Abstract
Study Design
Case-control study.
Objective
. To evaluate the effect of lumbar degenerative disc disease (DDD) on the disc deformation at the adjacent level and at the level one above the adjacent level during end ranges of lumbar motion.
Summary of Background Data
It has been reported that in patients with DDD, the intervertebral discs adjacent to the diseased levels have a greater tendency to degenerate. Although altered biomechanics have been suggested to be the causative factors, few data have been reported on the deformation characteristics of the adjacent discs in patients with DDD.
Methods
Ten symptomatic patients with discogenic low back pain between L4 and S1 and with healthy discs at the cephalic segments were involved. Eight healthy subjects recruited in our previous studies were used as a reference comparison. The in vivo kinematics of L3–L4 (the cephalic adjacent level to the degenerated discs) and L2–L3 (the level one above the adjacent level) lumbar discs of both groups were obtained using a combined magnetic resonance imaging and dual fluoroscopic imaging technique at functional postures. Deformation characteristics, in terms of areas of minimal deformation (defined as less than 5%), deformations at the center of the discs, and maximum tensile and shear deformations, were compared between the two groups at the two disc levels.
Results
In the patients with DDD, there were significantly smaller areas of minimal disc deformation at L3–L4 and L2–L3 than the healthy subjects (18% compared with 45% of the total disc area, on average). Both L2–L3 and L3–L4 discs underwent larger tensile and shear deformations in all postures than the healthy subjects. The maximum tensile deformations were higher by up to 23% (of the local disc height in standing) and the maximum shear deformations were higher by approximately 25% to 40% (of the local disc height in standing) compared with those of the healthy subjects.
Conclusion
Both the discs of the adjacent level and the level one above experienced higher tensile and shear deformations during end ranges of lumbar motion in the patients with DDD before surgical treatments when compared with the healthy subjects. The larger disc deformations at the cephalic segments were otherwise not detectable using conventional magnetic resonance imaging techniques. Future studies should investigate the effect of surgical treatments, such as fusion or disc replacement, on the biomechanics of the adjacent segments during end ranges of lumbar motion.
Keywords: adjacent segment, degenerative disc disease, imaging technique, intervertebral disc deformation, in vivo, lumbar spine, MRI
Low back pain (LBP) secondary to lumbar degenerative disc disease (DDD) is one of the most common causes of disability in working population.1,2 It has been reported that in patients with DDD, the intervertebral discs (IVD) adjacent to the diseased levels have a greater tendency to degenerate,3–5 especially after surgical fusion treatment of the diseased segments.6–9 Numerous studies have suggested that altered biomechanics, such as abnormal loading and/or motion patterns,10–12 are the causative factors of adjacent segment degeneration (ASD). However, it remains unclear whether these changes are due to the natural development triggered by the DDD13–16 or to the consequence of spinal surgeries.6,17,18 Therefore, a quantitative knowledge of the disc deformation at the adjacent segments under physiologic weight-bearing conditions is instrumental to delineate the biomechanical factors associated with ASD.
Many studies have examined the biomechanics of the adjacent segments after lumbar fusion or disc arthroplasty in vivo and in vitro. For example, segmental mobility19–23 and change in disc height9,18,19,24,25 have been measured using sagittal plane radiographs in patients after surgical treatments of the diseased discs. In vitro cadaveric tests and computational simulations have been used to investigate the effect of surgical treatments on loadings of the facet joints,26–30 intradiscal pressure,31–34 disc bulging,35 and stress-strain distribution.36–38 Few studies have investigated the effect of DDD on the biomechanics of the adjacent segments before surgical treatments. In finite element studies,35,39 disc degeneration was simulated by changing the disc height and its material properties, and adjacent segmental motions and disc stress-strain distributions were calculated under combined axial compressive forces and moments.35,39 However, the disc deformation at the segments adjacent to the DDD levels in living patients remains unclear.
We have recently developed a combined magnetic resonance imaging (MRI) and dual fluoroscopic imaging system (DFIS) technique to quantify the disc geometric deformation in vivo.40 The purpose of this study was to quantitatively evaluate the effect of lumbar DDD on the disc deformation at the adjacent level and the level one above the adjacent level during in vivo end ranges of lumbar spine motions, which corresponded to the extreme motions experienced during daily activities. In 10 patients with DDD with degenerated discs between L4 and S1, disc L3–L4 and L2–L3 were studied and compared with those of eight asymptomatic healthy subjects. We hypothesized that DDD can cause the healthy cephalic L3–L4 and L2–L3 segments to undergo larger deformation than normal subjects.
MATERIALS AND METHODS
Subject Recruitment
Ten patients with DDD (mean age, 51.8 ± 13.1 years; mean height, 169 ± 6.3 cm; mean weight, 65.7 ± 9.8 kg) who were diagnosed with discogenic LBP originated from L4–S1 were included consecutively in this study. Discogenic LBP was confirmed by both the treating surgeon and a neuroradiologist based on the clinical and radiographic assessments and discogram. On the basis of the assessment by the treating surgeon, patients were excluded when any of the following presents: previous spinal surgery, spinal pathology at segments other than L4-S1, facet joint arthritis, scoliosis, presence of metallic implants incompatible with the MRI, prior radiation within a year, and pregnancy. Approval of the experimental design by the authors’ institutional review board was obtained. A signed consent form was obtained from each patient.
