Abstract
Dasatinib is a second-line tyrosine kinase inhibitor used in patients with imatinib resistant or intolerant chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute leukemia. Gastrointestinal bleeding may occur in up to 7% of patients using dasatinib, although, severe dasatinib-related acute colitis had rarely been reported. Here, we present the case of a 36-year-old female who progressed to acute myeloid leukemia after fourteen months of receiving imatinib for CML in the chronic phase and was treated with a dasatinib-containing chemotherapy regimen. On day 34 of treatment, the patient developed moderate abdominal pain and bloody diarrhea with mucous. Analyses of stool specimens were negative for parasites, Clostridium difficile, and other pathogenic bacteria. The cytomegalovirus pp65 antigen was negative in her blood leukocytes. A colonoscopy revealed acute colitis, and a mucosal biopsy showed nonspecific colitis. The patient was treated with broad-spectrum antibiotics, bowel rest and hydration, and dasatinib treatment was stopped. Her bloody diarrhea improved within 72 h. After confirming cytological remission, the patient received initial course of consolidation, and dasatinib treatment was reinstated. However, hemorrhagic colitis recurred. After discontinuing dasatinib, herhemorrhagic colitis drastically improved and did not recur following the administration of nilotinib. The characteristics of our patient suggest that dasatinib treatment can lead to hemorrhagic colitis, which typically resolves after discontinuation of the drug.
Keywords: Philadelphia chromosome, Chronic myeloid leukemia, Dasatinib, Colitis
Core tip: Dasatinib is a second-line tyrosine kinase inhibitor used in imatinib resistant or intolerant chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute leukemia patients. Dasatinib, which binds to the active and inactive conformation of the BCR-ABL oncoprotein, demonstrates greater potency than imatinib for wild-type and mutant BCR-ABL cases, with the exception of the T315I mutation. The most frequent adverse effects include myelosuppression, diarrhea, nausea and peripheral edema. Severe dasatinib-relatedacute colitis without thrombocytopenia, coagulation abnormalities or colonic ulcers has rarely been reported. Here, we report the case of an adult patient with Philadelphia chromosome positive CML in the blastic phase who developed acute colitis after dasatinib use.
INTRODUCTION
Dasatinib, an oral inhibitor of ABL and SRC family tyrosine kinases, is an effective drug for patients with Philadelphia chromosome positive (Ph+) leukemia, especially for those who develop resistance or who are intolerant to imatinib[1]. Mild to moderate thrombocytopenia and neutropenia occurred in approximately 50% of patients, but these conditions are generally well tolerated. Other side effects include diarrhea, headache, weakness, pleural effusion, nausea and peripheral edema. In addition, gastrointestinal (GI) bleeding may occur in up to 7% of patients using dasatinib[2], although severe dasatinib-related hemorrhagic colitis without thrombocytopenia, coagulation abnormalities or colonic, ulcer has been rarely reported. Here, we report the case of an adult patient with Ph+ chronic myeloid leukemia (CML) in the blastic phase who suffered from acute colitis after dasatinib use.
