Abstract
Purpose
To investigate the changes in choroidal thickness (CT), axial length (AL), and ocular perfusion pressure (OPP) accompanying intraocular pressure (IOP) reduction after trabeculectomy.
Methods
Thirty-nine eyes of 39 patients with primary open-angle glaucoma uncontrolled by medical therapy were included in this prospective and interventional study. All patients underwent a fornix-based trabeculectomy. The CT was measured by enhanced depth imaging-optical coherence tomography. IOP, AL, and systolic/diastolic blood pressure were also measured, and OPP was calculated. All measurements were performed at baseline and 1 month after surgery.
Results
The mean IOP was 25.0±5.8 mm Hg at baseline and 11.7±2.6 mm Hg after trabeculectomy (P<0.001), and the mean subfoveal CT was 295±84 mm Hg at baseline and 331±82 mm Hg after trabeculectomy (P<0.001). The mean AL was 23.64±0.98 mm at baseline and 23.54±0.96 mm after trabeculectomy (P<0.001), whereas the mean OPP was 38.8±6.2 mm Hg preoperatively, and 51.1±7.3 mm Hg postoperatively (P<0.001). The change in CT negatively correlated with the change in IOP (r=−0.785, P<0.001) and AL (r=−0.693, P<0.001), whereas it positively correlated with the change in OPP (r=0.418, P=0.008).
Conclusion
These results suggest that the large IOP decrease following trabeculectomy causes choroidal thickening. In addition, CT changes are associated with IOP and AL reduction as well as OPP increase.
Keywords: choroidal thickness, axial length, ocular perfusion pressure, intraocular pressure, trabeculectomy
Introduction
Elevated intraocular pressure (IOP) has been associated with increased visual field loss in patients with open angle glaucoma, and current treatment strategies place primary importance on lowering IOP to decrease the rate of optic nerve damage. Trabeculectomy is commonly performed in patients with chronic open angle glaucoma when medical therapy fails to control IOP, because this procedure appears to be the best surgical method for preventing progressive optic disc damage and for preserving the visual field.
Trabeculectomy leads to a large decrease in IOP, especially during the early postoperative period. It results in well-known complications of hypotony, including choroidal effusion, hemorrhage, cataract, corneal decompensation, and hypotonous maculopathy.1, 2, 3 Other ocular changes after trabeculectomy, such as axial length (AL) decrease and improvement of ocular blood flow have also been reported previously.4, 5 As choroidal thickness (CT) is likely a dynamic parameter that is influenced by oscillations in IOP, a large decrease in IOP due to trabeculectomy might lead to significant CT changes. However, there has been no quantitative study on CT changes after large decreases in IOP.
Until recently, no imaging modality could gather accurate in vivo measurements of CT. Now, practitioners can use enhanced depth imaging-optical coherence tomography (EDI-OCT), which uses low signal strength and low resolution, to achieve greater depth on the conventional spectral domain (SD)-OCT, and thus acquire detailed cross-sectional images of the choroid as well as measure the choroid's thickness.6, 7 The purpose of the current study was to evaluate CT changes with EDI-OCT after a large IOP decrease had been achieved with trabeculectomy.
Materials and methods
Study population and design
This prospective and interventional study was performed at the Beyoglu Eye Research and Education Hospital, according to the principles of the Declaration of Helsinki. The study was approved by the local ethics committee, and the informed consent was obtained from each patient after explaining the nature of the study.
The patients were recruited from the glaucoma clinics. Eligibility criteria were the diagnosis of primary open angle glaucoma uncontrolled by medical therapy, a best-corrected visual acuity of 20/40 or better, a refractive error of <4 diopters of a sphere or 2 diopters of a cylinder, clear media, and no history of other ocular disease. The primary open angle glaucoma had been diagnosed on the basis of clinical examination, retinal nerve fiber layer analyses by SD-OCT, and visual field examination. Exclusion criteria included any history of retinal diseases, any systemic abnormalities (for example, vascular disease, hypertension, and diabetes mellitus), a history of previous intraocular surgery or laser therapy, and poor image quality because of unstable fixation or severe cataract. All patients underwent a fornix-based trabeculectomy with mitomycin-C (MMC) by the two experienced surgeons (CA, BS). The patients who did not achieve target IOP pressure or patients who had any peroperative and postoperative complications were also excluded from the study.