A group of eight age-, height-, and weight-matched healthy subjects (mean age, 54.4 ± 3.5 years; mean height, 163.5 ± 5.8 cm; mean weight, 63.5 ± 11.1 kg) who were recruited in our previous studies41 were used as a reference comparison. The subjects were recruited using advertisements placed within our institutional publications and internet network. The subjects were evaluated for the absence of LBP or any other spinal disorders using clinical history, physical examination, and radiographic findings, accessed by both a radiology specialist and an experienced spine surgeon. In addition, subjects were also excluded when any of the following was present: presence of metallic implants incompatible with the MRI, use of chronic pain medications, prior radiation within a year, and pregnancy.
The L3–L4 (the adjacent level to the degenerated discs) and L2–L3 (the level one above the adjacent level) lumbar discs of each subject were investigated, resulting in a total of 36 discs studied. The degrees of degeneration of the lumbar spine discs L2–S1 were graded from MR images using the five-level Pfirrmann’s scales,42 by both a radiology specialist and an experienced spine surgeon blinded to the group membership (Table 1). Both the patients and the normal subjects had nonstatistically different Pfirrmann’s scores of less than III at the L2–L3 and L3–L4 discs, where grade I and II represent minimal degeneration and grade V represents severe degeneration as a collapsed disc.42
TABLE 1.
Numbers of Subjects Fall into Each Disc Degeneration Grade of a Five-Level Pfirrmann’s Classification, with I, II for Minimal Degeneration and V for Severe Degeneration as a Collapsed Disc. The Grading Was Performed by Both a Radiologist and a Spine Surgeon
| Graded by Radiologist | Graded by Surgeon | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | I | II | III | IV | V | |
| DDD (n = 10) | DDD (n = 10) | |||||||||
| L2–L3 | 6 | 4 | 6 | 4 | ||||||
| L3–L4 | 5 | 5 | 5 | 4 | 1 | |||||
| L4–L5 | 3 | 3 | 4 | 1 | 5 | 4 | ||||
| L5–L1 | 5 | 5 | 5 | 5 | ||||||
| Normal (n = 8) | Normal (n = 8) | |||||||||
| L2–L3 | 7 | 1 | 6 | 2 | ||||||
| L3–L4 | 4 | 4 | 4 | 4 | ||||||
| L4–L5 | 2 | 6 | 2 | 4 | 2 | |||||
| L5–L1 | 1 | 7 | 6 | 2 | ||||||
Combined MRI and DFIS Technique41,43
For each subject, MRI of the lumbar spine was obtained using a 3 Tesla scanner (MAGNETOM Trio, Siemens, Erlangen, Germany) with a spine surface coil and a T2-weighted fat suppressed three-dimensional spoiled gradient recall (SPGR) sequence.44 Parallel digital images with a thickness of 1.5 mm (~85 images) without gap and with a resolution of 512 × 512 pixels were obtained (voxel size 0.45 × 0.45 × 1.5 mm). The parallel sagittal MRI of the spinal segments were imported into a solid modeling software (Rhinoceros v. 4.0, Robert McNeel & Associates, Seattle, WA) to construct three-dimensional anatomic vertebral models of L2, L3, and L4 using an established protocol43 (Figure 1A). Polygon mesh models of the vertebrae were created from the manually outlined contour lines of the vertebrae (Figure 1B).
Figure 1.
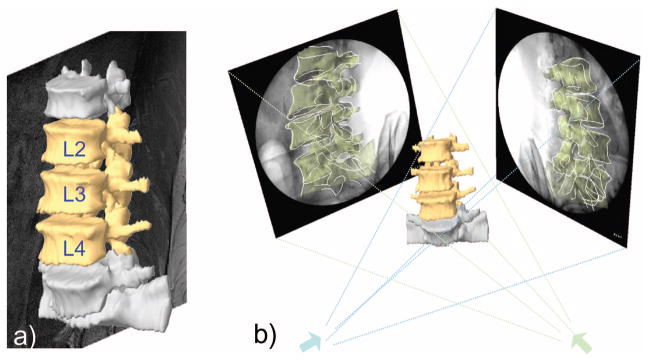
(A) Construction of three-dimensional vertebrae models from manually outlining parallel magnetic resonance images (~85 images per spine). (B) Reproduction of in vivo vertebrae positions by matching three-dimensional model projections to two-dimensional osseous contours.
The subject was then scanned using DFIS41 in standing position and at 6 end-ranges of motion: maximal left-right torsion, side-to-side bending, and flexion-extension of the torso, which corresponded to the motions experienced during daily activities. An orthopedic surgeon instructed the subject to perform these postures in a consistent way that minimized the motion of the pelvis. With a far larger modulus compare to the disc, the vertebrae were assumed to be rigid during motion. In each posture, the in vivo positions of the vertebrae L2, L3, and L4 were reproduced in a solid modeling software (Rhinoceros, Robert McNeel & Associates, Seattle, WA) by matching the projections of the three-dimensional MR image–based vertebral models at supine to their two-dimensional osseous contours in the fluoroscopic images at various end ranges of lumbar motion (Figure 1B). This system has been validated in its accuracy in determination of vertebral positions in space using their three-dimensional computer models43 where the accuracy in translation was within 0.3 mm and in orientation was within 0.7°.