CASE REPORT
A 36-year-old female, who has been treated with fourteen months imatinib for CML in the chronic phase, progressed to acute myeloid leukemia. The patient was given a course of systemic chemotherapy according to the protocol for AML, consisting of rubidomycin (45 mg/m2 daily for 3 d), cytosine arabinoside (200 mg/m2 continuous infusion for seven days) and dasatinib (140 mg once a day). After the end of chemotherapy, dasatinib was continued as maintenance therapy. On day 34 of treatment, the patient developed moderate abdominal pain and bloody diarrhea with mucous (4-6 bowel movements a day). Physical examination revealed the absence of fever and mild abdominal tenderness upon palpation. The laboratory results were as follows: hemoglobin 100 g/L, white blood cells 4 × 109/L with an absolute neutrophil count of 1.5 × 109/L, platelets 185 × 109/L, prothrombin time 15 s, active partial thromboplastin time 33 s and an international normalized ratio of 1.3. The analyses of stool specimens were negative for parasites, Clostridium difficile, and other pathogenic bacteria. The cytomegalovirus pp65 antigen was negative in her blood leukocytes. An abdominal ultrasound showed the presence of uniform circumferential thickening of the transverse colon and splenic flexure with pericolic fat infiltration, indicating potential colitis. An abdominal computed tomography scan revealed bowel wall thickening up to 1 cm, involving the entire colon with infiltration of the mesenteric fat and a pelvic peritoneal effusion consistent with pan-colitis. A total colonoscopy revealed no active bleeding, but there were multiple millimetric, nodular, hyperemic lesions on the mucosa involving the entire colon (Figure 1). A mucosal biopsy showed nonspecific colitis with a well-preserved crypt structure and lymphocytic infiltration in the lamina propria (Figure 2). Infiltrative lymphocytes expressed a high proportion of CD3 and sparse of CD20. No viral inclusion or apoptotic bodies were observed. The patient was treated with broad-spectrum antibiotics, bowel rest and hydration, and dasatinib treatment was stopped. Improvement in the bloody diarrhea was evident after 72 h, and a control colonoscopy was performed ten days later and showed that the colonic mucosa was quite normal. After confirming the achievement of cytological remission (4% of medullary blasts), the patient received the first course of consolidation treatment (cytosine arabinoside + etoposide + rubidomycin), and dasatinib was reinstated. On day 6 of treatment, the patient again developed severe diarrhea with a large amount of intestinal hemorrhage, and a repeat colonoscopy was consistent with acute colitis (Figure 3). Again, dasatinib treatment was stopped, and the hemorrhagic colitis drastically improved. A repeat colonoscopy was normal after discontinuing dasatinib treatment. Nilotinib (800 mg/d) was started as an alternative to dasatinib for CML treatment, and the hemorrhagic colitis did not recur. After confirming cytogenetic and molecular remission, the patient received a bone marrow transplantation from an HLA-identical, intrafamilial donor. She is still alive and in complete remission.
Figure 1.
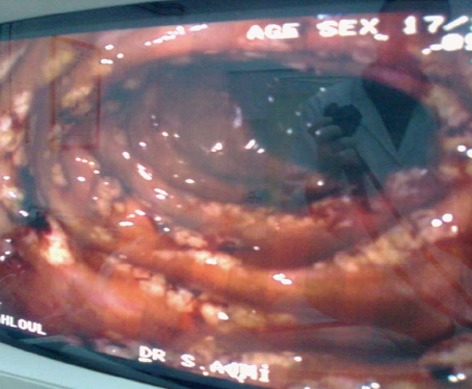
Colonoscopy showed multiple millimetric nodular hyperemic lesions on the mucosa.
Figure 2.
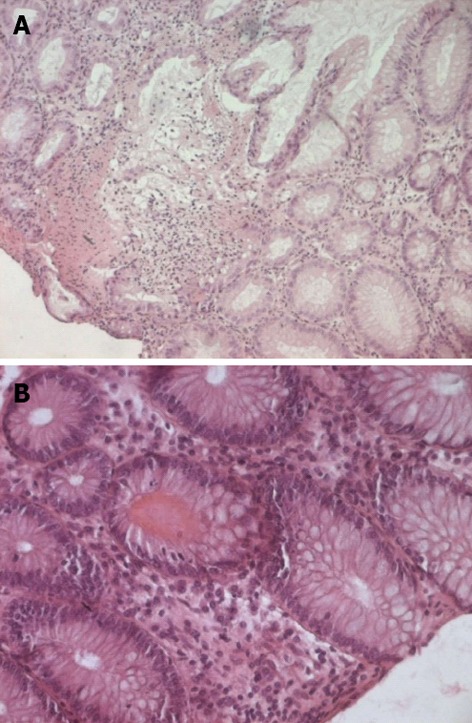
Mucosal biopsy of colon. A: A well-preserved crypt structure with lymphocytes infiltration in the lamina propria (original magnification × 100); B: Viral inclusions and apoptotic bodies were absent (original magnification × 200).
Figure 3.
