Abstract
Tumor angiogenesis and lymphangiogenesis are key features of tumor progression and metastasis. The role of tumor cells-derived factors in the promotion of associated angiogenesis and lymphangiogenesis is much studied and, no doubt, very important for the understanding of cancer progression. This review aims to present and discuss the work done on the pro-angiogenic and lymphangiogenic cellular interactions within the tumor microenvironment and the signaling pathways that regulate this cross talk. Such multifactor studies are critical for the development of future therapeutic approaches for cancer because they take into account the complexities of cellular interactions within the tumor microenvironment.
Keywords: Angiogenesis, angiogenesis stimulators, lymphangiogenesis, lymphangiogenesis stimulators, tumor microenvironment, endothelial cells, tumor cells, extracellular matrix
Introduction
It is well known that tumors require a microvasculature development in order to grow and metastasize (Hanahan and Folkman, 1996). The cancer cells migration to distant tissues occurs through blood and lymphatic vessels(He et al., 2005). The main metastatic route for oral squamous cell carcinoma is the lymphatic one (Tobler and Detmar, 2006). Blood and lymphatic vessels in tumor tissue are major components of the tumor microenvironment. These vessels are newly formed from pre-existing host vessels stimulated by pro-blood-angiogenic and pro-lymph-angiogenic (pro-blood/lymph-angiogenic) factors expressed in tumor cells (Onimaru et al., 2011).
Angiogenesis plays a important role not only in the tumor growth and its blood supply, but also in the tumoral metatstasis. The angiogenic processes are mediated mostly by cellular stimulation of diverse growth factors and secreted molecules. The main elucidated signaling mechanism involves the vascular endothelial growth factor-A (VEGF-A), produced by tumor cells, and the consequent proliferation activity of vascular endothelial cells (Tammela et al., 2005). Although these processes seem simple, in fact there are many complex mechanisms involving tumor cells, endothelial cells, as well as other stromal cells and microenviromental molecules working together in several signaling pathways.
In morphological terms, angiogenesis can present itself as a sprouting (Eilken and Adams, 2010), intussusseptive event (Burri et al., 2004), or even the tumor cells or other types of cells can turn into “endothelial-like” cells (Gottfried et al., 2007; Maniotis et al., 1999). In addition, both tumor and endothelial cell scan present different phenotypes in particular organs, tumor types and subtypes (Jung et al., 2002).
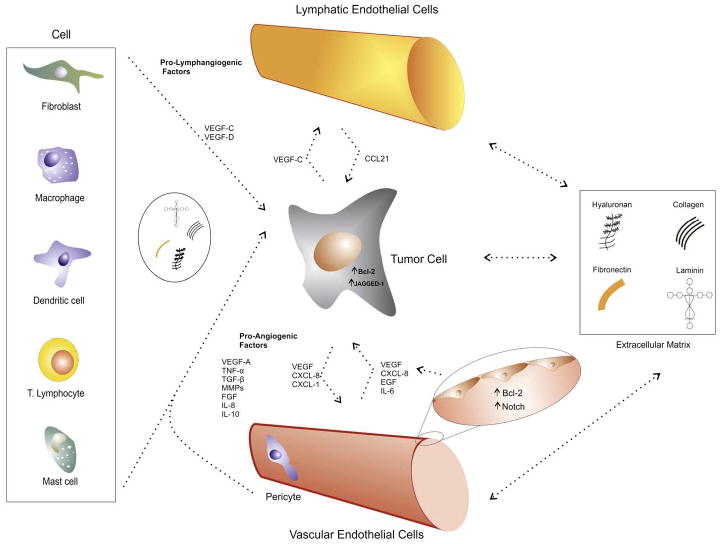
The lymphatic endotelial cells are distinct from vascular endotelial cells regarding their biological, functional and structural aspects, and also in terms of their protein secretion and expression. These cells can play an important role in the pathogenesis of cancer, changing the tumoral microinvironment.
Moreover, with the advances in research it becomes more clear the complexity of growth factors and receptors involved in angiogenic and lymphangiogenic processes. Several studies have shown that the growth factors which are more implied in lymphangiogenesis, such as vascular endothelial growth factor-C and -D(VEGF-C and VEGF-D), and its correspondent receptor vascular endothelial growth factor receptor-3(VEGFR-3), can also participate in angiogenesis. On the other hand, pro-angiogenic factors (ex: VEGF-A and angiopoetin-2) can also participate in lymphangiogenesis (Scavelli et al., 2004). Furthermore, recently there were discovered crosstalk mechanisms between tumor and endothelial cells (Issa et al., 2009; Kaneko et al., 2007; Neiva et al., 2009), which will be further elucidated in this review. With the research involving crosstalk mechanism, the literature started to point out to the existence of different intercellular crosstalk mechanisms involving tumor angiogenesis and lymphangiogenesis. Vascular endothelial cells, in a particular tumor microenvironment, can interact not only with tumor cells but also with immune cells, fibroblasts, pericytes and the extracellular matrix (Jung et al., 2002).