Calculation of IVD Deformation
The overall IVD deformation was calculated based on the positions and orientations of the disc endplates (L2–L3, L3–L4) from the reproduced kinematics of the vertebrae in each posture. As shown in Figure 2, local disc heights were determined by calculating the shortest distances between mesh vertices of the upper and lower endplates (about 1000 points per endplate) using a custom MATLAB code (MathWorks, Natick, MA). The disc height of each vertex in standing position was used as a reference to calculate the disc deformations at various end ranges of motion of the torso. To do this, a reference plane was created for each disc by automatically fitting a transverse plane through the lower disc endplate using Rhinoceros software (Figure 2). Tensile deformation at each vertex was defined as the component of the local height change that is perpendicular to the transverse plane. It was calculated in MATLAB and plotted on a color-coded map plot showing magnitudes with respect to the reference disc height (in standing, Figure 3). Similarly, shear deformation at each vertex was defined as the component parallel to the transverse plane and plotted on a gradient (quiver) plot showing both magnitudes and directions (Figure 4).
Figure 2.
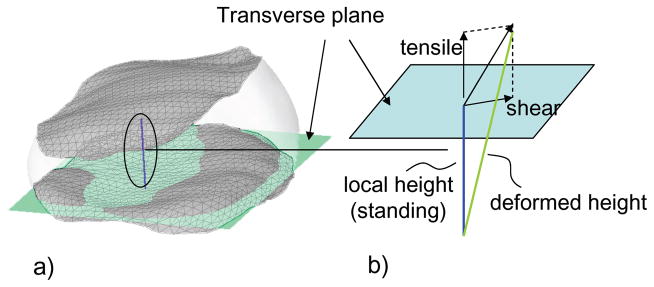
(A) Determination of local height at each mesh vertex. Transverse plane was fit through lower endplate of the disc. (B) Calculation of the tensile and shear deformations using local height in standing as a reference.
Figure 3.
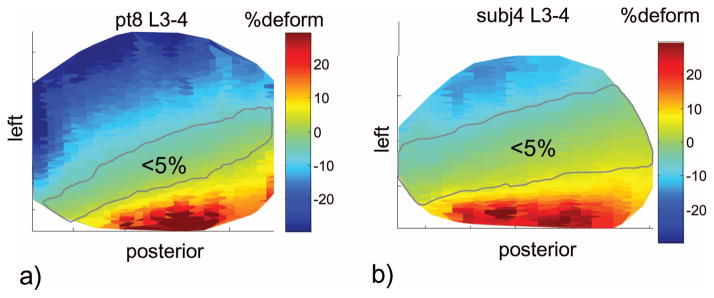
Typical disc tensile deformation of (A) a patient with degenerative disc disease and (B) a healthy subject at disc L3–L4. In the degenerative disc disease group, the maximum tensile deformations were larger. In addition, the areas of minimal deformation were smaller.
Figure 4.
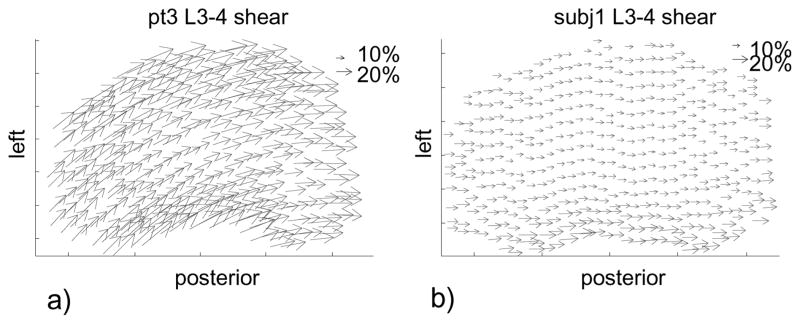
Typical disc shear deformation of (A) a patient with degenerative disc disease and (B) a healthy subject at disc L3–L4. In the degenerative disc disease group, the maximum shear deformation and the shear at the center of a disc were larger.
In this study, the characteristics of the disc deformation of the patients were compared with those of the normal subjects under various end ranges of motion of the torso. These included areas of minimal deformation (defined as <5% deformation), deformations at the center of the discs, and maximum tensile (tension and compression) and shear deformations. The areas of minimal deformation (defined as <5% deformation) were calculated in a custom MATLAB code. The 5% criterion was empirically picked on the basis of the magnitude of deformation near the center of a disc, which was observed to be the minimum among different portions of a disc in general. Two-way mixed model analyses of variance were used to compare the data of the two groups of subjects at the two disc levels, where disc levels was considered as a within-factor. A statistical significance was defined as P < 0.05. When a statistically significant difference was detected, a Newman-Keuls post hoc test was performed. The statistical analyses were performed in Statistica (Statsoft, Tulsa, OK).
