locations, such as hotel conference rooms or church halls, facilitated by an experienced facilitator (CL) and digitally recorded.
The topic guide explored participants’ views on: willingness to donate and acceptability of donating different types of HBSs, in what circumstances, for what purposes and to whom (see online supplementary appendix II). Recordings were transcribed and the software package NVivo V.9 (QSR International, Pty Ltd) used to facilitate data analysis. This comprised grouping responses to questions into broad thematic categories, which were then refined through subcodes. Coding was conducted by CL and verified by a second researcher to ensure inter-rater reliability. Any discrepancies were discussed between the two researchers until consensus was reached.
Survey
The findings from the focus groups were used to inform development of a quantitative survey used to canvas public opinion on the issues of interest across a representative sample of the UK population (see online supplementary appendix III). Key themes that were discussed or emerged from focus group discussions were reframed as survey questions; in a number of cases, answer options in the survey were informed by focus group discussions (eg, the different types of residual HBS participants were presented, which were raised by focus group participants). The survey was carried out by the market research company Research Now using their online panel community of UK residents. A stratified sampling method was used: quotas were set on sex, age, geographical location, SEG and ethnicity, in line with data provided by the Office of National Statistics (ONS) to ensure that the sample was as representative of the UK population as possible. Within each category, a random sample was selected from the Research Now database containing 451 185 active respondents. We aimed to recruit 1000 responders in total. In order to reduce any online bias in our sample, 100 face-to-face interviews with non-internet users were conducted. An additional ‘boost’ sample of 100 people (not included in the main sample analysis) was also conducted with people from three minority ethnic groups (‘Black’, ‘Chinese’, ‘S. Asian’), so that we could conduct subgroup analysis between the groups. The main survey was then conducted in September 2012. Survey participants were not given the background information sheet about the use of biosamples in research, which was given to all focus group participants. This was done so that the survey responses represented the attitudes of the general public as far as possible. They were, however, given information during the survey to enable them to make informed decisions when answering the survey questions.
Results
Study population
The participants’ characteristics are detailed in table 1.
Table 1.
Participants’ characteristics
| Characteristics | Focus group (N=81) | Survey (N=1110) |
|---|---|---|
| Gender | ||
| Male | 33; 41% | 504; 45% |
| Female | 48; 59% | 606; 55% |
| Age (years) | ||
| 18–24 | 13; 16% | 135; 12% |
| 25–34 | 18; 22% | 184; 17% |
| 35–44 | 19; 23% | 198; 18% |
| 45–54 | 10; 12% | 184; 17% |
| 55–64 | 16; 20% | 176; 16% |
| 65+ | 5; 6% | 233; 21% |
| Socioeconomic groups | ||
| A | 9; 11% | 41; 4% |
| B | 22; 27% | 215; 19% |
| C1 | 24; 30% | 311; 28% |
| C2 | 14; 17% | 233; 21% |
| D | 6; 7% | 145; 13% |
| E | 6; 7% | 165; 15% |
| Regions | ||
| East of England | 7; 7% | 92; 8% |
| East Midlands | – | 57; 5% |
| London | 18; 22% | 213; 19% |
| North East | – | 40; 4% |
| North West | – | 121; 11% |
| Northern Ireland | – | 30; 3% |
| Scotland | 14; 17% | 76; 7% |
| South East | 14; 17% | 165; 15% |
| South West | – | 81; 7% |
| Wales | – | 51; 5% |
| West Midlands | 14; 17% | 94; 8% |
| Yorkshire/Humberlands | 14; 17% | 90; 8% |
| Ethnicity | ||
| White or White British | 54; 67% | 1057; 95% |
| Mixed race | 1; 1% | 7; 1% |
| Asian or Asian British | 10; 12% | 18; 2% |
| Black or Black British | 9; 11% | 19; 2% |
| Chinese or Chinese British | 7; 9% | 2; 0% |
| Other ethnic groups | 0; 0% | 4; 0% |
| Prefer not to say | 0; 0% | 3; 0% |
| Religions | ||
| Christianity | 677; 61% | |
| Islam | 13; 1% | |
| Hinduism | 6; 1% | |
| Sikhism | 0; 0% | |
| Judaism | 6; 1% | |
| Buddhism | 11; 1% | |
| Other religions | 15; 1% | |
| No religion | 370; 33% | |
| Prefer not to say | 12; 1% | |
| Religiosity | ||
| Not at all religious | 234; 32% | |
| Moderately religious | 422; 58% | |
| Very religious | 64; 9% | |
| Prefer not to say | 8; 1% | |
| Education | ||
| No formal qualification | 15; 19% | 70; 6% |
| GCSE, O-level, Scottish Standard Grade or equivalent | 19; 23% | 264; 24% |
| GCE, A-level, Scottish Higher or similar | 17; 21% | 214; 19% |
| Vocational (BTEC/NVQ/Diploma) | – | 230; 21% |
| Degree level or above | 30; 37% | 317; 29% |
| Prefer not to say | – | 15; 1% |
| Self-reported knowledge of medical research process | ||
| No knowledge | 463; 42% | |
| Some knowledge | 603; 54% | |
| Good knowledge | 44; 4% | |
| Have you been affected by a disability or illness? | ||
| Yes | 399; 36% | |
| No | 711; 64% | |
| Has a close family member been affected by a disability or illness? | ||
| Yes | 767; 69% | |
| No | 343; 31% | |
| Have you had blood or tissue removed during a medical procedure? | ||
| Yes | 446; 40% | |
| No | 553; 50% | |
| Don't know | 111; 10% | |
| Have you ever been asked to donate blood or tissue for medical research? | ||
| Yes | 182; 16% | |
| No | 904; 81% | |
| Don't know | 24; 2% | |
| If so, did you agree to donate? | ||
| Yes | 155; 85% | |
| No | 23; 13% | |
| Don't know | 4; 2% | |
Focus groups
One hundred and eighty-two members of the public who were approached were eligible and 81 people agreed to participate (45% response rate; 48 women and 33 men).
