Abstract
This study was designed to describe the bone marrow features of multisystem Langerhans cell histiocytosis (LCH) at diagnosis in patients with or without hematologic dysfunction. A retrospective review of bone marrow biopsies from patients with multisystem LCH was performed. Cases were diagnosed at the Garrahan Hospital between 1987 and 2004. Routine and immunohistochemistry techniques (hemaloxylin-eosin, periodic acid-Schiff, Giemsa, Gomori reticulin, and CD1a, CD68, and CD61) were evaluated. Clinical outcome and laboratory data were obtained from the medical charts. Twenty-two bone marrow biopsies from patients with multisystem LCH were reviewed at onset of disease. Four patients had no hematologic dysfunction and the other 18 patients had monocytopenia (9), bicytopenia (7), or tricytopenia (2). Increased number and dysplasia of megakaryocytes were evident in 22/22 samples and emperipolesis was present in 21/22 (95%). Aggregates of histiocytes and hemophagocytosis were seen in 9/22 samples. Myelofibrosis was found in 16/17 (94%) evaluable samples at diagnosis. No association of myelofibrosis and cytopenias or clinical outcome was found. Positive CD1a confirmed the presence of LCH cells in 3/22 (14%) samples. Hemophagocytosis and poor outcome were significantly more common in patients with bilineage and trilineage cytopenias. Langerhans cell histiocytosis cells were rarely seen in the bone marrow of these patients (14%); increased histiocytes and hemophagocytosis were more commonly found (41 %). Hemophagocytosis was associated with severe cytopenias. Bicytopenia and tricytopenia were associated with poor outcome (death). Myelofibrosis, megakaryocytic dysplasia, and emperipolesis were common findings.
Keywords: cytopenias, emperipolesis, hemophagocytosis, histiocytosis, Langerhans, myelofibrosis
INTRODUCTION
Langerhans cells are bone marrow-derived antigenpresenting cells (dendritic cells) characterized by a unique cytoplasmic organelle, the Birbeck granule, and the expression of CD1a and class II major histocompatibility complex molecules. Langerhans cell histiocytosis (LCH) is a disorder in which accumulation of LCH cells occurs. Langerhans cell histiocytosis cells are immunohistochemically identified by the expression of CD1a in tissue sections. The etiology of LCH is unknown, and data supporting an immune dysregulatory disorder as well as a clonal neoplasm have been reported [1].
The clinical spectrum of LCH ranges from a self-healing unifocal/unisystem disease to a fatal multisystem disease. Bone marrow involvement can be suspected in patients with the multisystem form of LCH and single or multiple lineage cytopenias. In these patients, bone marrow examination is perfomled mainly to look for the presence of LCH cells [2]. However, the finding of LCH cells is not a universal feature [3–6]. The pathophysiologic explanation for cytopenias remains unclear, but myelodysplastic changes [7], myelofibrosis [8,9], and secondary macrophage activation syndrome previously have been reported [10].
This study was designed to better define the bone marrow features at diagnosis of multisystem LCH with risk-organ involvement in patients with or without cylopenia.
MATERIALS AND METHODS
A retrospective review of bone marrow biopsies obtained from patients meeting the criteria for multisystem LCH with risk-organ involvement with or without cytopenia was performed. Multisystem LCH is defined by the involvement of 2 or more organs. The liver, the lung, and the hematopoietic system are considered risk organs [11].
All cases were diagnosed at the Garrahan Hospital between January 1987 and December 2004. The biopsies were taken from the posterior iliac crest at the time of diagnosis as part of the initial workup and before any specific treatment had been initiated. Samples were collected, formalin-fixed, decalcified, and paraffin-embedded in a standardized fashion. Paraffin sections were stained with hematoxylin-eosin (H-E), periodic acid-Schiff, Giemsa, and Gomori reticulin techniques. Immunostaining with CD61 (DakoCytomation, Glostrup, Denmark), CD68 (PGM-I clone; DakoCytomation) and CD1a (Novocastra-Leika, Wetzlar, Germany) antibodies was also performed.
The following features were evaluated. Overall cellularity was determined by direct visualization method of an average of 5 sequential low-power fields (× 10). Subcortical and cartilaginous areas were excluded. The reported values represent a mean cellularity in cases of nonhomogeneous specimens. In agreement with the literature, in this pediatric group of patients we considered a biopsy as hypocellular when cellularity was <60% and hypercellular when it was >80% [12].
