Abstract
Background
In vitro data suggest that injury to the posterior cruciate ligament stresses the posterolateral structures of the knee, placing them at greater risk of secondary injury. However, it is not known how isolated posterior cruciate ligament deficiency affects these soft tissue stabilizers of the knee joint in vivo.
Hypothesis
Posterior cruciate ligament deficiency will alter the apparent length patterns of the lateral collateral ligament (LCL) and popliteus.
Study Design
Controlled laboratory study.
Methods
The apparent length changes in the lateral collateral ligament and popliteus muscle-tendon unit during weightbearing knee flexion were studied in 14 patients with isolated, unilateral posterior cruciate ligament deficiency using magnetic resonance imaging, dual-orthogonal fluoroscopy, and 3-dimensional modeling. Data of the injured and uninjured contralateral sides were compared.
Results
Posterior cruciate ligament deficiency caused significant increases in the apparent length of both posterolateral structures (P < .05). The differences between injured and uninjured contralateral side were greatest at 120° of knee flexion in the lateral collateral ligament (48.2 ± 6.1 mm and 51.6 ± 6.1 mm, respectively) and at 30° of knee flexion in the popliteus (101.2 ± 9.3 mm and 110.4 ± 10.2 mm, respectively).
Conclusion
Deficiency of the posterior cruciate ligament alters the length patterns of posterolateral structures in vivo and might place them at greater risk of secondary injury.
Clinical Relevance
Reestablishment of normal kinematics after posterior cruciate ligament injury is critical for restoring normal function of posterolateral structures of the knee.
Keywords: posterior cruciate ligament (PCL), lateral collateral ligament popliteus, posterolateral knee structures, PCL injury
Lately, there has been increasing evidence in the literature that isolated rupture of the posterior cruciate ligament (PCL) is not as benign as previously thought.7,10,14,35 Even though many patients do relatively well with nonoperative treatment fairly long term, several studies have shown that as PCL deficiency becomes chronic, there is an increased incidence of pain, swelling, and instability that may eventually result in joint degeneration.4–7,14,35,36
A potential explanation for poor patient outcome after PCL injury is that changes in tibiofemoral kinematics might disturb the normal function of other structures in the knee. Measurements of in situ forces in cadaveric human knee specimens indicate that transection of the PCL significantly increases the in situ forces in the lateral collateral ligament (LCL) and the popliteus complex.13 However, it is still unknown how PCL deficiency affects the posterolateral structures in vivo.
In this study, we investigate the effect of isolated PCL deficiency on the apparent length of the posterolateral structures of the knee (ie, LCL and popliteus muscle) during weight-bearing knee flexion in vivo. We use magnetic resonance (MR) imaging and dual-orthogonal fluoroscopy to measure the changes in the apparent length of posterolateral structures during weightbearing motion of the knee from 0° to 120° of flexion with contralateral uninjured knees used as controls.
MATERIALS AND METHODS
Patient Recruitment
Fourteen patients (10 men and 4 women; age range, 19–64 years; active on a moderate athletic level before injury and with no previous abnormal conditions of the knee or lower limb) with complaints of knee laxity were included in the study. The protocol was approved by the Institutional Review Board (IRB) at our hospital. Each patient signed an IRB-approved consent form before participating in the study. The included patients had diagnosed unilateral PCL injuries documented by clinical examination (<25-mm posterior drawer test result) and MR imaging. The patients had minimal associated injuries to other soft tissue structures and had healthy contralateral knees. Nine patients had isolated PCL injuries, 2 patients sustained medial meniscal tears requiring removal of <30% of the meniscus, 2 patients sustained tears of both medial and lateral menisci requiring removal of <40% of the meniscus on each side, and 1 patient showed intrasubstance signal abnormalities in the posterior horn of the medial meniscus. Patients with combined injuries to the posterolateral structures were excluded from the study. Each patient was carefully examined for posterolateral injury by an orthopaedic surgeon specializing in sports medicine, and posterolateral injury was also ruled out on MRI by a musculoskeletal radiologist. The average time from injury to testing was 22 months. We also evaluated the symptoms, function, and sports activity of the included patients at the time of scanning by the International Knee Documentation Committee (IKDC) scoring system.
