Abstract
Cardiac fibroblasts are the most abundant cell in the mammalian heart. While they have been historically overlooked in terms of functional contributions to development and physiology, cardiac fibroblasts are now front and center. They are currently recognized as key protagonists during both normal development and cardiomyopathy disease, and work together with cardiomyocytes through paracrine, structural, and potentially electrical interactions. However, the lack of specific biomarkers and their heterogeneous nature currently convolutes the study of this dynamic cell lineage; though, efforts to advance marker analysis and lineage mapping technologies are ongoing. These tools will help elucidate the functional significance of fibroblast-cardiomyocyte interactions in vivo and delineate the dynamic nature of normal and pathological cardiac fibroblasts. Since therapeutic promise lies in understanding the interface between developmental biology and the postnatal injury response, future studies to understand the divergent roles played by cardiac fibroblasts both in utero and following cardiac insult are essential.
Keywords: cardiac fibroblast, cardiomyocytes, embryo heart, cardiomyopathy, periostin, cell lineage marking
Introduction
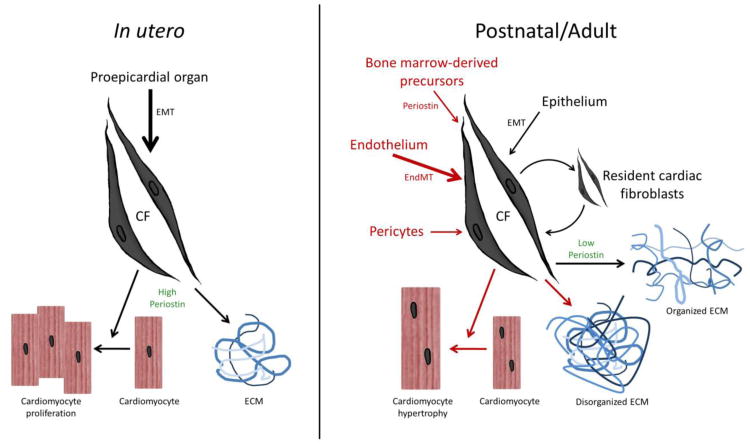
The composition of the mammalian heart is often described in the context of two categories: “cardiomyocytes” and “non-cardiomyocytes”—the majority of which are cardiac fibroblasts. Despite being lumped into the relatively nondescript “non-cardiomyocyte” category, cardiac fibroblasts make up anywhere from one-third to two-thirds of cells in the adult heart depending upon the species. But what are cardiac fibroblasts? Morphologically, they are spindle-shaped cells that often lack a basement membrane and feature a round, elongated nucleus surrounded by vast endoplasmic reticulum; however, there is still discussion surrounding the diverse origins of the cardiac fibroblast lineage. In the mammalian embryo, cardiac fibroblasts are thought to be primarily derived from the proepicardial organ via epithelial-to-mesenchymal transition (EMT) [1-7] although it has also been proposed that there may be some subsequent contribution from bone marrow derived precursors [8,9]. In the adult, it is not well established how the cardiac fibroblast population is maintained during normal homeostasis but it is generally thought that resident fibroblasts and epithelial cells undergoing EMT are the primary source of the low level of turnover within the adult heart [1,9,10]. Conversely, in the event of injury, there is a drastic increase in the number of fibroblasts, which can be derived from multiple sources including endothelial cells undergoing endothelial-to-mesenchymal transition (EndMT) [11], bone-marrow derived circulating progenitor cells (mainly monocytes and fibrocytes) [12-17], and pericytes [18] (reviewed in [9,10,19]). Thus, the cardiac fibroblasts present at birth are not necessarily the same as those present within the adult heart, which may in turn not be the same as those present following injury. Comparison of fibroblasts at these different stages could divulge clues as to the varying functional roles that cardiac fibroblasts play. For example, fibroblasts have been described as lacking a basement membrane [21], but it is unclear if this is universally the situation in embryonic, normal adult, and/or injured adult hearts? The more sophisticated our understanding of fibroblasts within a given environment becomes, the more capable we will be able to modulate their action. The fact that cardiac fibroblasts seem to be derived from different populations under different conditions (summarized in Figure 1) presents a novel potential therapeutic opportunity for the regulation of the fibrotic response following injury. Significantly, inhibiting EndMT following cardiac injury in mice has already been shown to decrease fibrosis throughout the repair process, whilst maintaining tissue homeostasis in the uninjured heart [11].
Figure 1. Comparison of in utreo and postnatal sources and interactions between cardiac fibroblasts, cardiomyocytes and the ECM.
In the developing embryo, the cardiac fibroblast lineage principally originate from the proepicardium via EMT. These in utero fibroblasts express components of the ECM (including fibronectin, collagen and high levels of matricellular proteins such as periostin), integrins and growth factors (i.e. heparin-binding EGF-like factor) that establish a microenvironment in which cardiomyocytes undergo coordinated growth and organogenesis in response to paracrine cardiac fibroblast stimuli [1,22,24]. In contrast, during both postnatal homeostasis and cardiomyopathy, fibroblast development is considerably more plastic. Significantly, postnatal cardiac fibroblasts can be derived from the endothelium via EMT and EndMT, as well as from perivascular cells, circulating monocytes and bone marrow-derived progenitors [19]. Resident cardiac fibroblasts undergo low level turnover and express diminished levels of periostin and ECM constituents. Once more numerous intracellular signaling pathways are activated, in part via paracrine signals from neighboring cardiac fibroblasts and interactions with the disorganized remodeling ECM (including robust deposition of periostin and ECM elements within the scar tissue), to affect cardiac fibroblast migration to the injury site and a coordinated hypertrophic and fibrotic responses within the injured adult heart [22,24].
Functionally, the notion of cardiac fibroblasts has transitioned over the past several years from the classification of fibroblasts as a cell lineage primarily responsible for contributing to the extracellular matrix (ECM) to our current understanding of cardiac fibroblasts as a dynamic, multi-functional lineage critical for both developmental and postnatal repair pathways. Cardiac fibroblasts not only produce and remodel the ECM in response to different physiological cues(reviewed in [1,20-23]), but also regulate cardiomyocyte proliferation and growth during development [24,25], directly connect to cardiomyocytes via connexins [21,26-29], electrically isolate various portions of the conduction system in the heart [1,23,30], secrete factors to regulate signaling of cardiomyocytes in a paracrine fashion (reviewed in[1,9,20,22,23,31]), as well as induce cardiomyocyte hypertrophic and fibrotic responses to injury in the adult heart (reviewed in [19,23]). Within the impressive breadth of cardiac fibroblast functions, this review focuses on the roles of cardiac fibroblasts in the context of development and injury, in order to compare and contrast these two physiologic processes and emphasize the dynamic nature of cardiac fibroblasts.
