SUMMARY
In 1942, when retinopathy of prematurity (ROP) first manifested as retrolental fibroplasia, the technology to monitor or regulate oxygen did not exist, and a fundus examination of preterm infants was not routinely performed. Supplemental, uncontrolled oxygen at birth has since been found to cause retrolental fibroplasia. At the same time, technological advances have made it possible to regulate oxygen and detect early forms of ROP. Nevertheless, despite our better understanding of ROP and ongoing investigations of supplemental therapeutic oxygen, including recent clinical trials (Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial [SUPPORT] and Benefits of Oxygen Saturation Targeting [BOOST]), the best oxygen profiles to reduce ROP risk while optimizing preterm infant health and development remain unknown. This article reviews major studies on oxygen use in preterm infants and the effects on the development of ROP.
Supplemental oxygen use to treat respiratory distress syndrome of prematurity and bronchopulmonary dysplasia has long been associated with retinopathy of prematurity (ROP). Following Terry’s description in 19421 of retrolental fibroplasia (RLF), now believed to have represented the worst stage of ROP, stage 5, studies were initiated to test the role of oxygen in ROP. Several studies, including a recent meta-analysis, provided evidence that maintaining lower saturations in infants of 24-28 weeks’ gestational age reduced the incidence of severe ROP as well as chronic lung disease. However, the effects of lower oxygen saturations on mortality are unclear.2-5 As ROP treatment has evolved with advances in neonatal care and technology, no consensus exists on optimal oxygen levels to reduce the risk of ROP and assure overall infant health.
Early Studies
Early investigations into the causes of RLF were conducted by Michaelson,6 Ashton and colleagues,7,8 and Patz and colleagues10 in animals that vascularize their retinas after birth; the damaging effects of high oxygen on developing retinal vessels in human preterm infants were extrapolated from these studies. Newborn animals were exposed to oxygen concentrations similar to those experienced by preterm infants. At that time, the available technology permitted neither fine control of oxygen delivery nor a detailed assessment of blood oxygen levels. Kittens no older than 12 days exposed to continuously delivered 70% to 80% oxygen experienced constriction of newly formed retinal capillaries, a phenomenon termed “vaso-obliteration” by Ashton and colleagues.8 (Since that time, it is questioned whether vaso-obliteration actually occurs in human infants not exposed to high oxygen at birth; rather, high oxygen levels can cause a vasoconstriction and “vaso-cessation”11 followed by a delay in physiologic retinal vascular development). On returning to room air, the kittens experienced a “vasoproliferative” effect.
Szewczyk,12 however, recognized that infants brought from high oxygen into room air developed dilated vessels, and vessel caliber could be restored when infants were again placed into oxygen. (This is now believed to be hypoxia-induced vascular dilatation that was reduced in supplemental oxygen.) Patz and colleagues10 summarized the findings of a number of investigators and concluded that high oxygen appeared to be a significant factor in vasoproliferation in RLF at that time.13 It was hypothesized that high oxygen caused avascular retina that became hypoxic once preterm infants were removed from supplemental oxygen and that the retinal hypoxia led to a release of angiogenic factor(s) that caused intravitreal blood vessel growth. Since then, vascular endothelial growth factor, in addition to many other angiogenic growth factors, such as stromal-derived factor and insulin-like growth factor, has been found to be important for the vascular activity in severe ROP.14,15