Survey
Four thousand six hundred and seven people were invited to take part in the survey; 2014 did not respond, 860 started to complete the survey but did not finish, 102 did not qualify to continue, 521 qualified for the survey but the quota was full and 1110 completed the questionnaire (28% response rate excluding those who did not qualify and where the quota was full). This response rate is comparable to similar studies on this topic.6 Our quota sample was close to but not exactly matching our set targets. For this reason, we carried out weighted as well as unweighted analyses. There was no difference in the conclusions we reached by either method. In this paper, we present the unweighted results (weighted results can be found from the online supplementary appendix IV).
Interest in being asked to donate
We began by providing a brief description of the use of HBSs in biomedical research and then asked survey participants whether, in general, they wanted to be asked to donate. Three quarters (75%) of survey participants wanted to be asked (29% definitely yes and 46% probably yes), 18% did not want to (14% probably not and 4% definitely not) and 7% did not know. When asked how important they thought it was to donate HBSs for biomedical research, 87% said it was either extremely important (50%) or important (37%). Less than 1% of participants (n=5) thought it was not at all important.
Respondents who wanted to be asked to donate HBSs were significantly more likely to be: either not religious or only moderately so (where they did have a religious affiliation, 79.7% vs 59.7%, χ2=36.56(1); p=0.001), from higher SEGs (A–D vs E, 83.8% vs 62.2%, χ2=36.55(1); p<0.001), had tissue removed during a medical or surgical procedure (87.2% vs 73.1%, χ2=27.13(1); p<0.001), had some or good knowledge of the medical research process (84.4% vs 75.1%, χ2=13.04(1); p<0.001), were under 55 years (84% vs 75.1%, χ2=11.56(1); p=0.001), were ‘White’ (81.7% vs 60.9%, χ2=10.9(1); p=0.001), had no religious affiliation (86.4% vs 77.9%, χ2=9.9(1); p=0.002) and had an education level of A-level or equivalent or higher (83.4% vs 76.1%, χ2=7.18(1); p=0.007). Using the boost sample for ethnic minorities, we found that ‘Black’ participants were significantly less likely to want to be asked to donate than ‘White’ participants (53.3% vs 81.7%, χ2=20.12(1); p<0.001). Participants who had a close family member affected by a condition were more willing to be asked to donate than those who had not, although the difference was not quite statistically significant (70.7% vs 63.3%, χ2=3.8(1); p=0.051).
Four independent variables were found to have a significant impact on participants’ interest in being asked to donate tissue as shown in the logistic regression model in table 2. The strongest predictor for wanting to be asked to donate was being from a higher SEG (A–D vs E, OR 3.52, 95% CI 2.19 to 5.66; p<0.001) followed by having had tissue removed during a medical or surgical procedure (OR 2.51, 95% CI 1.65 to 3.84; p=0.001), being either not at all or only moderately religious (OR 2.42, 95% CI 1.31 to 4.47; p=0.005) and having self-reported some or good knowledge of the medical research process (OR 2.01, 95% CI 1.33 to 3.03; p=0.001).
Table 2.
Multiple logistic regression examining a participant's willingness to donate tissue
| Participants’ characteristics | Coefficient | 95% CI | OR | p Value |
|---|---|---|---|---|
| Socioeconomic group | 1.26 | 2.19 to 5.66 | 3.52 | <0.001 |
| Religiosity | 0.89 | 1.31 to 4.47 | 2.42 | 0.005 |
| Knowledge of medical research process | 0.70 | 1.33 to 3.03 | 2.01 | 0.001 |
| Had tissue removed | 0.99 | 1.65 to 3.84 | 2.51 | <0.001 |
Demographic items were excluded from this table if they were not statistically significant. All variables were entered into the models as categorical variables.
Focus group participants also showed a strong willingness to donate HBSs for biomedical research. Four key reasons were provided by the participants. First, it was a good way of reciprocating for medical treatment received in the past, second, it was viewed as an ‘important way of developing drugs and treatments’, and third, that residual tissue, which participants did not have any strong emotional ties to, would otherwise go to waste. The fourth reason offered was one of personal benefit where participants themselves or a family member was affected by an illness or disease.
Someone in my family has got Alzheimer's so I'm particularly supportive. (Female, 18–24 focus group)
A minority of focus group participants did raise concerns. These included surgeons taking “liberties or advantage of the fact that you're out cold,” concerns about data privacy and mistrust of profit-making companies using donated HBSs or the government regulating their use.
The world's very corrupt, and if something needs to get pushed through, it gets pushed through. (Female, ‘Black’ focus group)
While interest in donating appeared to be high, it was evident that knowledge of the medical research process was low. In the survey, only 4% of respondents self-reported having a ‘good knowledge’ of the research process, 54% said they had ‘some knowledge’ and 42% said they had ‘no knowledge’. Similarly, a number of focus group participants commented that the information leaflet was the first time they had heard anything about the use of donated HBSs in medical research.
Types of HBSs
Residual HBSs
The majority of survey participants were either definitely or probably willing to donate residual blood (92%), cancerous tissue (90%), fat (89%), skin tissue (88%), bone or cartilage (84%) and liver tissue (84%) following a medical procedure (figure 1). Less than half of the participants were willing to donate spare eggs (women only, 48%) or spare embryos (44%) left over following in vitro fertilisation (IVF).