Cardiac fibroblast markers: attempts at identifying an elusive target
Although a substantial amount of research has been carried out to delineate the pathogenesis of fibrosis following injury in the adult heart and the role of cardiac fibroblasts within that process, we have only just begun to investigate the developmental role of cardiac fibroblasts and how their early role transitions into their adult role. It is the fibroblast's dual role both as a stimulus for growth and proliferation of cardiomyocytes during development and as the initiator of the injurious fibrotic response in pathology of the adult heart that begs the question: what is the key to modulating these two disparate responses? Understanding this physiologic switch could potentially lead to novel treatments aimed at minimizing the morbidity and mortality often associated with myocardial infarctions and heart failure. Despite a growing interest in the field, the study of cardiac fibroblasts has been hindered by the lack of definitive markers that are simultaneously sensitive and specific (reviewed in [1,10,19,32,33]). For example, vimentin exhibits tremendous sensitivity as a fibroblast marker; however, it is not specific as it labels other cell types such as the endothelial lineage. Other markers such as discoidin domain receptor 2 (Ddr-2), fibroblast-specific protein 1 (Fsp-1), Thy1 cell-surface antigen, fibroblast activation protein, and periostin all have similar limitations. For instance, they are also expressed in lineages other than cardiac fibroblasts, and some of these markers (Ddr-2 and Fsp-1 in particular) only mark a small subset of postnatal cardiac fibroblasts (reviewed in [10,19]). In terms of identifying activated fibroblasts (aka myofibroblasts), α-smooth muscle actin (α-SMA) is still the primary marker; however, it is also expressed in vascular smooth muscle cells and pericytes, which are both present in close proximity to fibroblasts, blighting its ability to distinguish fibroblasts in some cases [10,34]. The absence of comprehensive markers has greatly impeded the study of the dynamic functional nature of the cardiac fibroblast lineage as well as the study of the complex interactions between fibroblasts, ECM, and the surrounding cells. Our work suggests that, currently, periostin is the most consistent marker of in utero and early postnatal fibroblasts and is drastically upregulated within the cardiac fibroblast response to injury [1,20,35,36]. Conversely, Fsp-1 is the current best marker of resting and activated cardiac fibroblasts within the adult heart [11]. It may be that the diverse origins of cardiac fibroblasts preclude the discovery of a universal “one size fits all” marker, but by better understanding which markers are reliable under certain conditions and which combinations of markers best encompass the normal, quiescent cardiac fibroblast versus the transient, activated fibroblast, we may begin to probe this intricate system in greater detail and with increased precision.
The paucity of markers to definitively identify cardiac fibroblasts, unlike cardiomyocytes, endothelial, and pericyte lineages at a single time point is one issue facing the field. A related challenge involves finding a way to accurately lineage map cardiac fibroblasts to determine where they come from, how stable they are, and what they give rise to. Presently, there are two lineage reporter lines available for mouse transgenic manipulation and fibroblast identification purposes. Firstly, Fsp-1 (also called S100A4) is considered a marker of fibroblasts in various organs undergoing tissue remodeling, and Fsp1-Cre mice have been used to identify fibroblasts derived from EMT and EndMT in several organs including the adult heart [11]. Using combinatorial Tie1-Cre and Fsp1 lineage mapping approaches, it was elegantly demonstrated that Tie1-expressing endothelial cells undergo TGFβ-mediated EndMT and contribute to the total pool of adult cardiac fibroblasts in response to aortic banding [11]. Similarly, utilizing a partial periostin promoter to drive Cre recombinase expression in a subset of endogenous periostin-expressing cells, we demonstrated that 3.9kbPeriostin-Cre mice are a useful tool to lineage map these elusive cells [35,36]. In combination with the ROSA26-lacZ, ROSA26-YFP and ROSA26-Tomato reporter mouse lines (all readily available from Jackson Labs), 3.9kbPeriostin-Cre positive cardiac fibroblasts have been characterized in the context of the adult heart [35,36]. Significantly, within the heart itself this Cre is expressed (as indicated via ROSA26-lacZ reporter) in all four embryonic valves from E12 onwards, as well as in the suspensory apparatus and the annulus of fetal, newborn, and adult hearts of these mice (Conway lab, unpublished data). Subsequently, 3.9kbPeriostin-Cre is expressed in E16 cardiac fibroblasts, most of the newborn cardiac fibroblasts (∼90% as detected via fluorescence-activated cell sorting using Thy1+ marker), but it is only found in a minority of quiescent cardiac fibroblasts in the uninjured heart of an adult mouse. However, following injury, 3.9kbPeriostin-Cre is robustly induced in adult “activated cardiac fibroblasts.” Importantly, while this Cre is expressed in lineages other than cardiac fibroblasts, like endogenous periostin it is never expressed in cardiomyocytes [37,38] rendering it a useful tool for differentiating fibroblast versus cardiomyocyte interactions. In addition to marking fibroblasts for lineage mapping, the 3.9kbPeriostin-Cre can also be used to knockout or knock-in genes specifically within cardiac fibroblasts but not adjacent cardiomyocytes. Importantly, since 3.9kbPeriostin-Cre positive cardiac fibroblasts can be detected from E16 onward (Conway lab, unpublished data), this tool enables in utero conditional ablation of non-cardiomyocyte genetic targets prior to the postnatal transition. One example of the utility of 3.9kbPeriostin-Cre was demonstrated in Takeda et al. (2010) through the generation of a conditional knockout (cKO) of Klf5 (a transcription factor involved in tissue remodeling) to delineate the roles of fibroblasts versus cardiomyocytes in the context of injury-induced tissue remodeling. When exposed to low-intensity transverse aortic constriction, 3.9kbPeriostin-Cre mediated Klf5 cKO mice demonstrate a decreased level of hypertrophy and fibrosis compared to controls. However, when exposed to high-intensity transverse aortic constriction, the cardiac fibroblast-specific knockouts die more rapidly than controls because they are unable to compensate and manage acute mechanical stress [35]. As cardiomyocyte-specific Klf5 cKO deletion did not alter the hypertrophic responses, these findings clearly demonstrated that Klf5 expression and its resulting effects within fibroblasts can have both adaptive and maladaptive results; it merely depends upon the nature and duration of the insult. Modulation of cardiac fibroblast function may provide a novel strategy for treating patient heart failure, with KLF5 serving as an attractive target.
