Abstract
The purpose of this review paper is to update the current and potential future role of probiotics for Clostridium difficile-associated disease (CDAD). Included in this review, is an update on the testing of newer probiotics (e.g., Bacillus coagulans GBI-30, 6086) in animal models of CDAD. There is a focus on the modulation of signal transduction pathways (i.e., transcription factors like cAMP response element-binding, activator protein 1, and nuclear factor kappa B), as well as the inhibition of certain kinases (e.g., p38 mitogen activated protein kinases) by probiotics. Inhibition of signal transduction by probiotics, such as Saccharomyces boulardii, result in multiple effects on intestinal fluid secretion, neutrophil influx into the colon, inflammation, and colonocyte apoptosis that may positively impact CDAD. Recent clinical approaches with probiotics, for the prevention of primary and recurrent CDAD, are also summarized in this review paper. Future directions for the treatment of CDAD by probiotics are also mentioned in this review. In particular, the use of multi-strain probiotic formulations such as Ecologic® AAD and VSL #3® may represent a rationale pharmacological approach, particularly as adjunctive therapies for CDAD. Understanding the mechanistic basis of CDAD, and how probiotics interfere at ceratin steps in the pathogenic process, may also present the opportunity to design other multi-strain probiotics that could have a future impact on CDAD.
Keywords: Clostridium difficile, Colitis, Probiotics, Mechanisms of action, Immune modulation, Transcription factors, Saccharomyces boulardi, VSL#3
Core tip: Certain probiotics can inhibit signal transduction pathways (i.e., transcription factors like cAMP response element-binding, activator protein 1, and nuclear factor kappa B), as well as attenuate the activation of ceratin certain kinases (e.g., p38 mitogen activated protein kinases). Inhibition of these Intracellular signaling pathways by probiotics results in effects on intestinal fluid secretion, neutrophil influx into the colon, inflammation and colonocyte apoptosis that may positively impact Clostridium difficile-associated disease (CDAD). Understanding the mechanistic basis of CDAD, and how probiotics interfere at certain steps in the pathogenic process, may allow the development of novel probiotics that could have a future pharmacological impact on CDAD.
INTRODUCTION
Clostridium difficile (C. difficile) infection can cause nosocomial-related diarrhea and other distinct disease characteristics, which can affect the structural integrity of the intestine[1,2]. The spectrum of C. difficile-associated disease (CDAD) ranges from mild antibiotic associated diarrhea to severe pseudomembranous colitis that can lead to mortality[1,2]. CDAD is caused by the actions of two exotoxins (toxin A and toxin B), which are produced by various pathogenic strains of C. difficile[2,3].
CDAD is often treated successfully with standard antibiotics such as vancomycin and metronidazole[4-6]. However, recurrence occurs in many patients[4-6]. Some clinical studies have focused on combined treatment with vancomycin and probiotics such as Saccharomyces boulardii for the treatment of recurrence[7-10]. Therefore, initial treatment regimens with probiotics, or their use for prevention of recurrent disease, may be attractive as part of the overall therapeutic strategy for CDAD[11-13].
Probiotics are live microorganisms that when ingested can confer health benefits[14]. Typically, probiotics include various strains of Lactobacillus and/or Bifidobacteria species. They exist as either single entities, or as combination products (e.g., VSL#3)[15,16]. Other known probiotics include certain non-pathogenic Escherichia coli strains like Nissle 1917 and M-17[14,17].
Overall, the pertinent mechanisms explaining the potential role of probiotics as anti-colitis therapies have been reviewed in detail elsewhere[15,18-20]. The purpose of this review paper is to provide an update on the current and potential future role of probiotics for CDAD. Included in this review will be an update on the recent testing of some probiotics in animal models of CDAD, as well as how certain probiotics can modulate signal transduction pathways.
MECHANISMS OF ACTION FOR PROBIOTICS
Focus on modulation of signal transduction (immunomodulation)
In an excellent review paper, Hell et al[19] cited potential mechanisms by which probiotics could prevent or reverse CDAD. These mechanisms included: (1) competitive exclusion; (2) bacterial metabolic activity; (3) preservation of gut-barrier function; (4) influence on water and ion channels; (5) influence on the innate nervous system; (6) modulation of signal transduction; (7) stimulation of the innate immune system; and (8) induction of adaptive immunity[19]. Specific details on these mechanisms are provided elsewhere in the relevant literature[18,19].
