Abstract
Background
Cellular treatments for repairing diseased tissues represent a promising clinical strategy. Umbilical cord tissue-derived cells (UTC) are a unique source of cells with a low immunogenic profile and potential for tissue repair. By using UTC from miniature swine, we previously demonstrated that despite their low immunogenic phenotype, UTC could induce an immune response under certain inflammatory conditions and after multiple subcutaneous (SC) injections. Given that repeat dosing of cells may be necessary to achieve a lasting therapeutic benefit, in this study, we examined approaches to avoid an immune response to multiple SC injections of UTC.
Methods
By using in vitro and in vivo measures of sensitization to SC cellular injections, we assessed the effects of varying the location of administration site, prolongation of timing between injections, and use of immunosuppressive treatments on repeated cellular injections in Massachusetts General Hospital major histocompatibility complex-defined miniature swine.
Results
Although under normal conditions, a single SC injection of major histocompatibility complex-mismatched UTC did not induce a detectable immune response, multiple SC injections of UTC demonstrated rapid humoral and cell-mediated immune responses. Avoidance of an immune response to repeat SC injection was achieved by concurrent immunosuppression with each dose of UTC.
Conclusions
UTC and other similar cell types believed to be nonimmunogenic have the potential to induce immune responses under certain conditions. These studies provide important considerations and guidelines for preclinical studies investigating allogeneic cellular therapies.
Keywords: Umbilical cord tissue derived cells, Immunogenicity, Miniature swine, Cellular therapy
Umbilical cord tissue-derived cells (UTC) were targeted as a source of multipotent progenitor cells due to the immune-privileged nature of the maternal-fetal interface (1). UTC have been believed to have a low immunogenic profile because they lack major histocompatibility complex (MHC) II expression and have a low expression of MHC I (2). Several studies have suggested that cells with a similar MHC expression pattern engraft in tissues and cause functional improvement without the need for immunosuppression and without evidence of an immune response in vitro or in vivo across xenogeneic barriers and the “blood brain barrier” (3–7). Investigations using such sources as mesenchymal stem cells (MSC) often report that immune responses do not occur after administration based on the lack of T-cell proliferation in mixed lymphocyte reaction assays or the absence of cellular infiltrate at sites of injections using allogeneic and xenogeneic cells (3–8). In one animal model using porcine bone marrow-derived MSC, in vitro studies using measurement of T-cell proliferation and Th1 and Th2 cytokines by mixed lymphocyte reaction and enzyme-linked immunosorbent assay suggested that porcine bone marrow-derived MSC were nonimmunogenic. However, intracardiac injection of these same cells induced an immune response in vivo when administered across allogeneic barriers (9).
By using a porcine UTC (pUTC), we previously demonstrated that these cells induce an immune response when injected into an inflamed region, into the same region at 1-month intervals, or when stimulated with interferon-γ before injection (2). In this study, we evaluate approaches to avoid an immune response with repeated injections of pUTC. We test the effect of injecting pUTC in disparate sites, of extending the interval between injections, and of giving immunosuppression at the time of cellular administration.
MATERIALS AND METHODS
Porcine Umbilical Cord Tissue-Derived Cell Isolation and Culture
Umbilical cords were isolated from four Massachusetts General Hospital (MGH) MHC-defined miniature swine leukocyte antigen (SLA) dd (SLAdd) fetuses delivered at term through cesarean section, as previously described (2). pUTC were extracted by the same procedure as described for isolation of human UTC (10). Briefly, cords were drained of all blood, washed in phosphate-buffered saline, and then mechanically dissociated and digested using a mixture of the enzymes collagenase (10 U/mL, Sigma, St. Louis, MO), dispase (12.6 U/mL, Roche Diagnostics, Indianapolis, IN), and hyaluronidase (1 U/mL, Vitrase, ISTA Pharmaceuticals, Irvine, CA) diluted in Dulbecco’s minimum essential-low glucose medium (Invitrogen, Carlsbad, CA). After digestion at 37°C for 2 hr, the processed tissue was resuspended in growth medium consisting of Dulbecco’s minimum essential-low glucose medium, 15% fetal bovine serum (Hyclone, Logan, UT), 0.001% 2-mercaptoethanol (Sigma), and 1 mL antibiotic per 100 mL medium (10,000 U/mL penicillin, 10,000 μg/mL streptomycin). The resulting cells were seeded at a density of 3,000 or 5,000 viable cells/cm2 on tissue culture flasks (Corning Inc., Corning, NY) previously coated with 2% w/v gelatin (Gelita porcine Type A: 250 Bloom, Sigma) and grown at 37°C, 5% CO2 in growth medium.
MHC-Defined Miniature Swine Recipients
The Transplantation Biology Research Center of MGHmaintains swine of three different MHC haplotypes: SLAa, SLAc, and SLAd. Animals receiving injections of SLAdd pUTC were either SLAcc or SLAac to constitute a full, two-haplotype mismatch. Transplantation across full, two-haplotype MHC-mismatch barriers has been shown to cause vigorous rejection of kidney allografts in the absence of immunosuppression in this swine model (11–13). All animal care procedures were in compliance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (publication No. 86-23, revised 1985). A total of 13 animals between 3 and 7 months of age were used for these studies (Table 1). Approaches to avoid an immune response were investigated, and these included injection of pUTC in disparate sites, extending the interval between injections, and concurrent administration of immunosuppression with cellular injections (Table 1).