Reassessment of ROP in the 1940s Since Technological Advances
Since the original descriptions of RLF, more widespread use of indirect ophthalmoscopy16 and later efforts of the Committee for the International Classification of ROP have permitted ophthalmologists to screen for early forms of ROP before RLF.17,18 Also, technology to regulate oxygen and methods to implement its use were developed. Restricting high, unregulated supplemental oxygen to preterm infants dramatically reduced RLF, although it also increased mortality and morbidity.13,19,20 Today nurseries are still searching for the appropriate set of guidelines to minimize the risk of ROP/RLF while taking into consideration morbidities in other organs. It was with the ability to measure first transcutaneous oxygen in the 1980s and then oxygen saturation in the 1990s that an idea developed of what external, inspired oxygen translated to in the individual preterm infant.21 One of the gold standard studies addressing this issue is the randomized multicenter study, Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial (SUPPORT) from the Neonatal Research Network. The Network compared target ranges of oxygen saturation of 85% to 89% versus 91% to 95% among 1,316 infants born between 24 and 28 weeks’ gestational age. Severe retinopathy occurred less frequently in survivors of the 85% to 89% saturation group (relative risk [RR], 0.52; 95% CI, 0.37-0.73; P<0.001); however, death before discharge also occurred more frequently in this low saturation group (RR, 1.27; 95% CI, 1.01-1.60; P = 0.04).2
Oxygen Saturation, Partial Pressure of Arterial Oxygen, and Tissue Oxygenation
It is helpful to consider the oxyhemoglobin dissociation curve when relating oxygen saturation and partial pressure of oxygen in the adult and fetus (Figure 1). Generally, hemoglobin binds oxygen initially with low affinity, then with greater affinity as more oxygen molecules bind, until crowding of oxygen molecules occurs and hemoglobin is saturated. Once saturated, increased oxygen can be achieved by increasing hemoglobin molecules or with supplemental oxygen that dissolves into the plasma. The partial pressure of oxygen in the blood at which hemoglobin is 50% saturated is called the P50. P50 is decreased (indicated by a left shift in the oxyhemoglobin dissociation curve) when a lower partial pressure of oxygen is needed to maintain 50% saturation of hemoglobin.
FIG 1.
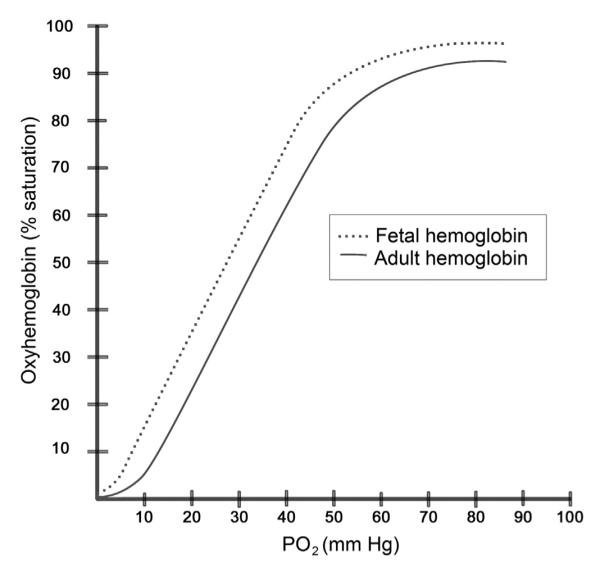
Oxyhemoglobin dissociation curve: relationship between oxygen saturation and partial pressure of oxygen for fetal and adult hemoglobin curve. Decreased 2,3-DPG and increased pH can shift the curve to the left, whereas increased 2,3-DPG or decreased pH can shift the curve to the right.
In the preterm infant, fetal hemoglobin (HbF) functions as the primary oxygen transport protein in red blood cells in the last 7 months of gestation and up to the first 6 months of postnatal life. A relatively high affinity for oxygen characterizes HbF based on a lower P50 value, 6–8 mm Hg [torr] lower than that of normal adult hemoglobin. This difference is due to decreased HbF affinity for 2,3-dephosphoglycerate (2,3-DPG). The consequence of the decreased affinity is that oxygen release from red blood cells requires a lower tissue PaO2 than it would for adult hemoglobin. This is also noted as a leftward shift in the oxyhemoglobin dissociation curve.