Furthermore, it has been shown that tumor and stromal cells can be responsible for compensatory pro-angiogenic signaling against anti-angiogenic therapies, and that these treatment approaches are still much directed against one or few pro-angiogenic factors (Abdollahi and Folkman, 2010). Specific anti-lymphangiogenic therapy in cancer is still not applied in clinical trials. However, several studies in animal models seem to be promising in inhibiting the lymphatic spread of cancer cells (Stacker and Achen, 2008). A recent review has focused on the cell-dependent molecular mechanisms that regulate tumor blood/lymph-angiogenesis, discussing the features tumor-associated blood/lymph-angiogenesis separately, and concerning the role of blood/lymph-angiogenic cascades in tumor microenvironment (Onimaru et al., 2011). In the present mini review we take in account this aspects and also the interactions among all the cells in the tumor microenvironment, pointing out the inter-cellular and matrix pro-angiogenic and lymphangiogenic mechanisms have been recognized as a result of interconnected factors, didactically separated into topics in this review.
Initial considerations
Hanahan and Weinberg published two very important articles entitled “The Hallmarks of Cancer” (Hanahan and Weinberg, 2000) and “Hallmarks of Cancer: the Next Generation” (Hanahan and Weinberg, 2011). These works show that there are tumor checkpoints, and that they can occur in different sequences within particular types of cancer (Hanahan and Weinberg, 2000). Such hallmarks are now eight: resisting cell death, deregulating cellular energetics, sustaining proliferative signaling, evading growth supressors, avoiding immune destruction, enabling replicative immortality, activating invasion and metastasis and inducing angiogenesis; mediated by two enabling characteristics: tumor-promoting inflammation and genome instability and mutation (Hanahan and Weinberg, 2011). Not all cancers need to activate all of these hallmarks and microenvironmetal and/or genetic alterations, but no cancer can develop itself without any of them. Taking this discussion to the study of signalling mechanisms involved in angiogenesis and lymphangiogenesis, it can be considered some cellular and environmental checkpoints as well.
1. Signaling pathways in tumor angiogenesis and lymphangiogenesis
The most important molecular pathway involved in both tumor angiogenesis and lymphangiogenesis is the one based on VEGFRs and Neuropilins (NRPs) signal transduction. There are, to date, five growth factors in the VEGF family: VEGF-A, B, C, D and the Placenta growth factor (PlGF) (Koch and Welsh, 2012). Moreover, these growth factors can suffer alternative splicing (VEGF-A, B and PlGF) and further processing (VEGF-A, C and D). Which are associated with differences in binding to receptors (more or less affinity towards VEGFRs and/or NRPs) and to the very own extracellular matrix (Koch and Welsh, 2012; Ferrara, 2010).
As mentioned before, the angiogenic process is most regulated by VEGF-A, VEGFR-1 and VEGFR-2. While the best described lymphangiogenic regulators are VEGF-C and VEGFR-3 (Tammela et al., 2005). There are, however, overlaps between growth factors and receptors involved in both processes (Scavelli et al., 2004).
Another well established participant in angiogenesis is the Delta-like-4 (Dll4)-Notch signaling axis. Notch is the receptor for the ligand Dll4 (among others), involved in proliferating signals in different cellular types (Artavanis-Tsakonas and Lake, 1999). Papers show that, during angiogenesis, the branching of pre-existing vessels occurs concomitantly with variations in Dll4 and Notch expression in endothelial cells (Adams and Alitalo, 2007). Where the tip cells (the ones leading the branching process) have an up-regulated expression of Dll4, and the stalk cells (that form the tube following tip formation) have an up-regulated expression of Notch (Dufraine et al., 2008). It has been shown that tumor cells can have an increased expression of another Notch ligand, Jagged-1, associated with pro-angiogenic effects (Zeng et al., 2005). And that a soluble form of the Notch 1 receptor (Notch 1 decoy) could have anti-angiogenic properties, thus, being a promising therapeutic approach towards cancer (Funahashi et al., 2008).