In this review, I will focus on the modulation of signal transduction pathways (i.e., immunomodulation) by probiotics, as related to CDAD[19,21,22]. As shown in Figure 1, endogenous colonic epithelial cells (colonocytes) seem to play an integral role in CDAD[23-25]. However, cells of the innante immune system (macrophages, neutrophils) also play a role in the etiology of CDAD[26,27]. In these cellular populations within the intestine, C. difficile associated toxins (particularly toxin A) result in the activation of three transcription factors (Figure 1). Nuclear factor-kappa B (NF-κB) is involved in chemokine production, and also plays a role in colonocyte apoptosis[23,28]. Activator protein-1 (AP-1) also plays a role in interleukin (IL)-8 production, in response to stimulation of colonocytes with toxin A[24]. Cyclic-AMP response binding protein (CREB) is critical for the production of Prostaglandin E2[23]. This prostaglandin plays an important role in the fluid secretion/diarrhea associated with CDAD (Figure 1). As shown in the figure, there is also cross talk between the various pathways. For example, prostaglandin E2 can stimulate Fas ligand expression and apoptosis in colonic epithelial cells[28,29].
Figure 1.
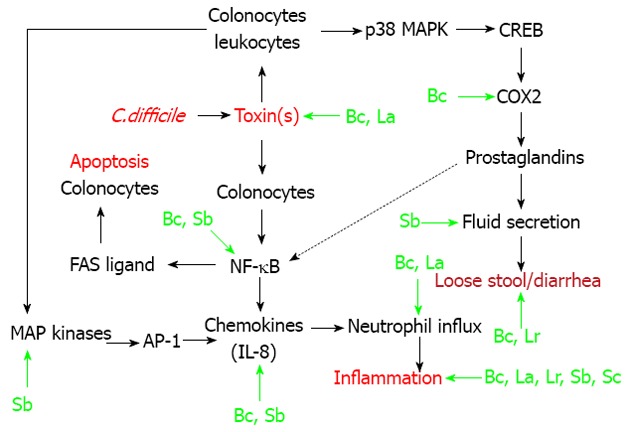
Immunomodulation by probiotics for Clostridium difficile-associated disease. Clostridium difficile (C. difficile) associated toxins (red font) engage colonic epithelial cells (colonocytes) leading to nuclear factor-kappa B (NF-κB) activation, interleukin (IL)-8 production, neutrophil influx and inflammation. These toxins also bind to receptors on colonocytes and leukocytes leading to p38 mitogen activated protein kinases (p38 MAPK) and cyclic-AMP response binding protein (CREB) activation. CREB, through cyclooxygenase 2 (COX2), is critical for the production of prostaglandin E2. In turn, this prostaglandin plays an important role in the fluid secretion/diarrhea associated with CDAD. C. difficile associated toxins also lead to the activation of other MAP kinases (ERK 1/2) and activator protein-1 (AP-1), which also plays a role in IL-8 production. There is also cross talk (dotted line) between the various pathways. For example, prostaglandin E2 can stimulate Fas ligand expression and apoptosis in colonic epithelial cells. The green arrows in this figure represent specific points of intervention by certain probiotics, resulting in immunomodulation by these agents. The abbreviations indicate the specific probiotics, which can modulate these signal transduction pathways. These probiotics (green font) include: Saccharomyces boulardii (Sb); Bacillus coagulans GBI-30, 6086 (Bc); Lactobacillus acidophilus (La); Lactobacillus rhamnosus (Lr); and Saccharomyces cerevisiae, strain 905 (Sc).
Specific points of intervention, resulting in immunomodulation by certain probiotics, are shown in Figure 1[21,30-39]. The non-pathogenic yeast probiotic, Saccharomyces boulardii has the most well described immunomodulatory actions[21]. Saccharomyces boulardii can inhibit toxin-A receptor binding to target cells, by release of a protease that digests both the exotoxin and its receptor binding sites[21,31,32]. Indirectly, this prevents the downstream activation of relevant MAP kinases, as well as transcription factor activation by toxin A (Figure 1). The same group of investigators showed that Saccharomyces boulardii supernatants could inhibit (in vitro or in vivo) toxin A-induced MAP kinase (ERK 1/2) activation, IL-8 production, fluid secretion, and intestinal inflammation[21,30]. Saccharomyces boulardii also reportedly inhibits activation of the key transcription factor NF-κB[21].