In addition, HbF content varies indirectly with gestational age and on the number of blood transfusions the infant receives. This is particularly significant in extremely premature infants, who often require packed red blood cell resuscitation from adult blood, containing adult hemoglobin, which has greater affinity for 2,3-DPG. The mix of adult and HbF thus affects the affinity of the preterm infant’s hemoglobin for oxygen and therefore oxygen delivery to tissue, based on 2,3-DPG levels.5
Estimates of the normal oxygenation of the blood in the retina are also complicated by dilution factors. Blood in the umbilical artery en route to the placenta has a PaO2 of 15–25 mm Hg; the level in blood returning via the umbilical vein can be as high as 55 mm Hg.22 However, as blood returns from the placenta via the ductus venosus, it mixes with blood from the inferior vena cava/superior vena cava in the right atrium, with preferential streaming via the persistent foramen ovale, and flows to the left atrium, where it again mixes with less oxygenated blood returning from the pulmonary veins before flowing to the ascending aorta, the brain and eyes. In addition, lung disease may reduce oxygenation of the blood. The eye may have PaO2 levels as high as 30–40 mm Hg or lower, depending on the dilution.
When the preterm infant is born, if resuscitated in high oxygen concentrations, there can be an increase in arterial oxygen saturation. PaO2 of ≥80 mm Hg is believed to be damaging to newly developed retinal capillaries.23 With older developmental age and the use of blood transfusions for anemia of prematurity, the fetal to adult hemoglobin ratio lowers and is in flux, varying with individual infants. Changes in cardiovascular parameters (heart rate, blood flow), pH, temperature, and anemia alter tissue extraction and delivery of oxygen by causing shifts in the oxyhemoglobin dissociation curve. Thus, it is virtually impossible to predict tissue oxygen levels at any given time for the individual preterm infant.
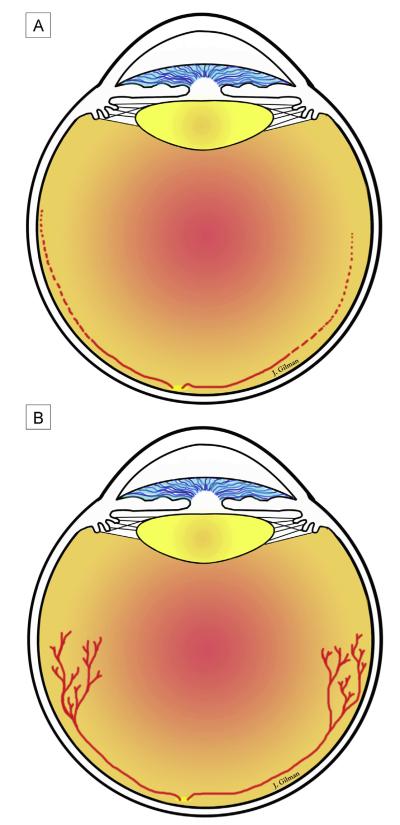
Another factor to consider is the ongoing development of the retinal vasculature in the preterm infant. ROP is believed to occur because of an increase in angiogenic factors caused after a preterm infant is no longer in supplemental oxygen and the avascular retina becomes hypoxic. Therefore, the extent of avascularized retina may be important. In the human infant the retina is not fully vascularized until about term (40 weeks’ gestation). The preterm human infant’s retina is vascularized through zone 1 at about 22 weeks’ gestation.24 After 22 weeks, there is little evidence in human infants how the retina is vascularized, but studies in other species suggest ongoing retinal vascular development occurs by angiogenesis, or the extension of existing blood vessels by proliferation and migration of endothelial cells toward a gradient of vascular endothelial growth factor.25,26 However, oxygen stresses, such as supplemental oxygen, and oxygen fluctuations can delay vascular development (Figure 2). In ROP, delayed physiologic retinal vascular development can increase the area of avascular retina and potentially increase retinal hypoxia, the stimulus for overexpressed angiogenic factors that lead to vascular activity and vasoproliferation in severe ROP.27
FIG 2.
Proposed retinal development.A, initially, there is impaired physiologic retinal vascular development from reduced growth factors from the placental circulation (line) and then a delay in retinal vascular development (dotted line). This leads to areas of avascular retina that become hypoxic once an infant is brought from supplemental oxygen into room air, given healthy lungs and other parameters. B, subsequently, hypoxic retina stimulates the production of angiogenic factors that cause vasoproliferation.