Three-Dimensional Knee Model
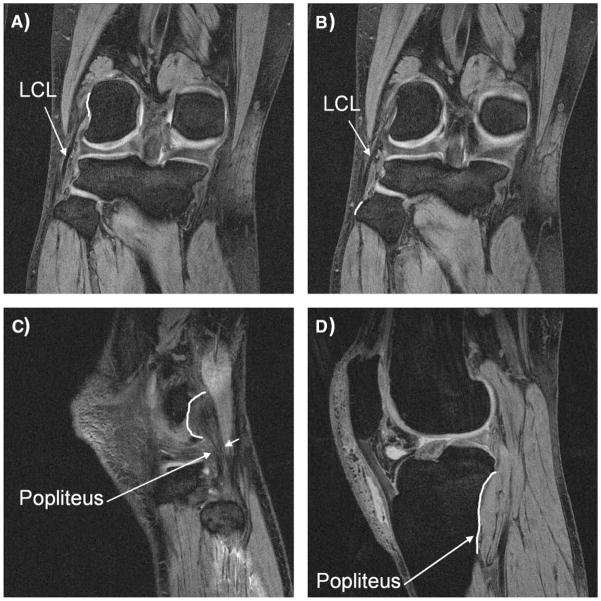
With the patients supine and the knee in a relaxed, extended position, both knees were imaged with an MR scanner using a 3-T magnet (MAGNETOM Trio, Siemens, Erlangen, Germany) and a fat-suppressed 3-dimensional (3-D) spoiled gradient-recalled echo sequence. The MR scans spanned the medial and lateral boundaries of the knee. Parallel sagittal and coronal plane images (resolution, 512 × 512 pixels) with a field of view of 16 × 16 cm and a spacing of 1 mm were taken. These images were then used to create 3-D models of the knees in a solid modeling software (Rhinoceros, Robert McNeal and Associates, Seattle, Washington). Each anatomical knee model included the geometry of the femur, tibia, and fibula, as well as the attachment sites of the lateral collateral ligament (LCL) and popliteus muscle. The ligament and muscle attachment sites were obtained from the MR images with the assistance of anatomical studies.† Before determining the insertion sites on the MR images, we reviewed studies by LaPrade et al16 and Brinkman et al2 that determined the insertion site geometry, quantified insertion areas, and quantified relationship to the surrounding bony landmarks. They showed that the LCL and popliteus have consistent attachment patterns and that the intersubject variations are less than the typical size of drill holes used in surgical reconstruction. Furthermore, in preparation for this study, we dissected 5 cadaveric knee specimens and meticulously identified the insertion site anatomy of the LCL and popliteus. Once the MR images of each studied knee were obtained, they were imported into a virtual environment of the solid modeling software where the insertions were digitally outlined on each MR cut. Thereafter, both an orthopaedic surgeon specializing in sports medicine and a musculoskeletal radiologist verified the outlined insertions on each studied knee. Representative MR images are shown on Figure 1. Furthermore, to test the repeatability of determining insertion site anatomy using this technique, we have measured the insertion areas and distance between their centroids 5 times in one knee. The repeatability represented by the standard deviation of measurements was <2 mm2 for the insertion site area and approximately 1 mm for the distance of centroids.
Figure 1.
Representative magnetic resonance (MR) images for the determination of the insertion sites of posterolateral structures of the knee. Proximal (A) and distal (B) insertion of the lateral collateral ligament (LCL) as well as proximal (C) and distal (D) insertion of the popliteus were determined on each MR slice.
Dual-Orthogonal Fluoroscopy and Reproduction of Knee Kinematics
After the MR image-based computer models were constructed, both knees of each patient were imaged using 2 orthogonally placed fluoroscopes (OEC 9800, General Electric Healthcare, Chalfont St Giles, United Kingdom) as the patient performed a quasistatic single-legged lunge at 0°, 30°, 60°, 75°, 90°, 105°, and 120° of flexion. The flexion angle was verified with a goniometer as patients stood upright on the platform with the fluoroscopes positioned in the horizontal plane. These images were used to quantify the in vivo knee position at each of the targeted flexion angles. The orthogonal images were imported into the solid modeling software and placed in the orthogonal planes based on the position of the fluoroscopes. The MR image-based knee models were viewed from 2 fluoroscopic directions corresponding to the views of the fluoroscopes. The models were manually manipulated in 6 degrees of freedom inside the software until the models matched the outlines of the tibia and femur on the fluoroscopic images. When the projections matched the outlines of the images taken during in vivo flexion, the model reproduced the in vivo position of the knee. A series of knee models that reproduced the knee position at all target flexion angles re-created the in vivo knee flexion from full extension to 120° of flexion. These imaging techniques have been described in detail in previous publications.21,29,30,38 This system has been rigorously validated and has an accuracy of 0.1 mm in translation and 0.2° in rotation.22
Measurement of Apparent Length of Posterolateral Structures
From the knee models, the relative position of the LCL and popliteus attachment sites on the femur, tibia, and fibula were determined (Figure 2). The apparent lengths of the LCL and popliteus were directly measured from these models at each flexion angle. The insertion areas were separated into 3 equal portions, creating 3 equal bundles for the LCL (anterior, middle, and posterior) and the popliteus (superior, middle, and inferior). The centroids of each portion were calculated. There was no bony interference with the LCL path, and the apparent length of each bundle was defined as a direct line between the centroids of the insertion areas. Because the popliteus courses over bony contours of the femur and tibia, the direct line connecting the area centroids was projected on the bony surfaces to create a curved path (Figure 3). The projected curves representing the fiber bundles of the popliteus were visually verified at each flexion angle so that they followed the behavior of the popliteus muscle-tendon unit described in cadaveric studies during knee flexion (eg, the relationship to the popliteal sulcus of the femur).16,17,33,37 The length of this projected curve was measured as apparent tendon and muscle length. This was done in a manner consistent with our previous studies investigating length change of other soft tissue structures of the knee that wrap around bones rather than having a straight path between 2 insertion sites (eg, medial collateral ligament).30,31,38 Knee models of a typical person at different flexion angles are shown in Figure 4.