Myeloid to erythroid lineage ratio of 3:1 was considered as normal.
A number of 4 to 6 megakaryocytes per high-power field were considered regular; the presence of dysplastic features (bizarre nuclear configuration, hypersegmented or hyposegmented nucleus, paratrabecular and grouped disposition) and “emperipolesis” (the passage of other hematopoietic cells through megakaryocytes) were also evaluated. CD61 immunostaining was used to confirm those cells were megakaryocytes (not histiocytes).
Histiocytes were considered elevated when they were easily detected in aggregates on H-E evaluation. Subsequent CD68 immunostaining was performed as a confirming test.
The presence of LCH cells was suspected on H-E evaluation and then confirmed by immunohistochemistry, in which LCH cells have a strongly membrane positivity for CD1a. (Immunostaining was also performed in samples without 1st-glance evidence of LCH cells.)
Hemophagocytosis was defined as red cells, lymphocytes, neutrophils, eosinophils, or their cellular debris visible in the macrophage cytoplasm.
Myelofibrosis
When the size and quality of the sample was adequate, a quantitative estimation of the reticulin fibers was performed according to the Bauermeister scale (from 0 to 4) [13].
Values of peripheral white blood cells, hemoglobin, and platelets at onset of disease as well as clinical outcome were obtained from the medical charts. Involvement of the hematopoietic system was defined, according to the criteria of Lahey [3], as the presence of peripheral white blood cells < 4000/mm3, hemoglobin < 10 g/dL, and platelets <100 000/mm3. According to these values, patients were considered as being with (monolineage, bilineage, or trilineage cytopenia) or without hematologic dysfunction. Lung and liver dysfunction were also defined according to the criteria of Lahey [3]. Death related to disease was the only marker of outcome considered in this study.
For each analyzed variable, differences between groups were evaluated using chi-square or Fisher exact probability test (based on sample size). Odds ratios (OR), 95% confidence intervals (CI), sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) were determined for those variables showing significant differences between groups.
RESULTS
Twenty-two bone marrow biopsies from 22 patients with multisystem LCH and risk-organ involvement at onset of disease were included. The male to female ratio was 13:9. Mean age at diagnosis was 10.5 months and median age was 14 months, with a range of 2 to 38 months.
Four of 22 patients had risk-organ dysfunction (liver), but without hematologic involvement. Eighteen patients had hematologic and other risk-organ dysfunction (liver and/or lung). Nine of these 18 patients had monocytopenia, 7 had bicytopenia, and the remaining 2 had tricytopenia.
All samples had patchy areas of marrow abnormality along the biopsy. Hypercellular areas were mixed with hypocellular ones, edema, and hemorrhage. The presence of fatty tissue was rare. Mean cellularity was 60%; 8/22 biopsies were hypocellular and 7/22 were hypercellular (range: 40% to 95%). All but 3 samples had abnormal myeloid to erythroid lineage ratios, with a median ratio of 1:1.
Increased numbers of megakaryocytes were observed in 15 patients. Megakaryocytic dysplasia was evident in all samples (the disposition in clusters being the most common). Emperipolesis was found in 21 biopsies (95%), engulfing neutrophils and lymphocytes (Fig. 1A–D).
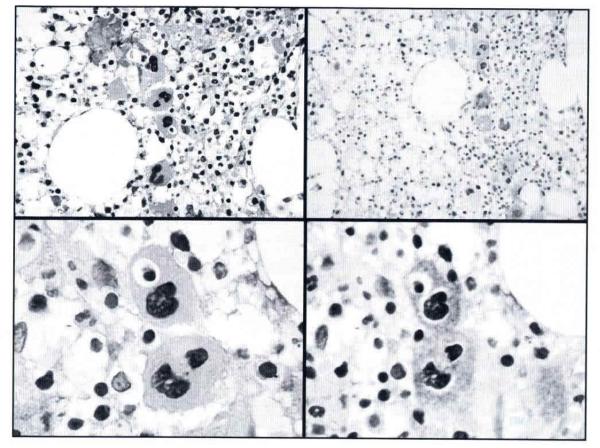
Figure 1.
Megakaryocytes: emperipolesis and cluster disposition. Megakaryocytes disclosing a line group; one is engulfing a hemopoietic cell (emperipolesis). Hematoxylin-eosin (H-E), original magnification ×400 and CD61, original magnification × 250. High magnification showing 2 megakaryocytes (cluster), one of them with emperipolesis. HE and CD61, original magnification ×100.