2. The interactions between the extracellular matrix (ECM) and the vasculature (blood and lymphatic vessels)
2.1. Tumor angiogenesis and the extracellular matrix
Angiogenesis is regulated by the homeostatic balance between pro- and anti-angiogenic factors (Carmeliet and Jain, 2000). The “angiogenic switch” occurs when an “imbalance” between those factors is established, pending to the side of greater concentrations of positive angiogenic regulators, usually such imbalance occurs at the transition between potentially malignant lesions and the cancer (Hanahan and Folkman, 1996). The literature points to at least 27 endogenous inhibitors of angiogenesis, such as proteins and small molecules (Nyberg et al., 2005). The matrix-derived angiogenesis inhibitors seem to be arresten, canstatin, collagen fragments, endostatin-like fragment from type XV collagen (EFC-XV), endorepellin, endostatin, fibronectin fragments, fibulin, thrombospondin-1 and -2 and tumstatin (Nyberg et al., 2005).
It has been shown that the cancer cells may escape from ECM endogenous inhibitors by up-regulating pro-angiogenic factors (Fernando et al., 2008). In vivo studies of cancer cells over expressing thrombospondin-1, endostatin and tumstatin showed that all cancers presented an ability to escape from these inhibitory situations by production of pro-angiogenic molecules such as VEGF, platelet-derived growth factor-A and -B (PDGF-A and – B, fibroblast) growth factor (FGF) and angiopoetin-2 (Fernando et al., 2008).
The ECM can play not just an anti-angiogenic role, but also, a pro-angiogenic influence in tumorigenesis (Campbell et al., 2010; Han et al., 2008). There were discovered different pro-angiogenic functions played by already known matrix-derived factors such as collagen IV, laminin and fibronectin (Campbell et al., 2010). Han et al. (2008) showed that the extracellular matrix protein-1 (ECM1) had a direct correlation with tumor angiogenesis and metastatic potential in laryngeal carcinoma. The very own pro-angiogenic growth factors and cytokines such as VEGF, FGF-2, interleukin-8 (IL-8 or CXCL8), tumor growth factor-β (TGF-β), PDGF and angiopoietins are constitutively present in the ECM and may be mobilized from it to exert their functions in favor of the tumor (Crivellato et al., 2008). It is fundamental to keep in mind that tumorigenesis is a dynamic process involving a network of molecular events related to each other. Therefore, the ECM can play different roles depending on several microenvironmental factors of the tumor, such as many different cell types (epithelial cells, endothelial cells and stromal cells), its proteic and glycoproteic components. The natural tissue homeostasis regulators can, in fact, prevent the processes involved in tumor progression. However, when these physiological regulators reduce homeostatic effectiveness, the tumor microenvironment may start to act in an opposite manner, promoting tumor growth and development (Bissell and Hines, 2011).
2.2. Tumor Lymphangiogenesis and the extracellular matrix
The interactions between lymphatic endothelial cells (LECs) and the ECM, are quite similar to those observed between the vascular endothelial cells and ECM. Lymphangiogenic tissue homeostasis is achieved through balance of pro- and anti-lymphangiogenic factors and one of the endogenous lymphangiogenic inhibitors that is shared with vascular endothelial cells is endostatin (Ji, 2006). It has been shown that the use of angiogenic inhibitors such as endostatin, platelet factor-4 (PF-4) and interferon-α (INF-α) had significant inhibitory effects on proliferative and migratory activities of lymphatic endothelial cells (Shao et al., 2008; Shao and Xie, 2005). LECs present an incomplete or absent basement membrane, with the presence of anchoring filaments serving as binding structures to the surrounding tissues (Leak and Burke, 1968). There is still much research to be conducted in the field of the crosstalk among LECs, the surrounding ECM, and associated stromal or cancer cells. Focusing on ECM signaling, basically the most important molecules involved in lymphangiogenesis are hyaluronan and integrins (Ji, 2006; Paupert et al., 2011).
Hyaluronan is a mucopolysaccharide, abundantly present in the extracellular matrix, which provides a favorable microenvironment for cell proliferation and migration (Laurent and Fraser, 1992). Lymphatic vessel endothelial hyaluronan receptor-1 (YVE-1) is the receptor for hyaluronan constituvely present in lymphatic endothelium, which is homologue to CD44 (Banerji et al., 1999). Itano et al. (2008) showed that tumor-derived hyaluronan serves as a scaffold for interactions between cancer and host cells, favoring promotion of both tumor angiogenesis and lymphangiogenesis.