Figure 2.
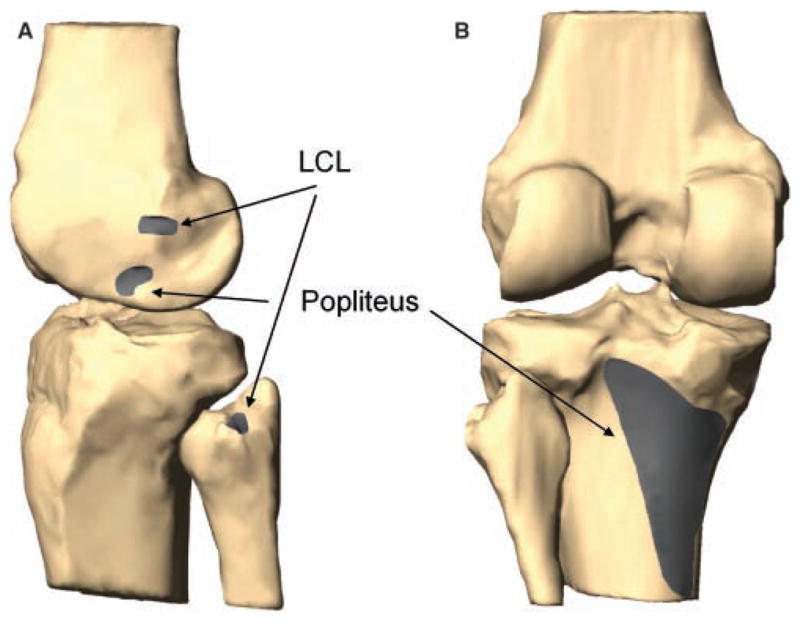
Digitized attachments of the lateral collateral ligament (LCL) and popliteus on a typical left knee. A, lateral view; B, posterior view.
Figure 3.
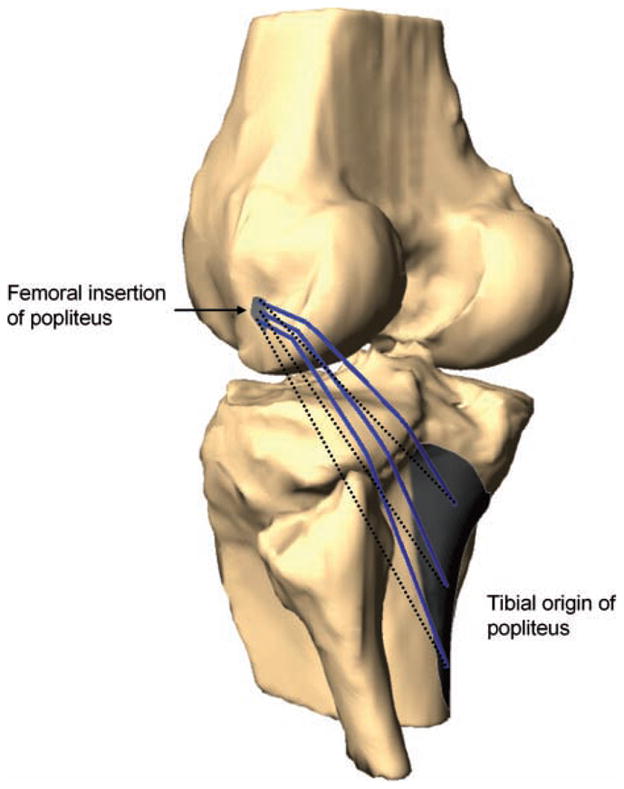
Lines connecting the centroids of the superior, middle, and inferior portions of the attachment sites of the popliteus muscle-tendon unit (dotted lines) were projected onto the surfaces of the femur and tibia to measure the apparent length (solid lines). A typical knee joint at 0° of flexion is shown.