Bacillus coagulans GBI-30, 6086 (Bc) is a novel probiotic, which can attenuate chemokine release both in vitro and in vivo[34,35]. Correspondingly, this probiotic reduced neutrophil influx and colonic inflammation associated with CDAD in mice[34,35]. Of note, Bacillus coagulans GBI-30 reduced the expression (by immunohistochemistry) of COX-2 in the colons of mice with CDAD (Figure 1)[34,35].
Lactobacillus acidophilus (L. acidophilus) substantially improved cyclosporine-induced C. difficile infection in mice[36,39]. Various parameters of infectious colitis were attenuate by probiotioc treatment, including myeloperoxidase and histopathology, as well as titers of toxins A and B derived from the cecal contents of mice (Figure 1)[36,39]. Another lactobacillus species, Lactobacillus rhamnosus (L. rhamnosus) improved C. difficile-induced inflammation and damage to the ileum of hamsters, with less evidence of diarrhea[37].
Martins et al[38] developed a screening paradigm for yeast probiotic strains, based upon protection against enteric pathogens including C. difficile. These investigators found that Saccharomyces cerevisiae, strain 905 protected the cecum of gnotobiotic mice from C. difficile-induced pathological changes in the cecum (Figure 1)[38].
PROBIOTICS AND PRECLINICAL MODELS OF CDAD
Table 1 shows a list of some probiotics that were tested in pre-clinical models of C. difficile-induced colitis. Early studies, which were conducted approximately 30 years ago, showed that Saccharomyces boulardii could prevent Clindamycin (and by association C. difficile)-induced mortality in hamsters, with improvement in the histological appearance of the intestine in these animals[40,41]. In the same time period, Corthier et al[42] found that Saccharomyces boulardii could limit mortality in gnotobiotic mice that were infected with C. difficile. Of note, this probiotic also modulated fecal cytotoxin production (Figure 1)[42].
Table 1.
Effects of probiotics in animal models of Clostridium difficile-induced colitis
| Probiotic | Species | Efficacy | Reference |
| Saccharomyces boulardii | Hamster | Yes | [40,41] |
| Saccharomyces boulardii | Mice | Yes | [42] |
| Saccharomyces cerevisiae 905 | Mice | Yes | [38] |
| Lactobacillus rhamnosus | Hamster | Yes | [37] |
| Lactobacillus acidophilus | Mice | Yes | [36,39] |
| Bacillus coagulans GBI-30, 6086 | Mice | Yes | [34,35] |
More recent studies, showed that Saccharomyces cerevisiae strain 905 and two lactobacillus strains (L. rhamnosus and acidophilus) were effective against CDAD in rodents (Figure 1 and Table 1)[36-39]. My laboratory found that the novel probiotic strain Bacillus coagulans GBI-30, 6086 could improve both the initial phase of colitis in mice following C. difficile infection, as well the recurrence of CDAD following vancomycin withdrawal[34,35]. This probiotic most profoundly affected the stool consistency in these mice (Figure 1)[34,35].
CLINICAL USE OF PROBIOTICS FOR CDAD
Since 2011, several comprehensive reviews have been published regarding the use of probiotics for CDAD. Specific details from these reviews can be found in the relevant literature[19,43-46]. Floch et al[43] gave probiotics a B/C recommendation for both the prevention of CDAD, and also the prevention of recurrent CDAD. Their somewhat arbitrary rating system suggested some positive clinical studies, but also the presence of some negative studies (B rating), or inadequate clinical experience (C rating). In their evaluations, the investigators focused mainly on studies involving Saccharomyces boulardii and Lactobacillus GG[43]. In another review, Hickson[44] suggested that the evidence supporting the use of probiotics for CDAD is overall equivocal. Musgrave et al[45] reported that probiotics could be considered for the prevention of C. difficile infection, or as an adjunctive therapy in otherwise healthy (non-immunocompromised) patients. Davidson et al[46] suggested the possible co-administration of probiotics for prevention of CDAD in patients at increased risk for developing disease. However, they did not recommend adjunctive probiotics for the routine treatment of CDAD[46]. The most recent Cochrane review (from 2008) on probiotics for CDAD in adults concluded that insufficient evidence existed to recommended probiotics as an adjunct to antibiotic therapy for C. difficile colitis[47]. Moreover, reportedly there was no evidence to support the use of probiotics alone for C. difficile colitis[47].
