Abstract
Background
Tumor-infiltrating lymphocyte (TIL) counts in colorectal cancer liver metastases (CRCLM) predict survival following resection. While CD4 and CD8 T cells have been correlated with outcome following CRCLM resection, the role of regulatory T cells (Treg) is not well defined.
Methods
TIL in 188 patients who underwent CRCLM resection between 1998 and 2000 were analyzed by immunohistochemistry using tissue microarrays. Correlation between TIL composition and outcome was determined while controlling for established prognostic factors. Total T cells (CD3), helper T cells (CD4), cytotoxic T cells (CD8), and Treg (FoxP3) were analyzed.
Results
Median follow-up time was 40 months for all patients and 95 months for survivors. Overall survival (OS) at 5 and 10 years was 40 and 25 %, respectively. The CD4 T cell count correlated with OS (p = .02) and recurrencefree survival (p = .04). A high number of CD8 T cells relative to total T cells (CD8:CD3 ratio) predicted longer OS times (p = .05). Analysis of Treg revealed that high FoxP3:CD4 (p = .03) and FoxP3:CD8 (p = .05) ratios were independent predictors of shorter OS. Patients with a high clinical risk score (CRS) were more likely to have a high number of intratumoral Treg, and patients ≥65 years old had a less robust CRCLM T cell infiltration.
Conclusions
A high number of Treg relative to CD4 or CD8 T cells predicted poor outcome, suggesting an immunosuppressive role for FoxP3 + TIL. The intratumoral immune response was an independent predictor of outcome in patients with colorectal liver metastases.
The immune response to neoplastic cells has been shown to predict survival in several human malignancies, including primary colorectal cancer.1–6 Our interest in the immune response to colorectal cancer liver metastases (CRCLM) is prompted by data indicating that the vast majority of patients recur despite curative resection and optimal adjuvant therapy.7,8 We speculate that the immunosuppressive nature of liver immune cells9–14 contributes to the propensity of colorectal cancer to metastasize to the liver and recur following resection. Tumor-infiltrating lymphocyte (TIL) counts were shown to predict outcome in a selected group of short- and long-term survivors following resection of CRCLM.15 More recently, our group reported that a high number of regulatory T cells (Treg) predicted decreased survival in patients with neuroendocrine tumor liver metastases.16 A better understanding of Treg in CRCLM may allow for the identification of patients who fail to mount an effective immune response and facilitate development of immunologic interventions designed to enhance intrahepatic immunity.
Our goals for this study were to determine the potential biologic importance of Treg in CRCLM patients and confirm that CD4 and CD8 T cell counts correlate with outcome. We characterized TIL from 188 patients who underwent liver resection. Tissue microarrays (TMA) were stained for CD3, CD4, CD8, and FoxP3 to quantify total T cells, helper T cells, cytotoxic T cells, and Treg, respectively. TIL subset counts and immune cell ratios were correlated with outcome. Several specific immunologic factors, including the Treg tumor infiltrate, were found to be independent predictors of outcome following CRCLM resection. Clinical correlates of TIL densities, including age and the clinical risk score (CRS), were demonstrated as well. These data support the biologic importance of the host immune response to intrahepatic metastases, including the intratumoral Treg infiltrate.
METHODS
Patients
A prospectively maintained hepatobiliary database was used with approval of the Institutional Review Board and in accordance with Health Insurance Portability and Accountability Act regulations. Of 293 consecutive patients who underwent resection of CRCLM at Memorial Sloan-Kettering Cancer Center (New York, NY) from September 1998 to 2000, we identified 188 who had sufficient tissue for TMA construction and analysis. Guidelines for resectability were medical fitness for major laparotomy and a resection encompassing all intrahepatic disease with an adequate remnant liver for recovery. Routine preoperative evaluation included chest X-ray, abdominal/pelvic computed tomography, and colonoscopy.