Other important molecules related to ECM-lymphatic endothelial interactions in lymphangiogenesis are the integrins, which are a large family of heterodimerictrans membrane glycoproteins (Paupert et al., 2011). Mishima s. (2007) have shown that prospero homeobox protein-1(PROX-1), through induction of α9 integrin expression on lymphatic endothelial cells, stimulated migration of LECs towards VEGF-C expressing cells. α4β1 integrin interaction with matrix fibronectin may be involved in tumor associated lymphangiogenesis and metastasis (Garmy-Susini et al., 2010).
3. Crosstalk between tumor and endothelial cells
Tumor cells establish a specific stromal microenvironment fostering tumor growth, in which blood/lymph-angiogenesis are involved. A complicated cytokine networks induces the tumor-associated blood/lymph-angiogenesis continually, providing several interactions among tumor cells and stromal cells, in which endothelial cells (ECs) are included (Onimaru et al., 2011).
3.1. Crosstalk between tumor and vascular endothelial cells
It is well known that the tumor cells can secrete growth factors and stimulating molecules such as VEGFs, PDGF, FGF-2, epidermal growth factor (EGF), angiopoietins, etc, leading to a proliferative activity of vascular endothelial cells. Research has been performed recently in order to comprehend the crosstalk mechanisms between tumor and vascular endothelial cells that can occur, in the tumor microenvironment. Recently, Nör et al. (2001) using an in vivo model of human tumors surrounded by human microvessels demonstrated that tumors with vascular endothelial cells (HDMECs) stably expressing Bcl-2, had a 3-fold increase in growth as well as an increase in microvascular density and survival of endothelial cells compared with control HDMECs and tumor cells. Bcl-2 can stimulate, in an autocrine manner, proangiogenic activities in vascular endothelial cells mediated by nuclear factor kappaB (NF-kB) and further production of angiogenic CXC chemokines (Karl et al., 2005). Tumor cells can also present the ability to up-regulate the expression of Bcl-2, as an antiapoptotic gene (Hanahan and Weinberg, 2011). Moreover, the crosstalk between tumor and vascular endothelial cells is mediated by Bcl-2, as Kaneko et al. (2007) showed in their study. They demonstrated that vascular endothelial cells expressing Bcl-2, via the transcription factor signal transducer and activator of transcription-3 (STAT3), could secrete VEGF, which induces the up-regulation of Bcl-2 and angiogenic chemokines such as chemokine ligand 1(CXCL1)and CXCL8 in head and neck squamous cell carcinoma (HNSCC) cells, increasing survival and proliferative activities in both cell types (Kaneko et al., 2007), and also enhancing the invasiveness potential of tumor cells (Warner et al., 2008).
Another study from the same laboratory established that vascular endothelial cells could secrete CXCL8, interleukin-6 (IL-6) and EGF into the extracellular medium (Neiva et al., 2009). These authors determined that these molecules could induce intra-cellular fosforilation of STAT3, protein kinase B (PKB or Akt) and extracellular signal-(ERK) in HNSCC cells, leading to increased survival and migration potential in the tumor cells (Neiva et al., 2009).
Zeng et al. (2005) identified a different crosstalk mechanism involving Notch activation in endothelial cells via the Notch ligand Jagged-1 expression in HNSCC cells. The over expression of Jagged-1 was due to the activation of the mitogen-activated protein kinase(MAPK) signaling pathway in tumor cells after the addition of growth factors such as hepatocyte growth factor (HGF), tumor growth factor-α (TGF-α and EGF. This suggests a possible crosstalk between tumor and vascular endothelial cells mediated by such secreted molecules (Zeng et al., 2005). Liu et al. (2010) demonstrated that tyrosine kinase receptor tie-2/TEK positive glioma and brain tumor stem cells had an increased adhesion potential to endothelial cells, which was observed in in vitro and in vivo studies. Such receptor had already been identified in normal and tumor associated endothelial cells and it is, positively related to an increase in tumor malignancy and angiogenesis (Peters et al., 1998). It has been shown, however, that this receptor is also present in brain tumor cells and that, stimulated by angiopoietin-1, tie-2 positive tumor cells increase the adhesion on vascular endothelial cells in vitro and in vivo, mediated by up-regulation of integrin β1 and N-cadherin (Liu et al., 2010).