FUTURE USE OF PROBIOTICS FOR CDAD
In a review article published in 2009, Imhoff et al[13] asked this question: Is there a future for probiotics in preventing clostridium difficile-associated disease and treatment of recurrent episodes? This statement remains a pertinent question in 2013. Recently, there has been a renewed interest in fecal microbiota transplantation therapy for recurrent CDAD, and new studies suggest efficacy for this indication[48-52]. Therefore, what about the future use of probiotics in CDAD beyond 2013? Because CDAD is a condition associated with disrupted endogenous gut flora, it is logical to employ treatment strategies that can reconstitute/restore the physiological intestinal flora. In a broad sense, both probiotics and fecal microbiota transplantation therapy attempt to accomplish this restoration of physiological bacterial species, but by different administration methods[13,53,54]. Certainly, fecal transplantation has yielded some interesting efficacy results[48-54]. However, it typically requires an invasive procedure (e.g., colonoscopy), as well as an overall technique that is still aesthetically displeasing to some patients[48-54]. In contrast, probiotics can be easily ingested, but are often not optimally formulated to survive transit through the GI tract for colonization in the colon[13,19]. Moreover, probiotics have demonstrated questionable efficacy for CDAD[43-47]. A recent publication further compares the pros and cons of probiotics versus fecal transplantation for intestinal diseases[55].
With respect to the future of probiotics for CDAD, Hell et al[19] have provided some good insights, as well as interesting initial clinical data. They postulated that a multi-strain probiotic, resembling a healthy human microbiotia, would be most effective for treating CDAD[19]. Therefore, these investigators developed a probiotic mixture (Ecologic® AAD) comprised of several Bifodabacterium and Lactobacillus strains, as well as Enterococcus faecum[19]. In a small series of 10 patients (five with recurrent disease) excellent results were obtained in all evaluable patients with C. difficile infection, following combined treatment with the multi-strain probiotic plus vancomycin[19]. This type of therapeutic paradigm seems to represent a logical future scientific approach for probiotic treatment in CDAD. Another probiotic preparation that could be tested for CDAD is VSL#3. This probiotic mixture contains 4 Lactobacillus strains, three strains of bifidobacteria and Streptococcus salivarus[56,57]. VSL#3 has been tested previously in both IBD and pouchitis patient populations, with some evidence of efficacy[56,57]. Moreover, the pertinent mechanism(s) of action for VSL#3 suggest that it would represent a rational pharmacological approach for CDAD[14,58]. Finally, it may be possible to utilize known mechanism of action diagrams, like in Figure 1, to create novel probiotic mixtures that could potentially be effective for CDAD.
While perhaps focusing on multi-strain probiotics, newer single strain probiotics of potential interest for CDAD could include Bifidobacterium animalis AHCT[59-61]. This probiotic can inhibit NF-κB activation, reduce C. difficile levels in the canine colon, and resolve idiopathic diarrhea in dogs. The pharmacological profile of Bifidobacterium animalis AHCT suggests that it could be an interesting candidate for further testing related to CDAD. Another single strain probiotic of interest is Clostridium butyricium MIYARI 588, which is being used for the prevention of CDAD in Japan[62].