Immunohistochemistry and Cell Count Analysis
TMA were constructed following pathologic review and diagnostic confirmation as previously described.15 Tissue blocks with minimal necrosis and fibrosis were selected to enable accurate acquisition of intratumoral lymphocyte counts, comparable among specimens. In brief, tumor tissue cores measuring 0.6 mm in diameter were made in triplicate from paraffin blocks and processed using the ATA-27 automated arrayer (Beecher Instruments, Sun Prairie, WI). TMA blocks were cut to 5-µm sections and deparaffinized, rehydrated in graded alcohol, and processed.17 Antibodies were used to recognize CD3 (F7.2.38, Dako, Carpinteria, CA), CD8 (c8/144B, Dako), CD4 (polyclonal goat, R&D Systems, Minneapolis, MN), and FoxP3 (236A/E7, Abcam Inc., Cambridge, MA). Biotinylated secondary immunoglobulins were added (Vector Laboratories, Inc., Burlingame, CA) followed by avidinbiotin peroxidase complexes (1:25; Vector Laboratories, Inc.). Diaminobenzidine was used as the chromogen, and hematoxylin was used as the nuclear counterstain. TMA slides were analyzed with the Aperio ScanScope XT and Aperio IHC analysis algorithm (Aperio, Vista, CA). Our automated counting method was previously validated.15 We excluded cases with fewer than 2 analyzable cores, which occurred in one instance each for CD3 and CD4 staining.
Statistical Analysis
The Kaplan–Meier method was used to estimate overall survival (OS) and recurrence-free survival (RFS). Cox regression multivariate models were used to identify independent prognostic factors. Separate multivariate models were constructed for each cell marker or cell marker ratio given the coexpression of the markers by single cell types and hence the potential for statistical interaction. The multivariate models for immunologic factors included the CRS and extrahepatic disease (EHD).7 Unpaired t tests and chi-square tests were used to compare TIL frequencies among subgroups. Optimal cutoff points for cell numbers were selected using the maximally selected chi-square method (maxχ2, R version 2.7, www.r-project.org).18 Use of the maxχ2 method enables selection of an outcome-dependent cutoff point that may be most clinically useful and avoids arbitrary cutoff points such as the median or a percentile-based value. A p value of ≤.05 was considered statistically significant (SPSS, version 15.0; Chicago, IL).
RESULTS
Patient Characteristics and Follow-Up
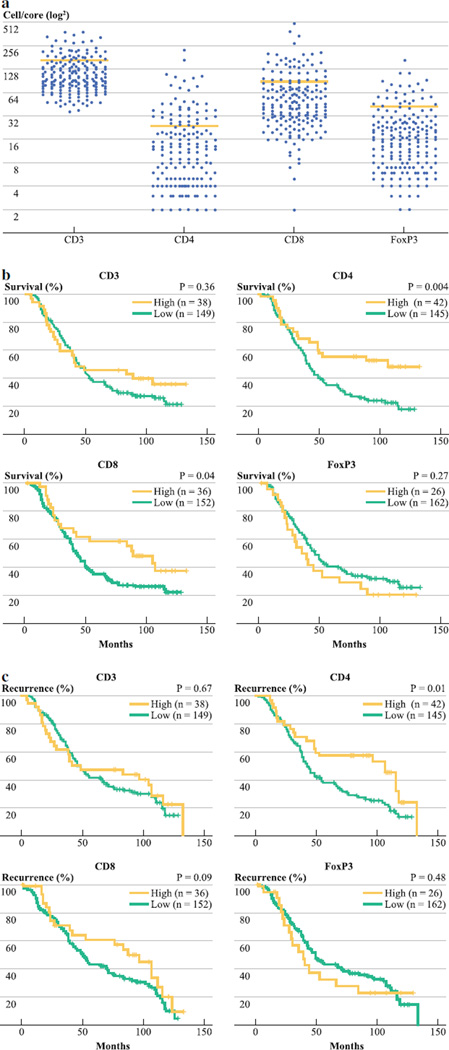
The average age was 63 years (range, 23–86 years) for 188 patients who underwent CRCLM resection (Table 1). The median follow-up time for all patients was 40 and 95 months for survivors (range, 0–133 months). A minority of patients had EHD (17 %), 66 % had evidence of primary tumor nodal metastases, and 26 % had a clinical risk score (CRS) ≥3. Chemotherapy was administered to 89 % of patients, with 25 % of patients receiving systemic therapy prior to resection and 26 % undergoing hepatic artery infusion. Systemic therapy regimens included irinotecan or oxaliplatin in 54 and 7 % of all patients, while 22 % received 5-fluorouracil based treatment without inclusion of a platinum agent or topoisomerase inhibitor. To determine whether TIL numbers and ratios correlated with outcome following liver resection, we stained TMA for CD3, CD4, CD8, and FoxP3. We found that 20, 23, and 19 % of patients had high levels of CD3+, CD4+, and CD8+ TIL counts, respectively. A high level of FoxP3+ TIL, which are putative Treg, was documented in 14 % of those treated in this series (Fig. 1a).