Examination protocol and study measurements
The participants underwent ophthalmological examinations, including visual acuity and refraction, slit-lamp biomicroscopy, gonioscopy, applanation tonometry, and dilated funduscopy. They also underwent a central corneal thickness (CCT) measurement with ultrasound pachymetry (DGH-550; DGH Technology Inc., Exton, PA, USA) and an AL measurement with partial optical coherence inferometry (IOLMaster; Carl Zeiss Meditec, La Jolla, CA, USA). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured 10 min before the CT measurement. Mean blood pressure (mBP) was calculated as the DBP plus one-third the difference between SBP and DBP. The ocular perfusion pressure (OPP) was calculated by measuring the difference between two out of three of the mBP and IOP values.8, 9 The subfoveal CT (SFCT) was measured using SD-OCT (Spectralis, wavelength: 870 nm; Heidelberg Engineering Co, Heidelberg, Germany) with enhanced depth-imaging modality. The procedure for EDI-OCT measurement has been described previously.7 SFCT was defined as the vertical distance from the hyperreflective line of Bruch's membrane to the hyperreflective line of the inner surface of the sclera. CT was measured at the fovea (SFCT), 1000 μ nasal to the fovea (N-CT), and 1000 μ temporal to the fovea (T-CT). One clinician (OB) took the images, and two ophthalmologists, who were masked in terms of measurement time (NK, CA), assessed the images. All measurements were performed within a limited time (0800 hours to 1100 hours) at baseline and at 1 month after surgery.
Data analyses
Statistical analysis was performed with SPSS software package version 17 (SPSS, Inc, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to identify the normality of the distribution and a paired t-test was used to compare the preoperative and postoperative values. Pearson's correlation was used to correlate SFCT with IOP, AL, and OPP. We performed a multivariate linear regression analysis, with SFCT as a dependent parameter and all other parameters as independent parameters. Statistical significance was P<0.05.
Results
Thirty-nine eyes of 39 patients were included in this study. The mean (SD) age of the enrolled patients was 62 (13) years (range, 28–72 years). Patient characteristics are described in Table 1.
Table 1. The demographic and clinical characteristics of patients.
| Variables | Values |
|---|---|
| Age, year | |
| Mean±SD | 62±13 |
| Range | 28–72 |
| Gender | |
| F/M | 24/15 |
| Central corneal thickness, μm | |
| Mean±SD | 542±40 |
| Range | 471–603 |
| Spherical equivalent, D | |
| Mean±SD | 0.87±1.49 |
| Range | −2.75 to +3.50 |
Abbreviation: D, diopter.
The CT, AL, IOP, and OPP changes following surgery are documented in Table 2. The mean IOP was 25.0±5.8 mm Hg (range 20–46 mm Hg) at baseline and 11.7±2.6 mm Hg (range 7–20 mm Hg) after trabeculectomy (P<0.001), and the reduction in IOP after trabeculectomy was 13.2±5.7 mm Hg (52.8%). The mean SFCT was 295±84 mm Hg (range 170–460 mm Hg) at baseline and 331±82 mm Hg (range 185–470 mm Hg) after trabeculectomy (P<0.001), and the increase in CT after trabeculectomy was 36±17 mm Hg (12.2%). The mean N-CT and T-CT were increased significantly after surgery (P<0.001 for both). The mean AL was 23.64±0.98 mm (range 21.91–25.74 mm) at baseline and 23.54±0.96 mm (range 21.76–24.97 mm) after trabeculectomy (P<0.001). The mean OPP was 38.8±6.2 mm Hg (range 19.5–53.5 mm Hg) preoperatively and 51.1±7.3 mm Hg (range 37.8–73.8 mm Hg) postoperatively (P<0.001). The increase in OPP after trabeculectomy was 12.2±8.9 mm Hg (31.4%).