Figure 1.
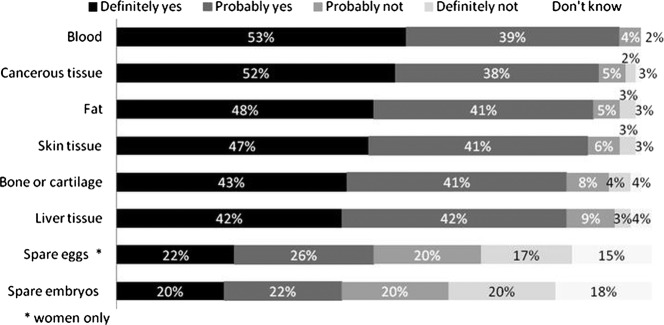
Would you donate the following types of samples for medical research if they were left over (after necessary medical tests had been done) following a medical procedure?
Note: percentages may not add up to 100% due to rounding. The χ2 test was conducted to examine effects of participants’ characteristics on willingness to donate spare eggs and spare embryos as these two tissue types caused the most divide among participants. Willingness in donating spare embryos was significantly associated with being: men (55.9% vs 48.3%, χ2=4.87(1); p=0.023), under 55 years (55.1% vs 45.3%, χ2=7.55(1); p=0.005), from a higher (A–D) socioeconomic group (54.9% vs 32%, χ2=22.05(1); p<0.001), White ethnicity (53% vs 27.7%, χ2=10.48(1); p=0.001), having a religious affiliation (63.5% vs 45.9%, χ2=24.13(1); p<0.001), being not at all or moderately religious where they did have a religious affiliation (48.1% vs 25.5%, χ2=9.38(1); p=0.002) and had tissue removed during a medical procedure (56.9% vs 46%, χ2=9.41(1); p=0.002). Women who were willing to donate spare eggs were significantly more likely to: be from a higher socioeconomic group (A–D, 58.5% vs 44.7%, χ2=4.45(1); p=0.035), White ethnicity (58.1% vs 17.4%, χ2=13.21(1); p<0.001), have no religious affiliation (71.5% vs 50.4%, χ2=18.47(1); p<0.001), be not at all or moderately religious where they did have a religious affiliation (53.5% vs 23.7%, χ2=10.88(1); p=0.001) and have had tissue removed during a medical procedure (62.8% vs 50.4%, χ2=6.77(1); p=0.009).
These results confirm our focus group findings, where most people were willing to donate residual tissue but donation of reproductive tissue raised concerns for a significant number of participants. A key concern was that reproductive tissue would be used for reproductive purposes without the knowledge of the donor.
Although they said it's [ethical approval process] very strict, I still in the back of my mind have a thing where someone could take my egg and have my child. (Female, had operation in past 2 years)
A further concern related to whether it was ‘right’ from an ethical or religious perspective to be conducting research on reproductive tissue.
I would be really worried...an embryo is a baby. I know it's still very, very early days, but you're playing God. (Female—patient affected by a condition)
Those people who were willing to donate reproductive tissue underscored the benefits that could result from such research.
You have to remove yourself from the situation and imagine yourself as an infertile person and maybe someone that could benefit immensely from that research. (Female—18–24 focus group)
Some did, however, highlight the importance of being informed as to how reproductive tissue would be used because of its sensitive nature.
‘Healthy’ volunteers
Survey participants were then presented with a scenario in which they were asked to imagine that they are in a hospital waiting room awaiting an appointment and are asked whether they would donate certain types of HBSs specifically for the purposes of medical research (figure 2). Most responders were either definitely or probably willing to donate urine (89%), saliva (89%) and blood (81%); however, fewer people would donate tissue taken during a local anaesthetic (67%) or sperm (men only, 58%).
Figure 2.
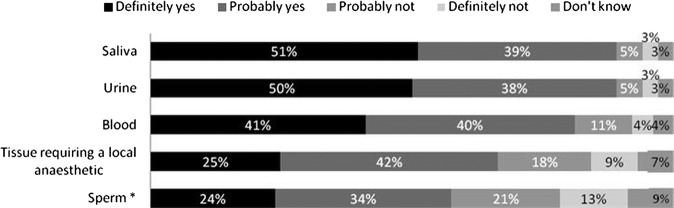
Would you agree to donate the following type of samples for medical research, that is, not as part of any medical procedure, but purely for the purposes of research?
Note: percentages may not add up to 100% due to rounding. The χ2 test was conducted to examine effects of participants’ characteristics on willingness to donate tissues requiring a local anaesthetic and sperm as these two tissue types caused the most divide among participants. Willingness to donate tissue requiring a local anaesthetic was significantly associated with: being over 25 years (72.9% vs 59.3%, χ2=9(1); p=0.003), from a higher socioeconomic group (A–D, 73.1% vs 60.9%, χ2=9.03(1); p=0.003), White ethnicity (72.3% vs 48.9%, χ2=10.87(1); p=0.001), being not at all or moderately religious where they had a religious affiliation (71.4% vs 49.2%, χ2=11.53(1); p=0.001), having good knowledge of the medical research process (75.8% vs 64.5%, χ2=14.96(1); p=0.001), having had tissue removed during a medical procedure (77.9% vs 64%, χ2=20.77(1); p<0.001) and having agreed to donate left over tissues (82.8% vs 45.5%, χ2=13.51(1); p<0.001). Men who were willing to donate sperm were significantly more likely to be: from a higher socioeconomic group (A–D, 66.8% vs 42%, χ2=14.47(1); p<0.001), White ethnicity (65.8% vs 22.2%, χ2=18.95%1); p<0.001) and be either not at all or moderately religious where they did have a religious affiliation (64.3% vs 36.4%, χ2=5.61(1); p=0.018).