TABLE 1.
Patient characteristics
| Median age, years (range) | 63 (23–86) |
|
|---|---|---|
| N | % | |
| Male sex | 102 | 54 |
| Extrahepatic disease | 32 | 17 |
| DFI <12 | 86 | 46 |
| Tumor>5 cm | 62 | 33 |
| Multiple tumors | 88 | 47 |
| Node + primary | 124 | 66 |
| Synchronous disease | 15 | 8 |
| CEA >200 | 26 | 14 |
| CRS ≥3 | 49 | 26 |
| Any chemotherapy | 168 | 89 |
| 5-FU based regimen | 42 | 22 |
| Oxaliplatin based regimen | 13 | 7 |
| Irinotecan-based regimen | 102 | 54 |
| Posthepatectomy chemotherapy | 166 | 88 |
| Prehepatectomy chemotherapy | 47 | 25 |
| Regional chemotherapy | 48 | 26 |
| CD3 High | 38 | 20 |
| CD4 High | 42 | 23 |
| CD8 High | 36 | 19 |
| CD4:CD3 High | 56 | 30 |
| CD4:CD8 High | 107 | 57 |
| CD8:CD3 High | 49 | 26 |
| FoxP3 High | 26 | 14 |
| FoxP3:CD4 High | 122 | 65 |
| FoxP3:CD8 High | 105 | 56 |
FIG. 1.
Tissue microarray samples from resected CRCLM were stained for various T cell markers. a The distributions of TIL counts for each marker are shown. The bars indicate the cutoff points (cells/tissue core) for each cell marker (CD3 = 174, CD4 = 26, CD8 = 95, FoxP3 = 44). Overall (b) and recurrence-free (c) survival were determined by the Kaplan–Meier method, and we used the log-rank test to compare groups
TIL Subset Numbers Correlate with Survival and Recurrence Following CRCLM Resection
OS at 5 and 10 years was 40 and 25 % for the entire group. We analyzed nonimmunologic factors previously shown to be of prognostic importance in patients undergoing CRCLM resection.7,19 Significant predictors of survival included the CRS and EHD (Table 2). The presence of EHD and a CRS ≥3 were significant predictors of poor recurrence-free survival (Table 2). Other correlates of OS and RFS included primary tumor lymph node metastases, tumor size, and CEA level (not shown).
TABLE 2.