Clusters of histiocytes and hemophagocytosis were seen in 9 biopsies (41%). These histiocytes were positive for CD68 (Fig. 2A,B) and negative for CD1a. In one case, mostly foamy histiocytes occupied large areas of the bone marrow.
Figure 2.
Hemophagocytosis, myelofibrosis, and Langerhans cell histiocytosis infiltration. A. Hemophagocytosis, red cells and cellular debris inside a histiocyte cytoplasm. Hematoxylin-eosin, original magnification × 100. B. Positive immunostaining for CD68 in histiocytes with hemophagocytosis, original magnification × 100. C. Increased reticulin fibers in a diffuse pattern, with coarse fibers (grade 3). Gomori reticulin technique, original magnification × 25. D. Aggregate of Langerhans cell histiocytosis cells with strongly positive immunostaining for CD1a. Original magnification ×40.
CD1a immunostaining was performed; positive cells for CD1a confirmed the presence of LCH cells in 3/22 samples (14%). CD1a staining highlighted the infiltration pattern, which consisted predominantly of patchy aggregates (Fig. 2D). The area of infiltration was quantified as 50%, 40%, and 25% of the 3 affected bone marrow samples. Increased reticulin fibers were evident in 16/17 (94%) of the evaluable samples: grade 1, 8; grade 2, 5 (Fig. 2C); grade 3, 3; and grade 4, 0 (Fig. 2C).
Nine patients (41%) died of the disease after a median survival of 5 months (range: 2–27 months). The remaining 13 patients are alive with a median follow-up of 8 years (range: 4–15 years).
Patients were classified into 3 groups according to their hematologic involvement: without cytopenia, with monocytopenia, and with bicytopenia or tricytopenia. Based on their clinical and histopathologic similarities, data from patients without cytopenia or with monocytopenia (group A; n = 13) were pooled together for comparison with data collected from patients with bicytopenia or tricytopenia (group B; n = 9) (Table 1).
Table 1.
Bone marrow and clinical findings
| Case no. | Sex/Age (months) | Cellularity(%) | Cytopenias | Emperipolesis | Hemophagocytosis | Myelofibrosis | LCH cells | Clinical outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | M/11 | 90 | − | + | − | + | − | Alive |
| 2 | M/17 | 40 | − | + | − | ++ | − | Alive |
| 3 | M/28 | 90 | − | + | − | +++ | +(25%) | Alive |
| 4 | M/24 | 40 | − | + | − | − | − | Dead |
| 5 | F/9 | 70 | Hb 8,6 | + | − | + | − | Alive |
| 6 | M/8 | 40 | Hb 5,8 | + | + | NE | − | Alive |
| 7 | F/22 | 70 | Hb 4,3 | + | − | NE | − | Alive |
| 8 | M/25 | 40 | Hb 5,8 | + | + | +++ | − | Alive |
| 9 | M/21 | 90 | Hb 5,4 | + | − | + | − | Alive |
| 10 | F/10 | 30 | Hb 4,2 | + | − | + | − | Alive |
| 11 | M/15 | 90 | Hb 4,7 | + | − | ++ | − | Alive |
| 12 | F/2 | 85 | Hb 7,2 | + | − | ++ | − | Dead |
| 13 | F/37 | 40 | Hb 8,5 | + | − | NE | − | Alive |
| 14 | F/12 | 40 | Hb 5,9 + PLT 45 000 | + | − | NE | − | Dead |
| 15 | M/3 | 60 | Hb 6,2 + PLT 38 000 | + | + | + | − | Dead |
| 16 | M/14 | 50 | Hb 5,9 + PLT 54 000 | + | + | + | − | Dead |
| 17 | M/6 | 40 | Hb 4,0 + PLT 13 000 | + | + | ++ | − | Dead |
| 18 | F/11 | 95 | Hb 7,2 + PLT 98 000 | + | − | +++ | +(40%) | Dead |
| 19 | M/15 | 60 | Hb 7,6 + PLT 6000 | − | + | NE | − | Alive |
| 20 | M/14 | 40 | Hb 6,8 + PLT 27 000 | + | − | ++ | +(50%) | Alive |
| 21 | F/11 | 60 | Hb 8,6 + PLT 25 000 + WBC 2900 | + | + | + | − | Dead |
| 22 | F/19 | 80 | Hb 7,9 + PLT 52 000 + WBC 3800 | + | + | + | − | Dead |
| Total | 21/22 | 9/22 | 16/17 | 3/22 | 9 Dead/13 alive |
Patients 1 to 13; cases with no cytopenias or unicytpenia (group A); patients 14 to 22; cases with bicytopenia/tricytopenia (group B). LCH indicates Langerhans cell histiocytosis; −, absent; +, Present; Hb, Hemoglobin (g/dL); NE, not evaluable; PLT, platelets (mm3); WBC, white blood count (mm3).