Focus group participants were also generally positive towards donating HBSs specifically for research purposes, although some commented that they would not want to undergo an invasive procedure.
Depends on what they wanted, if it's not invasive or nothing then I'd say yes. (Female—‘Black’ focus group)
A number of them said they would be more likely to donate HBSs if they did not have to travel somewhere specifically to do so. Focus group participants also appeared to want more information about how their sample would be used if they were donating HBSs as ‘healthy’ volunteers.
I'd want to know the purpose behind it but if I'm helping something then why not? (Male—‘Chinese’ focus group)
Additional HBSs during surgery
Finally, survey participants were presented with a scenario in which they are having surgery which requires a general anaesthetic, and asked whether they would be willing to have additional tissue taken that is not required to be removed for therapeutic benefits. Over three quarters of the responders (78%) were willing to donate HBSs taken from the same part of the body being operated on, 63% were willing to donate HBSs taken from an area close by and 44% would donate HBSs involving an additional procedure, for example, taking bone marrow while under the same general anaesthetic.
This scenario was not explored explicitly with focus group participants, but was included in the survey as a number of them had aired concerns about surgeons taking additional tissue during surgery without consent.
I would be worried about giving consent before they performed an operation in case the main task of the operation is to remove cancer...and they take some tissue for research. I think it would be OK but only if they asked. (Male, 18–24 group)
Donation of HBSs in the event of one's death
We explored whether the public were willing to donate tissue and whole organs in the event of their death. First, we compared survey responders’ views concerning the donation of tissue taken from an organ with donating a whole organ using the liver and brain as examples. We found that tissue type had a greater impact on people's willingness to donate than the amount of tissue: 89% of people were willing to donate liver tissue, 68% a whole liver, 66% brain tissue and 53% a whole brain. On presenting participants with a list of organs and asking them whether there were any whole organs they would not consider donating for medical research in the event of their death, 71% said they would not donate their brain, 65% would not donate their eyes, 27% would not donate their heart, 14% would not donate their liver, 14% would not donate their lungs and 13% would not donate their kidneys. Five people (0.5%) said they would not donate reproductive tissue in the free text box. Seventeen per cent would not donate any organs for medical research.
Donating whole organs for medical research in the event of one's death caused unease for a number of focus group participants. Some had concerns about the impact on family members, citing that it was a ‘sensitive subject’ that made them feel ‘uncomfortable’. A woman in the South Asian group cited religious reasons for not wanting to donate organs. Others did not like the idea of their body being ‘chopped up like on a butcher's board’ and preferred ‘to remain whole’ and ‘untouched’. A few participants erroneously believed that their organs would not be useful to researchers because they were old or unhealthy. When focusing on particular organs, eyes were found to be most contentious and made participants feel ‘funny’ or ‘squeamish’. They also had concerns that eyes were ‘identifiable’.
When asked whether they would be willing to donate whole organs not suitable for transplant for research purposes instead, 68% of survey responders said they would, 11% would prefer they were not used at all if they could not be used for transplant, 9% would not agree to donate an organ for transplant and 12% did not know.
Uses of HBSs
The most controversial types of research were research involving HBSs in combination with animals (only 34% of survey participants would donate for this purpose), research conducted outside the UK (35%) and research involving ‘cells from embryos’ (41%; figure 3). Research into understanding how our body fights disease was the least controversial (85%).
Figure 3.
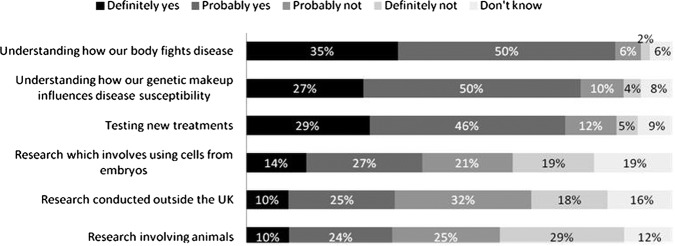
Would you be willing to donate samples for the following type of samples for research?
Note: percentages may not add up to 100% due to rounding. The χ2 test was conducted to examine effects of participants’ characteristics on willingness to donate samples for research outside the UK and research involving animals as these two research types had least support. Those participants who were less willing to donate samples for research outside the UK were significantly more likely to be: over 55 years (67.8% vs 53.8%, χ2=17.2(1); p=0.001), from a low socioeconomic group (E, 72.8% vs 56.6%, χ2=11.92(1); p=0.001), non-white ethnicity (78% vs 58%, χ2=5.7(1); p=0.017), have a religious affiliation (63.4% vs 49.8%, χ2=14.83(1); p=0.001), have a lower education level (GCSE or equivalent or lower, 65.9% vs 55.5%, χ2=8.18(1); p=0.004) and not having had tissue removed during a medical procedure (62.5% vs 55%, χ2=4.57(1); p=0.033). Those participants who were less willingness to donate samples for research involving animals were significantly more likely to be: women (69.5% vs 51.8%, χ2=30.74(1); p<0.001), from a low socioeconomic group (E, 78% vs 58.4%, χ2=19.9(1); p<0.001), non-white ethnicity (80% vs 60.5%, χ2=6.09(1); p=0.014), have a religious affiliation (63.6% vs 56.7%, χ2=4.02(1); p=0.045), be very religious whereby they did have a religious affiliation (78.7% vs 61.9%, χ2=5.99(1); p=0.014), have no knowledge of the medical research process (67.6% vs 57.1%, χ2=10.4(1); p=0.001) and not having agreed to donate left over tissue for medical research (81% vs 49.3%, χ2=6.12(1); p=0.013).