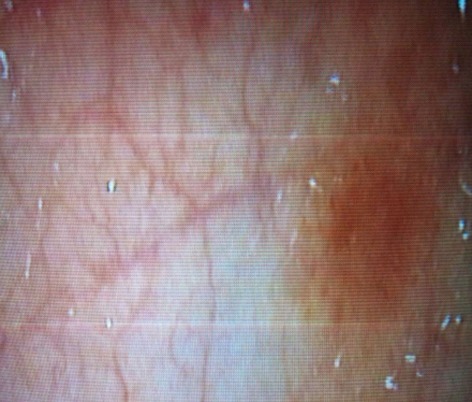
Macroscopic findings include erythema, edema and friability.
DISCUSSION
Diarrhea and nausea are generally observed in approximately about 30% and 20% of patients respectively, during dasatinib therapy[3]. GI bleeding may occur during dasatinib treatment, but is generally mild and easily handled. Data from 84 patients treated with dasatinib show that grade 3 or 4 GI bleeding occurred in approximately 6% of cases, although, detailed information on the mechanisms of action was not reported[4]. Herein, we reported the case of a patient with Ph+ CML in the blastic phase who had severe hemorrhagic colitis during dasatinib therapy. Acute colitis is a pathological phenomenon-characterized by the infiltration of inflammatory cells into the lamina propria. The pathogenesis of acute colitis is complex and many immune mediators and cells play roles in this poorly understood condition[5]. Moreover, the colitis induced by dasatinib may not be attributed simply to the tyrosine kinase inhibition because our patient did not experience similar GI symptoms during imatinib therapy. Although the precise mechanism is unknown, one possible explanations is that dasatinib may have a stronger inhibitory effect on tyrosine kinases and/or have some kinase-specific inhibitory effects on SRC-family kinases, BCR-ABL, c-KIT, EPHA2, and the PDGF receptor[6,7]. The SRC-family kinases are also expressed in normal B cells and are likely inhibited by dasatinib. Fei et al[8] showed that dasatinib inhibited the proliferation and function of T regulatory cells by decreasing the expression of box P3 transcription factor, 5-glucocorticoid induced tumor necrosis factor receptor, cytotoxic T-cell-associated protein 4 and inducing apoptosis in the G0/G1 phase of the cell cycle in T regulatory cells. Further-more, some studies have shown that dasatinib suppresses the function of natural killer cells and T cells by inhibiting SRC-family kinases[9,10]. Therefore, dasatinib may cause acute colitis by decreasing immune tolerance to intestinal microflora through reducing the number of immunoregulatory cells and inhibiting signal transduction pathways.
In conclusion, hemorrhagic colitis may develop during dasatinib treatment although this condition improves upon discontinuation of the drug. Additional studies with greater numbers of patients are necessary to elucidate the relationship between hemorrhagic colitis and dasatinib therapy.
Footnotes
P- Reviewer Quaresma J S- Editor Song XX L- Editor A E- Editor Li JY
References
- 1.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, de Souza CA, Lipton JH, Hochhaus A, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, Robak T, Khoroshko N, Masszi T, Skotnicki A, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 4.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 5.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 6.Piccaluga PP, Paolini S, Martinelli G. Tyrosine kinase inhibitors for the treatment of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Cancer. 2007;110:1178–1186. doi: 10.1002/cncr.22881. [DOI] [PubMed] [Google Scholar]
- 7.Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, Bokemeyer C, Deininger MW, Druker BJ, Heinrich MC. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 8.Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Götz M, Döhner H, Bunjes D, Schmitt M. Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol. 2009;144:195–205. doi: 10.1111/j.1365-2141.2008.07433.x. [DOI] [PubMed] [Google Scholar]
- 9.Blake SJ, Bruce Lyons A, Fraser CK, Hayball JD, Hughes TP. Dasatinib suppresses in vitro natural killer cell cytotoxicity. Blood. 2008;111:4415–4416. doi: 10.1182/blood-2008-02-138701. [DOI] [PubMed] [Google Scholar]
- 10.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111:1366–1377. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]