Table 2. Clinical measurements at baseline and after surgery.
| Preoperative | Postoperative | P-valuea | |
|---|---|---|---|
| IOP, mm Hg | |||
| Mean±SD | 25.0±5.8 | 11.7±2.6 | <0.001 |
| Range | 20–46 | 7–20 | |
| SFCT, μm | |||
| Mean±SD | 295±84 | 331±82 | <0.001 |
| Range | 170–460 | 185–470 | |
| N-CT, μm | |||
| Mean±SD | 270±64 | 293±73 | 0.001 |
| Range | 160–380 | 165–405 | |
| T-CT, μm | |||
| Mean±SD | 277±63 | 303±70 | <0.001 |
| Range | 160–385 | 174–420 | |
| AL, mm | |||
| Mean±SD | 23.64±0.98 | 23.54±0.96 | <0.001 |
| Range | 21.91–25.74 | 21.76–24.97 | |
| SBP, mm Hg | |||
| Mean±SD | 130±20 | 125±15 | 0.443 |
| Range | 90–160 | 90–150 | |
| DKB, mm Hg | |||
| Mean±SD | 78±9 | 76±11 | 0.422 |
| Range | 60–90 | 60–100 | |
| OPP, mm Hg | |||
| Mean±SD | 38.8±6.2 | 51.1±7.3 | <0.001 |
| Range | 19.5–53.5 | 37.8–73.8 | |
Abbreviations: AL, axial lenght; CT, choroidal thickness; IOP, intraocular pressure; N, nasal; OPP, ocular prefusion pressure; SF, subfoveal; T, temporal.
Paired-t-test.
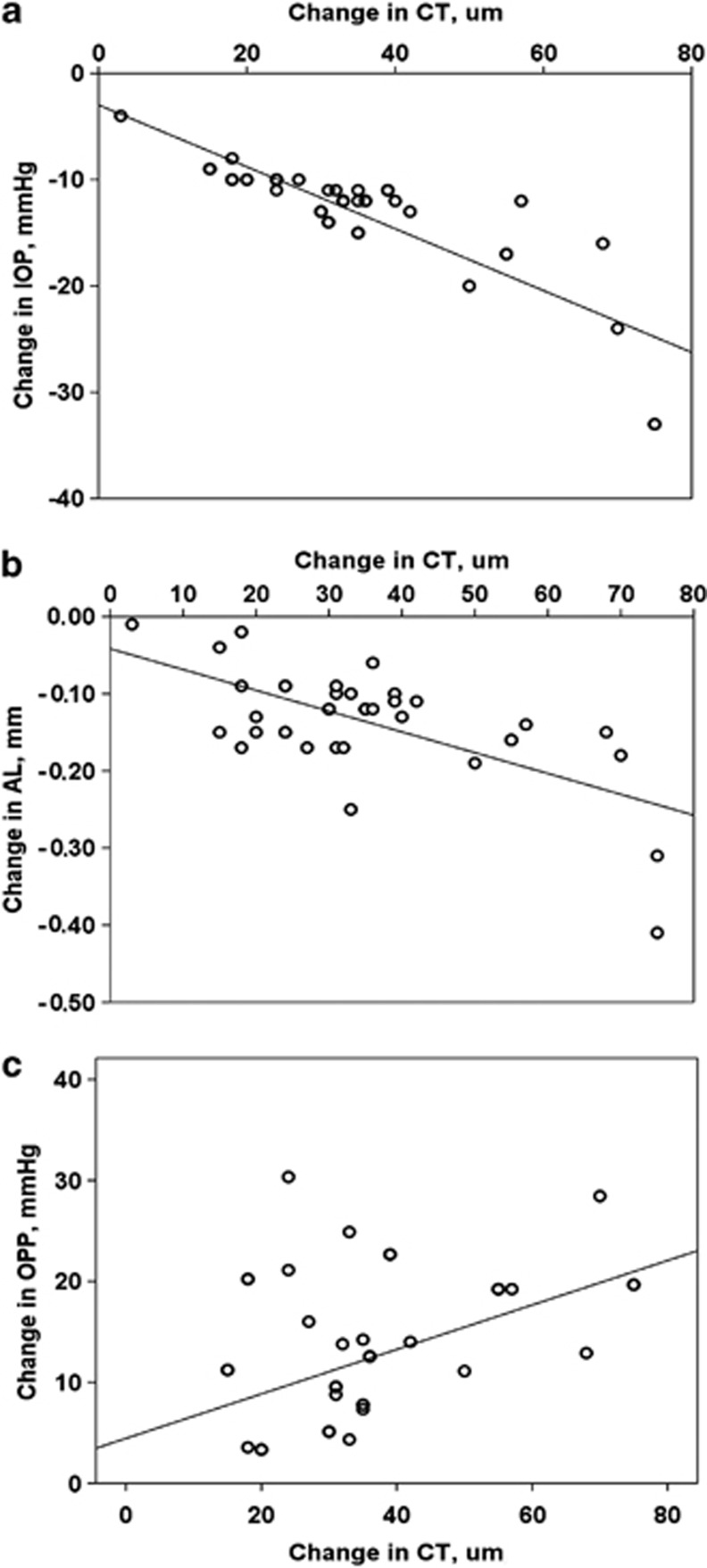
The change in CT was negatively correlated with the change in IOP (r=−0.785, P<0.001; Figure 1a) and in AL (r=−0.693, P<0.001; Figure 1b), yet positively correlated with the change in OPP (r=0.418, P=0.008; Figure 1c).
Figure 1.
Changes in IOP and CT before and after the trabeculectomy showed a significant negative correlation (r=−0.785, P<0.001; a). Changes in CT and AL before and after the trabeculectomy showed a significant negative correlation (r=−0.647, P<0.001; b). Changes in CT and OPP before and after the trabeculectomy showed a significant positive correlation (r=0.418, P=0.008; c).