Research involving animals was cited as a cause for concern by a number of focus group participants, particularly if the research caused the animals ‘excessive pain’. Others had concerns about the way animals were cared for in research settings. Nevertheless, a view held by a significant number of people was that research and drugs tested on animals was “not a nice thought...but in the long run the best option’ and that ‘the information gained from watching [an animal used in research] might help thousands of people.”
Research conducted outside the UK was a matter of concern for some focus group participants because other countries might not have similarly strict governance arrangements as those that exist in the UK, or because HBSs might be sold. Other types of research cited as being controversial included cloning, stem cell research, genetic engineering and ‘designer babies’.
Access to HBSs
Most survey responders were willing to donate HBSs to National Health Service (NHS) hospitals (84%), medical research charities (79%), universities (68%), diagnostic companies (63%) and pharmaceutical companies (56%; figure 4).
Figure 4.
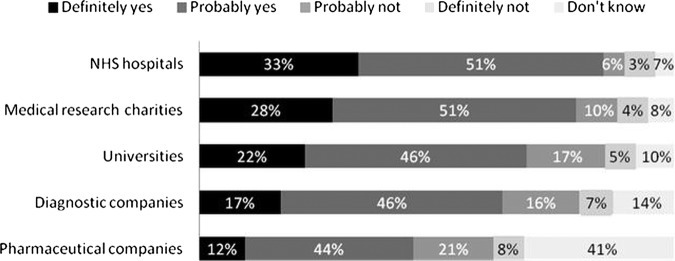
Would you be willing to donate samples to the following organisations to carry out approved research?
Note: percentages may not add up to 100% due to rounding. The χ2 test was conducted to examine effects of participants’ characteristics on willingness to donate samples for pharmaceutical companies as this organisation had the least support. Those participants who were less willing to donate samples to pharmaceutical companies were significantly more likely to be: over 55 years (39.4% vs 31.3%, χ2=6.16(1); p=0.013), from a low socioeconomic group (E, 43.4% vs 32.6%, χ2=5.91(1); p=0.015), non-white ethnicity (60.9% vs 32.9%, χ2=14(1); p=0.001), live in London (45.1% vs 31.7%, χ2=11.02(1); p=0.001), have a religious affiliation (38.1% vs 26.9%(1), χ2=11.1(1); p=0.001), be very religious whereby they had a religious affiliation (54.5% vs 36.7%, χ2=6(1); p=0.014), have no knowledge of the medical research process (38.3% vs 31.6%, χ2=4.14(1); p=0.042) and not having had tissue removed during a medical procedure (38% vs 29.8%, χ2=6(1); p=0.014).
Focus group discussions highlighted that there was generally a high level of faith in the benefits of science, and trust towards the NHS, charities and universities, who were seen as contributing positively towards society. The role of ethics review boards and legal systems in providing oversight and control of medical research was considered important; nevertheless, it was acknowledged that most people are unaware of these safeguards.
I do now know something about the process, and like the ethics side and presenting to a board, but before I wouldn't have known anything about the research process. So I guess I'm just thinking, how would I feel if I didn't know about those procedures? (Female—pilot group)
Some initial negativity was found in relation to pharmaceutical companies conducting research because of their commercial, profit-making nature and concerns that they ‘exploit patients’. However, such concerns were often addressed by other members of the group who acknowledged that commercialisation of research was ‘a fact of life’ and that pharmaceutical companies ‘need to make money to keep their research going’.
Medical records and lifestyle information
We asked participants whether they would be willing to have medical records and lifestyle information linked but deidentified (so that the sample is anonymous to the researcher but contains codes that would allow others to identify an individual from it) to their biosample. Sixty-eight per cent of survey responders would, 22% would not and 10% did not know. Survey responders were more willing to have their deidentified lifestyle information linked to their biosample; 82% would, 12% would not and 6% did not know.
Concerns about linking medical records with HBSs were raised by focus group participants. Data protection and privacy were two key concerns; for example, participants were worried that their personal data might be ‘hacked or mislaid’. Others cited concerns about data being accessed by the police or insurance companies. Some participants felt uncomfortable about sensitive medical details such as sexual diseases or illegal drug use being seen by people unconnected with their health. Nevertheless, most participants understood the importance of linking medical records and lifestyle information to HBSs as long as they were not identifiable.
You want the sample to be as useful as possible so you want to give them the most complete picture. You want to give them all the information that is available. (Male—had operation in past 2 years)
Discussion
Results from this study are consistent with the findings from other empirical research that there is a high level of public support for biomedical research and willingness to donate HBSs for this purpose.3–5 7 17–19 However, by presenting participants with scenarios consisting of a variety of HBS types across a number of settings, and by using qualitative as well as quantitative methods, we have been able to build a richer understanding of public attitudes. While it is important to bear in mind that the opinions expressed are hypothetical and therefore do not necessarily correspond with how people would actually behave in practice, they still offer an intriguing insight into public attitudes which can help inform policy and practice.