Analysis of the association of tumor-infiltrating lymphocyte numbers and other clinicopathologic variables with overall survival
| Factor | 5-year OS (%) |
Median OS (months) |
UV P value |
MV P value |
OR (95 % CI) |
5-year RFS (%) |
Median RFS (months) |
UV P value |
MV P value |
OR (95 % CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| CD3+ | ||||||||||
| High | 46 | 42 | .360 | – | – | 48 | 49 | .67 | – | – |
| Low | 38 | 45 | 42 | 49 | ||||||
| CD4+ | ||||||||||
| High | 55 | 107 | .004 | .02** | 1.8 (1.1–2.9) | 58 | 107 | .01 | .04 | 1.6 (1.0–2.6) |
| Low | 35 | 40 | 39 | 43 | ||||||
| CD8+ | ||||||||||
| High | 59 | 89 | .04 | .08 | 1.5 (0.9–2.5) | 59 | 97 | .09 | – | – |
| Low | 35 | 43 | 39 | 46 | ||||||
| CD4+:CD3+ | ||||||||||
| High | 53 | 68 | .01 | .07 | 1.5 (1.0–2.2) | 55 | 71 | .05 | .23 | 1.3 (0.9–1.9) |
| Low | 35 | 39 | 39 | 43 | ||||||
| CD8+:CD3+ | ||||||||||
| High | 53 | 84 | .05 | .05 | 1.6 (1.0–2.4) | 60 | 95 | .10 | – | – |
| Low | 35 | 42 | 38 | 45 | ||||||
| CD4+:CD8+ | ||||||||||
| High | 46 | 52 | .02 | .11 | 1.3 (0.9–1.9) | 50 | 55 | .05 | .23 | 1.3 (0.9–1.8) |
| Low | 31 | 37 | 34 | 40 | ||||||
| FoxP3+ | ||||||||||
| High | 31 | 35 | .27 | – | – | 34 | 39 | .48 | – | – |
| Low | 41 | 46 | 46 | 49 | ||||||
| FoxP3+:CD4+ | ||||||||||
| High | 34 | 39 | .007 | .03 | 1.6 (1.1–2.3) | 38 | 43 | .03 | .09 | 1.4 (0.9–2.0) |
| Low | 51 | 65 | 54 | 71 | ||||||
| FoxP3+:CD8+ | ||||||||||
| High | 35 | 40 | .03 | .05 | 1.5 (1.0–2.1) | 39 | 43 | .09 | – | – |
| Low | 46 | 53 | 49 | 56 | ||||||
| Clinical risk score | ||||||||||
| 1–2 | 46 | 52 | <.001 | <.001** | 2.1 (1.5–3.1) | 51 | 66 | <.001 | <.001 | 2.2 (1.5–3.3) |
| >3 | 21 | 27 | 22 | 29 | ||||||
| Extrahepatic disease | ||||||||||
| No | 44 | 49 | <.001 | .002 | 2.0 (1.3–3.1) | 48 | 55 | .003 | .01 | 1.8 (1.1–3.0) |
| Yes | 15 | 24 | 21 | 37 | ||||||
UV univariate analysis, MV multivariate analysis (separate MV models constructed for each cell marker or cell ratio, which also included CRS and extrahepatic disease)
Statistically significant when analyzed as a continuous variable
Analysis of CD4 and CD8 staining demonstrated that OS was significantly greater among patients with a high level of TIL expressing either marker (Fig. 1b). The 5-year OS among those with a high level of CD4+ TIL was 55 % compared with 35 % for patients with a low CD4+ TIL count (p = .004). A significant 5-year survival advantage was also found for patients with a high number of CD8+ TIL (59 vs 35 %, p = .04). When multivariate analyses were performed, a high CD4+ TIL count proved to be a significant independent predictor of OS [odds ratio (OR) =1.8, p = .02], while a high CD8+ TIL count approached statistical significance (OR = 1.5, p = .08). Superior RFS at 5 years was associated with a high CD4+ TIL count on univariate (Fig. 1c) and multivariate analysis (58 vs 39 %, p = .04).