We performed multiple linear regression analysis to determine the factors most associated with change in SFCT among patient age, baseline SBP, baseline DBP, baseline AL, baseline IOP, changes in SBP and DBP, change in OPP, change in AL, and change in IOP. Change in IOP was the only factor that is significantly associated with SFCT (P<0.001; regression coefficient: −2.55; 95% CI, −3.79 to −1.32; standardized coefficient beta: −0.86); other factors were not significantly correlated with change in SFCT. Table 3 shows the results of multiple regression analysis.
Table 3. Multivariate analysis of associations between changes in SFCT and ocular and general parameters.
|
Coefficientsa | ||||||
|---|---|---|---|---|---|---|
| Parameters |
Unstandardized coefficients |
95% CI for B |
Standardized coefficients | t | P-value | |
| B | Lower bound | Upper bound | Beta | |||
| Age, years | 0.023 | −0.29 | 0.34 | 0.016 | 0.15 | 0.881 |
| Gender | 2.56 | −10.59 | 15.72 | 0.055 | 0.40 | 0.691 |
| SBP, mm Hg | 0.28 | −0.12 | 0.69 | 0.21 | 1.45 | 0.159 |
| DBP, mm Hg | −0.22 | −0.78 | 0.33 | −0.12 | −0.84 | 0.405 |
| IOP, mm Hg | −1.019 | −2.54 | 0.50 | −0.15 | −1.37 | 0.181 |
| AL, mm | −1.32 | −6.67 | 4.02 | −0.073 | −0.51 | 0.614 |
| Changes in SBP | 0.092 | −0.16 | 0.34 | 0.10 | 0.75 | 0.460 |
| Changes in DBP | −0.14 | −0.58 | 0.29 | −0.088 | −0.68 | 0.500 |
| Changes in OPP | 0.17 | −0.43 | 0.78 | 0.093 | 0.60 | 0.551 |
| Changes in IOP | −2.55 | −3.79 | −1.32 | −0.86 | −4.27 | 0.000 |
| Changes in AL | −17.53 | −137.89 | 102.81 | −0.055 | −0.30 | 0.766 |
Abbreviations: AL, axial length; CI, confidence interval; DBP, diastolic blood pressure; IOP, intraocular pressure; OPP, ocular perfusion pressure; SBP, systolic blood pressure; SFCT, subfoveal choroidal thickness.