TABLE 1.
Experimental treatments for multiple injections of porcine umbilical cord tissue-derived cells
| Animal | No. UTC injections | Interval between injections | Injection sites | Immunosuppression | Skin graft placement |
|---|---|---|---|---|---|
| 17476 | 0 | N/A | N/A | N/A | Yes |
| 17506 | 0 | N/A | N/A | N/A | Yes |
| 17472 | 1 | N/A | N/A | N/A | No |
| 17471 | 2 | 1 mo | Disparate | N/A | Yes |
| 17472 | 2 | 1 mo | Disparate | N/A | Yes |
| 17164 | 2 | 3 mo | Same | N/A | Yes |
| 17165 | 2 | 3 mo | Same | N/A | Yes |
| 17162 | 2 | 6 mo | Same | N/A | Yes |
| 17163 | 2 | 6 mo | Same | N/A | Yes |
| 17307 | 2 | 1 mo | Same | Prednisolone (second injection only) | Yes |
| 17308 | 2 | 1 mo | Same | Prednisolone (second injection only) | Yes |
| 17375 | 2 | 1 mo | Same | Prednisolone and CyA (both injections) | Yes |
| 17377 | 2 | 1 mo | Same | Prednisolone and CyA (both injections) | Yes |
UTC, umbilical cord tissue-derived cells; CyA, cyclosporine A; N/A, not applicable or available.
Control Animals
Two animals (17476 and 17506) served as naïve controls and were given no pUTC injections. After placement of a pUTC haplotype-matched SLAdd skin graft, these animals were assessed for serum SLAdd IgG production and by time course of skin graft rejection.
Surveillance of pUTC at Injection Site
To determine whether pUTC persist at the injection site, one female animal (17472) was injected with male-derived pUTC in four separate locations, which were biopsied weekly for 1 month. Samples were assessed for the presence of the male-specific gene by polymerase chain reaction (PCR) for sex-determining region Y (SRY). Along with SRY analysis, samples were assessed by histologic staining with hematoxylineosin to identify the presence of infiltrating leukocytes. For skin biopsies, the skin surface was carefully cleaned with alcohol and betadine. Dermal biopsies of 6.0 mm3 were removed and frozen immediately at −80°C. No skin graft was placed on this animal.
Repeat Injection at Disparate Site
To assess whether varying the location of injection sites would inhibit an immune response, two animals (17471 and 17475) were injected twice, 1 month apart in disparate sites. The first injection was administered behind the ear, and the second round was given in the contralateral flank. Prolonging the time period between pUTC injections was investigated to determine whether increasing the timing between the first and second injections would inhibit an immune response. Four animals were injected twice, with the second injection either 3 months (n=2: 17164 and 17165) or 6 months (n=2: 17162 and 17163) after the first.
Concurrent Immunosuppression With Cellular Injection
For immunosuppressive treatments, four animals were injected twice, at 1 month intervals, and given prednisolone solution at the time of the second injection (n=2: 17307 and 17308) or prednisolone solution and cyclosporine A (CyA; Neoral, Novartis, Hanover, NJ) at the time of both injections (n=2: 17375 and 17377). Prednisolone (6.25 mg/kg body weight) was administered orally twice per day for 7 consecutive days, followed by a 7-day tapering dose. CyA (7.5–15 mg/kg body weight) was given twice per day to maintain a trough level of 400 to 600 ng/mL for 7 consecutive days, followed by a 7-day tapering dose. This level of immunosuppression was chosen based on our experience with therapeutic levels of CyA and steroids used to induce tolerance to renal transplants in miniature swine (14, 15). Immunosuppressive treatments were administered beginning 1 day before pUTC injections.
Injections of pUTC
SLAdd pUTC were thawed, cultured for 4 to 5 days, harvested, and resuspended in Lactated Ringer’s solution at a concentration of 1×107 cells/mL and injected into fully allogeneic SLAcc or SLAac recipient pigs at a total dose of 1×108 cells, as previously described (11). Subcutaneous (SC) injections were administered at four adjacent sites behind the ear or in the contralateral flank with 2.5 mL of the cell suspension (0.25×108 cells per site) using a 25-gauge needle.
Detection of Sex-Determining Region Y in Skin
To determine whether pUTC persist at the site of injection in recipients, quantitative PCR analysis for identification of the male-specific gene, SRY, was assessed. DNA was isolated from skin biopsy specimens. Briefly, 25 to 30 mg of each skin sample was homogenized (Omni International GLH homogenizer; Omni International, Kennesaw, GA) in buffer ATL (Qiagen; Valencia, CA). The homogenate was centrifuged, supernatant was removed, and digested with proteinase K. DNA was isolated using a DNeasy Kit 50 as per manufacturer’s protocol (Qiagen). DNA concentration was measured at OD260 using a spectrophotometer (ND-1000, NanoDrop; Thermo Scientific, Wilimington, DE).