In utero cardiac fibroblasts and cardiomyocytes: setting the stage for proliferation and heart morphogenesis
Cardiac fibroblasts have been hypothesized to be involved in cardiomyocyte proliferation due to the temporal link between the developmental début of cardiac fibroblasts in the compact myocardium (at E12) and the critical phase of cardiomyocyte proliferation involved in ventricular formation (beginning at E11.5 and continuing throughout gestation) [1,7,24]. Several factors have been implicated in regulating fibroblast differentiation via EMT and colonization of the adjacent myocardium during development including: platelet derived growth factor (PDGF)-B, Sox9, Tbx5, thymosin β4, Ets factors, and fibroblast growth factors (FGFs) [6,22,39,40]. In particular, Fgf10 regulates the migration of epicardially-derived cells destined to become cardiac fibroblasts into the compact myocardium during development by stimulating the fibroblast growth factor receptors: FGFR1 and FGFR2b. The exact downstream mechanism leading to this migration has not been fully elucidated, but FGFs are known to act through Ras/MAK, phospholipase Cγ/Ca2+, and the PI3 kinase/Akt pathways [41]. Inactivation of the critical Fgf10 signaling pathway at either the ligand or receptor level leads to both a decreased number of fibroblasts within the compact myocardium and a smaller heart size overall [41]. Conversely, overexpression of Fgf10 results in an increase in the number of cardiac fibroblasts in the compact myocardium and an increase in heart size [41]. This indicates that the number of cardiac fibroblasts present in the compact myocardium controls overall heart size, possibly via regulation of cardiomyocyte proliferation. While much remains to be uncovered regarding the reciprocal interactions between cardiac fibroblasts and cardiomyocytes leading to the proliferation of the latter, detailed studies by Ieda et al. (2009) implicated a role of fibroblast-secreted factors in regulating the mitotic activity of cardiomyocytes during late embryonic development. Specifically, fibronectin, heparin-binding epidermal growth factor-like growth factor (HBEGF), and the matricellular protein periostin were all found to stimulate cardiomyocyte proliferation through β1 integrin signaling [24]. These factors are of particular interest because of their temporal expression pattern, namely high expression during development and relatively low expression within the adult heart. Due to the fact that cardiomyocytes lose the ability to proliferate at the same developmental time point that the levels of periostin, fibronectin, and HBEGF are decreasing (i.e. postnatally), it is possible that these cardiac fibroblast factors help to regulate the physiologic shift of cardiomyocytes from a proliferative cell type during development to a postnatal lineage that responds to stress with a hypertrophic growth pattern complicated by fibrosis. Similarly, insulin-like growth factor-1 (Igf1), produced and released mainly from in utero cardiac fibroblasts, promotes collagen synthesis by cardiac fibroblasts and can function as a paracrine factor to modulate subsequent cardiac myocyte hypertrophy [42]. As hypertrophy and fibrosis are hallmarks of many cardiac pathologies, understanding how the microenvironment of the heart influences the phenotype of cardiomyocytes could be key to future treatments of various cardiomyopathies.
Postnatal cardiac fibroblasts and cardiomyocytes: potential for reprogramming and regeneration
After birth, tremendous plasticity is observed within the early postnatal heart. The heart adapts to the substantial increase in systolic pressures by increasing the thickness of the ventricular wall as well as its tensile strength; the latter of which is thought to be largely achieved by a two-fold increase in the number of cardiac fibroblasts [43]. The ECM components also reorganize to better distribute the force that is subsequently generated by the postnatal ventricle [44]. This period of active remodeling lasts for the first week of life and then the mouse heart transitions relatively rapidly to the adult phenotype which becomes fully apparent 30 days after birth [8]. During this dynamic restructuring process, the ECM and cardiomyocytes are not merely adapting to the changing environment independently, but are synergistically modifying how they interact with each other. For example, neonatal cardiomyocytes are capable of adhering to ECM components of either the basement membrane or the interstitial matrix, whereas adult cardiomyocyte attachments are restricted to basement membrane specific components [44]. In rats, the switch from a hyperplastic (increase in cell number) to a hypertrophic (increase in cell size) response in injured/stressed cardiomyocytes occurs between postnatal day 3 and 4 [45]. This finding has been corroborated in mice by demonstrating the capacity of 1-day-old mice to recover from apical cardiac resection via full regeneration of functionally and histologically normal cardiomyocytes by 21 days post resection [46]. This regenerative ability was lost by 7 days after birth as apical cardiac resection of 7-day-old pups resulted in massive fibrosis without any evidence of attendant cardiomyocyte proliferation. The one-week window of cardiac regeneration correlates with the time-point at which rodent cardiomyocytes are reported to exit the cell cycle and undergo proliferative arrest [45].