Footnotes
P- Reviewers Hokama A, Schwarz SM S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Johnson S, Gerding DN. Clostridium difficile-associated diarrhea. Clin Infect Dis. 1998;26:1027–1034; quiz 1035-1036. doi: 10.1086/520276. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 3.Pothoulakis C, Lamont JT. Microbes and microbial toxins: paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280:G178–G183. doi: 10.1152/ajpgi.2001.280.2.G178. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett JG. Treatment of antibiotic-associated pseudomembranous colitis. Rev Infect Dis. 1984;6 Suppl 1:S235–S241. doi: 10.1093/clinids/6.supplement_1.s235. [DOI] [PubMed] [Google Scholar]
- 5.Leffler DA, Lamont JT. Treatment of Clostridium difficile-associated disease. Gastroenterology. 2009;136:1899–1912. doi: 10.1053/j.gastro.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 6.Martinez FJ, Leffler DA, Kelly CP. Clostridium difficile outbreaks: prevention and treatment strategies. Risk Manag Healthc Policy. 2012;5:55–64. doi: 10.2147/RMHP.S13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surawicz CM, McFarland LV, Elmer G, Chinn J. Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Am J Gastroenterol. 1989;84:1285–1287. [PubMed] [Google Scholar]
- 8.McFarland LV, Surawicz CM, Greenberg RN, Fekety R, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL, Noorani Z. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–1918. [PubMed] [Google Scholar]
- 9.Surawicz CM, McFarland LV, Greenberg RN, Rubin M, Fekety R, Mulligan ME, Garcia RJ, Brandmarker S, Bowen K, Borjal D, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31:1012–1017. doi: 10.1086/318130. [DOI] [PubMed] [Google Scholar]
- 10.Kimmey MB, Elmer GW, Surawicz CM, McFarland LV. Prevention of further recurrences of Clostridium difficile colitis with Saccharomyces boulardii. Dig Dis Sci. 1990;35:897–901. doi: 10.1007/BF01536805. [DOI] [PubMed] [Google Scholar]
- 11.Tung JM, Dolovich LR, Lee CH. Prevention of Clostridium difficile infection with Saccharomyces boulardii: a systematic review. Can J Gastroenterol. 2009;23:817–821. doi: 10.1155/2009/915847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, Guyatt GH. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878–888. doi: 10.7326/0003-4819-157-12-201212180-00563. [DOI] [PubMed] [Google Scholar]
- 13.Imhoff A, Karpa K. Is there a future for probiotics in preventing Clostridium difficile-associated disease and treatment of recurrent episodes? Nutr Clin Pract. 2009;24:15–32. doi: 10.1177/0884533608329232. [DOI] [PubMed] [Google Scholar]
- 14.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Fedorak RN, Madsen KL. Probiotics and prebiotics in gastrointestinal disorders. Curr Opin Gastroenterol. 2004;20:146–155. doi: 10.1097/00001574-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Shanahan F. Physiological basis for novel drug therapies used to treat the inflammatory bowel diseases I. Pathophysiological basis and prospects for probiotic therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G417–G421. doi: 10.1152/ajpgi.00421.2004. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick LR, Small J, Hoerr RA, Bostwick EF, Maines L, Koltun WA. In vitro and in vivo effects of the probiotic Escherichia coli strain M-17: immunomodulation and attenuation of murine colitis. Br J Nutr. 2008;100:530–541. doi: 10.1017/S0007114508930373. [DOI] [PubMed] [Google Scholar]
- 18.Parkes GC, Sanderson JD, Whelan K. The mechanisms and efficacy of probiotics in the prevention of Clostridium difficile-associated diarrhoea. Lancet Infect Dis. 2009;9:237–244. doi: 10.1016/S1473-3099(09)70059-3. [DOI] [PubMed] [Google Scholar]
- 19.Hell M, Bernhofer C, Stalzer P, Kern JM, Claassen E. Probiotics in Clostridium difficile infection: reviewing the need for a multistrain probiotic. Benef Microbes. 2013;4:39–51. doi: 10.3920/BM2012.0049. [DOI] [PubMed] [Google Scholar]
- 20.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 21.Pothoulakis C. Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol Ther. 2009;30:826–833. doi: 10.1111/j.1365-2036.2009.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigón G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol. 2007;14:485–492. doi: 10.1128/CVI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Rhee SH, Kokkotou E, Na X, Savidge T, Moyer MP, Pothoulakis C, LaMont JT. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J Biol Chem. 2005;280:21237–21245. doi: 10.1074/jbc.M413842200. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Park HR, Oh YK, Kim YJ, Youn J, Han JS, Kim JM. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J Mol Med (Berl) 2007;85:1393–1404. doi: 10.1007/s00109-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 25.Lica M, Schulz F, Schelle I, May M, Just I, Genth H. Difference in the biological effects of Clostridium difficile toxin B in proliferating and non-proliferating cells. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:275–283. doi: 10.1007/s00210-010-0595-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Alcantara C, Stenson WF, Steiner TS, Guerrant RL. Role of inducible cyclooxygenase and prostaglandins in Clostridium difficile toxin A-induced secretion and inflammation in an animal model. J Infect Dis. 2001;184:648–652. doi: 10.1086/322799. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Rhee SH, Pothoulakis C, Lamont JT. Inflammation and apoptosis in Clostridium difficile enteritis is mediated by PGE2 up-regulation of Fas ligand. Gastroenterology. 2007;133:875–886. doi: 10.1053/j.gastro.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 29.O’Callaghan G, Kelly J, Shanahan F, Houston A. Prostaglandin E2 stimulates Fas ligand expression via the EP1 receptor in colon cancer cells. Br J Cancer. 2008;99:502–512. doi: 10.1038/sj.bjc.6604490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S, O’Brien M, Pothoulakis C, Kelly CP. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem. 2006;281:24449–24454. doi: 10.1074/jbc.M605200200. [DOI] [PubMed] [Google Scholar]
- 31.Pothoulakis C, Kelly CP, Joshi MA, Gao N, O’Keane CJ, Castagliuolo I, Lamont JT. Saccharomyces boulardii inhibits Clostridium difficile toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 32.Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64:5225–5232. doi: 10.1128/iai.64.12.5225-5232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen GS, Benson KF, Carter SG, Endres JR. GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 2010;11:15. doi: 10.1186/1471-2172-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzpatrick LR, Small JS, Greene WH, Karpa KD, Keller D. Bacillus Coagulans GBI-30 (BC30) improves indices of Clostridium difficile-Induced colitis in mice. Gut Pathog. 2011;3:16. doi: 10.1186/1757-4749-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzpatrick LR, Small JS, Greene WH, Karpa KD, Farmer S, Keller D. Bacillus coagulans GBI-30, 6086 limits the recurrence of Clostridium difficile-Induced colitis following vancomycin withdrawal in mice. Gut Pathog. 2012;4:13. doi: 10.1186/1757-4749-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaur S, Vaishnavi C, Ray P, Kochhar R, Prasad KK. Effect of biotherapeutics on cyclosporin-induced Clostridium difficile infection in mice. J Gastroenterol Hepatol. 2010;25:832–838. doi: 10.1111/j.1440-1746.2009.06135.x. [DOI] [PubMed] [Google Scholar]
- 37.Naaber P, Mikelsaar RH, Salminen S, Mikelsaar M. Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection. J Med Microbiol. 1998;47:591–598. doi: 10.1099/00222615-47-7-591. [DOI] [PubMed] [Google Scholar]
- 38.Martins FS, Nardi RM, Arantes RM, Rosa CA, Neves MJ, Nicoli JR. Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol. 2005;51:83–92. doi: 10.2323/jgam.51.83. [DOI] [PubMed] [Google Scholar]
- 39.Kaur S, Vaishnavi C, Prasad KK, Ray P, Kochhar R. Effect of Lactobacillus acidophilus & amp; epidermal growth factor on experimentally induced Clostridium difficile infection. Indian J Med Res. 2011;133:434–441. [PMC free article] [PubMed] [Google Scholar]
- 40.Massot J, Sanchez O, Couchy R, Astoin J, Parodi AL. Bacterio-pharmacological activity of Saccharomyces boulardii in clindamycin-induced colitis in the hamster. Arzneimittelforschung. 1984;34:794–797. [PubMed] [Google Scholar]
- 41.Toothaker RD, Elmer GW. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob Agents Chemother. 1984;26:552–556. doi: 10.1128/aac.26.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corthier G, Dubos F, Ducluzeau R. Prevention of Clostridium difficile induced mortality in gnotobiotic mice by Saccharomyces boulardii. Can J Microbiol. 1986;32:894–896. doi: 10.1139/m86-164. [DOI] [PubMed] [Google Scholar]
- 43.Floch MH, Walker WA, Madsen K, Sanders ME, Macfarlane GT, Flint HJ, Dieleman LA, Ringel Y, Guandalini S, Kelly CP, et al. Recommendations for probiotic use-2011 update. J Clin Gastroenterol. 2011;45 Suppl:S168–S171. doi: 10.1097/MCG.0b013e318230928b. [DOI] [PubMed] [Google Scholar]
- 44.Hickson M. Probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Therap Adv Gastroenterol. 2011;4:185–197. doi: 10.1177/1756283X11399115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musgrave CR, Bookstaver PB, Sutton SS, Miller AD. Use of alternative or adjuvant pharmacologic treatment strategies in the prevention and treatment of Clostridium difficile infection. Int J Infect Dis. 2011;15:e438–e448. doi: 10.1016/j.ijid.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Davidson LE, Hibber PL. Clostridium difficile and probiotics. Up to date 2013, published online, Wolters Kluwer, Alphen aan den Rijn, the Netherlands. Available from: http://www.uptodate.com/contents/clostridium-difficile-and-probiotics.