Primers and Probe
The complete swine SRY gene sequence (accession number GI: 1330325) was analyzed to determine optimal primers/probe combination (PrimerQuest, Integrated DNA Technologies; Coralville, LA). Primer sequences were SRY1101 forward, 5′-TCCCTTCACATACACAGTCGCCAA-3′; SRY1306 reverse, 5′-TGAGAAAGTCCCGGCTGTAAACCA-3′; SRY1146 probe, 6Fam-5′-AAGC-CAGTTAAGTCACTCACAGCCCA-3′-TAMRA. The swine class I gene was used to determine the amount of starting template in each well. The primer sequences were as follows: forward, 5′-GCCCTG GGCTTCTACCCTAA-3′; reverse, 5′-TCTCAGGGTGAGTGGCTCCT-3′; probe, 6FAM-5′-CCAGGACCAGAGC-CAGGACATGGAGCTCGT-3′-TAMRA. The PCR-amplified portion of the SRY gene and class I gene were each cloned into a pCR2.1-TOPO plasmid using a TOPO TA Cloning kit (Invitrogen) for use in generation of the standard curves.
Quantitative Real-Time PCR Analysis
Quantitative real-time PCR (Q-PCR) analysis was performed with a Stratagene MX3005P QPCR System software version 3.0. Analysis was performed with the MxPro v3.0 software package. The experimental type used was quantitative PCR (multiple standards). The cycle threshold (Ct) value of amplification for the SRY gene and the class I gene was determined in triplicate and averaged for each sample. The number of copies of the SRY gene in each sample was determined by comparing Ct values from a standard curve of a serially diluted plasmid containing the cloned fragment of the swine SRY gene. The amount of initial template in each sample was determined by comparing Ct values from a standard curve of a serially diluted plasmid containing the cloned fragment of the swine class I gene. The standard curves were derived by serial 10-fold dilutions of plasmid from 1×108 copies to 1×101 copies. Each dilution was measured in triplicate, and the average Ct value was used for each dilution point. Only data present on the linear portion of the standard curves were used for analysis. Data were calculated as copies of SRY per 1×105 copies of class I and expressed as percent of male cells relative to the pUTC control.
Antibody Detection by Flow Cytometry
Antibody response to pUTC was assayed by flow cytometry using sera collected at serial time points from injected animals to stain peripheral blood mononuclear cells (PBMC) that were haplotype-matched to pUTC (SLAdd), as previously described (2). Briefly, 10 μL of heat-inactivated decomplemented serum from each recipient was added to 1×106 cells of SLAdd PBMC. After 30-min incubation, cells were washed twice before incubation with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (goat anti-swine IgM-FITC and goat anti-swine IgG-FITC; KPL: cat. 02-14-03 and 02-14-02). Sera from previously immunized and tested animals were used as positive controls. Negative controls consisted of SLAcc PBMC binding by SLAd serum antibody and preinjection sera from the same animals. Detection of antibody was reported as a difference in mean fluorescence intensity when compared with the pretreatment sample.
Antibody-Complement-Mediated Cytotoxicity Assay
Detection of cytotoxic antibodies to cell surface antigens was performed using an antibody-complement reaction, followed by a dye exclusion assay and fluorescence-activated cell sorter acquisition. A negative control of media alone and a positive control consisting of serum from a previously immunized animal were used for each assay. Negative controls also consisted of both SLAc PBMC killing by SLAd serum and complement, and assessment of sera from the same animals before injection of pUTC. Serum samples previously decomplemented were serially diluted in a 96-well round bottom plate and incubated with 25 μL of 5×106 cells/mL of target SLAd PBMC at a total volume of 50 μL per well. After 15 min of sera incubation at 37°C, each well was washed with 125 μL of media and then centrifuged at 1200g for 5 min at 4°C. Diluted rabbit complement was added to each well and incubated at 37°C for 30 min before addition of 10 μL of 10 μg/mL 7-amino-actinomycin D (7-AAD, Sigma A9400) for 30 min at 4°C. 7-AAD positive cells were gated for flow cytometric analysis to compare live versus dead populations.
Skin Grafting
A pUTC haplotype-matched SLAdd skin graft was placed at least 30 days after pUTC injection to confirm whether the animal was sensitized by the pUTC injection. Split-thickness skin grafts were obtained from both an SLAdd donor animal and the experimental animal for an autologous “self” graft using a dermatome. Skin was then placed on a graft bed, also prepared with the dermatome on the dorsal surface of the recipient animal. Allogeneic (SLAdd) and self skin grafts were biopsied on days 4 and 7 for histopathologic analysis to determine acceptance or rejection; skin grafts were also monitored daily based on three visual indicators of rejection criteria: texture, color, and temperature. The time course of rejection was measured on a scale of 1 to 3 for each of these criteria (3 is most severe). The tempo of skin graft rejection was determined as the start day (any of 3 criteria greater than 1) until the day of completion (all criteria equal 3).