Clinical Studies on Oxygen in ROP
Table 1 provides an overview of several large-scale randomized controlled studies that investigated the relationship of oxygen level or oxygen saturation and ROP risk. Castillo and colleagues28 published the results of a seven-center prospective study comparing arterial oxygen and pulse oxygen saturation values in 122 preterm infants. The average gestational age was 29.2 ± 5.2 weeks; average birth weight, 1338 ± 871.5 g; and median age, 3 days after birth (range, 1-38 days). Arterial oxygen measurements were obtained from indwelling arterial catheters and simultaneous pulse oximetry measurements were made. Infants breathing supplemental oxygen and having pulse oximetry values between 85% and 93% had on average arterial oxygen levels of 56 ± 14.7 mm Hg (median, 54 mm Hg) and were between 40 mm Hg and 80 mm Hg 86% of the time, whereas oxygen saturation measurements >93% aligned with mean arterial oxygen measurements of 107.3 ± 59.3 mm Hg (median, 91 mm Hg) and were >80 mm Hg 60% of the time.
Table 1.
Major studies on supplemental oxygen in preterm infants
| Studya | Infant characteristics | Intervention or study comparison | Outcome |
|---|---|---|---|
| ELGAN (2002-2004)29 | Infants born <28 wks GA | Blood gases on first 2 of 3 postnatal days |
Infants in greatest quartile for PaO2 or PCO2 or lowest quartile for pH had increased risk of zone 1 and severe ROP |
| Pulse Oxygen Saturation Levels and Arterial Oxygen Tension Values (7/2005-11/2006)28 |
Prospective comparison of PaO2 and SaO2 values (mean GA, 29.2 ± 5.2 wks; mean birth weight, 1,338 ± 871.5 g) |
PaO2 and SaO2 measurements | SaO2 of 85%-93% aligned with mean PaO2 of 56 ± 14.7 mm Hg; SaO2 >93% aligned with mean PaO2 of 107.3 ± 59.3 mm Hg and >80 mm Hg 60% of time |
| STOP-ROP (2/94-3/99)37 | Prethreshold ROP one eye | 96%-99% SaO2 vs 89%-94% SaO2 | No difference in ROP, adverse pulmonary outcomes in 96%-99% SaO2 |
| SUPPORT (2/2005-2/2009)2,42 |
24 to <28 wks’ GA | 85%-89% SaO2 vs 91%-95% SaO2 | Increased mortality in 85%-89% SaO2; of survivors, less ROP in 85%-89% SaO2 |
| BOOST II (2006-2011)43 | <28 wks’ GA | 85%-89% SaO2 vs 91%-95% SaO2 | Greater survival rate in 91%-95% SaO2 |
GA, gestational age.
BOOST, Benefits of Oxygen Saturation Targeting Study; ELGAN, Extremely Low Gestational Age Newborn; STOPROP, Supplemental Therapy with Oxygen to Prevent ROP Trial; SUPPORT, Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial.
In the Extremely Low Gestational Age Newborn (ELGAN) study,29 investigators tested the hypothesis that preterm infants who had blood gas derangements on 2 of the first 3 postnatal days of life would be at risk for severe ROP. This was an association study of 1,042 infants with multivariable analyses accounting for confounders associated with blood gas derangements or severe ROP, including gestational age, birth weight, use of maternal aspirin, and presence of maternal preeclampsia. Median arterial oxygen levels of 100 mm Hg and PCO2 of approximately 50 mm Hg were found in the greatest quartiles. Being in the greatest quartile of PaO2 or PCO2 or the lowest quartile of pH increased the risk of zone 1 ROP and severe ROP.