3.2. Crosstalk between tumor and lymphatic endothelial cells
It is known that tumor cells can produce and secrete VEGF-C and VEGF-D. VEGF-C is the most important growth factor in mediating tumor-associated lymphangiogenesis, which can be followed by migration of cancer cells through these newly formed lymphatic vessels, leading to the invasion and metastasis (Achen et al., 2005). As mentioned above, pro-angiogenic molecules can also participate in promoting tumor lymphangiogenesis (Scavelli et al., 2004). Issa et al.2009 have brilliantly shown that tumor-secreted VEGF-C could not only induce, in an autocrine manner, an increased matrix-degradation and migration in a three dimensional model (mainly by consequent production of matrix metalloproteinase 9 [MMP-9]), but also, VEGF-C was capable of up-regulating chemokine ligand 21(CCL21) secretion in the lymphatic endothelium. This up regulation could also enhance the chemoatraction of C-C chemokine receptor type-7 (CCR7)-expressing tumoral cells, which could move toward the surrounding lymphatics (Issa et al., 2009). Moreover, Tarquinio et al. (2011) observed that VEGF-C was responsible for the up-regulation of Bcl-2 on lymphatic endothelial cells through VEGFR-3 and consequent PI3k/Aktphosphorylation. And that there’s an increased expression of Bcl-2 on endothelial cells from human oral tumors with lymph node metastasis as compared to stage-matched oral squamous cell carcinomas without metastatic spread (Tarquinio et al., 2011).
4. Interactions between tumor angiogenesis and lymphangiogenesis with associated non-endothelial stromal cells
It is increasingly important to study how stromal cells can affect tumor angiogenesis and lymphangiogenesis. The literature highlights very interesting data concerning the pro-angiogenic and lymphangiogenic molecules secreted by such cells, some of it further described in this review. It is also important to acknowledge that, during the co-evolution between tumor cells and their microenvironment, those two interconnected mechanisms are frequently involved. Tumor cells can alter the surrounding stroma and stromal cells can strongly influence tumorigenesis and tumor progression (Polyak et al., 2009). Therefore, crosstalk between all cellular types such as dendritic cells, fibroblasts, macrophages, pericytes, lymphocytes and tumor cells is crucial for cancer establishment and development.
4.1. Role of non-endothelial stromal cells in tumor angiogenesis and lymphangiogenesis
4.1.1. Dendritic cells
Dendritic cells (DCs) are observed in peripheral tissues, being the sentinel of the innate immune system. Once in contact with an invading pathogen and being stimulated through inflammatory cytokines, those cells can ingest antigens, become mature, and after they circulate in the afferent lymphatics reaching the lymph nodes with consequent antigen presentation to lymphocytes, what characterizes DCs as a pivotal cell in the regulation of adaptive responses (Sozzani et al., 2007). Within the tumor microenvironment, DCs can produce different pro-angiogenic factors, such as tumor necrosis factor-α (TNF-α), TGF-β, and granulocyte macrophage colony-stimulating factor (GM-CSF), as well as they can response to different mediators related to angiogenesis (Sozzani et al., 2007). Tumor-secreted factors, suppressor myeloid cells, and tumor-associated macrophages have been demonstrated to inhibit DCs maturation in cancer, mainly by activation of the transcriptional factor STAT3, leading to a decreased effectiveness in immune responses, such as tolerance and immune suppression, facilitating the development of some malignancies (Fainaru et al., 2010). Fainaru et al. (2010) have demonstrated in vivo that xenografted tumors with an increased level of angiogenesis were infiltrated by an immature population of DCs, whereas dormant a vascular tumors had an infiltration of mature DC population. Moreover, such as some tumoral cells, DCs can also “transform” into endothelial-like cells (Gottfried et al., 2007).
4.1.2. Fibroblasts
Cancer-associated fibroblasts (CAFs) have been shown to exert pro-angiogenic and developmental activities in melanoma (Li et al., 2003) and prostate cancer (Niu and Xia, 2009). Quiescent fibroblasts can be activated in a paracrine manner when present in the tumor microenvironment (Lorusso and Ruegg, 2008). Like some immune cells, stromal fibroblasts can also inhibit cancer in its initial stages (Rasanen and Vaheri, 2010).