The allure of harnessing cardiac regenerative capacity as a means to alleviating the damage caused by myocardial infarction and other fibrotic pathologies that all too often affect the heart has been a long-time aspiration that is slowly being realized. Developmental biology research has opened important avenues for converting fully differentiated cells into various lineages via reprogramming technologies. As a crucial player in cardiac development, the cardiac fibroblast has recently been exploited as a key target to accomplish cardiac regeneration. Expression of three transcription factors (Gata4, Mef2c, Tbx5, collectively referred to as GMT) mediated by retroviral gene transfer was shown to be sufficient to directly reprogram adult fibroblasts to become adult cardiomyocytes both in vitro [47], and, most significantly, in vivo [36]. Identifying the derivatives of these reprogrammed cardiac fibroblasts was accomplished using the 3.9kbPeriostin-Cre and Fsp1-Cre lineage reporters, in concert with various lacZ and fluorescent indicator mice [36]. Importantly, it was demonstrated that in the absence of genetic reprogramming, no cardiomyocytes expressed any lacZ either before or after myocardial infarct; however, the retroviral-induced GMT cardiac fibroblasts were able to give rise to lacZ-positive cardiomyocyte-like cells, suggesting that these cells were derived from reprogrammed cardiac fibroblasts [36]. Not only did the reprogrammed fibroblasts differentiate into cardiomyocyte-like cells, but these in vivo studies revealed that cellular reprogramming post-myocardial infarction resulted in improved cardiac function [36]. More recently, miRNAs have been used to facilitate conversion of neonatal and adult cardiac fibroblasts into adult cardiomyocytes [48]. While both of these techniques show promise for increasing the regenerative capacity of the heart following ischemic injury, their in vitro efficiencies are low. Interestingly, both groups observed an enhancement in vivo either through increased reprogramming efficiency [48] or through the presence of a more “fully reprogrammed” phenotype [36]. A better understanding of the paracrine and ECM interactions of cardiac fibroblast, cardiomyocyte, and endothelial lineages is crucial to regenerating a stable myocardium that responds appropriately to stimuli. Further work is needed to evaluate the safety of both of these approaches particularly in the context of arrhythmogenesis; however, an understanding of which cardiac fibroblast-specific factors are capable of enhancing the success of regeneration following ischemic injury will undoubtedly be valuable in optimizing these exciting technologies and therapeutic opportunities.
Pathology: when well-intentioned repair goes wrong
Dynamic cross-talk between cardiomyocytes and cardiac fibroblasts is a prominent feature of both development as well as injury-induced remodeling [31,49]. Throughout life, cardiac fibroblasts are responsible for controlling many aspects of the heart's microenvironment. During development, this includes secreting factors conducive to cardiomyocyte proliferation and establishment of a functionally competent ventricle, providing the structural stability required for transitioning from pre- to postnatal life, and directly coupling to cardiomyocytes via gap junctions [26,50]. The paracrine, structural, and possibly electrical interactions between fibroblasts and cardiomyocytes that underlie normal development also modulate the pathological responses to injury in the adult heart. During adult heart failure, fibroblasts secrete several proinflammatory cytokines that directly promote hypertrophy of cardiomyocytes including: IL-1β, IL-6, TNF-α and TGFβ [31]. Cardiomyocytes also secrete some of these same cytokines, which induce fibroblast migration, stimulate transformation of fibroblasts into myofibroblasts (TGFβ1 in particular), and increase synthesis of several ECM components [1,22,31]. Similarly, angiotensin II type-1 receptors on neighboring cardiomyocytes play an important role in determining the behavior of cardiac fibroblasts in the early phase of cardiac remodeling [51]. This role of activated cardiomyocytes driving fibrosis is in agreement with transgenic overexpression studies using activated forms of calcineurin or calcium-dependent signal-transducing molecules [52]. Thus, intracellular signaling crosstalk creates an environment where cardiac fibroblasts and cardiomyocytes reciprocally influence each other's phenotype [19]. Fibroblasts have also been shown to have extensive electrical coupling to each other as well as to cardiomyocytes via gap junctions in vitro during both developmental stages as well as following injury [26-29]; however, the functional significance of these interactions has yet to be established in vivo. Increasingly available information relating to the dynamic nature of the cardiac microenvironment further alludes to the possibility that cardiac fibroblasts may be a key regulatory cell capable of mediating in vivo communication to cardiomyocytes, which is critical for both normal heart development and facilitating pharmacological therapeutic approaches within the heart [31].
Electrophysiological coupling
Although cardiac fibroblasts have traditionally been thought of as electrically inert, in vitro data suggests that they may be intimately involved in the electrophysiological coupling of the heart [26-29]. Cardiac fibroblasts can express connexin (Cx) 43, 45, and by some reports Cx40 as well [31]. Each of these connexins can facilitate cellular coupling and communication of fibroblasts to other fibroblasts and to cardiomyocytes [26,50] although Cx43 seems to be the most functionally relevant in the context of development [53] and injury [28,31,54]. Significantly, the main connexins expressed within the heart are Cx40, 43 and 45. During development, altered distribution of Cx43 correlates with alterations of the velocity and directionality of action potential propagation in the mammalian heart [53]. Less is known about direct electrical effects of cardiomyocytes on cardiac fibroblasts. This could be due to the fact that it is more technically feasible to measure alterations in an electrically dynamic system such as a cardiomyocyte undergoing an action potential than in the more subtle electrical system of a fibroblast or perhaps this simply has not been investigated. In the context of injury, cardiac fibroblasts respond to increases in TGFβ levels via an increase in their expression of Cx43 in vitro. As a result, these Cx43-high fibroblasts are able to more aptly modulate electrophysiological parameters of cocultured cardiomyocytes [27,28,31]. The resulting increase in cellular coupling actually decreases the conduction velocity and induces longer action potential durations at high densities of fibroblasts, thus the electrophysiological role of fibroblasts appears to be highly density dependent [27]. Whether these in vitro findings are representative of the actual electrophysiological environment in vivo has yet to be established [55], but the level of Cx expression in fibroblasts does seem to be physiologically relevant in mouse models. During post-myocardial infarction, increased expression of Cx43 in cardiac fibroblasts coupled with the decreased Cx43 expression in cardiomyocytes is thought to produce a labile electrical environment that is prone to potentially fatal arrhythmogenesis [28]. The risk of arrhythmia can be decreased by reintroducing embryonic cardiomyocytes (expressing Cx43) post-infarction [56], thus modulating Cx43 expression levels may be clinically advantageous. In addition to the electrical implications of Cx43 expression, Cx43 levels have also been established to influence cellular proliferation and alter cellular behavior through paracrine signaling. When cocultured fibroblasts and cardiomyocytes are exposed to an antibody targeting Cx43, an increase in TNF-α was observed while IL-6 levels decreased [57]. Moreover, reduced Cx43 levels have also been shown to increase proliferation of fibroblasts whereas increased expression leads to reduced proliferation [54], which could indirectly affect cardiomytocytes. While a direct electrical exchange between fibroblasts and cardiomyocytes has yet to be recorded in vivo, as techniques for identifying and transgenically manipulating cardiac fibroblasts improve, it will become feasible to address the functional significance of Cx43-mediated electrical milieu both in utero and within the post-injury state.