Several studies have provided evidence that oxygen fluctuations are now a recognized risk for ROP,9,30-34 as supported by earlier basic research.35 York and colleagues34 provided strong evidence that fluctuations in oxygen at several time durations up to 30 days following birth increased the odds of a preterm infant developing severe ROP. Fluctuations in oxygen delivered to the retina in the preterm infant can be due to, for example, apnea and bradycardia, changes in fetal/adult hemoglobin, shunting of blood in the lungs, and changes in CO2 and temperature.
Several authors have examined the role of supplemental oxygen in ROP. Investigators have studied whether providing supplemental oxygen would reduce the hypoxic stimulus that increased angiogenic factors.36 In the Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP) Trial,37 investigators tested the hypothesis that among preterm infants with prethreshold ROP in one or both eyes supplemental oxygen to maintain 96% to 99% SaO2, determined through pulse oximetry, in contrast to conventional levels of 89% to 94% SaO2, would reduce the rate of progression to threshold ROP. This hypothesis was based on studies in kittens after hyperoxic insult that recovery in supplemental oxygen led to less severe retinopathy than recovery in hypoxemia.38 In the STOP-ROP study, there were 649 enrolled infants of mean gestational age 25.4 weeks and mean postmenstrual age of 35.4 ± 2.5 weeks (range, 30-48 weeks). There was no difference in progression of ROP from prethreshold to threshold disease in the supplemental oxygen compared with conventional groups.37 However, a post hoc subgroup analysis found that infants with no plus disease were less likely to progress to threshold with supplemental oxygen than did those in the conventional group. Supplemental oxygen increased adverse pulmonary events during treatment and follow-up through corrected age of 3 months.37 Some investigators have recommended considering oxygen changes at times other than the STOP-ROP’s protocol.36,39
Other investigators studied the role of lower oxygen saturation limits and the risk of severe ROP.40,41 Several reported that lower oxygen saturation targets at young post-gestational ages with increased targets at older postgestational ages reduced the incidence of severe ROP.36,40,41
The Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial (SUPPORT) is considered a seminal study by many neonatologists.2,42 The Neonatal Research Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development performed this randomized multicenter study of 1,316 infants born at 24 to <28 weeks’ gestational age who received intubation and surfactant within 1 hour of birth or continuous positive airway pressure and a protocol-limited ventilation strategy. Infants were assigned to either target oxygen saturation of 85% to 89% or 91% to 95%. Those in the lower SaO2 group were more likely to experience death before discharge (19.9% vs 16.2%; RR, 1.27; P = 0.04). Among survivors, those in the low SaO2 group were less likely to develop severe ROP (8.6% vs 17.9%; RR, 0.52; P < 0.001).2,42 Infants receiving continuous positive airway pressure were less likely to experience pulmonary morbidity.42 The Benefits of Oxygen Saturation Targeting Study II (BOOST-II) in the UK and Australia tested the same oxygen targets as SUPPORT in 3,631 infants born at <28 weeks’ gestational age. Those in the greater oxygen group had a greater survival rate even after introduction of a new calibration algorithm for SaO2, and therefore, cessation of enrollment in the two trials has been discussed by the Data and Safety Monitoring Committee.43 Some believe that studies to reduce the target oxygen saturation may in fact be reducing the fluctuations in oxygen by decreasing the wide swings between high and low oxygen levels.29
In conclusion, the best oxygen levels for reducing ROP, reducing pulmonary morbidity, and increasing survival and cognitive development have not been determined. Without more information, clinicians continue to have concerns about limiting oxygen. Many nurseries do not obtain routine blood gas measurements; therefore, assessments are made on the basis of SaO2 and not on PaO2. Recently a group of investigators from five multicenter trials convened to form a consensus. Results are not available at this time, but a general conclusion was to not target oxygen levels below 90% saturation.44 Actual intervention by oxygen may not provide the safest and optimal means to manage the pathology in ROP. However, the use of suitable models that test the effects of oxygen stresses on molecular pathomechanisms may lead to more effective and safer strategies. In addition, more research is needed to determine whether a combination of approaches may reduce morbidity seen in SUPPORT and Boost II2,42,43 while reducing the occurrence of severe ROP.