However, tumor progression involves CAFs, which can promote tumor development (Orimo and Weinberg, 2006). In a 4T1 murine breast cancer model, CAFs where shown to promote tumor growth and metastasis by modulating the tumor immune microenvironment (Liao et al., 2009). Through DNA vaccine targeting the activated-CAFs proteins, these cells were eliminated resulting, in a shift of tumor immune polarization, followed by a suppressed recruitment of cancer associated macrophages and reduced tumor angiogenesis and lymphangiogenesis (Liao et al., 2009). Interestingly, fibroblasts may initiate their pro-angiogenic activity through VEGF secretion by tumor cells. The release of VEGF into the stromal microenvironment induces microvascular permeability, leading to extra vasation of plasma proteins such as fibrin, which in turn leads to a generated influx of fibroblasts, inflammatory cells and endothelial cells (Kalluri and Zeisberg, 2006). Fibroblasts can produce fibronectin and type I collagen rich extracellular matrices, VEGF and matrix metalloproteinasis (MMPs which are capable of conducting and initiating tumor angiogenesis and progression (Kalluri and Zeisberg, 2006).
In relation to lymphangiogenesis, Itano’s research group has extensively worked with the pivotal role that ECM rich in hyaluronan may play in inducing tumor initiation and progression as well as intratumoral lymphangiogenesis (Itano et al., 2008; Koyama et al., 2008). Using an in vivo model of hyaluronan synthase2 (Has2) transgenic mice, it was observed that an overproduction of hyaluronan facilitated intratumoral lymphangiogenesis, suggesting that cancer cells could maintain favorable conditions for lymphangiogenesis through hyaluronan-induced CAFs recruitment with consequent VEGF-D secretion (Koyama et al., 2008).
4.1.3. Macrophages
These cells are phagocitary antigen-presenting cells, which originated from the bone-marrow, circulating briefly in the blood as monocytes and later differentiating into macrophages in the tissues or in inflammatory foci (Guruvayoorappan, 2008). Tumor-associated macrophages (TAMs) have been shown to affect not only tumor angiogenesis, but also tumor cell invasion onto surrounding stroma with degradation of the basement membrane by MMP production; immuno supression by expression of prostaglandin E2 (PGE2), interleukin-10 (IL-10) and TGF-β and metastasis by secretion of factors such as EGF that can guide tumor cells toward blood vessels (Lewis and Pollard, 2006). TAMs can secrete a number of potent pro-angiogenic growth factors and cytokines such as VEGF, TNF-α, bFGF and other angiogenesis modulating enzymes, including matrix metalloproteinase 2 (MMP-2), matrix metalloproteinase 7(MMP-7), matrix metalloproteinase 9(MMP-9), matrix metalloproteinase 12 (MMP-12) and cyclo-oxygenase-2 (COX-2) (Lewis and Pollard, 2006). Lamagna et al. (2006) stated that TAMs can have dual roles within the tumor microenvironment. They can act together with mature dendritic and NK cells, fighting against tumor cells (Lamagna et al., 2006). However, when cancer cells begin to escape from immune surveillance, TAMs may act promoting tumor progression and angiogenesis (Lamagna et al., 2006).
As reviewed in angiogenic processes linked to cancer, tumor-associated macrophages can promote tumor lymphangiogenesis as well (Schoppmann et al., 2002; Skobe et al., 2001). Schoppmann et al. (2002) have discovered populations of TAMs in samples of cervix squamous intraepithelial lesions, that expressed VEGF-C and D, as well as their receptor VEGFR-3. They have also isolated VEGFR-3 expressing monocytes in the circulation, suggesting that peritumoral lymphangiogenesis may occur, in part, due to the infiltration of such sub fraction of monocytes and the consequent differentiation into VEGF-C and D expressing tumor-associated macrophages (Schoppmann et al., 2002). Also, it has been demonstrated that VEGF-C could have a macrophage-recruitment activity, despite its lymphangiogenic and angiogenic activities (Skobe et al., 2001). Infiltration of TAMs has been correlated with increase of peritumoral lymphatics and poor prognosis in lung adenocarcinoma (Zhang et al., 2010). Also, in cutaneous squamous cell carcinoma, it was described that the microenvironment consisted of increased lymphatic density with high levels of macrophage-derived VEGF-C (Moussai et al., 2011).
4.1.4. Pericytes
Pericytes are cells that surround endothelial cells, in the formation of blood vessels. Reinmuth et al. (2001) have demonstrated that both pericytes and tumor cells could stimulate, in a paracrine manner, endothelial cells through VEGF secretion. Tumor and endothelial cells could produce PDGF towards pericytes, enabling them to secrete VEGF (Reinmuth et al., 2001).