TGFβ signaling and periostin
TGFβ signaling is known to play a key prominent role in both cardiac development and in response to injury. Developmentally, the TGFβ superfamily signaling pathway is important for regulating structural aspects of heart development including valvulogenesis and outflow tract remodeling, as well as integration of several key canonical and non-conical developmental gene pathways [58,59]. In terms of cardiac fibroblast lineage, a significant TGFβ-responsive downstream effector of TGFβ signaling is the matricellular protein periostin [1,12,20,60-63]. Matricelluar proteins are classed as proteins secreted into the ECM that modulate cell-to-cell as well as cell-to-matrix interactions but are not directly involved in ECM structure and mechanical organization [62]. Periostin is an inducible regulator of ECM homeostasis, cellular differentiation, cellular migration, and tissue maturation (reviewed in [20,62,65,66]). It is highly expressed during development (beginning at E9.5 and peaking in the early postnatal period), then levels drop and are maintained at a low levels throughout adult life until an injury is sustained [20,65,66]. Upon injury, TGFβ1 which is also normally expressed at low levels in the adult heart is rapidly secreted. This increase in TGFβ signaling causes an 8-40 fold increase in periostin expression levels and deposition within the injury site [12,60-63]. Once expressed, periostin appears to functions in the adult heart as it did during development: it regulates ECM homeostasis, induces cellular migration, and is involved in establishing the balance between cardiomyocytes and fibroblasts by promoting differentiation to the fibroblast lineage while inhibiting differentiation to the cardiomyocyte lineage [8,12,66]. In what has been coined the “periostin hypothesis,” researchers have speculated that when periostin is induced by TGFβ following a myocardial infarction, it channels the hematopoietic stem cells (HSCs) migrating from the bone marrow into the cardiac fibroblast lineage as opposed to the cardiomyocyte lineage [12,67-69]. Since HSCs are capable of differentiating into cardiomyocytes and repairing myocardial infarctions [70-75], periostin may be impeding a naturally regenerative process and could potentially be targeted for pharmacologic intervention post-myocardial infarction. As a caveat to altering periostin levels with the intent of achieving a physiological effect, it is essential to remember that because periostin modulates ECM remodeling following injury, a certain level of periostin is required to maintain structural integrity of the infarcted wall [8,76]. Systemic Periostin null mice die from post-myocardial infarction cardiac rupture at a drastically increased rate compared to wild type controls due to a structurally weakened ventricular wall facilitated by the decreased number of cardiac fibroblasts as well as the decreased collagen fiber amount, cross-sectional area, and cross-linking observed in the infarcts of Periostin null mice [76].
In addition to upregulating periostin expression, TGFβ is also responsible for initiating the cardiac fibroblast-myofibroblast transition (CMT) [9,77-80]. Myofibroblasts, a term introduced by Gabbiani, are not present in the healthy heart (with the exception of the valve leaflets) but are efficiently derived from cardiac fibroblasts and their precursors (epithelial cells (via EMT) and bone marrow progenitor cells) following injury [9]. Myofibroblasts are not dissimilar to fibroblasts morphologically with their ruffled membranes and highly active endoplasmic reticulum; however, these two cell populations are distinguished by the expression of α-SMA present in myofibroblasts but not in resting cardiac fibroblasts [1,10,78,81]. Upon injury, TGFβ1 is secreted by infiltrating lymphocytes, platelets, and activated macrophages as well as injured cardiomyocytes and cardiac fibroblasts [79]. This cytokine induces a signaling cascade within fibroblasts that result in increased transcription of genes such as those encoding α-SMA, vimentin, the embryonic form of smooth muscle myosin heavy chain (Smemb), focal adhesion proteins including paxillin, tensin, and fibronectin, as well as collagen and collagenase [78,79]. Thus, CMT is initiated and a myofibroblast is born. The significance of myofibroblasts in the context of injury lies in the fact that they are extremely responsive to chemokines released at the site of injury, migrate into injured myocardium, and deposit large amounts of collagen. The timeline of this dynamic subpopulation is not well characterized but myofibroblasts are reported to be activated by 48 hours, peak in number anywhere from 5-14 days, and decrease 21-28 days after injury [78].
TGFβ regulates injury response through its dual role in reactivating developmental genes such as periostin as well as by inducing CMT and its associated physiological alterations, including ECM remodeling and increased collagen production [20,78,79]. Initially, this response is protective. In fact, it has been discovered that delivering exogenous TGFβ coinciding with the reperfusion step of an ischemia-reperfusion model in rats results in decreased levels of creatine kinase indicating that fewer cardiomyocytes are lost as a result of the injury itself [79]. TGFβ initially stimulates its own synthesis and secretion until the excess begins binding to proteoglycans which then terminates the self-inducing loop by ceasing TGFβ production [82]. However, chronic increases in TGFβ levels can inactivate this protective feedback mechanism and the response can become maladaptive and lead to overt fibrosis, hypertrophy, and eventual heart failure [21,79,81]. Therefore, as with most key signaling pathways, an intricate balance of TGFβ levels must be achieved in order to maximize the benefit and minimize the damage to the heart. Contemplating pharmacological intervention is further complicated by the presence of three different isoforms of TGFβ. Unfortunately, delineating isoform specific roles of TGFβ family members is presently difficult [59]. Currently a lot of published work addressing cardiac insult either does not specify which isoform of TGFβ is acting or only investigates TGFβ1 [81]. There are several reasons for the present limitations, including the lack of isoform specific antibodies, the difficulties regulating TGFβ levels, and cross-talk with the BMP pathway. Additionally, the study of TGFβ1 is more robust due to the availability of genetic mouse models. Systemic knockouts of TGFβ1 are viable postnatally if kept on immunodeficient genetic background while knockouts of isoforms β2 (die due to cardiac defects) and β3 (die due to cleft palate defects) are not (reviewed in [59]). Also, a conditional knockout model of TGFβ1 [81,83] has been available for some time, whereas a cKO model for TGFβ3 was recently published this year [84], and there is still no published cKO of TGFβ2. Due to the fact that TGFβ2 and 3 are also highly expressed in cardiac fibroblasts during development [85], it is possible that these isoforms play a more prominent role in heart disease and injury repair than currently appreciated. As new tools continue to be developed, perhaps these questions will begin to be answered and will further emphasize the importance of using developmental biology to inform our therapeutic approaches to targeting postnatal cardiovascular disease.