Figure 4.
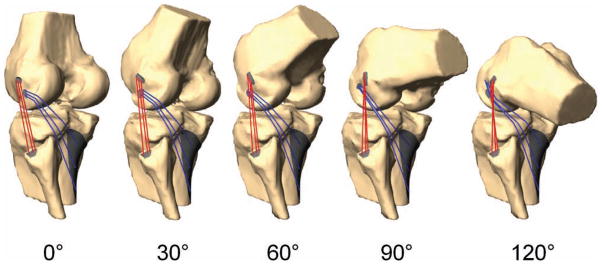
Posterolateral structures in a typical person after virtual reproduction of kinematics at 0°, 30°, 60°, 90°, and 120° of flexion during a quasistatic single-legged lunge.
Statistical Analysis
At each flexion angle, a Student t test was used to compare the length of the bundles between the control and PCL-injured knees. Statistical significance was set at P < .05.
RESULTS
Lateral Collateral Ligament
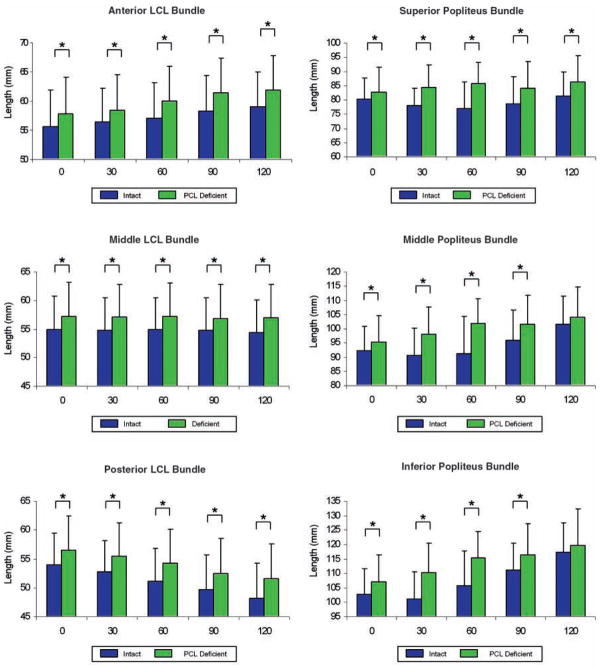
A significant increase in the apparent length of the anterior, middle, and posterior fiber bundles of the LCL was observed at all flexion angles when the PCL-injured and -uninjured contralateral knees were compared (P < .01). In the uninjured contralateral knee, the apparent length of the anterior bundle increased from 55.6 ± 6.2 mm at 0° to 59.0 ± 6.1 mm at 120°. In the PCL-deficient knee, the apparent length of the anterior bundle increased from 57.8 ± 6.2 mm at 0° to 61.9 ± 5.7 mm at 120° (Figure 5). In the uninjured knee, the apparent length of the middle bundle remained relatively unchanged throughout the flexion path of the knee from 54.9 ± 5.7 mm at 0° to 54.4 ± 5.5 mm at 120°. In the PCL-deficient knee, the apparent length of the middle bundle also remained relatively unchanged throughout the flexion path of the knee from 57.3 ± 5.9 mm at 0° to 56.9 ± 5.7 mm at 120° (Figure 5). In the uninjured contralateral knee, the apparent length of the posterior bundle decreased from 53.9 ± 5.5 mm at 0° to 48.2 ± 6.1 mm at 120°. On the injured side, the apparent length of the posterior bundle decreased from 56.5 ± 5.9 mm at 0° to 51.9 ± 6.1 mm at 120° (Figure 5).
Figure 5.
Apparent length of anterior, middle, and posterior bundles of the lateral collateral ligament (LCL) and superior, middle, and inferior bundles of the popliteus in posterior cruciate ligament–deficient knees and uninjured contralateral knees during a weightbearing lunge from 0° to 120° of knee flexion. Comparison at 75° and 105° of flexion is not shown here. *Denotes statistically significant difference (P < .05).
The maximal significant difference between the uninjured contralateral and injured knee was at 120°, at which angle the apparent length of the posterior bundle measured 48.2 ± 6.1 mm and 51.6 ± 6.1 mm, respectively. This represents a 6.0% increase in apparent length compared with that of the same bundle in the uninjured contralateral knee at 120° of flexion.