The general willingness of the UK public to donate residual HBSs is consistent with findings from other studies conducted in the UK, the USA and Scandinavia where willingness to donate varied from 67% to 88%.3–5 7 17 18 Interestingly, people who themselves had had tissue removed were significantly more likely to want to be asked to donate than those that had not (87% compared to 73%). A number of other studies conducted in the UK and elsewhere have found patients’ willingness to be high, between 83% and 99%.20–25 This is likely to be so because donation of surplus tissue provides patients with an opportunity to reciprocate or demonstrate gratitude towards those involved in the therapeutic process22 or because they have had the medical need explained to them and can relate to the experience more closely. Those responders who self-reported having some or good knowledge of the medical research process were also significantly more likely to want to be asked to donate. This finding supports the need for public education to improve understanding of the research process and the contribution made to this by HBSs. We identified that more people saw a biosample donation as important (87%) than wanted to be asked to donate (75%). It may be that although people see a donation as important, other concerns, for example, around issues such as data privacy, or other ethical considerations, such as commercial use of HBSs, may prevent some people from donating; concerns have been identified in this and other empirical studies.5 16 20 26
Lower levels of support for HBSs donation were identified among minority ethnic groups, a finding that has been seen elsewhere, particularly among African-Americans19 27–29 and Asian-American women.30 A study conducted in China also found that the public and patients’ willingness to donate residual tissue was low compared to studies conducted in the UK, Scandinavia and the USA, at only 65%.31 These differences may stem from different cultural attitudes towards donation, religious beliefs or low levels of trust in public institutions (which may stem from previous breaches of trust as highlighted by Ma et al31 among the ‘Chinese’ population). Mistrust of profit-making companies and the government was identified during focus group discussions with ethnic minority groups in this study, although not exclusively so. Information about the role of ethics review boards in safeguarding participants’ interests is therefore vital for ensuring public trust.
A large proportion of people were unwilling to donate reproductive tissue. This type of HBSs donation raised a number of unique moral, ethical and social concerns, as exemplified by focus group discussions. Interestingly, the survey showed that men were more likely to donate semen than women were likely to donate excess eggs following an IVF procedure (58% vs 48%), which may indicate that egg donation is a more contentious issue or that women feel a greater attachment to eggs than men to sperm.32 Another possible reason may be the limited number of eggs that a woman has, and the greater effort and risk required to make them available ex vivo, resulting in a more judicious approach to their use; for example, women may prefer to keep excess eggs following IVF for future uses rather than donate them for research. More controlled forms of consent (tiered or specific) may be one way of alleviating concerns people may have about donating sensitive HBSs.
When asked to consider post mortem donation, eyes and brains were considered the least desirable organs to donate for research purposes, a finding that has been reported elsewhere.7 As the donation of eyes is crucial for vision research and drug testing, and with donated brains being essential for research into conditions such as multiple sclerosis, Alzheimer's and Parkinson's disease, different ways of raising awareness and motivating donation need to be considered. For example, completely transparent discussion with families on the day prior to forensic post mortem examination, conducted in a sensitive manner, has led to research authorisation and donation in a very high proportion of cases to the Sudden Death Brain and Tissue Bank in Edinburgh.33 It may also be worth considering incentives to donation for research, as have already been discussed in depth elsewhere, although with a greater emphasis on donation for transplant.34 Our research highlights that a significant number of people (68%) would be willing to donate whole organs not suitable for transplant for research purposes instead. Such soft incentives are likely to be welcomed by families, a finding which has also been reported by Womack and Jack35 where over 70% of family members consented to the retrieval of blood and tissue at the time of post mortem examination.
The finding that 67% of people were willing to undergo a local anaesthetic to donate tissue seems unusually high at first glance. Nevertheless, such a finding should not be dismissed; women have been known to undergo local aesthetic to donate healthy breast tissue for breast cancer research, as evidenced by the 2800 women who have donated to the Komen Tissue Bank in the USA.36 The finding that a significant proportion of the public are willing to donate as ‘healthy volunteers’ also supports the premise that there is a strong altruistic desire to contribute to medical research and a high level of trust in and support for the research process.8
Research involving animals and research conducted outside the UK were the least supported research types in this study. Animal research remains a controversial topic and much empirical and ethical debate has focused on this issue.37–39 However, the finding that a large proportion of the public do not want their HBSs used for research outside the UK is intriguing. Our qualitative data show that concerns exist around regulation and commodification of HBSs, findings supported elsewhere in the literature.40 41 To address these concerns, potential donors should be provided with information related to the specific issues that relate to those countries where HBSs are likely to be sent.
Regarding access to donated HBSs, the overall findings indicate high levels of trust towards research organisations conducting biomedical research. The lowest trust was afforded to pharmaceutical companies, primarily because of their profit-making nature, a finding which concurs with other studies.5 10 11 Greater transparency and education of the public by the research community about the role pharmaceutical companies play in research and drug development, (as is currently being conducted through initiatives such as EUPATI; http://www.patientsacademy.eu), will help to highlight the collaborations that frequently exist between private and public enterprises. Emphasising the safeguards that exist in research through regulation and ethics review boards is also likely to alleviate some of these concerns.
Finally, our research reinforces the concerns held by the public regarding the linking of deidentified clinical data to HBSs.20 26 Discussion during the consent procedure around the value of associated clinical data, and the safeguards in place to ensure data security, may go some way to reducing these worries. Similarly, the strict governance arrangements around access to personal information by third parties, including the police, insurance companies and employers, should be made clear.