RESULTS
Deformation Patterns
In the patients with DDD, the areas of minimal IVD deformation (<5%) at the adjacent level (L3–L4) and the level one above (L2–L3) were smaller than the normal subjects (Table 2). The differences were statistically significant except for L3–L4 and L2–L3 under left torsion. On average, in the normal subjects, approximately 45% of the discs were minimally deformed at the two disc levels. In the patients with DDD, the areas of minimal deformation was only about 18% of the disc area at the two disc levels (Figure 3). Although not quantitatively evaluated, the areas of minimal deformation were observed to locate near the centers of the discs in the healthy group while shifted off the central axis in the DDD group. At the center of the discs, both the patients with DDD and the normal subjects had similar tensile deformations with average magnitudes less than 6% (Table 3). No statistically significant difference was observed between the two groups, except for L3–L4 disc during extension, where the average tensile deformation was −6% ± 6% (compression) for the patients with DDD and 1% ± 4% for the normal subjects. At the center of the discs, shear deformations were generally larger in the patients with DDD than those in the healthy subjects for all postures (Table 3). Significant differences were observed at the L3–L4 level during left bending (26% DDD vs. 9% healthy) and at the L2–L3 level during right torsion (21% DDD vs. 9% healthy), during left bending (25% DDD vs. 9% healthy), and during right bending (28% DDD vs. 10% healthy).
TABLE 2.
Percentage Area (Average ± SD) of the Discs Under Minimal Deformation (<5%) During End Ranges of Motion of the Torso.
| Left Torsion | Right Torsion | Left Bend | Right Bend | Extension | Flexion | |
|---|---|---|---|---|---|---|
| L2–L3 | ||||||
| DDD | 23% ± 29% | 14% ± 14%* | 15% ± 11%* | 19% ± 16%* | 20% ± 12%* | 18% ± 8%* |
| Normal | 37% ± 15% | 57% ± 22%* | 41% ± 24%* | 43% ± 19%* | 54% ± 24%* | 34% ± 12%* |
| L3–L4 | ||||||
| DDD | 28% ± 25% | 12% ± 15%* | 15% ± 8%* | 18% ± 8%* | 19% ± 19%* | 14% ± 7%* |
| Normal | 48% ± 27% | 61% ± 15%* | 50% ± 14%* | 48% ± 21%* | 44% ± 15%* | 44% ± 19%* |
Using Mixed Model Analysis of Variance, Statistically Significant Differences Between DDD and Normal Subjects Were Marked as *, P < 0.05. No Statistically Significant Difference Was Found Between L2–L3 and L3–L4 Discs in All Case
TABLE 3.
Tensile and Shear Deformations (Average ± SD) at the Center of the Discs in the Patients with DDD and in the Normal Subjects During End Ranges of Motion of the Torso.
| Left Torsion | Right Torsion | Left Bend | Right Bend | Extension | Flexion | ||
|---|---|---|---|---|---|---|---|
| L2–L3 | |||||||
| Tensile | DDD | 3% ± 19% | 4% ± 16% | 1% ± 16% | −4% ± 21% | 3% ± 13% | 4% ± 12% |
| Normal | −1% ± 6% | 0% ± 4% | −1% ± 7% | 1% ± 3% | 0% ± 6% | −1% ± 5% | |
| Shear | DDD | 26% ± 14% | 21% ± 13%* | 25% ± 14%* | 28% ± 16%* | 26% ± 18% | 27% ± 17% |
| Normal | 16% ± 10% | 9% ± 6%* | 9% ± 5%* | 10% ± 4%* | 15% ± 8% | 14% ± 7% | |
| L3–L4 | |||||||
| Tensile | DDD | 3% ± 7% | −1% ± 10% | −4% ± 8% | 2% ± 10% | −6% ± 6%* | −2% ± 10% |
| Normal | 1% ± 5% | −2% ± 4% | 1% ± 3% | 0% ± 6% | 1% ± 4%* | −1% ± 3% | |
| Shear | DDD | 19% ± 12% | 20% ± 21% | 26% ± 13%* | 32% ± 24% | 20% ± 19% | 32% ± 31% |
| Normal | 9% ± 7% | 12% ± 6% | 9% ± 5%* | 15% ± 12% | 14% ± 4% | 17% ± 9% | |
Using Mixed Model Analysis of Variance, Statistically Significant Differences Between DDD and Normal Subjects were Marked as *, P < 0.05. No Statistically Significant Difference was Found Between L2–L3 and L3–L4 Discs in All Cases
Maximum Tensile and Shear Deformations
At the adjacent level (L3–L4), in all postures, maximum tension deformations were larger in the patients with DDD (ranging from 18% to 45%, on average) compared with the healthy subjects (ranging from 10% to 26%) in all postures (Table 4). Significant differences were observed during right torsion (26% DDD vs. 10% healthy), during right bending (35% DDD vs. 12% healthy), and during flexion (47% DDD vs. 22% healthy). Maximum compressive deformations were also larger in the patients with DDD (ranging from −7% to −41%) than in the healthy subjects (ranging from −9% to −17%), except for left torsion. Significant differences were only observed during left bending (−31% DDD vs. −13% healthy). Maximum shear were larger in the patients with DDD (ranging from 53 to 66%) than in the healthy subjects (ranging from 15% to 34%) in all postures. Significant differences between the patients with DDD and the healthy subjects were observed in most postures, except during right torsion and during flexion.
TABLE 4.