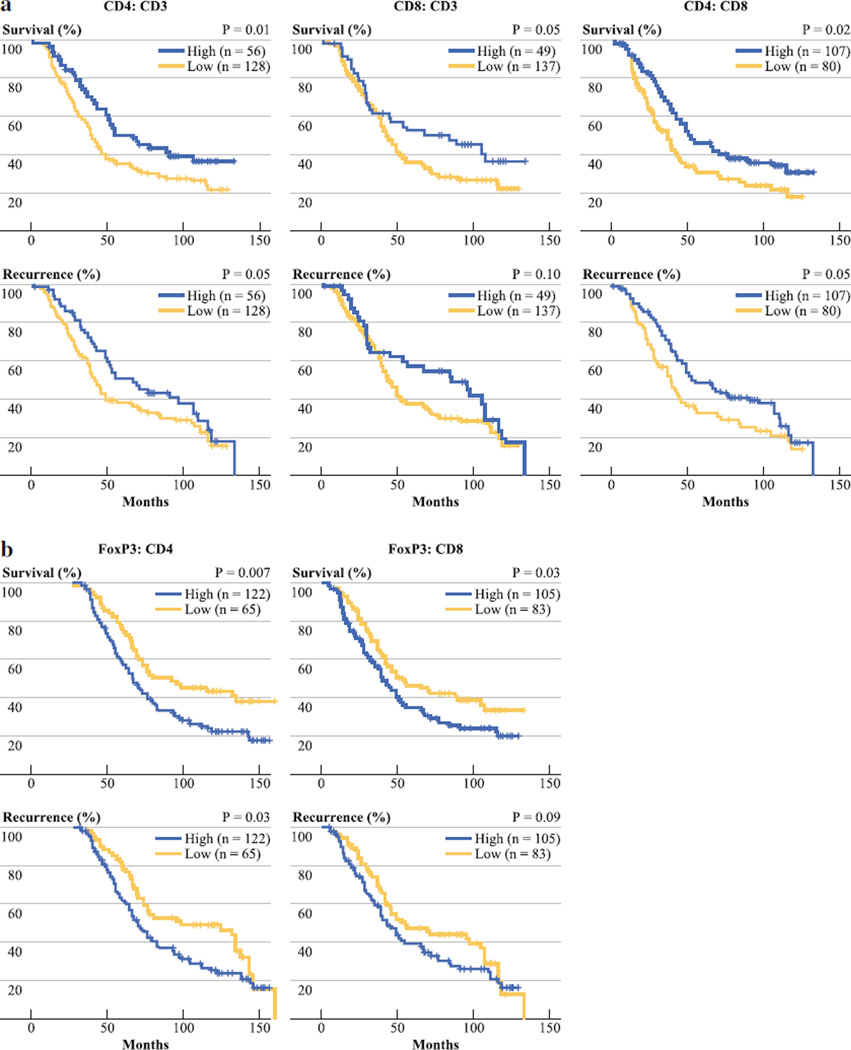
CD4 and CD8 T cell Ratios and Outcome Following Resection of CRCLM
As previously reported, immune cell ratios may be more informative than individual TIL subtype counts.15 High CD4:CD8 and CD4:CD3 ratios were significant predictors of favorable OS and RFS on univariate analyses (Fig. 2a; Table 2). Although the absolute number of CD8 T cells was not an independent predictor of outcome, the CD8:CD3 ratio was a significant independent predictor of OS (OR = 1.6, p = .05). The median OS time for patients with a high CD8:CD3 ratio was 84 months, compared with 42 months for those with a low ratio.
FIG. 2.
Tissue microarray samples from resected CRCLM were stained for various T cell markers and cell ratios were calculated. We analyzed various ratios based on CD3, CD4, and CD8 staining of TIL (a). In addition, we examined ratios of FoxP3+ TIL to CD4 and CD8 T cells (b). Overall and recurrence-free survival were determined by the Kaplan–Meier method and we used the log-rank test to compare groups
Infiltration of CRCLM by Regulatory T Cells Predicts Poor Outcome Following Resection
FoxP3 is a marker for Treg, which can suppress the ability of CD4 and CD8 liver T cells to mount an effective immune response. The absolute number of FoxP3+ cells did not predict OS or RFS (Fig. 1b, c), although patients with a high FoxP3+ TIL count had a 5-year OS rate of only 31 % (Table 2). We also analyzed ratios of FoxP3+ cells to other TIL types, as the presence of Treg relative to CD4 and CD8 T cells has been well documented to influence their suppressive effects.20 High FoxP3:CD4 and FoxP3:CD8 ratios were significant independent predictors of shorter OS (Fig. 2b; Table 2). At 5 years following CRCLM resection, 51 % of those with a low FoxP3:CD4 ratio were alive compared with 34 % for those with a high ratio (OR = 1.6, p = .03). Similarly, 5-year survival was significantly greater in those with a low FoxP3:CD8 ratio (46 %) when compared with those with relatively more FoxP3+ cells (35 %, HR = 1.5, p = .05).