Strengths and limitations
As with any qualitative research, the findings from this study rely on the researcher's interpretation of comments made by focus group participants. Nevertheless, we have used a methodology grounded in the data and ensured inter-rater reliability through cross-checking coding to ensure that the interpretation was as close to the intended meaning as possible. Moreover, we have been able to verify focus group findings through the results from the survey. A major limitation of this study is its hypothetical nature; hence, the results need to be interpreted with caution. Nevertheless, where possible, we presented questions as scenarios to try and make them as ‘real’ as possible. We also provided focus group participants with a background information sheet so that they had some knowledge about the subject matter before the discussion took place and, as such, were likely to be more well informed than the general public. Survey participants were not given this information sheet and were only given selective background information that it was felt (by the authors) was necessary to enable them to make informed decisions when answering questions. This in itself, however, may have impacted the representativeness of the findings as they may have responded differently if no background information had been provided. A further limitation is that the dropout rate was relatively high; participants who did complete the survey may have done so because of a strong attachment to the issues raised and this may have skewed the results. However, every effort was made to ensure that the results were as representative of the UK population as possible. Finally, the focus groups and survey were conducted in English, which excluded those people who were not competent English speakers and/or readers. Our findings are therefore not necessarily representative of the non-English speaking community living in the UK.
Conclusion
There is a high level of public support for, and willingness to contribute to, biobanking and the research process. In particular, people appear to be keen to contribute to research above and beyond the donation of residual tissue. Nevertheless, underlying concerns exist regarding the use of certain types of HBSs, the conditions under which they are used and data security, although these issues did not necessarily preclude the willingness to participate. Improved public education in these areas, for example, through the development of a ‘Frequently Asked Questions’ document which includes information on the ethics infrastructure that exists in the UK may mitigate some of these concerns. More controlled forms of consent and focused communication for sensitive types of HBSs may also positively impact the public's willingness to donate infrequently donated tissue types. More focused communication may also address the finding that certain subgroups, such as particular minority ethnic groups, are less likely to donate. Finally, greater transparency in the biomedical research process and the fostering of trust in those organisations involved throughout that process is vital to ensure that the process of donating tissue to biobanks is satisfactory to all parties involved. These suggestions should be considered by the research community and policy makers.
Supplementary Material
Acknowledgments
The authors would like to thank Lisa Bennett, Sarah Dickson, Catherine Elliott, James Ironside and Chris Womack for their helpful comments on this manuscript; Sarah Dickson, Jim Elliott and the late Neil Formstone for their contribution to the design of the study and development of the focus group and survey questions; and Samantha Reeve and Zheng Lei for helping with data analysis. Focus group recruitment was conducted by the company The Focus Group and the survey was conducted through the market research company Research Now.
Footnotes
Contributors: JC conceived the study. All authors contributed to the study design and development of the focus group and survey questions. CL facilitated the focus groups and conducted data analysis and interpretation with the help of Samantha Reeve and Zheng Lei. The initial draft of the manuscript was prepared by CL and then circulated repeatedly among the authors for critical revision. All authors approved the final version of the manuscript.
Funding: This work was supported by a grant from the Technology Strategy Board through the Stratified Medicines Competition grant number 101021; direct financial contributions were made by AstraZeneca and resources in kind by AstraZeneca, GlaxoSmithKline and Lab 21.
Competing interests: LJS is an employee of GlaxoSmithKline. MJR is an employee of AstraZeneca.
Ethics approval: This study was approved by the Ethics Review Board of the University of Manchester, reference 11459.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Transcripts from the focus groups and full results of the survey are available from CL at celine@geneticalliance.org.uk. Supplementary material is also available at http://www.geneticalliance.org.uk/projects/stratum_docs.htm
References
- 1.Kaiser J. Biobanks. Population databases boom, from Iceland to the U.S. Science 2002;298:1158–61 [DOI] [PubMed] [Google Scholar]
- 2.Tutton R, Kaye J, Hoeyer K. Governing UK Biobank: the importance of ensuring public trust. Trends Biotechnol 2004;22:284–5 [DOI] [PubMed] [Google Scholar]
- 3.Kaufman D, Bollinger J, Dvoskin R, et al. Preferences for opt-in and opt-out enrollment and consent models in biobank research: a national survey of Veterans Administration patients. Genet Med 2012;14:787–94 [DOI] [PubMed] [Google Scholar]
- 4.Kettis-Lindblad Å, Ring L, Viberth E, et al. Genetic research and donation of tissue samples to biobanks. What do potential sample donors in the Swedish general public think? Eur J Public Health 2006;16:433–40 [DOI] [PubMed] [Google Scholar]
- 5.Tupasela A, Sihvo S, Snell K, et al. Attitudes towards biomedical use of tissue sample collections, consent, and biobanks among Finns. Scand J Public Health 2010;38:46–52 [DOI] [PubMed] [Google Scholar]
- 6.Simon CM, L'Heureux J, Murray JC, et al. Active choice but not too active: public perspectives on biobank consent models. Genet Med 2011;13:821–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodson ML, Vernon BG. A study of public opinion on the use of tissue samples from living subjects for clinical research. J Clin Pathol 2004;57:135–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell B, Lipworth W, Axler R, et al. Cancer as rubbish: donation of tumor tissue for research. Qual Health Res 2011;21:75–84 [DOI] [PubMed] [Google Scholar]