Dependent variable: changes are SFCT. P<0.001 are indicated in bold.
Discussion
In light of previous studies, it can be concluded that large IOP changes influence ocular biometrics.4, 10, 11, 12, 13, 14 Leydolt et al10 investigated the effect of short-term mechanical IOP elevation on AL, anterior chamber depth, CCT, and lens thickness. They found a significant reduction in AL, but did not find any significant changes in anterior chamber depth, CCT, and lens thickness during IOP elevation. Nemeth et al14 reported an AL decrease and ocular wall thickness increase 4 days after trabeculectomy. Cashwell et al4 found a significant decrease in AL at various times after trabeculectomy by using B-scan ultrasonography. In a study by Kook et al15, AL changes after trabeculectomy with MMC in glaucomatous eyes were associated with a high preoperative IOP and a low postoperative target IOP. Although the exact cause of the AL decrease after trabeculectomy remained unknown, the authors speculated that a thickened choroid accompanied a lowered IOP. However, there is no quantitative study that shows the CT changes and its association with AL after trabeculectomy.
In this study, we investigated the CT and AL changes in relation to IOP reduction after trabeculectomy. We found that the CT increased significantly after trabeculectomy and that this increase correlated with changes in IOP, AL, and OPP. CT changes associated with the increase in IOP have been previously investigated by Hata et al.16 They evaluated the changes in CT and AL that accompany IOP increase, and investigated the changes in axial eye dimensions, which are induced by IOP increase. In their study, patients with primary angle closure underwent the darkroom-prone provocative test for 1 h. They found that an IOP increase induces immediate choroidal thinning and axial elongation.
One can expect a significant increase in OPP after trabeculectomy owing to large IOP reduction. In our study, OPP significantly increased after trabeculectomy. These results were similar to those of the studies by Yamazaki et al17 and Berisha et al,5 both of whom found a significant increase after trabeculectomy. Also, many previous studies measured SFCT with EDI-OCT and they found that it was significantly associated with OPP.18, 19 Similarly, we found a positive and significant correlation between the changes in CT and changes in OPP following trabeculectomy.
Many factors may influence CT. It has been reported that CT was negatively correlated with older age, longer AL, higher IOP, and thicker CCT.20, 21, 22, 23, 24 In our study, multiple linear regression analysis showed that the SFCT was significantly associated with only the change in IOP. This may be due to the change in IOP, which was the most marked factor after surgery. Moreover, the increase in OPP was more associated with the decrease in IOP rather than the increase in blood pressure.