Statistics
The difference in immune response rates between experimental groups was assessed by Fisher’s exact test. The Wilcoxon rank-sum test was used to assess the association between antibody response before skin graft placement and the time to skin graft rejection. The exact two-sided P values were computed by StatXact (Cytel, Cambridge, MA).
RESULTS
Detection of Cellular Infiltrate and Remaining pUTC at the Site of Injection
Histopathology was performed to determine whether the pUTC persist and whether infiltrating leukocytes are observed at the site of injection. Analysis of weekly biopsies identified pUTC after injections as SC aggregates of mononuclear cells with abundant foamy cytoplasm that resemble histiocytes at postinjection day 0 only (Fig. 1). In skin biopsies from postinjection day 7, 14, 21, and 28, no cells resembling pUTC were detected, and no leukocyte infiltration was noted. As a more sensitive assessment of whether pUTC persist at the site of injection, quantitative PCR for identification of the male-specific gene, SRY, was performed. The SRY Q-PCR assay was designed to detect the Y chromosome present in male cells. Because all donor pUTC were of male origin and recipients of pUTC were female, the SRY Q-PCR assay was able to quantitatively detect the presence of injected pUTC (Table 2). The percent male cells in the negative control female skin biopsy (animal 17471) was just above the lower limit of detection, likely representing minimal skin contamination by material containing male cells. The lower limit of detection of the SRY Q-PCR assay with 95% confidence was 10 copies with a sensitivity of 0.01%, as determined by serial dilution of mixed male/female cells (data not shown). The positive controls of male pUTC and male skin biopsy (animal 17475) were clearly detected by the SRY Q-PCR assay. A skin biopsy sample of the pUTC injection site of recipient animal 17472, taken immediately postinjection, contained 6.8% male cells relative to the pUTC control (Table 2). Subsequent biopsies of injection sites of the recipient animal (17472) at days 7, 14, 21, and 28 showed no evidence of detectable pUTC in that all biopsies resulted in SRY counts below the limit of detection of the Q-PCR assay. Thus, pUTC represented less than 0.01% of the total biopsy tissue in these samples.
FIGURE 1.
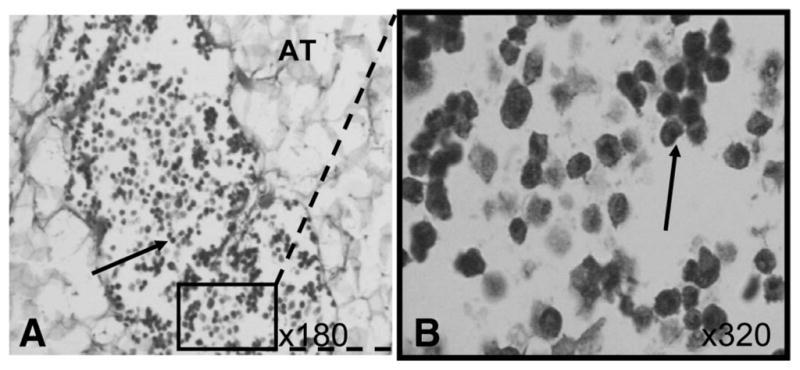
Representative histopathologic samples from day 0 of porcine umbilical cord tissue-derived cell (pUTC) injections. Hematoxylineosin staining illustrates subcutaneous aggregates of mononuclear cells with abundant foamy cytoplasm that cytologically resemble histiocytes. (A) pUTC (arrow) within adipose tissue (AT); ×180. (B) Close-up image of “A,” more clearly demonstrating pUTC in situ.
TABLE 2.
Sex-determining region Y quantitative PCR—male pUTC injected into female skin
| Sample | Sample obtained | Percent male cells (% average) | ±2 SD | n |
|---|---|---|---|---|
| 17471 skin (female) | Preinjection | 0.0427 | 0.0228 | 3 |
| 17475 skin (male) | Preinjection | 96.4 | 5.09 | 2 |
| pUTC (male) | Preinjection | 100 | 0 | 4 |
| 17472 (day 0) | Postinjection | 6.75 | 3.09 | 2 |
| 17472 (day 7) | Postinjection | 0.00114 | 0.000458 | 2 |
| 17472 (day 14) | Postinjection | 0.00508 | 0.00397 | 2 |
| 17472 (day 21) | Postinjection | 0.00789 | 0 | 1 |
| 17472 (day 28) | Postinjection | 0 | 0 | 1 |
Quantitative SRY PCR analysis was performed to detect the presence and persistence of pUTC from day 0 through day 28 of pUTC injections. The number of copies of the SRY gene in each sample was determined in triplicate by comparison of the Ct of SRY amplification to a standard curve of a serially diluted plasmid containing the amplified portion of the SRY gene. Initial template concentration of each sample was determined in triplicate by comparison of the Ct value of class I amplification for each sample to a standard curve of a serially diluted plasmid containing the amplified portion of the class I gene. The data were calculated as the number of copies of SRY per 1×105 copies of class I and expressed as a percent of male cells relative to the male pUTC control. The standard deviation with 95% confidence limits was determined for “n” number of each sample.