- 47.Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;23:CD004611. doi: 10.1002/14651858.CD004611.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly CP. Fecal microbiota transplantation--an old therapy comes of age. N Engl J Med. 2013;368:474–475. doi: 10.1056/NEJMe1214816. [DOI] [PubMed] [Google Scholar]
- 49.Suwantarat N, Bobak DA. Fecal Bacteriotherapy for Recurrent Clostridium difficile Infection: What’s Old Is New Again? Curr Infect Dis Rep. 2013;15:101–103. doi: 10.1007/s11908-013-0314-8. [DOI] [PubMed] [Google Scholar]
- 50.Zucca M, Scutera S, Savoia D. Novel avenues for Clostridium difficile infection drug discovery. Expert Opin Drug Discov. 2013;8:459–477. doi: 10.1517/17460441.2013.770466. [DOI] [PubMed] [Google Scholar]
- 51.Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med. 2013;80:101–108. doi: 10.3949/ccjm.80a.12110. [DOI] [PubMed] [Google Scholar]
- 52.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 53.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–149. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 54.Kleger A, Schnell J, Essig A, Wagner M, Bommer M, Seufferlein T, Härter G. Fecal transplant in refractory Clostridium difficile colitis. Dtsch Arztebl Int. 2013;110:108–115. doi: 10.3238/arztebl.2013.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruis W. Specific probiotics or ‘fecal transplantation’. Dig Dis. 2012;30 Suppl 3:81–84. doi: 10.1159/000342611. [DOI] [PubMed] [Google Scholar]
- 56.Chapman TM, Plosker GL, Figgitt DP. VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases. Drugs. 2006;66:1371–1387. doi: 10.2165/00003495-200666100-00006. [DOI] [PubMed] [Google Scholar]
- 57.Chapman TM, Plosker GL, Figgitt DP. Spotlight on VSL#3 probiotic mixture in chronic inflammatory bowel diseases. BioDrugs. 2007;21:61–63. doi: 10.2165/00063030-200721010-00007. [DOI] [PubMed] [Google Scholar]
- 58.Petrof EO. Probiotics and Gastrointestinal Disease: Clinical Evidence and Basic Science. Antiinflamm Antiallergy Agents Med Chem. 2009;8:260–269. doi: 10.2174/187152309789151977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Mahony D, Murphy S, Boileau T, Park J, O’Brien F, Groeger D, Konieczna P, Ziegler M, Scully P, Shanahan F, et al. Bifidobacterium animalis AHC7 protects against pathogen-induced NF-κB activation in vivo. BMC Immunol. 2010;11:63. doi: 10.1186/1471-2172-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelley RL, Park JS, O’Mahony L, Minikhiem D, Fix A. Safety and tolerance of dietary supplementation with a canine-derived probiotic (Bifidobacterium animalis strain AHC7) fed to growing dogs. Vet Ther. 2010;11:E1–14. [PubMed] [Google Scholar]
- 61.O’Mahony D, Murphy KB, MacSharry J, Boileau T, Sunvold G, Reinhart G, Kiely B, Shanahan F, O’Mahony L. Portrait of a canine probiotic Bifidobacterium--from gut to gut. Vet Microbiol. 2009;139:106–112. doi: 10.1016/j.vetmic.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Woo TD, Oka K, Takahashi M, Hojo F, Osaki T, Hanawa T, Kurata S, Yonezawa H, Kamiya S. Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J Med Microbiol. 2011;60:1617–1625. doi: 10.1099/jmm.0.033423-0. [DOI] [PubMed] [Google Scholar]