Popliteus Muscle-Tendon Unit
The PCL injury caused an increase in the apparent length of the 3 fiber bundles of the popliteus (Figure 5). In the uninjured contralateral knee, the apparent length of the superior bundle at 0° was 80.4 ± 6.2 mm, decreased to 77.0 ± 9.2 mm at 60°, and then increased to 81.5 ± 10.2 mm at 120°. In the PCL-injured knee, the apparent length of the superior bundle was measured as 82.9 ± 8.7 mm at 0° and increased to 86.2 ± 8.6 mm at 120°. The difference in apparent length of the superior fiber bundle was statistically significant at all flexion angles (P < .05). In the uninjured contralateral knee, the apparent length of the middle bundle was 92.3 ± 8.6 mm at 0°, decreased to 90.8 ± 9.4 mm at 30°, and then increased to 101.7 ± 10.9 mm at 120°. In the injured knee, the apparent length of the middle bundle was 95.5 ± 9.2 mm at 0° and increased to 104.0 ± 10.4 mm at 120°. The difference in apparent length of the middle fiber bundle was statistically significant at 0°, 30°, 60°, 75°, 90°, and 105° of flexion (P < .01). The apparent length of the inferior bundle in the uninjured contralateral knee was 102.9 ± 8.9 mm at 0°, decreased to 101.2 ± 9.3 mm at 30°, and then increased to 117.3 ± 11.1 mm at 120°. In the injured knee, the apparent length of the inferior bundle was 107.1 ± 9.5 mm at 0° and increased to 118.6 ± 11.5 mm at 120°. The difference in apparent length of the superior fiber bundle was statistically significant at 0°, 30°, 60°, 75°, and 90° of flexion (P < .01).
The maximal significant difference between the uninjured contralateral and the injured side was at 30° (P < .01), at which angle the apparent length of the inferior bundle was 101.2 ± 9.3 mm and 110.4 ± 10.2 mm, respectively. This represents a 9.2% increase in apparent length compared with that of the same bundle in the uninjured contralateral knee at 30° of flexion.
The average IKDC score at the time of scanning (22 months after injury) was 51.4.
These values include the data of 4 patients with partial meniscectomy and 1 patient with MR-documented intra-substance signal abnormality in the posterior horn of the medial meniscus. In this subgroup of patients, similar trends were detected in the change of apparent length of the posterolateral structures at all studied flexion angles. The differences between uninjured contralateral and the injured side were greatest at 120° in the posterior bundle of the LCL (46.5 ± 6.2 mm and 49.1 ± 6.1 mm, respectively) and at 30° in the inferior bundle of the popliteus (98.0 ± 9.5 mm and 106.5 ± 10.2 mm, respectively).
DISCUSSION
This study investigated the effects of injury to the PCL on the apparent length change of the posterolateral structures of the knee in vivo during a quasistatic single-legged lunge from 0° to 120° of knee flexion. The apparent lengths of the LCL and popliteus were measured as the distance between the insertion sites (surface projected). The uninjured contralateral knees were used as controls. The results showed that PCL deficiency significantly increased the apparent length of the fiber bundles of the LCL and popliteus. In the LCL, the difference between the uninjured contralateral and the PCL-injured knees was highest at 120° of flexion in the posterior bundle. The apparent lengths of the fiber bundles of the popliteus muscle were also found to be significantly increased, but the highest difference between the PCL-deficient and uninjured contralateral knees was observed at 30° of flexion in the inferior fiber bundle. On average, the highest differences between the uninjured contralateral and the PCL-injured knees were observed at high flexion angles when apparent lengths of LCL bundles were compared and at lower flexion angles when apparent lengths of the popliteus were compared.
The altered elongation patterns of the posterolateral structures could be explained by changes in tibiofemoral kinematics occurring in vivo during weightbearing flexion of the knee. Numerous studies have demonstrated that the PCL plays a pivotal role in resisting posterior translation and external rotation of the tibia.‡ Furthermore, Li et al21 recently observed that PCL deficiency causes not only a statistically significant increase in posterior tibial translation but also an increase in lateral shift of the tibia (approximately 1 mm) at 75° and 90° of flexion. These changes in kinematics might help explain the increase in apparent length of the posterolateral structures during weightbearing knee flexion. We observed that the orientation of the LCL is such that the ligament passes from anterior proximally to posterior distally. The popliteus tendon traveled from its distal origin on the posterior wall of the tibia obliquely to its proximal insertion at the anterior end of the popliteal sulcus of the femur. Based on the orientation of the posterolateral structures of the knee, the increased posterior translation combined with increased lateral translation and external rotation of the tibia will elongate both the popliteus and the LCL.