In our study, we evaluated the eyes with open angle glaucoma. A previous study by Arora et al25 investigated the change in CT after water drinking test, comparing angle closure with open angle eyes. They found that a significant increase in CT after water drinking test was observed in angle closure eyes but not in open angle eyes. Their study showed that eyes with angle closure have significant differences in the baseline and dynamic behavior of the choroid compared with open angle eyes. Abnormal CT increase has been hypothesized to be a contributing feature to primary angle closure.26
Some studies have put several hypotheses for CT increase.27 First, increase in CT might come from increased synthesis of large, osmotically active proteoglycans, and these proteoglycans help to pull water into the choroid. Second, the choroid might be thickening because of an increase in the size or number of the fenestrations in the choriocapillaris, which might also increase the amount of molecules in the choroidal matrix that encourage osmosis. Third, the fluid might come from the retina by transporting across the retina pigment epithelium. Finally, changes in the tonus of the nonvascular smooth muscle that spans the width of the choroid also thicken the choroid. The choroid contains abundant nonvascular smooth muscle, so these muscles might squeeze fluid out of the choroid whenever they contract, thereby thinning the choroid. Whenever they contract, the choroid becomes thinner, and whenever they relax the choroid becomes thicker. Therefore, lowering the IOP drastically might cause choroidal expansion. More importantly, increase in OPP may cause the increase in CT. Our findings revealed that CT is influenced by OPP. Previous studies have also shown that the choroid was thicker in eyes with higher blood pressure, and relatively lower IOP seems logical if the blood volume of the choroid increased with higher perfusion pressure.28 These quantitative results support the previous studies where an increase in choroidal blood flow in response to increase in OPP was concluded.29, 30
To the best of our knowledge, this study is the first work to assess CT after trabeculectomy and its association with changes in IOP, AL, and OPP. The strengths of this study include its prospective design and sufficient study population. In addition, all measurements were performed within a limited time (0800–1100 hours), which minimized the possibility of CT change caused by the diurnal variations that have been reported in numerous studies.31, 32, 33 In addition, our OCT device has eye-tracking and image-averaging capabilities, resulting in improved signal-to-noise ratio and improved visualization of the choroid. On the other hand, the present study has several limitations. The manual measurement of SFCT is one of the principal drawbacks of this study. Current OCT equipment does not provide software for the automated measurement of CT, so all identifications of Bruch's membrane and the inner scleral border were conducted manually. It is our hope that software for segmenting the choroid automatically will be available in the near future. Moreover, the reproducibility of CT measurements using OCT is still debatable. However, some studies found high interobserver correlation, high repeatability, and high intersystem, interexaminer, and intervisit reproducibility in CT measurements.7, 34, 35 Another limitation is that we investigated only the changes that occurred at postoperative 1 month; so, how long the change in CT persists after the trabeculectomy remains unknown. Further long-term research is required to establish which CT changes continue and for how long.
In conclusion, reduction in IOP following trabeculectomy affects the CT measurement, which indicates that IOP measurement is necessary for choroid evaluation. In addition, the IOP changes that are related to CT could cause axial shortening.
The authors declare no conflict of interest.
References
- Pederson JE.HypotonyIn: Duane TD, (ed.)Clinical ophthalmology 3rd VolChapter 58Harper & Row: Philadelphia; 19841–8. [Google Scholar]
- Stamper RL, McMenemy MG, Lieberman MF. Hyptonous maculopathy after trabeculectomy with subconjunctival 5-fluorouracil. Am J Ophthalmol. 1992;114:544–553. doi: 10.1016/s0002-9394(14)74481-2. [DOI] [PubMed] [Google Scholar]
- Suner IJ, Greenfield DS, Miller MP, Nicolela MT, Palmberg PF. Hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment. Ophthalmology. 1997;104:207–214. doi: 10.1016/s0161-6420(97)30332-7. [DOI] [PubMed] [Google Scholar]
- Cashwell LF, Martin CA. Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology. 1999;106:2307–2311. doi: 10.1016/S0161-6420(99)90531-6. [DOI] [PubMed] [Google Scholar]