Conclusion
Cardiac fibroblasts comprise a substantial component of the mammalian heart and are intimately involved in both normal cardiac development and injury through paracrine, mechanical, and potentially electrical interactions with cardiomyocytes. While there has been a steady increase in research investigating these interactions, further in vivo work is critical for addressing the functional contribution of each element both in utero and following injury to more aptly describe the dynamic roles of cardiac fibroblasts in development and disease. Obstacles such as the absence of a comprehensive cardiac fibroblast marker have hindered in vivo analysis of these interactions to date; however, promising new techniques such as utilizing the 3.9kbPeriostin-Cre and Fsp1-Cre lines for lineage mapping and genetic modification of in utero and adult cardiac fibroblasts, as well as an increasing number of fibroblast markers are emerging to help address these challenges. Harnessing these new tools to examine the developmental origins of these cardiac fibroblast-cardiomyocyte and cardiomyocyte-cardiac fibroblast interactions and how they influence injury response may uncover methods of shifting pharmacologic interventions from a reactionary focus designed merely to consolidate damage to a more proactive approach aimed at regeneration and undoing the damage caused by injury.
Acknowledgments
We thank the members of the Conway lab, Dr. Mohamad Azhar and the reviewers for their insightful comments. These studies were supported, in part, by Riley Children's Foundation, and Indiana University Department of Pediatrics (Neonatal-Perinatal Medicine) and National Institute of Health [HL60714] to SJC.
References
- 1.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105(10):934–947. doi: 10.1161/CIRCRESAHA.109.201400. 105/10/934 [pii] 10.1161/CIRCRESAHA.109.201400 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gittenberger-de Groot AC VPM, Mentink MMT, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 3.Kolditz DP, Wijffels MC, Blom NA, van der Laarse A, Hahurij ND, Lie-Venema H, Markwald RR, Poelmann RE, Schalij MJ, Gittenberger-de Groot AC. Epicardium-derived cells in development of annulus fibrosis and persistence of accessory pathways. Circulation. 2008;117(12):1508–1517. doi: 10.1161/CIRCULATIONAHA.107.726315. CIRCULATIONAHA.107.726315 [pii] 10.1161/CIRCULATIONAHA.107.726315 [doi] [DOI] [PubMed] [Google Scholar]
- 4.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174(2):221–232. doi: 10.1006/dbio.1996.0068. S0012-1606(96)90068-8 [pii] 10.1006/dbio.1996.0068 [doi] [DOI] [PubMed] [Google Scholar]
- 5.Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46(8):1005–1013. [PubMed] [Google Scholar]
- 6.Lie-Venema H, van den Akker NM, Bax NA, Winter EM, Maas S, Kekarainen T, Hoeben RC, deRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. ScientificWorldJournal. 2007;7:1777–1798. doi: 10.1100/tsw.2007.294. 10.1100/tsw.2007.294 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, Dettman RW, Burch JB. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol. 366(2):111–124. doi: 10.1016/j.ydbio.2012.04.020. S0012-1606(12)00209-6 [pii] 10.1016/j.ydbio.2012.04.020 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. 1123/1/30 [pii] 10.1196/annals.1420.005 [doi] [DOI] [PubMed] [Google Scholar]
- 9.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105(12):1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. 105/12/1164 [pii] 10.1161/CIRCRESAHA.109.209809 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 107(11):1304–1312. doi: 10.1161/CIRCRESAHA.110.231910. 107/11/1304 [pii] 10.1161/CIRCRESAHA.110.231910 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. nm1613 [pii] 10.1038/nm1613 [doi] [DOI] [PubMed] [Google Scholar]
- 12.Visconti RP, Markwald RR. Recruitment of new cells into the postnatal heart: potential modification of phenotype by periostin. Ann N Y Acad Sci. 2006;1080:19–33. doi: 10.1196/annals.1380.003. 1080/1/19 [pii] 10.1196/annals.1380.003 [doi] [DOI] [PubMed] [Google Scholar]
- 13.Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, Drake CJ, Ogawa M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp Hematol. 2006;34(2):219–229. doi: 10.1016/j.exphem.2005.10.008. S0301-472X(05)00502-3 [pii] 10.1016/j.exphem.2005.10.008 [doi] [DOI] [PubMed] [Google Scholar]
- 14.Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98(5):690–696. doi: 10.1161/01.RES.0000207384.81818.d4. 01.RES.0000207384.81818.d4 [pii] 10.1161/01.RES.0000207384.81818.d4 [doi] [DOI] [PubMed] [Google Scholar]
- 15.van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214(3):377–386. doi: 10.1002/path.2281. 10.1002/path.2281 [doi] [DOI] [PubMed] [Google Scholar]
- 16.Endo J, Sano M, Fujita J, Hayashida K, Yuasa S, Aoyama N, Takehara Y, Kato O, Makino S, Ogawa S, Fukuda K. Bone marrow derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation. 2007;116(10):1176–1184. doi: 10.1161/CIRCULATIONAHA.106.650903. CIRCULATIONAHA.106.650903 [pii] 10.1161/CIRCULATIONAHA.106.650903 [doi] [DOI] [PubMed] [Google Scholar]
- 17.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103(48):18284–18289. doi: 10.1073/pnas.0608799103. 0608799103 [pii] 10.1073/pnas.0608799103 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24(7):909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 19.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 225(3):631–637. doi: 10.1002/jcp.22322. 10.1002/jcp.22322 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102(7):752–760. doi: 10.1161/CIRCRESAHA.107.159517. CIRCRESAHA.107.159517 [pii] 10.1161/CIRCRESAHA.107.159517 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291(3):H1015–1026. doi: 10.1152/ajpheart.00023.2006. 00023.2006 [pii] 10.1152/ajpheart.00023.2006 [doi] [DOI] [PubMed] [Google Scholar]