Findings of the present study support the existing literature that suggest that the posterolateral structures may be at a greater risk of secondary injury after an isolated PCL rupture.13 Höher et al13 studied the effect of PCL deficiency on in situ forces in the posterolateral structures (LCL and popliteus complex) of 8 cadaveric knees using a robotic force-moment sensor testing system. They measured forces in response to 110 N of posterior load and 44 N of simulated popliteus muscle load and found that transection of the PCL increased the forces in the posterolateral structures up to 6 times from 0° to 90° of flexion in both loading conditions. These findings support observations from our study in which the LCL and popliteus were significantly more elongated in the PCL-deficient knees.
LaPrade et al18 observed in an animal model that the posterolateral structures have minimal healing potential when injured. In their study of 14 New Zealand White rabbits, only 1 LCL and none of the popliteus tendons healed at 12 weeks. They also demonstrated inferior mechanical properties of the healing posterolateral structures. The excessive elongation of the posterolateral structures observed in our study might have a negative effect on the biomechanical function of the posterolateral structures, potentially hindering healing in the event of injury to the posterolateral structures of the knee.
There are limitations of this study that should be noted. (1) We measured the apparent length of the LCL and popliteus fiber bundles as the distance between the bundle insertion centroids on the tibia and femur. Because the popliteus wraps around the lateral condyles of the femur and tibia, a straight line connecting the centroids was projected onto the surfaces of the bones to create a curved path of the tendon. The length of the projected curve was measured as length of the popliteus muscle-tendon unit. A similar technique was used in our previous studies investigating the apparent length of the medial collateral ligament (MCL) of the knee.30,31,38 (2) Another possible limitation is that the posterolateral structures are not as well delineated on MRI. However, it has been shown that their insertion sites can be reliably detected based on bony landmarks.§ (3) Because patients were scanned, on average, 22 months after the injury, there is a possibility that patients might have suffered mild associated injuries to the posterolateral structures that were either mild and not detectable at the time of scanning or healed. Because isolated PCL injuries are relatively rare, we did include some patients who had partial tears of one of the menisci. Our current study number of 14 PCL-deficient patients did not have enough statistical power to analyze the effect of partial removal of the meniscus as well. The findings from this study might therefore have been affected by the meniscal damage. (4) These data cannot be directly related to strains of the posterolateral structures because the reference length of the structures (zero load length) is unknown. (5) We did not investigate the length changes of other posterolateral structures such as the popliteofibular ligament, arcuate ligament, or fabellofibular ligament. One reason for this was that these structures do not have bony attachments on either end. Moreover, there is variability in the anatomy of these structures. (6) The effect of PCL deficiency on the apparent length of posterolateral structures was studied during a quasistatic lunge, and a goniometer was used to control the flexion angles. Future research is needed to quantify the behavior of posterolateral structures during dynamic functional activities.
In conclusion, deficiency of the PCL alters the apparent lengths of the posterolateral structures (ie, LCL and popliteus muscle-tendon unit) during in vivo weightbearing flexion of the knee. The PCL injury caused a significant increase in apparent length of the posterolateral structures for most flexion angles when compared with the uninjured contralateral knees. The findings of altered length patterns of the posterolateral structures along with alterations in tibiofemoral kinematics in knees with PCL deficiency demonstrate that deficiency of the PCL upsets the in vivo knee homeostasis and puts the remaining joint soft tissue environment at greater risk of secondary injury. Therefore, it would be ideal to restore the knee kinematics to the preinjury level when a safe and effective surgical technique to do so is developed. By restoring the normal 6 degrees of freedom kinematics, secondary injury might be prevented and healing of the combined ligamentous lesions improved.
Acknowledgments
The authors gratefully acknowledge the financial support of the National Institutes of Health (R01 AR 052408) and National Football League Charities Foundation. We would also like to thank the patients for their participation in this study and Ramprasad Papannagari, Louis E. DeFrate, Kyung Wook Nha, Jong Keun Seon, and Bijoy Thomas for technical assistance.