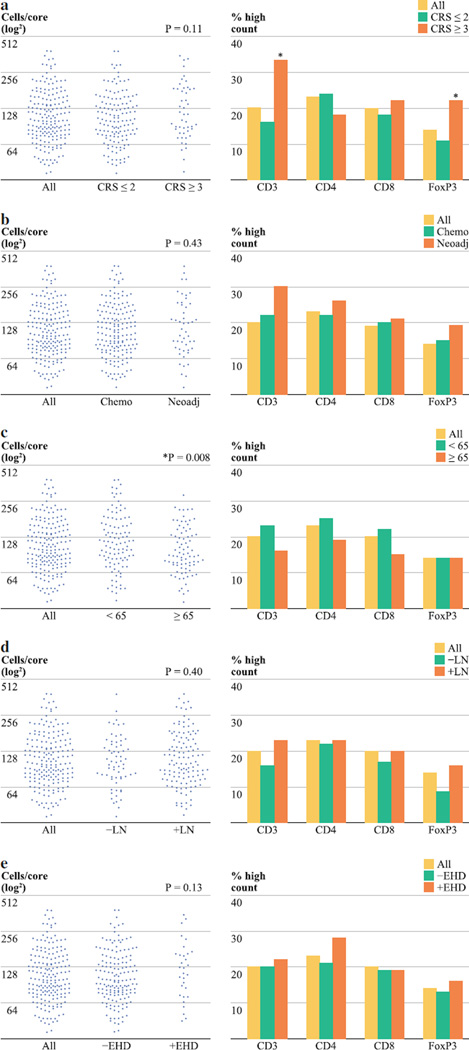
Predicting the Extent of CRCLM T Cell Infiltration
To assess potential predictors of T cell counts within tumors, we compared CRCLM TIL frequencies among patient subsets defined by well-accepted clinical predictors of outcome (Fig. 3). Patients with a high CRS were more likely to have high FoxP3+ TIL counts (Fig. 3a). Since the majority of our patients (89 %) received some form of perioperative chemotherapy, we were interested in determining whether chemotherapy before resection impacted TIL frequency. A trend toward a higher T cell count was noted among patients who received neoadjuvant chemotherapy (30 %) compared with those who did not receive systemic therapy prior to resection (18 %, p = .08, not shown). However, when individuals who received irinotecan or oxaliplatin were analyzed separately, we found that administration of these drugs prior to liver resection correlated with a greater percentage of patients with high levels of favorable TIL counts. Among individuals who were given irinotecan or oxaliplatin before liver resection, 43, 34, and 36 % had high CD3+, CD4+, and CD8+ TIL counts, compared with 13, 15, and 18 % for those who did not receive these agents preoperatively (p ≤ .01 for each comparison, data not shown). When the mean CD3+ TIL counts were compared among subgroups, older patients (≥65 years) had significantly lower levels of T cell infiltration (Fig. 3c, p = .01).
FIG. 3.
The frequencies of CD3+ TIL counts and proportion with high TIL subset counts is shown for subgroups defined by clinical risk score (a), systemic therapy (b), age (c), primary tumor node status (d), and presence of extrahepatic disease (e). Neoadjuvant therapy refers to treatment given prior to hepatic resection. The distribution plots on the left reveal the CD3+ TIL counts for all patients, and the p values represent comparisons of the mean cell numbers among subgroups. The bar graphs on the right demonstrate the proportion of patients within each subgroup who had high TIL counts for each marker. The cutoff points (cells/ tissue core) for each cell marker are as follows: CD3 = 174, CD4 = 26, CD8 = 95, FoxP3 = 44
DISCUSSION
We have demonstrated an association between the degree of tumor T cell infiltration and outcome following resection of CRCLM. After controlling for EHD and the CRS, individual T cell counts and subset ratios were significant predictors of survival and recurrence following CRCLM resection. High ratios of CD4 and CD8 T cells relative to the total infiltrate were significant predictors of favorable outcomes following CRCLM resection. Patients with high FoxP3:CD4 and FoxP3:CD8 ratios had shorter survival times. The balance between Treg and conventional CD4 or CD8 T cells may be a determinant of a patient’s capacity to mount an effective immune response to intrahepatic metastases.