Ct, cycle threshold; SRY, sex-determining region Y; pUTC, porcine umbilical cord tissue-derived cell.
Assessment of Alloantibody Production Induced by pUTC Injections
As a first measure of immune response to pUTC, serum antibody response to SLAd was assessed by flow cytometry. Serum was also evaluated for the presence of cytotoxic antibodies by complement-mediated cytotoxicity using samples taken at a pretreatment time point and 2 weeks after the second injection. Naïve control animals (17476 and 17506) demonstrated anti-SLAd IgG production within 10 days after placement of SLAdd skin grafts (Fig. 2A). These animals demonstrated a normal pattern of SLAdd skin graft rejection beginning on day 4 and reaching complete rejection by day 8 (Table 3), consistent with historical controls (16) and indicating no prior sensitization. Cytotoxic antibody directed at SLAdd PBMC was detected in sera of all animals that had detectable levels of induced anti-SLAdd IgG (Fig. 3). The presence of detectable serum antibody to SLAd before placement of skin grafts was associated with accelerated skin graft rejection based on the start of clinical signs and the completion of rejection (P=0.002; Table 3).
FIGURE 2.
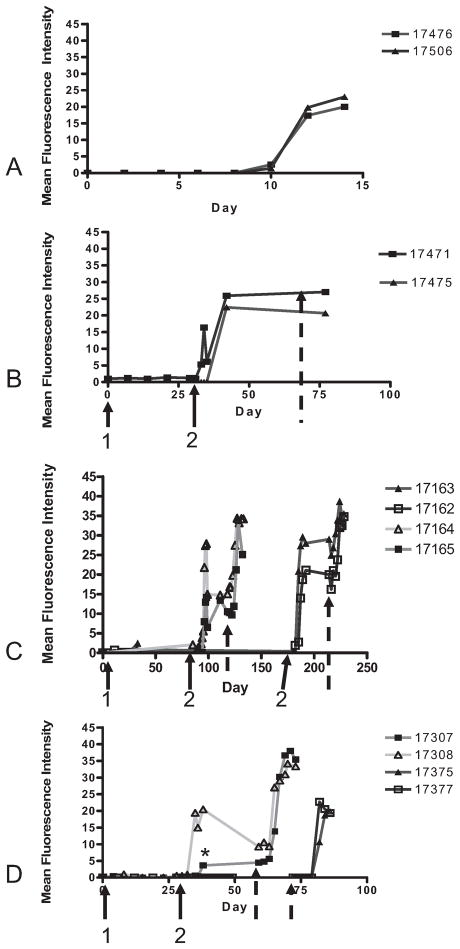
Flow cytometric analysis of serum anti-swine leukocyte antigen dd (SLAdd) IgG produced after porcine umbilical cord tissue-derived cell (pUTC) injections. Naïve control animals (17476 and 17506) demonstrated IgG production 10 days after placement of SLAdd skin grafts (A), whereas animals injected twice, 1 month apart in disparate locations (17471 and 17475), showed SLAdd IgG on days 5 and 7 after the second injection of pUTC (B). Animals injected a second time at both 3 months (17164 and 17165) and 6 months (17162 and 17163) after the first injection demonstrated anti-SLAdd IgG within the first 5 days after the second injection (C). Immunosuppression treatments showed that animals injected twice and given prednisolone cover at the time of the second injection (17307 and 17308) produced anti-SLAdd IgG 10 and 7 days after the second injection (D), whereas animals injected twice and given prednisolone and cyclosporine A cover at the time of both injections (17375 and 17377) showed no detectable antibody production (D). Animal 17307 demonstrated a lower titer of IgG after the second injection of pUTC (*), possibly due to prednisolone treatment. 1, first injection. 2, second injection; broken arrow, skin graft placement.
TABLE 3.
Tempo of skin graft rejection in comparison with SLAd IgG production
| Animal | SLAd lgG postinjection 1 (d) | SLAd lgG postinjection 2 (d) | SLAd lgG postskin graft (d) | Time course of skin graft rejection (start/completion) | Rejection according to pathology (d) |
|---|---|---|---|---|---|
| 17476 | None | N/A | 10 | 4/8 | 7 |
| 17506 | None | N/A | 10 | 4/8 | 7 |
| 17471 | None | 5 | N/A | 1/7 | N/A* |
| 17475 | None | 7 | N/A | 1/7 | 4 |
| 17164 | None | 1 | N/A | 1/7 | 4 |
| 17165 | None | 5 | N/A | 2/7 | 4 |
| 17162 | None | 2 | N/A | 1/7 | 4 |
| 17163 | None | 5 | N/A | 1/7 | 4 |
| 17307 | None | 10 | N/A | 2/7 | 4 |
| 17308 | None | 7 | N/A | 1/7 | 4 |
| 17375 | None | None | 10 | 4/8 | 7 |
| 17377 | None | None | 10 | 4/8 | 7 |
In vitro assessment of SLAd IgG production after second pUTC injection is compared with the tempo of skin graft rejection in vivo (start-to-completion of rejection d 1–7). A technical error occurred with skin grafting of animal 17471, so that day 4 pathologic observation of the graft was impossible (*). Because day 7 rejection was identical to animal 17475 with IgG production after second injection was identical and with visual criteria analysis of the graft demonstrating accelerated rejection, it is likely that this animal would have demonstrated an accelerated course of rejection by histology.