Footnotes
References
- 1.Alpert JM, McCarty LP, Bach BR., Jr The posterolateral corner of the knee: anatomic dissection and surgical approach. J Knee Surg. 2008;21(1):50–54. doi: 10.1055/s-0030-1247792. [DOI] [PubMed] [Google Scholar]
- 2.Brinkman JM, Schwering PJ, Blankevoort L, Kooloos JG, Luites J, Wymenga AB. The insertion geometry of the posterolateral corner of the knee. J Bone Joint Surg Br. 2005;87(10):1364–1368. doi: 10.1302/0301-620X.87B10.16536. [DOI] [PubMed] [Google Scholar]
- 3.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 4.Clancy WG, Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament: report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310–322. [PubMed] [Google Scholar]
- 5.Cross MJ, Powell JF. Long-term followup of posterior cruciate ligament rupture: a study of 116 cases. Am J Sports Med. 1984;12(4):292–297. doi: 10.1177/036354658401200409. [DOI] [PubMed] [Google Scholar]
- 6.Dandy DJ, Pusey RJ. The long-term results of unrepaired tears of the posterior cruciate ligament. J Bone Joint Surg Br. 1982;64(1):92–94. doi: 10.1302/0301-620X.64B1.7068728. [DOI] [PubMed] [Google Scholar]
- 7.Dejour H, Walch G, Peyrot J, Eberhard P. The natural history of rupture of the posterior cruciate ligament. Rev Chir Orthop Reparatrice Appar Mot. 1988;74(1):35–43. [PubMed] [Google Scholar]
- 8.Fanelli GC, Giannotti BF, Edson CJ. The posterior cruciate ligament arthroscopic evaluation and treatment. Arthroscopy. 1994;10(6):673–688. doi: 10.1016/s0749-8063(05)80067-2. [DOI] [PubMed] [Google Scholar]
- 9.Fuss FK. An analysis of the popliteus muscle in man, dog, and pig with a reconsideration of the general problems of muscle function. Anat Rec. 1989;225(3):251–256. doi: 10.1002/ar.1092250311. [DOI] [PubMed] [Google Scholar]
- 10.Geissler WB, Whipple TL. Intraarticular abnormalities in association with posterior cruciate ligament injuries. Am J Sports Med. 1993;21(6):846–849. doi: 10.1177/036354659302100615. [DOI] [PubMed] [Google Scholar]
- 11.Gill TJ, DeFrate LE, Wang C, et al. The biomechanical effect of posterior cruciate ligament reconstruction on knee joint function: kinematic response to simulated muscle loads. Am J Sports Med. 2003;31(4):530–536. doi: 10.1177/03635465030310040901. [DOI] [PubMed] [Google Scholar]
- 12.Gill TJ, DeFrate LE, Wang C, et al. The effect of posterior cruciate ligament reconstruction on patellofemoral contact pressures in the knee joint under simulated muscle loads. Am J Sports Med. 2004;32(1):109–115. doi: 10.1177/0095399703258794. [DOI] [PubMed] [Google Scholar]
- 13.Höher J, Harner CD, Vogrin TM, Baek GH, Carlin GJ, Woo SL. In situ forces in the posterolateral structures of the knee under posterior tibial loading in the intact and posterior cruciate ligament-deficient knee. J Orthop Res. 1998;16(6):675–681. doi: 10.1002/jor.1100160608. [DOI] [PubMed] [Google Scholar]
- 14.Keller PM, Shelbourne KD, McCarroll JR, Rettig AC. Nonoperatively treated isolated posterior cruciate ligament injuries. Am J Sports Med. 1993;21(1):132–136. doi: 10.1177/036354659302100122. [DOI] [PubMed] [Google Scholar]
- 15.Krudwig WK, Witzel U, Ullrich K. Posterolateral aspect and stability of the knee joint. II. Posterolateral instability and effect of isolated and combined posterolateral reconstruction on knee stability: a biomechanical study. Knee Surg Sports Traumatol Arthrosc. 2002;10(2):91–95. doi: 10.1007/s00167-001-0269-4. [DOI] [PubMed] [Google Scholar]
- 16.LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854–860. doi: 10.1177/03635465030310062101. [DOI] [PubMed] [Google Scholar]
- 17.LaPrade RF, Morgan PM, Wentorf FA, Johansen S, Engebretsen L. The anatomy of the posterior aspect of the knee: an anatomic study. J Bone Joint Surg Am. 2007;89(4):758–764. doi: 10.2106/JBJS.F.00120. [DOI] [PubMed] [Google Scholar]