4.1.5. T cells
Very interesting work has been done concerning VEGF interactions with T cells, although little is known about such associations. Shin et al. (2009) have demonstrated that T lymphocytes activated by monoclonal antibodies anti-CD3, anti-CD28 or by some antigens, transcribed mRNA for VEGFR-1 and 2, where only VEGFR-1 was present in the cell surface. Furthermore, the addition of VEGF to either non-activated or activated T lymphocytes led to up-regulation of IL-10 and slight down-regulation of interferon-γ (INF-γ ) and anti-angiogenic cytokines. This suggest that high concentrations of VEGF may cause T cells to migrate towards a tumor microenvironment, and that this interaction could play a role in IL-10 mediated immune evasion by tumor cells (Shin et al., 2009).
4.1. 6. Mast cells
Mast cells (MCs) are immune cells that mediate the transition between innate and adaptive responses, orchestrating inflammatory responses against bacteria, parasites and viruses (Metz and Maurer, 2007). MCs present dual roles in tumorigenesis. They can display a suppressor activity by secreting different inhibitory interleukines and also the TNF-α or they can help tumor progression by promoting angiogenesis, ECM degradation and immuno suppression (Theoharides and Conti, 2004). Concerning the pro-angiogenic capacity, MCs can secrete molecules such as VEGF, FGF-2, IL-8 and TGF-β (Crivellato et al., 2008). The recruitment of these cells toward the tumor microenvironment is mainly due to the stem cell factor (SCF) produced by cancer cells (Crivellato et al., 2008).
MCs may play a very important role not just in angiogenesis, but also in tumor lymphangiogenesis. It was shown, in vivo, that endostatin was capable of decreasing the infiltration levels of VEGF-C expressing MCs in induced skin squamous cell carcinoma. The capacity of endostatin in inhibiting adhesion and migration of MCs on fibronectin has also been shown in vitro, suggesting that the reduction in aggressiveness caused by endostatin could be, in part, due to the decrease in levels of VEGF-C, which is consequence of MC migration and adhesion inhibition (Brideau et al., 2007).
5. Tumor-stroma interactions and therapeutic applications
The increasing knowledge of the signaling mechanisms between cancer cells and the surrounding stroma has opened room for therapeutic approaches targeting the tumor microenvironment. It is true, for example, that the studies about the VEGF signaling have allowed the development of the so called therapeutic agent Bevacizumab (a monoclonal antibody against VEGF-A). The effects of this drug will be also targeted to stroma-derived VEGF (Hofmeister et al., 2008). Still concerning anti-angiogenic therapy, the soluble Notch1 decoy (Funahashi et al., 2008) seems to be an elegant approach towards tumor/endothelial crosstalk. The most studied cell component of the tumor microenvironment is the associated endothelial cell. In this respect, very interesting work have also targeted an endothelial specific membrane molecule, CD105 (endoglin). Moreover, in vivo studies are being carried out in order to suppress the anti-apoptotic effects of this surface protein using these antibodies (Hofmaister et al., 2008). Maybe the future in stroma-directed therapies is to develop new strategies targeting molecular surface markers of the cells composing the tumor microenvironment.
6. Concluding remarks
The purpose of this review was to present, in a dynamic topic format, a concise and updated review about the work that has been done concerning microenvironmental mechanisms and intercellular interactions in tumor angiogenesis and lymphangiogenesis. Such processes involving neo vasculature have been well described as important keys in tumor growth and metastasis and, for that reason, it is increasingly necessary to enlarge the knowledge of these interactions and signaling pathways, with the interest of pursuing new and more complete therapeutic approaches to treat cancer.
Figure 1.