SLA, swine leukocyte antigen; N/A, not applicable or available.
FIGURE 3.
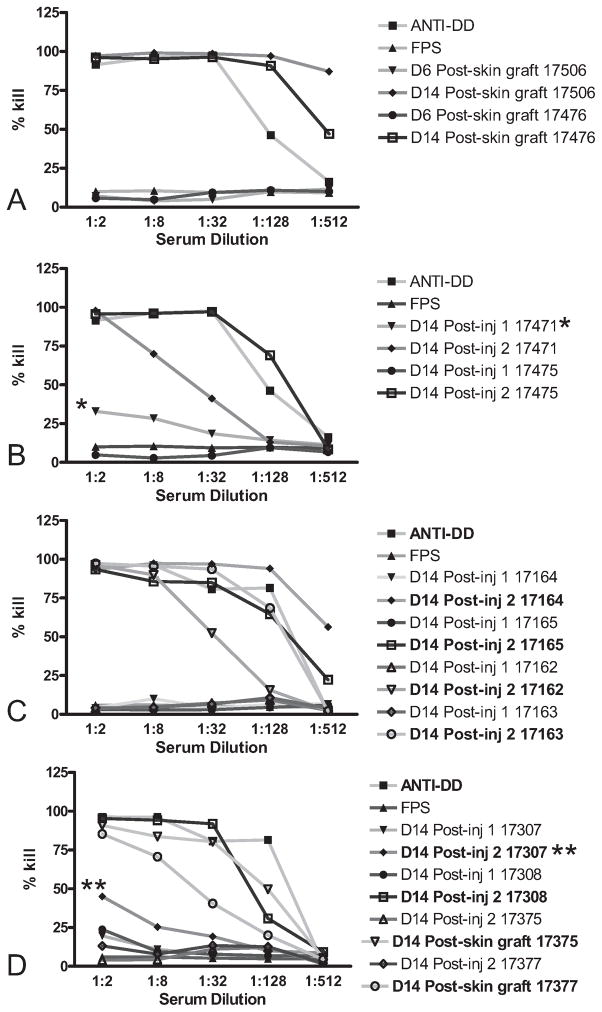
Complement-mediated killing of swine leukocyte antigen dd (SLAdd) peripheral blood mononuclear cells (PBMC) by sera of all animals that had detectable levels of SLAdd IgG. Negative controls consisted of media alone, treatment of SLAcc PBMC with all serum samples, assessment of preinjection sera (data not shown), and fetal porcine serum (FPS) treatment of SLAdd PBMC (FPS). Positive control consisted of SLAdd PBMC killing by serum from a previously SLAdd-immunized animal (anti-DD). Cytotoxic antibody was detected in sera of all animals that had detectable levels of IgG directed at SLAdd PBMC. (B) Animal 17471 demonstrated numerous pulmonary abscesses, indicating consolidated pneumonia on pathologic analysis. These abscesses were surrounded by fibrosis, chronic inflammation, and a notable presence of eosinophils. The presence of cytotoxicity after the first injection of porcine umbilical cord tissue-derived cells (pUTC; *) may relate to nonspecific binding by IgM present in this animal (data not shown) as a result of this infection. (D) Animal 17307 demonstrated a decreased cytotoxic effect (**), which correlates with a decreased IgG titer (Fig. 2), after the second injection of pUTC possibly due to prednisolone treatment.
Injecting pUTC in a Disparate Site Does Not Prevent an Immune Response
When animals (17471 and 17475) were injected twice, 1 month apart in disparate locations, behind the ear and then in the contralateral flank without immunosuppression, anti-SLAd IgG production was observed within 5 and 7 days after the second injection, respectively (Fig. 2B). These animals demonstrated initial signs of SLAdd skin graft rejection by day 1 after the graft was placed, and rejection was complete by day 7 (Table 3).
Extending the Interval Between pUTC Injections Does Not Prevent an Immune Response
Animals injected with SLAdd pUTC at both 3-month (17164 and 17165) and 6-month (17162 and 17163) time points after the first injection demonstrated production of anti-SLAd IgG antibody within the first 5 days after the second injection (Fig. 2C). These animals demonstrated initial signs of SLAdd skin graft rejection by day 1 (17164, 17162, and 17163) or 2 (17165) after the graft was placed, and rejection was complete by day 7 (Table 3).
Administration of Immunosuppression at the Time of Repeat Injection of pUTC Does Not Prevent an Immune Response
The two animals injected twice with SLAdd pUTC and given Prednisolone at the time of the second injection (17307 and 17308) demonstrated the production of anti-SLAd IgG within 10 and 7 days after the second injection, respectively (Fig. 2D). These animals demonstrated initial signs of SLAdd skin graft rejection by day 2 or 1, respectively, after the graft was placed. Rejection was complete by day 7 (Table 3). One of these animals (17307) demonstrated a lower titer of anti-SLAd IgG after the second injection (Fig. 2D) and also showed a decreased cytotoxic effect (Fig. 3D).