- 9.Barr M. ‘I'm not Really Read up on Genetics’: biobanks and the social context of informed consent. BioSocieties 2006;1:251–62 [Google Scholar]
- 10.Haddow G, Cunningham-Burley S, Bruce A, et al. Generation Scotland: consulting publics and specialists at an early stage in a genetic database's development. Crit Public Health 2008;18:139–49 [Google Scholar]
- 11.Budimir D, Polasek O, Marusic A, et al. Ethical aspects of human biobanks: a systematic review. Croat Med J 2011;52:262–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipworth W, Morrell B, Irvine R, et al. An empirical reappraisal of public trust in biobanking research: rethinking restrictive consent requirements. J Law Med 2009;17:119–32 [PubMed] [Google Scholar]
- 13.Lewis C, Clotworthy M, Hilton S, et al. Consent for the use of human biological samples for biomedical research: a mixed methods study exploring the UK public's preferences. BMJ Open 2013;3:e003022 doi:10.1136/bmjopen-2013-003022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitzinger J. Focus groups. In: Pope C, Mays N. eds. Qualitative research in health care. 3rd edn Oxford: Blackwell Publishing Ltd, 2006:21–31 [Google Scholar]
- 15.Tutton R. Constructing participation in genetic databases. Sci Technol Hum Values 2007;32:172–95 [Google Scholar]
- 16.Haddow G, Bruce A, Sathanandam S, et al. ‘Nothing is really safe’: a focus group study on the processes of anonymizing and sharing of health data for research purposes. J Eval Clin Pract 2010;17:1140–6 [DOI] [PubMed] [Google Scholar]
- 17.Kaufman DJ, Murphy-Bollinger J, Scott J, et al. Public opinion about the importance of privacy in biobank research. Am J Hum Genet 2009;85:643–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ipsos MORI Social Research Institute. 2010. Human Tissue Authority General Public Survey. http://www.hta.gov.uk/_db/_documents/2007–09–10_Ipsos_MORI_general_public_qual_and_quant_report_final_200709101952.pdf (accessed 26 Feb 2013)
- 19.Chen DT, Rosenstein DL, Muthappan P, et al. Research with stored biological samples: what do research participants want? Arch Intern Med 2005;165:652–5 [DOI] [PubMed] [Google Scholar]
- 20.Jack AL, Womack C. Why surgical patients do not donate tissue for commercial research: review of records. BMJ 2003;327:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant RJ, Harrison RF, Start RD, et al. Ownership and uses of human tissue: what are the opinions of surgical in-patients? J Clin Pathol 2008;61:322–6 [DOI] [PubMed] [Google Scholar]
- 22.Start RD, Brown W, Bryant RJ, et al. Ownership and uses of human tissue: does the Nuffield bioethics report accord with opinion of surgical inpatients? BMJ 1996;313:1366–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler J, Agarwal M, Sugden J, et al. Experiences from the front-line routine consenting of surplus surgically removed tissue: without investment by the National Health Service fully informed consent for all is not available. J Clin Pathol 2007;60:351–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeulen E, Schmidt MK, Aaronson NK, et al. A trial of consent procedures for future research with clinically derived biological samples. Br J Cancer 2009;101:1505–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamajima N, Tajima K, Oya H, et al. Patients’ views on residual blood use for research purposes. Jpn J Cancer Res 1998;89: 341–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeyer K, Olofsson BO, Mjorndal T, et al. Informed consent and biobanks: a population-based study of attitudes towards tissue donation for genetic research. Scand J Public Health 2004;32:224–9 [DOI] [PubMed] [Google Scholar]
- 27.Wang SS, Fridinger F, Sheedy KM, et al. Public attitudes regarding the donation and storage of blood specimens for genetic research. Community Genet 2001;4:18–26 [DOI] [PubMed] [Google Scholar]
- 28.Moorman PG, Skinner CS, Evans JP, et al. Racial differences in enrolment in a cancer genetics registry. Cancer Epidemiol Biomarkers Prev 2004;13:1349–54 [PubMed] [Google Scholar]
- 29.McQuillan GM, Porter KS, Agelli M, et al. Consent for genetic research in a general population: the NHANES experience. Genet Med 2003;5:35–42 [DOI] [PubMed] [Google Scholar]
- 30.Lee CI, Bassett LW, Leng M, et al. Patients’ willingness to participate in a breast cancer biobank at screening mammogram. Breast Cancer Res Treat 2012;136:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Dai H, Wang L, et al. Consent for use of clinical leftover biosample: a survey among Chinese patients and the general public. PLoS ONE 2012;7:e36050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkman M. Egg and embryo donation and the meaning of motherhood. Women Health 2003;38:1–18 [DOI] [PubMed] [Google Scholar]
- 33.Millar T, Walker R, Arango JC, et al. Tissue and organ donation for research in forensic pathology: the MRC Sudden Death Brain and Tissue Bank. J Pathol 2007;213:369–75 [DOI] [PubMed] [Google Scholar]
- 34.Nuffield Council on Bioethics. 2011. Human bodies: donations for medicine and research. http://www.nuffieldbioethics.org/sites/default/files/Donation_full_report.pdf (accessed 26 Feb 2013)
- 35.Womack C, Jack AL. Family attitudes to research using samples taken at coroner's postmortem examinations: review of records. BMJ 2003;327:781–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. http://blog.cookcountygov.com/2012/09/12/stroger-hospital-of-cook-county-hosts-komen-cure-tissue-bank-event-september-29th/. (accessed 27 Feb 2013) [Google Scholar]
- 37.Jones DA. What does the British public think about human-animal hybrid embryos? J Med Ethics 2009;35:168–70 [DOI] [PubMed] [Google Scholar]
- 38.Hobson-West P. The role of ‘public opinion’ in the UK animal research debate. J Med Ethics 2010;36:46–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringach DL. The use of nonhuman animals in biomedical research. Am J Med Sci 2011;342:305–13 [DOI] [PubMed] [Google Scholar]
- 40.Abou-Zeid A, Silverman H, Shehata M, et al. Collection, storage and use of blood samples for future research: views of Egyptian patients expressed in a cross-sectional survey. J Med Ethics 2010;36:539–47 [DOI] [PubMed] [Google Scholar]
- 41.Capron AM, Mauron A, Elger BS, et al. Ethical norms and the international governance of genetic databases and biobanks: findings from an international study. Kennedy Inst Ethics J 2009;19:101–24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