In our prior study of highly selected 2- and 10-year survivors, we noted that patients with a more robust CD8 T cell response were more likely to survive long-term following CRCLM resection.15 Another group recently substantiated our initial report of the prognostic importance of CRCLM TIL.21 Presently, we confirmed that CRCLM CD8 T cell infiltration correlates with survival time following resection in a larger, more recent series of patients, consistent with what has been reported for primary colorectal cancer.3,4,22 These findings fit well with the role cytotoxic CD8 T cells play as direct mediators of tumor killing and the enhanced activation status of CRCLM TIL compared with T cells in nontumor liver.23
CD4 T cells are essential for the generation of effective antitumor immune responses, as they provide the necessary help for cytotoxic CD8 T cells.24 In the present study, we found that a high CD4 count was an independent predictor of survival and recurrence. We previously reported that CD4 T cells correlated with a decreased chance of longterm survival.15 The patient groups from the present and prior studies are difficult to compare and differ in ways that have the potential to influence the nature of the immune response. As noted earlier, our previous work was confined to 2- and 10-year survivors and thus a limited spectrum of disease biology. The present series includes a more recent group of patients who were exposed to more modern cytotoxic therapy regimens. The differences in chemotherapeutic agents may have influenced the impact of the CD4+ infiltrate among the two studies. Oxaliplatin and irinotecan have been demonstrated to have significant effects on CD4 T cells and our patients who received these agents prior to hepatic resection had significantly higher levels of CD4+ TIL counts.25–27 In this series, 25 % of patients received systemic therapy preoperatively. Only 19 % of subjects in our previous report were recipients of prehepatectomy systemic treatment, and none received oxaliplatin- or irinotecan-based regimens. When considering the functional plasticity of CD4 T cells, it is not surprising that the prognostic implications of CD4+ TIL vary among patient groups treated in different eras with alternative regimens.
Treg are a small subset of CD4 T cells and are identified by their expression of FoxP3, a transcription factor critical to their differentiation and suppressive function.28 FoxP3+ cell counts have been associated with a higher likelihood of disease progression in several malignancies.2,16,29,30 Although patients with a high number of FoxP3+ cells had lower OS and RFS rates, the results were not significant. The functional importance of Treg lies in their ability to interact with and suppress helper CD4 and cytotoxic CD8 T cells.20,31,32 As such, we analyzed ratios of Treg to CD4 and CD8 T cells. High FoxP3:CD4 and FoxP3:CD8 ratios were independent predictors of shorter survival following CRCLM resection (Fig. 2b; Table 2). These findings suggest that a high number of Treg in relation to helper and cytotoxic T cells leads to a more suppressive tumor microenvironment and hence decreased survival.
In several instances, TIL ratios proved to be better predictors of outcome than individual cell counts, indicating the importance of immune cell interactions and networks within the liver.32 The CD8:CD3, FoxP3:CD4, and FoxP3:CD8 ratios were independent predictors of outcome, whereas the individual CD8 and Foxp3 counts were not. Immune cell ratios may provide more insight into the functional impact of a subset of TIL, as the ultimate clinical impact of a TIL subset will depend on the ability of the cells to influence the immunologic response as a whole. In addition, TIL ratios allowed for the identification of a greater number of patients with favorable outcomes. TIL ratios may be more biologically relevant and of greater use in clinical decision making.
To enhance the potential utility of TIL counts and ratios, the identification of correlates of an effective immune response to CRCLM is of interest. We found that patients with poor prognoses, as defined by high CRS, were more likely to have a high level of suppressive Treg. The presence of a high number of Treg may have contributed to poor outcomes in those with a high CRS, although we cannot infer causation based on our data. In addition, patients over the age of 65 years were less likely to have a high level of CRCLM T cell infiltration and age-related declines in immunity have been described.33 Confirmation of these correlations and identification of other surrogates of immune responses to CRCLM will increase the potential clinical applicability of our findings.