Maximum Tensile (Tension and Compression) and Shear Deformations (Average ± SD) of the Discs in the Patients with DDD and in the Normal Subjects During End Ranges of Motion of the Torso.
| Left Torsion | Right Torsion | Left Bend | Right Bend | Extension | Flexion | ||
|---|---|---|---|---|---|---|---|
| L2–L3 | |||||||
| Tension | DDD | 29% ± 20%* | 38% ± 33%* | 25% ± 22% | 18% ± 19% | 32% ± 21%* | 45% ± 11%* |
| Normal | 10% ± 8%* | 11% ± 9%* | 12% ± 9% | 18% ± 6% | 12% ± 11%* | 26% ± 9%* | |
| Compression | DDD | −7% ± 24% | −14% ± 20% | −41% ± 32%* | −22% ± 22% | −20% ± 13% | −18% ± 13% |
| Normal | −12% ± 11% | −9% ± 6% | −13% ± 16%* | −14% ± 10% | −13% ± 14% | −17% ± 8% | |
| Shear | DDD | 53% ± 27% | 53% ± 24%* | 65% ± 32%* | 56% ± 23%* | 55% ± 34% | 66% ± 30%* |
| Normal | 34% ± 17% | 20% ± 12%* | 15% ± 8%* | 23% ± 11%* | 31% ± 19% | 18% ± 16%* | |
| L3–L4 | |||||||
| Tension | DDD | 26% ± 26% | 26% ± 19%* | 24% ± 24% | 35% ± 25%* | 23% ± 20% | 47% ± 25%* |
| Normal | 18% ± 16% | 10% ± 8%* | 15% ± 9% | 12% ± 10%* | 16% ± 13% | 22% ± 13%* | |
| Compression | DDD | −11% ± 10% | −17% ± 21% | −31% ± 17%* | −24% ± 20% | −24% ± 20% | −28% ± 21% |
| Normal | −13% ± 9% | −10% ± 7% | −13% ± 10%* | −12% ± 7% | −14% ± 9% | −15% ± 10% | |
| Shear | DDD | 62% ± 23%* | 50% ± 31% | 63% ± 26%* | 64% ± 27%* | 62% ± 28%* | 54% ± 32% |
| Normal | 26% ± 17%* | 29% ± 21% | 21% ± 14%* | 28% ± 18%* | 26% ± 10%* | 29% ± 17% | |
Using Mixed Model Analysis of Variance, Statistically Significant Differences Between DDD and Normal Subjects were Marked as *, P < 0.05. No Statistically Significant Difference was Found Between L2–L3 and L3–L4 Discs in All Cases
At the level one above (L2–L3) the adjacent segment, maximum tension deformations were larger in the patients with DDD (ranging from 23% to 47%, on average) than in the healthy subjects (ranging from 10% to 22%) in all postures (Table 4). Significant differences were observed in most postures, except during left bending and right bending. Maximum compressive deformations were also larger in the patients with DDD (ranging from −11% to −31%) than in the healthy subjects (ranging from −10% to −15%), except for left torsion. Significant difference was only observed during left bending (−41% DDD vs. −13% healthy). Maximum shear deformations were larger in the patients with DDD (ranging from 50 to 64%) than in the healthy subjects (ranging from 21% to 29%) in all postures. Significant differences between the patients with DDD and the healthy subjects were observed in most postures, except during left torsion and during extension.
Difference Between L3–L4 and L2–L3 Discs
No statistically significant difference (P > 0.05) was found between L3–L4 and L2–L3 in any of the studied postures, in either groups, in terms of the areas of minimal deformation, tensile and shear deformations at the center of the discs, or maximum tensile and shear deformations.
DISCUSSION
This study investigated and compared the lumbar IVD deformation of the adjacent level (L3–L4) and the level one above (L2–L3) the adjacent level between the patients with DDD at L4 to S1 and the healthy subjects in end ranges of motion of the torso, using a previously described noninvasive imaging technique.40,41,43 The results showed that in the patients with DDD, IVDs of both L3–L4 and L2–L3 underwent larger tensile and shear deformations in all postures compared with the normal subjects. The maximum tensile deformations were larger by up to 23% (of the local disc height in standing) and the maximum shear deformations were larger by approximately 25% to 40% (of the local disc height in standing) when compared with the deformation of the healthy subjects at the same levels during the same in vivo postures. On the other hand, the deformation patterns were also different, as the areas bearing minimal deformation (<5%) were significantly smaller in the patients with DDD by approximately 25% of the total disc areas. Although not quantitatively evaluated, in the patients with DDD these areas were observed to shift away from the disc centers. At the center of the discs, both groups experienced similar small tensile deformations of <6%. However, shear deformations in the patients with DDD were larger than those of the normal subjects by approximately 10% during all end ranges of motion. Despite these differences between the two groups, no statistically significant difference was found between L3–L4 and L2–L3 discs within each group.
These differences can be directly related to the increased motion/loading at the adjacent levels of the DDD discs as observed by others.35,39 Kim et al35 developed a two motion segment (L3–L4 and L4–L5) finite element model and investigated the effects of disc degeneration (simulated at the L4–L5 level) on the adjacent intact L3–L4 level. They found increased maximum stress-strain, intradiscal pressure, and disc bulging at the L3–L4 disc under axial compressive load. They concluded that these changes may trigger the degenerative process at the L3–L4 disc over time. More recently, Ruberte et al39 modified a finite element model of lumbar spine (L1–S1) to simulate degeneration at the L4–L5 disc. Under compressive preload and moments in three principal planes, they found that the motion at the cephalic adjacent level (L3–L4) increased by 26% (of the normal motion) under axial torsion, 21% under lateral bending, and 28% under flexion/extension. They also reported increases in stress range from 30% to 10-fold and suggested that degeneration can increase the risk for injury at the adjacent levels. Although there are substantial differences between the experimental setups of our in vivo patient measurements and these finite element studies, our study and the finite element models showed similar trends of the effects of DDD on the deformation of the discs at the adjacent segments.