- 18.LaPrade RF, Wentorf FA, Crum JA. Assessment of healing of grade III posterolateral corner injuries: an in vivo model. J Orthop Res. 2004;22(5):970–975. doi: 10.1016/j.orthres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Gill TJ, DeFrate LE, Zayontz S, Glatt V, Zarins B. Biomechanical consequences of PCL deficiency in the knee under simulated muscle loads: an in vitro experimental study. J Orthop Res. 2002;20(4):887–892. doi: 10.1016/S0736-0266(01)00184-X. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Most E, DeFrate LE, Suggs JF, Gill TJ, Rubash HE. Effect of the posterior cruciate ligament on posterior stability of the knee in high flexion. J Biomech. 2004;37(5):779–783. doi: 10.1016/j.jbiomech.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Papannagari R, Li M, et al. Effect of posterior cruciate ligament deficiency on in vivo translation and rotation of the knee during weightbearing flexion. Am J Sports Med. 2008;36(3):474–479. doi: 10.1177/0363546507310075. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Wuerz TH, DeFrate LE. Feasibility of using orthogonal fluoroscopic images to measure in vivo joint kinematics. J Biomech Eng. 2004;126(2):314–318. doi: 10.1115/1.1691448. [DOI] [PubMed] [Google Scholar]
- 23.Lovejoy JF, Jr, Harden TP. Popliteus muscle in man. Anat Rec. 1971;169(4):727–730. doi: 10.1002/ar.1091690411. [DOI] [PubMed] [Google Scholar]
- 24.Markolf KL, O’Neill G, Jackson SR, McAllister DR. Effects of applied quadriceps and hamstrings muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32(5):1144–1149. doi: 10.1177/0363546503262198. [DOI] [PubMed] [Google Scholar]
- 25.Markolf KL, Slauterbeck JL, Armstrong KL, Shapiro MM, Finerman GA. Effects of combined knee loadings on posterior cruciate ligament force generation. J Orthop Res. 1996;14(4):633–638. doi: 10.1002/jor.1100140419. [DOI] [PubMed] [Google Scholar]
- 26.Murthy CK. Origin of popliteus muscle in man. J Indian Med Assoc. 1976;67(4):97–99. [PubMed] [Google Scholar]
- 27.Noyes FR, Grood ES, Butler DL, Raterman L. Knee ligament tests: what do they really mean? Phys Ther. 1980;60(12):1578–1581. doi: 10.1093/ptj/60.12.1578. [DOI] [PubMed] [Google Scholar]
- 28.Noyes FR, Stowers SF, Grood ES, Cummings J, VanGinkel LA. Posterior subluxations of the medial and lateral tibiofemoral compartments: an in vitro ligament sectioning study in cadaveric knees. Am J Sports Med. 1993;21(3):407–414. doi: 10.1177/036354659302100314. [DOI] [PubMed] [Google Scholar]
- 29.Papannagari R, DeFrate LE, Nha KW, et al. Function of posterior cruciate ligament bundles during in vivo knee flexion. Am J Sports Med. 2007;35(9):1507–1512. doi: 10.1177/0363546507300061. [DOI] [PubMed] [Google Scholar]
- 30.Park SE, DeFrate LE, Suggs JF, Gill TJ, Rubash HE, Li G. The change in length of the medial and lateral collateral ligaments during in vivo knee flexion. Knee. 2005;12(5):377–382. doi: 10.1016/j.knee.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Park SE, DeFrate LE, Suggs JF, Gill TJ, Rubash HE, Li G. Erratum to “The change in length of the medial and lateral collateral ligaments during in vivo knee flexion”. Knee. 2006;13(1):77–82. doi: 10.1016/j.knee.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Reis FP, de Carvalho CA. Anatomical study on the proximal attachments of the human popliteus muscle. Rev Bras Pesqui Med Biol. 1975;8(5–6):373–380. [PubMed] [Google Scholar]
- 33.Seebacher JR, Inglis AE, Marshall JL, Warren RF. The structure of the posterolateral aspect of the knee. J Bone Joint Surg Am. 1982;64(4):536–541. [PubMed] [Google Scholar]
- 34.Staubli HU, Birrer S. The popliteus tendon and its fascicles at the popliteal hiatus: gross anatomy and functional arthroscopic evaluation with and without anterior cruciate ligament deficiency. Arthroscopy. 1990;6(3):209–220. doi: 10.1016/0749-8063(90)90077-q. [DOI] [PubMed] [Google Scholar]
- 35.Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Arthroscopic evaluation of articular cartilage lesions in posterior-cruciate-ligament-deficient knees. Arthroscopy. 2003;19(3):262–268. doi: 10.1053/jars.2003.50037. [DOI] [PubMed] [Google Scholar]
- 36.Torg JS, Barton TM, Pavlov H, Stine R. Natural history of the posterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1989;246:208–216. [PubMed] [Google Scholar]
- 37.Tria AJ, Jr, Johnson CD, Zawadsky JP. The popliteus tendon. J Bone Joint Surg Am. 1989;71(5):714–716. [PubMed] [Google Scholar]
- 38.Van de Velde SK, DeFrate LE, Gill TJ, Moses JM, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency on the in vivo elongation of the medial and lateral collateral ligaments. Am J Sports Med. 2007;35(2):294–300. doi: 10.1177/0363546506294079. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe Y, Moriya H, Takahashi K, et al. Functional anatomy of the posterolateral structures of the knee. Arthroscopy. 1993;9(1):57–62. doi: 10.1016/s0749-8063(05)80344-5. [DOI] [PubMed] [Google Scholar]