Our data must be interpreted in the context of study design limitations. Use of TMA to study TIL introduces the potential for sampling error. To minimize the influence of sampling error, 3 tissue cores were included for each patient and we calculated the mean cell counts. Our tissue cores included tumor only, without normal liver or the tumor-liver interface. A prior report indicated that the total number TIL is of greater prognostic importance than separate analyses of intratumoral T cells and those at the margin.3 In addition, we recognize that FoxP3 is not a perfect marker for Treg in humans.34,35 Finally, treatment-related variables such as liver-directed therapy following resection or systemic regimens prior to referral for treatment of CRCLM may have impacted the outcomes of certain patients. While our data suggest a favorable T cell response may prolong survival following CRCLM resection, we cannot be certain that antitumor immunity was responsible for improved OS or if both simply reflect unidentified surrogates of tumor biology.
The present study demonstrates that T cell subset and ratio analyses provide independently significant prognostic information following resection of CRCLM. TIL analysis may be useful for refining prognoses following CRCLM resection. More importantly, our findings suggest that immunomodulatory therapy designed to enhance the endogenous antitumor response may be a valuable approach. Further work is needed to determine if agents that block T cell regulatory checkpoints, such as the PD-1 and CTLA-4 pathways, will enhance intrahepatic immune responses to CRCLM.36–38 Future studies should focus on the immunologic network within the liver, in addition to individual cell type and their specific phenotypic and functional properties.
REFERENCES
- 1.Clemente CG, Mihm MC, Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagés C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 6.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for meta-static colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 9.Katz SC, Pillarisetty VG, Bleier JI, Kingham TP, Chaudhry UI, Shah AB, et al. Conventional liver CD4 T cells are functionally distinct and suppressed by environmental factors. Hepatology. 2005;42:293–300. doi: 10.1002/hep.20795. [DOI] [PubMed] [Google Scholar]
- 10.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173:230–235. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 11.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445–454. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 12.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 13.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:2524–2530. doi: 10.1245/s10434-009-0585-3. [DOI] [PubMed] [Google Scholar]
- 16.Katz SC, Donkor C, Glasgow K, Pillarisetty VG, Gönen M, Espat NJ, et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB (Oxford) 2010;12:674–683. doi: 10.1111/j.1477-2574.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen T, Prus D, Shia J, Abu-Wasel B, Pinto MG, Freund HR, et al. Expression of P53, P27 and KI-67 in colorectal cancer patients of various ethnic origins: clinical and tissue microarray based analysis. J Surg Oncol. 2008;97:416–422. doi: 10.1002/jso.20989. [DOI] [PubMed] [Google Scholar]
- 18.Miller R, Siegmund D. Maximally selected chi square statistics. Biometrics. 1982;38:1011–1016. [Google Scholar]
- 19.House MG, Ito H, Gonen M, Allen PJ, DeMatteo RP, Brennan MF, et al. Survival after hepatic resection for metastatic colo-rectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. doi: 10.1016/j.jamcollsurg.2009.12.040. 752-5. [DOI] [PubMed] [Google Scholar]
- 20.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8 +/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 23.Wagner P, Koch M, Nummer D, Palm S, Galindo L, Autenrieth D, et al. Detection and functional analysis of tumor infiltrating T-lymphocytes (TIL) in liver metastases from colorectal cancer. Ann Surg Oncol. 2008;15:2310–2317. doi: 10.1245/s10434-008-9971-5. Erratum in: Ann Surg Oncol. 2009;16:1084. Rahbari, Nuh [added]. [DOI] [PubMed] [Google Scholar]
- 24.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8 + T cell immunity against a tumor/self-antigen is augmented by CD4 + T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–123. doi: 10.1038/sj.bjc.6605465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melichar B, Touskova M, Vesely P. Effect of irinotecan on the phenotype of peripheral blood leukocyte populations in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002;49:967–970. [PubMed] [Google Scholar]
- 28.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 29.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 31.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 34.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 35.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4+ CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]