We also found that the disc deformations in two cephalic levels were different in the patients with DDD than the healthy subjects. There was no statistically significant difference between the two cephalic levels. The results showed that DDD can affect the levels other than the immediately adjacent levels. Ruberte et al39 used a finite element model of lumbar spine L1-S1 to simulate degeneration at the L4–L5 disc and had only reported the findings on the degenerated level and the immediately adjacent level. To the best of our knowledge, our study is the first showing multilevel biomechanics above the degenerated discs in living human subjects.
Most previous studies have investigated ASD in patients after surgical treatments and some have suggested a correlation between fusion and the development of radiographic and symptomatic ASD.9,17,45 In a literature review by Park et al,12 the incidence of lumbar ASD after arthrodesis has been reported to range from 5.2% to 100%, whereas the incidence of symptomatic ASD range from 5.2% to 18.5%. Although the early result of total disc replacement are satisfactory, the basic premise that motion preservation will diminish ASD is yet to be proven.36,46 A recent review by Harrop et al17 noted that the incidence of ASD is approximately 9% after arthroplasty, whereas the incidence of symptomatic ASD is approximately 1%. Abnormal biomechanical changes at the adjacent segments after surgical treatments of the DDD have been reported in both arthrodesis and arthroplasty patients, in terms of mobility,19–23 change in disc height,18,19,24,25 loading on the facet joints,26–30 intradiscal pressure,31–34 disc bulging,35 and stress-strain.36–38 All of these suggest surgical treatments can have an adverse effect on ASD.10–12 However, no studies have reported on the quantitative effect of the spine surgeries on the disc deformation at the adjacent segments in living patients and under physiologic motions of the spine. Fusion or other surgical treatments may further change the adjacent discs deformation in a way that maybe related to the mechanism of high occurrence of ASD. Our study indicated that the disc deformation characteristics at the adjacent level and at the level one above in the patients with DDD were different from the healthy subjects even before the surgeries. Our results warrant a further investigation on the correlation between the deformation of the adjacent discs and the development of ASD in this group of patients after surgical treatments, which may provide invaluable information for prosthesis designs and surgical plans to include their effects on the entire lumbar spine, rather than focus merely on the DDD levels.
Controversially, several studies have suggested that ASDs are subsequent to the natural development instead of the surgical intervention, based on comparing radiographic changes between age and gender matched surgical and control groups.13–15,47 In a recent biomechanical study, Axelsson et al16 observed hypermobility of the segments adjacent to fusions in nine patients both before and 5 years after surgery. The hypermobility was found not to significantly change over time. They therefore concluded that the abnormal biomechanics at the adjacent level may not associate with progressive degeneration because of fusion. In our study, we found in the patients with DDD, both the adjacent level and the level one above had different disc deformation patterns and larger maximum deformations before surgery than the healthy subjects. We therefore postulate that the adjacent discs might have gradually adapted to the changing environment during the DDD development in the L4–L5–S1 levels, although they were rather healthy on the basis of MRI findings in this group of patients. Whether this may or may not further trigger radiographic or clinical ASD over time even without surgical intervention is unclear. It would be of clinical interests to perform a long-term follow-up study of these patients to longitudinally examine how disc deformation may change at the adjacent levels and correlate to the development of ASD, or even LBP, if eventually surgical treatments were not performed.
There are certain limitations of this study. The sample sizes in the two groups were relatively small, which might limit our ability to detect differences. This may also explain why some of the differences were not statistically significant as well as the relatively large SDs that were observed. Even though we have tried to standardize the motion of the torso, patients may be more or less likely to perform combined movements. However, we would expect little effect of the combined movements on the deformation results reported, since the differences were generally observed between the two groups, not among different postures. As reported in the previous studies,40,43 the maximum error in calculation of the geometric deformation was 4% when considering both the accuracy of the imaging technique and the deformation of the endplates. Simplifications in calculation of the deformation were made as we only determined the overall geometric deformation throughout the thickness of the disc. The results were only overall strains of the discs. In the future, a finite element study using these results as boundary conditions should be carried out to further investigate the in vivo stress-strain distributions inside the discs. In addition, we will follow up this patient group, whether or not they will have surgical treatments, to further study the adjacent discs longitudinally and to investigate the biomechanical mechanism of ASD.
In summary, disc deformations were studied using a novel combined MRI and DFIS imaging technique. In patients with lumbar DDD, the discs at the adjacent level and at the level one above experienced higher deformations during various end ranges of motion of the torso when compared with those of the normal subjects. Both tensile and shear deformations were larger at the adjacent segment and the segment one above the adjacent level. Disc areas bearing minimal deformation were significantly smaller. These differences in disc deformations were otherwise not detectable using conventional MRI techniques that classify the degeneration of the discs. Future studies should quantify how surgical treatments, such as fusion and total disc replacement, would further alter the disc deformation at the adjacent segments.
Key Points.
Before surgery, the patients with degenerated discs between L4 and S1 had larger disc deformations at the adjacent level (L3–L4) and at the level one above the adjacent level (L2–L3) than healthy subjects.
In the patients with degenerative disc disease, L3–L4 and L2–L3 discs experienced higher tensile and shear deformations during end ranges of motion of the lumbar spine than healthy subjects.