- Berisha F, Schmetterer K, Vass C, Dallinger S, Rainer G, Findl O, et al. Effect of trabeculectomy on ocular blood flow. Br J Ophthalmol. 2005;89:185–188. doi: 10.1136/bjo.2004.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence omography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Barbosa CP, Stefanini FR, Penha F, Góes MÂ, Draibe SA, Canziani ME, et al. Intraocular pressure and ocular perfusion during hemodialysis. Arq Bras Oftalmol. 2011;74:106–109. doi: 10.1590/s0004-27492011000200007. [DOI] [PubMed] [Google Scholar]
- Schmidl D, Weigert G, Dorner GT, Resch H, Kolodjaschna J, Wolzt M, et al. Role of adenosine in the control of choroidal blood flow during changes in ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2011;52:6035–6039. doi: 10.1167/iovs.11-7491. [DOI] [PubMed] [Google Scholar]
- Leydolt C, Findl O, Drexler W. Effects of change in intraocular pressure on axial eye length and lens position. Eye. 2008;22:657–661. doi: 10.1038/sj.eye.6702709. [DOI] [PubMed] [Google Scholar]
- Read SA, Collins MJ. The short-term influence of exercise on axial length and intraocular pressure. Eye. 2011;25:767–774. doi: 10.1038/eye.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SA, Collins MJ. Water drinking influences eye length and IOP in young healthy subjects. Exp Eye Res. 2010;91:180–185. doi: 10.1016/j.exer.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Uretmen O, Ates H, Andac K, Deli B. Axial length changes accompanying successful nonpenetrating glaucoma filtration surgery. Ophthalmologica. 2003;217:199–203. doi: 10.1159/000068977. [DOI] [PubMed] [Google Scholar]
- Nemeth J, Horoczi Z. Changes in the ocular dimensions after trabeculectomy. Int Ophthalmol. 1992;16:355–357. doi: 10.1007/BF00917990. [DOI] [PubMed] [Google Scholar]
- Kook MS, Kim HB, Lee SU. Short-term effect of mitomycin-C augmented trabeculectomy on axial length and corneal astigmatism. J Cataract Refract Surg. 2001;27:518–523. doi: 10.1016/s0886-3350(00)00646-5. [DOI] [PubMed] [Google Scholar]
- Hata M, Hirose F, Oishi A, Hirami Y, Kurimoto Y. Changes in choroidal thickness and optical axial length accompanying intraocular pressure increase. Jpn J Ophthalmol. 2012;56:564–568. doi: 10.1007/s10384-012-0173-0. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hayamizu F. Effect of trabeculectomy on retrobulbar circulation and visual field progression in patients with primary open-angle glaucoma. Clin Ophthalmol. 2012;6:1539–1545. doi: 10.2147/OPTH.S36331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim SS, Kwon HJ, Koh HJ, Lee SC. Association between choroidal thickness and ocular perfusion pressure in young, healthy subjects: enhanced depth imaging optical coherence tomography study. Invest Ophthalmol Vis Sci. 2012;53:7710–7717. doi: 10.1167/iovs.12-10464. [DOI] [PubMed] [Google Scholar]
- Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118:1571–1579. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeelpour M, Povazay B, Hermann B, Hofer B, Kajic V, Kapoor K, et al. Three-dimensional 1060-nm OCT: choroidal thickness maps in normal subjects and improved posterior segment visualization in cataract patients. Invest Ophthalmol Vis Sci. 2010;51:5260–5266. doi: 10.1167/iovs.10-5196. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148:445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Ikuno Y, Tano Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:3876–3880. doi: 10.1167/iovs.08-3325. [DOI] [PubMed] [Google Scholar]
- Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3430–3435. doi: 10.1167/iovs.10-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51:2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- Arora KS, Jefferys JL, Maul EA, Quigley HA. Choroidal thickness change after water drinking ıs greater in angle closure than in open angle eyes. Invest Ophthalmol Vis Sci. 2012;53:6393–6402. doi: 10.1167/iovs.12-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Friedman DS, Congdon NG. Possible mecha-nisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12:167–180. doi: 10.1097/00061198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Li J, Liu R, Chen X, Pan J, Tang S, et al. Choroidal thickness in both eyes of patients with unilateral ıdiopathic macular hole. Ophthalmology. 2012;119:2328–2333. doi: 10.1016/j.ophtha.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Invest Ophthalmol Vis Sci. 1997;38:2338–2343. [PubMed] [Google Scholar]
- Polska E, Simader C, Weigert G, Doelemeyer A, Kolodjaschna J, Scharmann O, et al. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest Ophthalmol Vis Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]
- Brown JS, Flitcroft DI, Ying GS, Francis EL, Schmid GF, Quinn GE, et al. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:2267–2271. doi: 10.1167/iovs.10-6024. [DOI] [PubMed] [Google Scholar]
- Ikuno Y, Maruko I, Yasuno Y, Miura M, Sekiryu T, Nishida K, et al. Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:5536–5540. doi: 10.1167/iovs.10-6811. [DOI] [PubMed] [Google Scholar]