Hemophagocytosis was significantly more common in patients in group 8 in comparison to patients in group A (P = 0.007; OR, 19.25; 95% CI, 2.18–169.79; Se, 0.77; Sp, 0.84; PPV, 0.77; NPV, 0.84). Moreover, in this small cohort, death related to disease was also significantly more frequent in patients in group B compared with those in group A (P = 0.007; OR, 19.25; 95% CI, 2.18–169.79; Se, 0.77; Sp, 0.84; PPV, 0.77; NPV, 0.84). Interestingly, hemophagocytosis alone was not significantly associated with poor outcome (death). Although myelofibrosis was a highly prevalent feature in this study (94%), its presence was not statistically associated with either of the cytopenias or with poor clinical outcome.
DISCUSSION
In the past, the main aim of performing a bone marrow biopsy in multisystem LCH was the detection of LCH cells. Even if hematologic involvement is frequently seen in the initial clinical picture, LCH cells are rarely found in these biopsies. We hypothesized that other events could be responsible for those cytopenias.
Abnormal bone marrow features were frequently and easily found in all analyzed samples. The presence of aggregates of cells with eosinophilic cytoplasm was a common finding in >50% (12/22) of the samples. In our study, 3 of these biopsies (14%) stained positive for CD1a, and the remaining 9 were CD1a-negative/CD68-positive histiocytes. Minkov and colleagues [6] reported the prevalence of 34% of CD1a-positive histiocytes by immunocytochemistry in LCH cases. However, in their study, when LCH cases were stratified into those with “single system” or “multisystem” disease, abundance of CD1a positive cells was found only in 14.3% (2/14) cases of severe multisystem LCH. This prevalence agrees with our findings of 14% (3 /22).
When histiocyte aggregates were present, they consistently showed hemophagocytosis, probably a sign of macrophage activation. Interestingly, whenever hemophagocytosis was detected, single or multiple cytopenias were present. On the other hand, when LCH cells were detected, hemophagocytosis was absent. Activated macrophages and LCH cells were not found in the same sample.
Statistical analysis showed that hemophagocytosis was significantly more common in patients with bilineage and trilineage cytopenia (group B), and these patients were significantly more prone to die of disease. Besides, 4/7 patients with confirmed multisystem LCH and bicytopenia or tricytopenia (group B) who died of disease had also presented with fever, hepatosplenomegaly, and hemophagocytosis. Thus, secondary hemophagocytic syndrome could not be ruled out in these cases. Unfortunately, other diagnostic criteria for hemophagocytic syndrome (e.g., hypertriglyceridemia or hypofibrinogenemia, natural killer cell function, ferritin levels, or soluble interleukin-2 receptor levels) were not systematically searched. Besides, because of their clinical condition, 8/9 patients with hemophagocytosis had been transfused with packed red blood cells (mean, 5.75; range, 2 to 11), prior to bone marrow biopsies were performed. Transfusions are a common cause for hemophagocytosis. Based on our findings, the presence of secondary hemophagocytic syndrome and its potential role as a concurrent cause of death in multisystem LCH cannot be confirmed or specifically determined, but it certainly warrants further studies.
The megakaryocytic lineage consistently showed dysplastic characteristics, but emperipolesis was an unexpected common feature. Emperipolesis, a term coined in 1956 to describe the “inside round about wandering” of lymphocytes within cells [14], is not only a feature frequently described in myelodysplastic and chronic myeloproliferative syndromes, but also in other neoplastic or stress-related conditions [15].
In order to evaluate the strength of these data, our cases were compared with 12 age-matched bone marrow biopsies from individuals with nonhematologic malignancies (median age: cases, 14 months vs controls, 12.5 months, difference not significant). The prevalence of emperipolesis was compared between groups based on H-E routine technique. Even though emperipolesis was more prevalent in cases than in controls (95% vs 66%), its presence is far from being specific of LCH. On the other hand, the absence of emperipolesis was very rare among cases (only 1/22 cases).