Concurrent Administration of Immunosuppression With Each pUTC Injection Successfully Prevents an Immune Response
No detectable anti-SLAdd IgG production was observed in serum from the two animals (17375 and 17377) injected twice with SLAdd pUTC and given prednisolone and cyclosporine A at the time of both injections (Fig. 2D). These animals demonstrated a pattern of rejection like that of controls, beginning on day 4 and complete by day 8 (Table 3).
DISCUSSION
In recent years, cells derived from the umbilical cord matrix of humans, pigs, rats, and mice have been isolated and used for preclinical studies. These UTC have been shown to improve symptoms of retinal disease (10). We have previously established a preclinical swine model to investigate the immunology of pUTC (11). In this prior work, we found that a single injection of pUTC across allogeneic barriers did not produce a measurable immune response. However, when given under certain conditions such as injection into an inflamed region or repeated injection in the same region at 1 month intervals, we found that these cells are immunogenic. For the purpose of repairing damaged organs and tissues, cellular transplantation may likely require injection of allogeneic cells into inflamed areas, and repeated doses of cells may be necessary to achieve the desired benefits. Because these approaches may simulate clinical applications, we sought to evaluate the approaches to avoid immune responses under these conditions.
In our previous work, our results demonstrated no detectable immune response with the injection of unactivated pUTC. However, it was not clear whether this was due to a lack of response or to the inability of our methods to identify such. From the data in this current study, it seems that the latter possibility of a “subclinical” immune response below the level of detection is more likely. This is supported by both in vitro and in vivo observations after repeated pUTC injections. Rapid peripheral accumulation of cytotoxic pUTC-specific SLAd IgG followed the second dose of pUTC and suggested a secondary immune response. Further, in vivo assessment of skin grafts demonstrated an accelerated tempo of rejection more consistent with prior sensitization in animals with detectable SLAd IgG.
Neither administration of pUTC in disparate locations nor extending the time interval between injections prevented an immune response. However, the addition of immunosuppression with pUTC injections did affect responses. In the animals receiving prednisolone only with the second set of pUTC injections, immunosuppression failed to prevent the an immune response one while leading in the other (17307) to a decreased SLAd IgG titer and decreased complement-mediated cytotoxicity in vitro found after this repeat administration of cells. Treatment with prednisolone and cyclosporine A at both injections did result in inhibition of SLAd IgG production and in a normal course of skin graft rejection, consistent with naïve control animals. These results indicate that currently available oral immunosuppressive medications can be used to modulate immune responses after pUTC injections and are also consistent with recent studies that used oral tacrolimus in suppression of immune response after intracardiac injection of MSC (17). Further studies investigating to tailor the immunosuppression regimen to minimize dosages and side effects of these medications may be helpful toward development of clinical protocols.
To date, aside from our previously published work, the only measure of immune response induced from injection of UTC has been evaluation of local injection sites. In murine studies, using similar types of cells from umbilical cord tissue have been shown to increase in number and migrate throughout the brain after injection, and to the kidney parenchyma when administered intravenously without apparent stimulation of an immune response (3, 18). Analysis of the male-specific gene, SRY, to detect male pUTC injected subcutaneously into female recipients, indicate that pUTC do not persist at the site of injection and could only be detected in the skin biopsies taken immediately postinjection. At this time point, the percentage of donor cells detected was only 6.8% of the positive control, likely because of the injected pUTC volume intersperses with the recipient tissues immediately, so that obtaining a pure biopsy containing the entire injection site, and therefore, all donor cells is difficult. Despite the fact that pUTC were undetectable at the injection sites at any time point other than immediately postinjection either by histopathologic or SRY assessment, an immune response was stimulated after injections. The inability to detect pUTC at the injection site could be due to migration to other sites during the first week postinjection or rejection by the immune system. Even in cases where an immune response occurred, histologic analysis failed to demonstrate any infiltration of leukocytes after SC injections. These data suggest that lack of cellular infiltrate at the injection site is not a good indicator for absence of an immune response.
Although statistical comparisons are limited by the small animal numbers, the consistency of observed data across experimental conditions assessed by in vitro (antibody fluorescence-activated cell sorter and cytotoxicity) and in vivo (skin grafting) assays supports the interpretation of pUTC immunogenicity and immunosuppressive remediation of immunogenicity. Further, if a single SC injection of MHC-mismatched pUTC were capable of eliciting an immune response at least half (50%) of the time, it is statistically unlikely that all of the eight recipient animals lacked an immune response in the four treatment groups that did not receive immunosuppressive treatments with their first SC pUTC injection (P=0.004). Statistical comparison of the undetected immune response in the prednisolone and cyclosporine A treated animals (n=2) with the other four treatment groups that did demonstrate an immune response after the second SC injection yields a significant difference (P=0.022).