Literature Search
PubMed and Google were searched, most recently on April 15, 2013, using the following terms: ROP AND oxygen, RLF AND oxygen, prematurity AND oxygen; and combinations of oxyhemoglobin dissociation, fetal hemoglobin, fetal circulation. Results were restricted to English-language articles from 1942 to the present. Some reviews on fetal circulation were obtained from standard neonatal teaching articles.
Acknowledgments
The authors acknowledge James Gilman, CRA, for Figures 1 and 2.
The following grant support is acknowledged: MEH (R01EY015130, PI; R01EY017011, PI) and RHL (R01DK080558, PI; R01 DK081756, co-PI; 1R01HL110002-01, co-PI).
References
- 1.Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens: (1) preliminary report. Am J Ophthalmol. 1942;25:203–4. doi: 10.1016/j.ajo.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 2.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finer N, Leone T. Oxygen saturation monitoring for the preterm infant: The evidence basis for current practice. Pediatr Res. 2009;65:375–80. doi: 10.1203/PDR.0b013e318199386a. [DOI] [PubMed] [Google Scholar]
- 4.Saugstad OD, Speer CP, Halliday HL. Oxygen saturation in immature babies: Revisited with updated recommendations. Neonatology. 2011;100:217–18. doi: 10.1159/000329845. [DOI] [PubMed] [Google Scholar]
- 5.Oski FA. Clinical implications of the oxyhemoglobin dissociation curve in the neonatal period. Crit Care Med. 1979;7:412–18. doi: 10.1097/00003246-197909000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Michaelson IC. The mode of development of the vascular system of the retina. With some observations on its significance for certain retinal diseases. Trans Ophthal Soc UK. 1948;68:137–80. [Google Scholar]
- 7.Ashton N, Ward B, Serpell G. Role of oxygen in the genesis of retrolental fibroplasia: A preliminary report. Br J Ophthalmol. 1953;37:513–20. doi: 10.1136/bjo.37.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–430. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McColm JR, Fleck BW. Retinopathy of prematurity—causation. Semin Neonatol. 2001;6:453–60. doi: 10.1053/siny.2001.0079. [DOI] [PubMed] [Google Scholar]
- 10.Patz A, Eastham A, Higginbotham DH, Kleh T. Oxygen studies in retrolental fibroplasia. Am J Ophthalmol. 1953;36:1511–22. [PubMed] [Google Scholar]
- 11.Reynolds JD. The management of retinopathy of prematurity. Paediatr Drugs. 2001;3:263–72. doi: 10.2165/00128072-200103040-00003. [DOI] [PubMed] [Google Scholar]
- 12.Szewczyk TS. Retrolental fibroplasia and related ocular diseases: classification, etiology, and prophylaxis. Am J Ophthalmol. 1953;36:1336–61. doi: 10.1016/0002-9394(53)90792-2. [DOI] [PubMed] [Google Scholar]
- 13.Patz A. The role of oxygen in retrolental fibroplasia. Trans Am Ophthalmol Soc. 1968;66:940–85. [PMC free article] [PubMed] [Google Scholar]
- 14.Sonmez K, Drenser KA, Capone A, Jr, Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology. 2008;115:1065–70. doi: 10.1016/j.ophtha.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 15.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–9. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schepens CL. A new ophthalmoscope demonstration. Trans Am Acad Ophthalmol Otolaryngol. 1947;51:298–301. [PubMed] [Google Scholar]
- 17.International Committee An international classification of retinopathy of prematurity. Br J Ophthalmol. 1984;68:690–97. doi: 10.1136/bjo.68.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn JT. An international classification of retinopathy of prematurity. In: Silverman WA, Flynn JT, editors. Contemporary Issues in Fetal and Neonatal Medicine: Retinopathy of Prematurity. 2nd ed Blackwell Scientific; Boston: 1985. pp. 1–17. [Google Scholar]
- 19.Askie LM, Henderson-Smart DJ, Ko H. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD001077. doi: 10.1002/14651858.CD001077.pub2. 2009;Jan 21:CD001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsey VE. Cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol. 1956;56:481–543. [PubMed] [Google Scholar]