Myelofibrosis was present in 94% of the evaluable samples in the present study. Pinckney and colleagues [16], Sartori and Resnick [17], and Piguet and colleagues [8] previously reported the presence of myelofibrosis in patients with LCH, but these features were detected after several months of treatment. Myelofibrosis was reported after treatment initiation by our group as well [9]. To the best of our knowledge, this study is the 1st report of myelofibrosis as part of the initial clinical picture in multisystem LCH.
We reviewed 19 bone marrow biopsies from 10 patients included in this study, evaluated at 2 to 24 months after diagnosis. Interestingly, the results regarding myelofibrosis during follow-up (not part of our aim) were extremely variable. Different explanations could be hypothesized for these findings, the most simplistic suggesting that myelofibrosis may follow a patched pattern and its presence or absence would depend on the sampling site.
Megakaryocytes have been largely implicated in the development of myelofibrosis [18–24]. The pathophysiologic mechanism proposed involves an abnormal subcellular P-selectin distribution in these megakaryocytes, which appeared to correlate with excessive and pathologic emperipolesis of polymorphonuclear leukocytes. This leads to the destruction of megakaryocyte storage organelles and leakage of their alpha-granular contents (platelet-derived growth factor and transforming growth factor-beta) into the bone marrow microenvironment. Both growth factors are essential for the megakaryocyte-dependent fibroblast proliferation. The mutual cellular interaction of megakaryocytes and polymorphonuclear leukocytes may participate, through fibroblast activation, in the generation of myelofibrosis. This might also be the mechanism for the genesis of myelofibrosis in LCH disease.
In summary the presence of LCH cells is rarely seen in the bone marrow of multisystem LCH patients with risk-organ involvement at diagnosis (14%). However, its presence was negatively associated with hemophagocytosis. Increased histiocytes and hemophagocytosis are a more common finding (41%), and hemophagocytosis is positively associated with severe cytopenias. Moreover, patients with bilineage or trilineage cytopenias were significantly associated with poor outcome (death). Although myelofibrosis is a highly prevalent feature in this study (94%), its presenee was associated neither with cytopenias nor with poor outcome. Considering the high prevalence of megakaryocytic dysplasia and emperipolesis in the analyzed samples and their relationship with myelofibrosis, we hypothesize that this might be a feasible pathway underlying myelofibrosis in multisystem LCH.
REFERENCES
- 1.Bechan GI, Meeker AK, De Marzo AM, et al. Telomere length shortening in Langerhans cell histiocytosis. Br J Haematol. 2008;140:420–428. doi: 10.1111/j.1365-2141.2007.06904.x. [DOI] [PubMed] [Google Scholar]
- 2.Broadbent Y, Gadner H, Komp DM, Ladisch S. Histiocytosis syndromes in children: II. Approach to the clinical and laboratory evaluation of children with Langerhans cell histiocytosis. Med Pediatr Oncol. 1989;17:492–495. doi: 10.1002/mpo.2950170527. [DOI] [PubMed] [Google Scholar]
- 3.Lahey ME. Histiocytosis X: an analysis of prognostic factors. J Pediatr. 1975;87:184–189. doi: 10.1016/s0022-3476(75)80576-2. [DOI] [PubMed] [Google Scholar]
- 4.Nezelof C, Frileux-Herbet F, Cronier-Sachot J. Disseminated histiocytosis X: analysis of prognostic factors based on a retrospective study of 50 cases. Cancer. 1979;44:1824–1838. doi: 10.1002/1097-0142(197911)44:5<1824::aid-cncr2820440542>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.McClain K, Ramsay NKC, Robinson L, Sundberg RD, Nesbit M., Jr. Bone marrow involvement in histiocytosis X. Med Pediatr Oncol. 1983;11:167–171. doi: 10.1002/mpo.2950110307. [DOI] [PubMed] [Google Scholar]
- 6.Minkov M, Pötschger U, Grois N, Gadner H, Dworzak MN. Bone marrow assessment in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2007;49:694–698. doi: 10.1002/pbc.21227. [DOI] [PubMed] [Google Scholar]