The patients with degenerative disc disease had smaller disc areas minimally deformed (<5%) at end ranges in L3–L4 and L2–L3 compared with healthy subjects
The differences in the disc deformations were otherwise not detectable using conventional magnetic resonance imaging techniques.
Acknowledgments
This work is supported by ECOR fund from MGH and NIH R21AR057989.
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Footnotes
Approval by the authors’ institutional review board was obtained.
Each subject signed an approved consent form.
References
- 1.Adams MA. Biomechanics of back pain. Acupunct Med. 2004;22:178–88. doi: 10.1136/aim.22.4.178. [DOI] [PubMed] [Google Scholar]
- 2.Luoma K, Riihimaki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Elfering A, Semmer N, Birkhofer D, et al. Risk factors for lumbar disc degeneration: A 5-year prospective MRI study in asymptomatic individuals. Spine (Phila Pa 1976) 2002;27:125–34. doi: 10.1097/00007632-200201150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Waris E, Eskelin M, Hermunen H, et al. Disc degeneration in low back pain: A 17-year follow-up study using magnetic resonance imaging. Spine (Phila Pa 1976) 2007;32:681–4. doi: 10.1097/01.brs.0000257523.38337.96. [DOI] [PubMed] [Google Scholar]
- 5.Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86-A:1497–503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 1988;13:375–7. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Goel VK, Weinstein JN, editors. Biomechanics of the Spine–-Clinical and Surgical Perspective. Boca Raton, FL: CRC press; 1990. [Google Scholar]
- 8.Kumar MN, Jacquot F, Hall H. Long-term follow-up of functional outcomes and radiographic changes at adjacent levels following lumbar spine fusion for degenerative disc disease. Eur Spine J. 2001;10:309–13. doi: 10.1007/s005860000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg Br. 2002;84:289–94. doi: 10.1302/0301-620x.84b2.11937. [DOI] [PubMed] [Google Scholar]
- 10.Adams MA, Freeman BJ, Morrison HP, et al. Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976) 2000;25:1625–36. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila Pa 1976) 2004;29:2724–32. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine (Phila Pa 1976) 2004;29:1938–44. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 13.Seitsalo S, Schlenzka D, Poussa M, et al. Disc degeneration in young patients with isthmic spondylolisthesis treated operatively or conservatively: A long-term follow-up. Eur Spine J. 1997;6:393–7. doi: 10.1007/BF01834066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Horn JR, Bohnen LM. The development of discopathy in lumbar discs adjacent to a lumbar anterior interbody spondylodesis. A retrospective matched-pair study with a postoperative follow-up of 16 years. Acta Orthop Belg. 1992;58:280–6. [PubMed] [Google Scholar]
- 15.Hambly MF, Wiltse LL, Raghavan N, et al. The transition zone above a lumbosacral fusion. Spine (Phila Pa 1976) 1998;23:1785–92. doi: 10.1097/00007632-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson P, Johnsson R, Stromqvist B. Adjacent segment hypermobility after lumbar spine fusion: no association with progressive degeneration of the segment 5 years after surgery. Acta Orthop. 2007;78:834–9. doi: 10.1080/17453670710014635. [DOI] [PubMed] [Google Scholar]
- 17.Harrop JS, Youssef JA, Maltenfort M, et al. Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1701–7. doi: 10.1097/BRS.0b013e31817bb956. [DOI] [PubMed] [Google Scholar]
- 18.Ekman P, Moller H, Shalabi A, et al. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18:1175–86. doi: 10.1007/s00586-009-0947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frymoyer JW, Hanley EN, Jr, Howe J, et al. A comparison of radiographic findings in fusion and nonfusion patients ten or more years following lumbar disc surgery. Spine (Phila Pa 1976) 1979;4:435–40. doi: 10.1097/00007632-197909000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Stokes IA, Wilder DG, Frymoyer JW, et al. 1980 Volvo award in clinical sciences. Assessment of patients with low-back pain by bi-planar radiographic measurement of intervertebral motion. Spine (Phila Pa 1976) 1981;6:233–40. doi: 10.1097/00007632-198105000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Axelsson P, Johnsson R, Stromqvist B. The spondylolytic vertebra and its adjacent segment. Mobility measured before and after posterolateral fusion. Spine (Phila Pa 1976) 1997;22:414–7. doi: 10.1097/00007632-199702150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Panjabi M, Malcolmson G, Teng E, et al. Hybrid testing of lumbar CHARITE discs versus fusions. Spine (Phila Pa 1976) 2007;32:959–66. doi: 10.1097/01.brs.0000260792.13893.88. discussion 67. [DOI] [PubMed] [Google Scholar]
- 23.Auerbach JD, Jones KJ, Milby AH, et al. Segmental contribution toward total lumbar range of motion in disc replacement and fusions: A comparison of operative and adjacent levels. Spine (Phila Pa 1976) 2009;34:2510–7. doi: 10.1097/BRS.0b013e3181af2622. [DOI] [PubMed] [Google Scholar]
- 24.Guigui P, Wodecki P, Bizot P, et al. Long-term influence of associated arthrodesis on adjacent segments in the treatment of lumbar stenosis: a series of 127 cases with 9-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2000;86:546–57. [PubMed] [Google Scholar]