- 22.Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 106(1):47–57. doi: 10.1161/CIRCRESAHA.109.207456. 106/1/47 [pii] 10.1161/CIRCRESAHA.109.207456 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda N, Manabe I. Cellular Interplay between Cardiomyocytes and Nonmyocytes in Cardiac Remodeling. Int J Inflam. 2011:535241. doi: 10.4061/2011/535241. 10.4061/2011/535241 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16(2):233–244. doi: 10.1016/j.devcel.2008.12.007. S1534-5807(08)00517-0 [pii] 10.1016/j.devcel.2008.12.007 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noseda M, Schneider MD. Fibroblasts inform the heart: control of cardiomyocyte cycling and size by age-dependent paracrine signals. Dev Cell. 2009;16(2):161–162. doi: 10.1016/j.devcel.2009.01.020. S1534-5807(09)00048-3 [pii] 10.1016/j.devcel.2009.01.020 [doi] [DOI] [PubMed] [Google Scholar]
- 26.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93(5):421–428. doi: 10.1161/01.RES.0000089258.40661.0C. 10.1161/01.RES.0000089258.40661.0C [doi] 01.RES.0000089258.40661.0C [pii] [DOI] [PubMed] [Google Scholar]
- 27.Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC. Morley GE Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res. 107(8):1011–1020. doi: 10.1161/CIRCRESAHA.110.227421. CIRCRESAHA.110.227421 [pii] 10.1161/CIRCRESAHA.110.227421 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol. 19(6):e233–240. doi: 10.1016/j.carpath.2009.12.002. S1054-8807(09)00154-9 [pii] 10.1016/j.carpath.2009.12.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98(6):801–810. doi: 10.1161/01.RES.0000214537.44195.a3. 01.RES.0000214537.44195.a3 [pii] 10.1161/01.RES.0000214537.44195.a3 [doi] [DOI] [PubMed] [Google Scholar]
- 30.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20(2 Pt 2):397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 31.Ottaviano FG, Yee KO. Communication signals between cardiac fibroblasts and cardiac myocytes. J Cardiovasc Pharmacol. 57(5):513–521. doi: 10.1097/FJC.0b013e31821209ee. 10.1097/FJC.0b013e31821209ee [doi] [DOI] [PubMed] [Google Scholar]
- 32.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. nrc1877 [pii] 10.1038/nrc1877 [doi] [DOI] [PubMed] [Google Scholar]
- 33.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99(20):12877–12882. doi: 10.1073/pnas.162488599. 10.1073/pnas.162488599 [doi] 162488599 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber KT. Monitoring tissue repair and fibrosis from a distance. Circulation. 1997;96(8):2488–2492. [PubMed] [Google Scholar]
- 35.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 120(1):254–265. doi: 10.1172/JCI40295. 40295 [pii] 10.1172/JCI40295 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD. Srivastava D In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. doi: 10.1038/nature11044. nature11044 [pii] 10.1038/nature11044 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JB, Firulli AB, Conway SJ. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol. 2007;307(2):340–355. doi: 10.1016/j.ydbio.2007.04.041. S0012-1606(07)00864-0 [pii] 10.1016/j.ydbio.2007.04.041 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev. 2001;103(1-2):183–188. doi: 10.1016/s0925-4773(01)00356-2. S0925477301003562 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Lie-Venema H, Gittenberger-de Groot AC, van Empel LJ, Boot MJ, Kerkdijk H, de Kant E, DeRuiter MC. Ets-1 and Ets-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circ Res. 2003;92(7):749–756. doi: 10.1161/01.RES.0000066662.70010.DB. 10.1161/01.RES.0000066662.70010.DB [doi] 01.RES.0000066662.70010.DB [pii] [DOI] [PubMed] [Google Scholar]
- 40.Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 108(12):e15–26. doi: 10.1161/CIRCRESAHA.110.235531. CIRCRESAHA.110.235531 [pii] 10.1161/CIRCRESAHA.110.235531 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vega-Hernandez M, Kovacs A, De Langhe S, Ornitz DM. FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development. 138(15):3331–3340. doi: 10.1242/dev.064410. 138/15/3331 [pii] 10.1242/dev.064410 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horio T, Maki T, Kishimoto I, Tokudome T, Okumura H, Yoshihara F, Suga S, Takeo S, Kawano Y, Kangawa K. Production and autocrine/paracrine effects of endogenous insulin-like growth factor-1 in rat cardiac fibroblasts. Regul Pept. 2005;124(1-3):65–72. doi: 10.1016/j.regpep.2004.06.029. S0167-0115(04)00233-2 [pii] 10.1016/j.regpep.2004.06.029 [doi] [DOI] [PubMed] [Google Scholar]
- 43.Borg TK, Ranson WF, Moslehy FA, Caulfield JB. Structural basis of ventricular stiffness. Lab Invest. 1981;44(1):49–54. [PubMed] [Google Scholar]
- 44.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984;104(1):86–96. doi: 10.1016/0012-1606(84)90038-1. 0012-1606(84)90038-1 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol. 1996;271(5 Pt 2):H2183–2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 46.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 331(6020):1078–1080. doi: 10.1126/science.1200708. 331/6020/1078 [pii] 10.1126/science.1200708 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 142(3):375–386. doi: 10.1016/j.cell.2010.07.002. S0092-8674(10)00771-3 [pii] 10.1016/j.cell.2010.07.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-Mediated In Vitro and In Vivo Direct Reprogramming of Cardiac Fibroblasts to Cardiomyocytes. Circ Res. doi: 10.1161/CIRCRESAHA.112.269035. CIRCRESAHA.112.269035 [pii] 10.1161/CIRCRESAHA.112.269035 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. CIRCULATIONAHA.110.982777 [pii] 10.1161/CIRCULATIONAHA.110.982777 [doi] [DOI] [PubMed] [Google Scholar]
- 50.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn. 2004;230(4):787–794. doi: 10.1002/dvdy.20095. 10.1002/dvdy.20095 [doi] [DOI] [PubMed] [Google Scholar]
- 51.Matsusaka T, Katori H, Inagami T, Fogo A, Ichikawa I. Communication between myocytes and fibroblasts in cardiac remodeling in angiotensin chimeric mice. J Clin Invest. 1999;103(10):1451–1458. doi: 10.1172/JCI5056. 10.1172/JCI5056 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–228. doi: 10.1016/s0092-8674(00)81573-1. S0092-8674(00)81573-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Litchenberg WH, Norman LW, Holwell AK, Martin KL, Hewett KW, Gourdie RG. The rate and anisotropy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern. Cardiovasc Res. 2000;45(2):379–387. doi: 10.1016/s0008-6363(99)00363-6. S0008-6363(99)00363-6 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, Yamada KA. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15(3):289–303. doi: 10.1080/15419060802198736. 904168828 [pii] 10.1080/15419060802198736 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camelliti P, Green CR, Kohl P. Structural and functional coupling of cardiac myocytes and fibroblasts. Adv Cardiol. 2006;42:132–149. doi: 10.1159/000092566. 92566 [pii] 10.1159/000092566 [doi] [DOI] [PubMed] [Google Scholar]