- 7.Surico G, Muggeo P, Rigillo N, Gadner H. Concurrent Langerhans cell histiocytosis and myelodysplasia in children. Med Pediatr Oncol. 2000;35:421–425. doi: 10.1002/1096-911x(20001001)35:4<421::aid-mpo5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Piguet C, Ketterer S, Gilbert B, De Lumley L. Langerhans’ histiocytosis and medullar fibrosis [in French] Arch Pédiatr. 1998;5:1045–1048. [PubMed] [Google Scholar]
- 9.Braier J, Chantada G, Rosso D, et al. Langerhans cell histiocytosis: retrospective evaluation of 123 patients at a single institution. Pediatr Hematol Oncol. 1999;16:377–385. doi: 10.1080/088800199276921. [DOI] [PubMed] [Google Scholar]
- 10.Favara BE, Jaffe R, Egeler RM. Macrophage activation and hemophagocytic syndrome in Langerhans cell histiocytosis: report of 30 cases. Pediatr Dev Pathol. 2002;5:130–140. doi: 10.1007/s10024001-0159-2. [DOI] [PubMed] [Google Scholar]
- 11.Gadner H, Grois N, Pötschger U, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–2562. doi: 10.1182/blood-2007-08-106211. [DOI] [PubMed] [Google Scholar]
- 12.Friebert SE, Shepardson LB, Shurin SB, Rosenthal GE, Rosenthal NS. Pediatric bone marrow cellularity: are we expecting too much? J Pediatr Hematol Oncol. 1998;20:439–443. doi: 10.1097/00043426-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bauermeister DE. Quantification of bone marrow reticulin: abnormal range. Am J Clin Pathol. 1971;56:24–31. doi: 10.1093/ajcp/56.1.24. [DOI] [PubMed] [Google Scholar]
- 14.Humble JG, Jayne WHW, Pulvertaft RJ. Biological interaction between lymphocytes and other cells. Br J Haematol. 1956;2:283–294. doi: 10.1111/j.1365-2141.1956.tb06700.x. [DOI] [PubMed] [Google Scholar]
- 15.Cashell AW, Buss DH. The frequency and significance of megakaryocytic emperipolesis in myeloproliferative and reactive states. Ann Haematol. 1992;64:273–276. doi: 10.1007/BF01695470. [DOI] [PubMed] [Google Scholar]
- 16.Pinckney L, Parker B. Myelosclerosis and myelofibrosis in treated histiocytosis-X. Am J Roentgenol. 1977;129:521–523. doi: 10.2214/ajr.129.3.521. [DOI] [PubMed] [Google Scholar]
- 17.Sartori DJ, Resnick D. Myelofibrosis arising in treated histiocytosis X. Em J Pediatr. 1985;144:200–202. doi: 10.1007/BF00451914. [DOI] [PubMed] [Google Scholar]
- 18.Thiele J, Steinberg T, Hoeppner B, et al. Histo and immunomorphometry of megakaryopoieses in chronic myeloid leukaemia with myelofibrosis and so-called primary (idiopathic) osteo-myelofibrosis/sclerosis. Anal Cell Pathol. 1990;2:215–227. [PubMed] [Google Scholar]
- 19.Thiele J, Kuemmel T, Sander C, Fischer R. Ultrastructure or bone marrow tissue in so-called primary (idiopathic) myelofibrosis-osteomyelosclerosis (agnogenic myeloid metaplasia). I Abnormalities of megakaryopoiesis and thrombocytes. J Submicrosc Cytol Pathol. 1991;23:93–107. [PubMed] [Google Scholar]
- 20.Bobik R, Podolak-Dawidziak M, Kiełbiński M, Jeleń M, Wróbel T. Emperipolesis in megakaryocytes in patients with thrombocytosis in the course of myeloproliferative disorders. Acta Haematol Pol. 1995;26:179–183. [PubMed] [Google Scholar]
- 21.Schmitt A, Jouault H, Guichard J, Wendling F, Drouin A, Cramer EM. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood. 2000;96:1342–1347. [PubMed] [Google Scholar]
- 22.Schmitt A, Drouin A, Massé JM, Guichard J, Shagraoui H, Cramer EM. Polymorphonuclear neutrophil and megakaryocyte mutual involvement in myelofibrosis pathogenesis. Leuk Lymphoma. 2002;43:719–724. doi: 10.1080/10428190290016809. [DOI] [PubMed] [Google Scholar]
- 23.Centurione L, Di Baldassarre A, Zingariello M, et al. Increased and pathologic emperipolesis of neutrophils with megakaryocytes associated with marrow fibrosis in GATA (low) mice. Blood. 2004;104:3573–3580. doi: 10.1182/blood-2004-01-0193. [DOI] [PubMed] [Google Scholar]
- 24.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23:8520–8530. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]