- 25.Schulte TL, Leistra F, Bullmann V, et al. Disc height reduction in adjacent segments and clinical outcome 10 years after lumbar 360 degrees fusion. Eur Spine J. 2007;16:2152–8. doi: 10.1007/s00586-007-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CK, Langrana NA. Lumbosacral spinal fusion. A biomechanical study. Spine (Phila Pa 1976) 1984;9:574–81. doi: 10.1097/00007632-198409000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Bastian L, Lange U, Knop C, et al. Evaluation of the mobility of adjacent segments after posterior thoracolumbar fixation: a biomechanical study. Eur Spine J. 2001;10:295–300. doi: 10.1007/s005860100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denoziere G, Ku DN. Biomechanical comparison between fusion of two vertebrae and implantation of an artificial intervertebral disc. J Biomech. 2006;39:766–75. doi: 10.1016/j.jbiomech.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 29.Rohlmann A, Burra NK, Zander T, et al. Comparison of the effects of bilateral posterior dynamic and rigid fixation devices on the loads in the lumbar spine: a finite element analysis. Eur Spine J. 2007;16:1223–31. doi: 10.1007/s00586-006-0292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park CK, Ryu KS, Jee WH. Degenerative changes of discs and facet joints in lumbar total disc replacement using ProDisc II: Minimum two-year follow-up. Spine (Phila Pa 1976) 2008;33:1755–61. doi: 10.1097/BRS.0b013e31817b8fed. [DOI] [PubMed] [Google Scholar]
- 31.Chow DH, Luk KD, Evans JH, et al. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine (Phila Pa 1976) 1996;21:549–55. doi: 10.1097/00007632-199603010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Chen CS, Cheng CK, Liu CL. A biomechanical comparison of posterolateral fusion and posterior fusion in the lumbar spine. J Spinal Disord Tech. 2002;15:53–63. doi: 10.1097/00024720-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Weinhoffer SL, Guyer RD, Herbert M, et al. Intradiscal pressure measurements above an instrumented fusion. A cadaveric study. Spine (Phila Pa 1976) 1995;20:526–31. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham BW, Kotani Y, McNulty PS, et al. The effect of spinal destabilization and instrumentation on lumbar intradiscal pressure: An in vitro biomechanical analysis. Spine (Phila Pa 1976) 1997;22:2655–63. doi: 10.1097/00007632-199711150-00014. [DOI] [PubMed] [Google Scholar]
- 35.Kim YE, Goel VK, Weinstein JN, et al. Effect of disc degeneration at one level on the adjacent level in axial mode. Spine (Phila Pa 1976) 1991;16:331–5. doi: 10.1097/00007632-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Chen SH, Zhong ZC, Chen CS, et al. Biomechanical comparison between lumbar disc arthroplasty and fusion. Med Eng Phys. 2009;31:244–53. doi: 10.1016/j.medengphy.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Chiang MF, Zhong ZC, Chen CS, et al. Biomechanical comparison of instrumented posterior lumbar interbody fusion with one or two cages by finite element analysis. Spine (Phila Pa 1976) 2006;31:E682–9. doi: 10.1097/01.brs.0000232714.72699.8e. [DOI] [PubMed] [Google Scholar]
- 38.Kumar N, Judith MR, Kumar A, et al. Analysis of stress distribution in lumbar interbody fusion. Spine (Phila Pa 1976) 2005;30:1731–5. doi: 10.1097/01.brs.0000172160.78207.49. [DOI] [PubMed] [Google Scholar]
- 39.Ruberte LM, Natarajan RN, Andersson GB. Influence of single-level lumbar degenerative disc disease on the behavior of the adjacent segments–afinite element model study. J Biomech. 2009;42:341–8. doi: 10.1016/j.jbiomech.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Xia Q, Passias P, et al. Measurement of geometric deformation of lumbar intervertebral discs under in-vivo weight bearing condition. J Biomech. 2009;42:705–11. doi: 10.1016/j.jbiomech.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Wang S, Passias P, et al. Segmental in vivo vertebral motion during functional human lumbar spine activities. Eur Spine J. 2009;18:1013–21. doi: 10.1007/s00586-009-0936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Passias P, Li G, et al. Measurement of vertebral kinematics using noninvasive image matching method-validation and application. Spine (Phila Pa 1976) 2008;33:E355–61. doi: 10.1097/BRS.0b013e3181715295. [DOI] [PubMed] [Google Scholar]
- 44.Disler DG, Peters TL, Muscoreil SJ, et al. Fat-suppressed spoiled GRASS imaging of knee hyaline cartilage: technique optimization and comparison with conventional MR imaging. AJR Am J Roentgenol. 1994;163:887–92. doi: 10.2214/ajr.163.4.8092029. [DOI] [PubMed] [Google Scholar]
- 45.Yang JY, Lee JK, Song HS. The impact of adjacent segment degeneration on the clinical outcome after lumbar spinal fusion. Spine (Phila Pa 1976) 2008;33:503–7. doi: 10.1097/BRS.0b013e3181657dc3. [DOI] [PubMed] [Google Scholar]
- 46.Anderson PA, Rouleau JP. Intervertebral disc arthroplasty. Spine (Phila Pa 1976) 2004;29:2779–86. doi: 10.1097/01.brs.0000146460.11591.8a. [DOI] [PubMed] [Google Scholar]
- 47.Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar inter-body fusion. Spine (Phila Pa 1976) 1995;20:743–7. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]