In summary, repeated SC injection of pUTC into a disparate site or delayed administration of the subsequent pUTC dose in the same site up to 6 months later seems to elicit a rapid humoral immune response and accelerated rejection of allogeneic skin grafts. The immune response stimulated by SC pUTC injection can be prevented by concurrent immunosuppression with each dose of pUTC. These studies in the MHC-defined MGH miniature swine illustrate the importance of immunogenic assessment and provide a framework for further preclinical investigations of immunogenicity of allogeneic cellular therapies. Because these cells are ultimately intended for clinical use as repair strategies for damaged tissues, the inclusion of immunosuppression for treatment protocols should be considered as it may be beneficial to optimize efficacy and patient safety.
Acknowledgments
This work was supported in part by Centocor R&D Inc., Stem Cell Internal Venture (sponsored research agreement).
The authors thank Kazuhiko Yamada and Shuji Nobori for harvesting the porcine umbilical cords and Adam Griesemer for providing SLAdd skin grafts. They also thank Benjamin Horner, M.D., for the surgical support and Raimon Duran-Struuck, D.V.M., and Richard Hurley, D.V.M., for their dedicated assistance in the medical care of the animals. The swine class I forward primer and probe sequences for SRY Q-PCR were kindly provided by Jay Fishman, M.D.
Footnotes
The first two authors contributed equally to this study and manuscript.
BVL, PSC, SHP, DHS, and CAH participated in research design; BVL, PSC, ELH, KKF, AGST, JSH, NC, SNG, DJM, and SH performed research and collected data; BVL, PSC, DJM, NC, SHP, CAH, and BYP analyzed data; and BVL, PSC, JSH, BYP, SHP, DHS, and CAH participated in manuscript preparation.
References
- 1.Sindram-Trujillo A, Scherjon S, Kanhai H, et al. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol. 2003;49:261. doi: 10.1034/j.1600-0897.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 2.Cho PS, Messina DJ, Hirsh EL, et al. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111:430. doi: 10.1182/blood-2007-03-078774. [DOI] [PubMed] [Google Scholar]
- 3.Weiss ML, Mitchell KE, Hix JE, et al. Transplantation of porcine umbilical cord matrix cells into the rat brain. Exp Neurol. 2003;182:288. doi: 10.1016/s0014-4886(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 4.Zimmet JM, Hare JM. Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy. Basic Res Cardiol. 2005;100:471. doi: 10.1007/s00395-005-0553-4. [DOI] [PubMed] [Google Scholar]
- 5.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 6.Odeberg J, Piao JH, Samuelsson EB, et al. Low immunogenicity of in vitro-expanded human neural cells despite high MHC expression. J Neuroimmunol. 2005;161:1. doi: 10.1016/j.jneuroim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Koc ON, Day J, Nieder M, et al. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 8.McTaggart SJ, Atkinson K. Mesenchymal stem cells: Immunobiology and therapeutic potential in kidney disease. Nephrology (Carlton) 2007;12:44. doi: 10.1111/j.1440-1797.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 9.Poncelet AJ, Vercruysse J, Saliez A, et al. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 10.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 11.Gianello PR, Sachs DH. Effect of major histocompatibility complex matching on the development of tolerance to primarily vascularized renal allografts: A study in miniature swine. Hum Immunol. 1996;50:1. doi: 10.1016/0198-8859(96)00059-6. [DOI] [PubMed] [Google Scholar]
- 12.Pescovitz MD, Thistlethwaite JR, Jr, Auchincloss H, Jr, et al. Effect of class II antigen matching on renal allograft survival in miniature swine. J Exp Med. 1984;160:1495. doi: 10.1084/jem.160.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkman RL, Colvin MW, Flye GS, et al. Transplantation in miniature swine. VI. Factors influencing survival of renal allografts. Transplantation. 1979;28:18. [PubMed] [Google Scholar]
- 14.Fishbein JM, Rosengard BR, Gianello P, et al. Development of tolerance to class II mismatched renal transplants following a short course of cyclosporine therapy in miniature swine. Transplantation. 1994;57:1303. doi: 10.1097/00007890-199405150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. II. Effect of steroids and age on the induction of tolerance to class I mismatched renal allografts. Transplantation. 1999;67:458. doi: 10.1097/00007890-199902150-00020. [DOI] [PubMed] [Google Scholar]
- 16.Mathes DW, Yamada K, Randolph MA, et al. In utero induction of transplantation tolerance. Transplant Proc. 2001;33:98. doi: 10.1016/s0041-1345(00)01924-2. [DOI] [PubMed] [Google Scholar]
- 17.Poncelet AJ, Nizet Y, Vercruysse J, et al. Inhibition of humoral response to allogeneic porcine mesenchymal stem cell with 12 days of tacrolimus. Transplantation. 2008;86:1586. doi: 10.1097/TP.0b013e31818bd96f. [DOI] [PubMed] [Google Scholar]
- 18.Medicetty S, Bledsoe AR, Fahrenholtz CB, et al. Transplantation of pig stem cells into rat brain: Proliferation during the first 8 weeks. Exp Neurol. 2004;190:32. doi: 10.1016/j.expneurol.2004.06.023. [DOI] [PubMed] [Google Scholar]