- 56.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Doran R, Becher UM, Hwang SM, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450(7171):819–824. doi: 10.1038/nature06321. nature06321 [pii] 10.1038/nature06321 [doi] [DOI] [PubMed] [Google Scholar]
- 57.Bowers SL, Borg TK, Baudino TA. The dynamics of fibroblast-myocyte-capillary interactions in the heart. Ann N Y Acad Sci. 1188:143–152. doi: 10.1111/j.1749-6632.2009.05094.x. NYAS5094 [pii] 10.1111/j.1749-6632.2009.05094.x [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conway SJ, Doetschman T, Azhar M. The inter-relationship of periostin, TGF beta, and BMP in heart valve development and valvular heart diseases. ScientificWorldJournal. 2011;11:1509–1524. doi: 10.1100/tsw.2011.132. 10.1100/tsw.2011.132 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doetschman T, Barnett JV, Runyan RB, Camenisch TD, Heimark RL, Granzier HL, Conway SJ, Azhar M. Transforming growth factor beta signaling in adult cardiovascular diseases and repair. Cell Tissue Res. 2012;347(1):203–223. doi: 10.1007/s00441-011-1241-3. 10.1007/s00441-011-1241-3 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110(13):1806–1813. doi: 10.1161/01.CIR.0000142607.33398.54. 10.1161/01.CIR.0000142607.33398.54 [doi] 01.CIR.0000142607.33398.54 [pii] [DOI] [PubMed] [Google Scholar]
- 61.Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SW, Chen YF. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42(1):88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. 10.1161/01.HYP.0000074905.22908.A6 [doi] 01.HYP.0000074905.22908.A6 [pii] [DOI] [PubMed] [Google Scholar]
- 62.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 68(19):3201–3207. doi: 10.1007/s00018-011-0784-5. 10.1007/s00018-011-0784-5 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT. Altered patterns of gene expression in response to myocardial infarction. Circ Res. 2000;86(9):939–945. doi: 10.1161/01.res.86.9.939. [DOI] [PubMed] [Google Scholar]
- 64.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14(5):608–616. doi: 10.1016/s0955-0674(02)00361-7. S0955067402003617 [pii] [DOI] [PubMed] [Google Scholar]
- 65.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9(8):548–555. doi: 10.2174/138920208786847917. 10.2174/138920208786847917 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal. 2009;3(3-4):275–286. doi: 10.1007/s12079-009-0063-5. 10.1007/s12079-009-0063-5 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5(4):329–339. doi: 10.1016/s1535-6108(04)00081-9. S1535610804000819 [pii] [DOI] [PubMed] [Google Scholar]
- 68.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002;62(18):5358–5364. [PubMed] [Google Scholar]
- 69.Kim CJ, Yoshioka N, Tambe Y, Kushima R, Okada Y, Inoue H. Periostin is down-regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells. Int J Cancer. 2005;117(1):51–58. doi: 10.1002/ijc.21120. 10.1002/ijc.21120 [doi] [DOI] [PubMed] [Google Scholar]
- 70.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. discussion 229-230. [DOI] [PubMed] [Google Scholar]
- 71.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. 10.1038/35070587 [doi] 35070587 [pii] [DOI] [PubMed] [Google Scholar]
- 72.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. 10.1172/JCI12150 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. 10.1038/86498 [doi] 86498 [pii] [DOI] [PubMed] [Google Scholar]
- 74.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108(17):2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. 10.1161/01.CIR.0000099501.52718.70 [doi] 01.CIR.0000099501.52718.70 [pii] [DOI] [PubMed] [Google Scholar]
- 75.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. 10.1038/nature00870 [doi] nature00870 [pii] [DOI] [PubMed] [Google Scholar]
- 76.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205(2):295–303. doi: 10.1084/jem.20071297. jem.20071297 [pii] 10.1084/jem.2007.1297 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan YH, Dong H, Pan Q, Cao YJ, Li H, Wang HC. Notch signaling may negatively regulate neonatal rat cardiac fibroblast-myofibroblast transformation. Physiol Res. 60(5):739–748. doi: 10.33549/physiolres.932149. 932149 [pii] [DOI] [PubMed] [Google Scholar]
- 78.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 57(4):376–379. doi: 10.1097/FJC.0b013e3182116e39. 10.1097/FJC.0b013e3182116e39 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71(1-2):418–435. doi: 10.1006/mgme.2000.3032. 10.1006/mgme.2000.3032 [doi] S1096-7192(00)93032-4 [pii] [DOI] [PubMed] [Google Scholar]
- 80.Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 301(6):G950–955. doi: 10.1152/ajpgi.00132.2011. ajpgi.00132.2011 [pii] 10.1152/ajpgi.00132.2011.[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, Ahamed J. Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119(4):1064–1074. doi: 10.1182/blood-2011-09-377648. blood-2011-09-377648 [pii] 10.1182/blood-2011-09-377648 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bai D, Gao Q, Li C, Ge L, Gao Y, Wang H. A conserved TGFbeta1/HuR feedback circuit regulates the fibrogenic response in fibroblasts. Cell Signal. 24(7):1426–1432. doi: 10.1016/j.cellsig.2012.03.003. S0898-6568(12)00080-0 [pii] 10.1016/j.cellsig.2012.03.003 [doi] [DOI] [PubMed] [Google Scholar]
- 83.Azhar M, Yin M, Bommireddy R, Duffy JJ, Yang J, Pawlowski SA, Boivin GP, Engle SJ, Sanford LP, Grisham C, Singh RR, Babcock GF, Doetschman T. Generation of mice with a conditional allele for transforming growth factor beta 1 gene. Genesis. 2009;47(6):423–431. doi: 10.1002/dvg.20516. 10.1002/dvg.20516 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doetschman T, Georgieva T, Li H, Reed TD, Grisham C, Friel J, Estabrook MA, Gard C, Sanford LP, Azhar M. Generation of mice with a conditional allele for the transforming growth factor beta3 gene. Genesis. 50(1):59–66. doi: 10.1002/dvg.20789. 10.1002/dvg.20789 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]