- 21.Hay WWJ. Physiology of oxygenation and its relation to pulse oximetry in neonates. J Perinatol. 1987;7:309–19. [PubMed] [Google Scholar]
- 22.Murphy PJ. The fetal circulation. Cont Educ Anaesth. 2005;5:107–12. [Google Scholar]
- 23.Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. Trans Am Ophthalmol Soc. 1991;89:77–95. [PMC free article] [PubMed] [Google Scholar]
- 24.McLeod DS, Hasegawa T, Prow T, Merges C, Lutty G. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn. 2006;235:3336–47. doi: 10.1002/dvdy.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division: Evidence that “physiological hypoxia” is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–14. [PubMed] [Google Scholar]
- 26.Stone J, Itin A, Alon T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity. Trans Am Ophthalmol Soc. 2010;108:96–119. [PMC free article] [PubMed] [Google Scholar]
- 28.Castillo A, Sola A, Baquero H, et al. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: Is 85% to 93% an acceptable range? Pediatrics. 2008;121:882–9. doi: 10.1542/peds.2007-0117. [DOI] [PubMed] [Google Scholar]
- 29.Hauspurg AK, Allred EN, Vanderveen DK, et al. Blood gases and retinopathy of prematurity: The ELGAN Study. Neonatology. 2011;99:104–11. doi: 10.1159/000308454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham S, Fleck BW, Elton RA, Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–5. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Omoto T, Cho Y, Hatsukawa Y, Fujimura M, Takeuchi T. The progression of retinopathy of prematurity and fluctuation in blood gas tension. Graefes Arch Clin Exper Ophthalmol. 1993;231:151–6. doi: 10.1007/BF00920938. [DOI] [PubMed] [Google Scholar]
- 32.Tin W, Milligan DWA, Pennefather PM, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonat Ed. 2001;84:106–10. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–45. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 34.York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24:82–7. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 35.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995;36:2063–70. [PubMed] [Google Scholar]
- 36.Gaynon MW. Rethinking STOP-ROP: Is it worthwhile trying to modulate excessive VEGF levels in prethreshold ROP eyes by systemic intervention? A review of the role of oxygen, light adaptation state, and anemia in prethreshold ROP. Retina. 2006;26:S18–23. doi: 10.1097/01.iae.0000244292.86627.1e. [DOI] [PubMed] [Google Scholar]
- 37.The STOP-ROP Multicenter Study Group Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 38.Phelps DL. Reduced severity of oxygen-induced retinopathy in kittens recovered in 28% oxygen. Pediatr Res. 1988;24:106–9. doi: 10.1203/00006450-198807000-00024. [DOI] [PubMed] [Google Scholar]
- 39.Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009;116:513–18. doi: 10.1016/j.ophtha.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 40.Wallace DK, Veness-Meehan KA, Miller WC. Incidence of severe retinopathy of prematurity before and after a modest reduction in target oxygen saturation levels. J AAPOS. 2007;11:170–74. doi: 10.1016/j.jaapos.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanderveen DK, Mansfield TA, Eichenwald EC. Lower oxygen saturation alarm limits decrease the severity of retinopathy of prematurity. J AAPOS. 2006;10:445–8. doi: 10.1016/j.jaapos.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Early CPAP versus Surfactant in Extremely Preterm Infants. N Eng J Med. 2010;229:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Eng J Med. 2011;364:1680–82. doi: 10.1056/NEJMc1101319. [DOI] [PubMed] [Google Scholar]
- 44.Askie LM. Optimal oxygen saturations in preterm infants: A moving target. Curr Opin Pediatr. 2013;25:188–92. doi: 10.1097/MOP.0b013e32835e2c00. [DOI] [PubMed] [Google Scholar]