Acknowledgments
Support for this work was provided by grant P50-CA-97248 (University of Michigan Head and Neck SPORE) from the NIH/NCI; grants - R01DE15948, and R01-DE21139 from the NIH/NIDCR; and CNPq/Brazil.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13 (1–2):16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7 (2):121–7. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake R. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144 (4):789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17 (3):320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, et al. Endostatin over expression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67 (24):11528–35. doi: 10.1158/0008-5472.CAN-07-1458. [DOI] [PubMed] [Google Scholar]
- Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231 (3):474–88. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- Campbell NE, Kellenberger L, Greenaway J, Moorehead RA, Linnerth-Petrik NM, Petrik J. Extracellular matrix proteins and tumor angiogenesis. J Oncol. 2010;2010:586905. doi: 10.1155/2010/586905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407 (6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: new insight from experimental carcinogenesis. Cancer Lett. 2008;269 (1):1–6. doi: 10.1016/j.canlet.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132–5137. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22 (5):617–25. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A, et al. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. FASEB J. 2010;24 (5):1411–8. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando NT, Koch M, Rothrock C, Gollogly LK, D’Amore PA, Ryeom S, et al. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin Cancer Res. 2008;14 (5):1529–39. doi: 10.1158/1078-0432.CCR-07-4126. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Molecular Biology of the Cell. 2010;21(5):687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikina N, Sharma A, Kanamaru E, Borisenko V, Desilva DM, Suzuki A, Wang X, Shawber CJ, Kandel JJ, Yamashiro DJ, Kitajewski CJ. A notch1 ectodomain inhibits endothelial signaling, tumor growth, and angiogenesis. 2008;68(12):4327–35. doi: 10.1158/0008-5472.CAN-07-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, et al. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70 (8):3042–51. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E, Kreutz M, Haffner S, Holler E, Iacobelli M, Andreesen R, et al. Differentiation of human tumour-associated dendritic cells into endothelial-like cells: an alternative pathway of tumour angiogenesis. Scand J Immunol. 2007;65 (4):329–35. doi: 10.1111/j.1365-3083.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- Guruvayoorappan C. Tumor versus tumor-associated macrophages: how hot is the link? Integr Cancer Ther. 2008;7 (2):90–5. doi: 10.1177/1534735408319060. [DOI] [PubMed] [Google Scholar]
- Han Z, Lin GJ, Chi FL, Wang SY, Huang JM, Liu HJ, et al. The relationship between the extracellular matrix and the angiogenesis and metastasis of laryngeal carcinoma. ORL J Otorhinolaryngol Relat Spec. 2008;70 (6):352–8. doi: 10.1159/000163030. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86 (3):353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100 (1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144 (5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Herttuala SY, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular Endothelial Cell Growth Factor Receptor 3–Mediated Activation of Lymphatic Endothelium Is Crucial for Tumor Cell Entry and Spread via Lymphatic Vessels. Cancer Res. 2005;65(11):4739–46. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- Hofmeister V, Schrama D, Becker JC. Anti-cancer therapies targeting the tumor stroma. Cancer ImmunoIImunother. 2008;57:1–17. doi: 10.1007/s00262-007-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69 (1):349–57. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- Itano N, Zhuo L, Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 2008;99 (9):1720–5. doi: 10.1111/j.1349-7006.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol. 2006;4 (2):83–100. doi: 10.1089/lrb.2006.4.83. [DOI] [PubMed] [Google Scholar]
- Jung YD, Ahmad SA, Liu W, Reinmuth N, Parikh A, Stoeltzing O, et al. The role of the microenvironment and intercellular cross-talk in tumor angiogenesis. Semin Cancer Biol. 2002;12 (2):105–12. doi: 10.1006/scbi.2001.0418. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6 (5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Zhang Z, Mantellini MG, Karl E, Zeitlin B, Verhaegen M, et al. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;67 (20):9685–93. doi: 10.1158/0008-5472.CAN-07-1497. [DOI] [PubMed] [Google Scholar]
- Karl E, Warner K, Zeitlin B, Kaneko T, Wurtzel L, Jin T, et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-kappaB and CXC chemokines. Cancer Res. 2005;65 (12):5063–9. doi: 10.1158/0008-5472.CAN-05-0140. [DOI] [PubMed] [Google Scholar]
- Koch S, Welsh LC. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2(7):1–21. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Kobayashi N, Harada M, Takeoka M, Kawai Y, Sano K, et al. Significance of tumor-associated stroma in promotion of intratumoral lymphangiogenesis: pivotal role of a hyaluronan-rich tumor microenvironment. Am J Pathol. 2008;172 (1):179–93. doi: 10.2353/ajpath.2008.070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80 (4):705–13. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6 (7):2397–404. [PubMed] [Google Scholar]
- Leak LV, Burke JF. Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol. 1968;36 (1):129–49. [PMC free article] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66 (2):605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Meier F, Berking C, Bogenrieder T, Herlyn M. Function and regulation of melanoma-stromal fibroblast interactions: when seeds meet soil. Oncogene. 2003;22 (20):3162–71. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4 (11):e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Martin V, Fueyo J, Lee OH, Xu J, Cortes-Santiago N, et al. Tie2/TEK modulates the interaction of glioma and brain tumor stem cells with endothelial cells and promotes an invasive phenotype. Oncotarget. 2010;1 (8):700–9. doi: 10.18632/oncotarget.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso G, Ruegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130 (6):1091–103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155 (3):739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Maurer M. Mast cells--key effector cells in immune responses. Trends Immunol. 2007;28 (5):234–41. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, et al. Prox1 induces lymphatic endothelial differentiation via integrin alpha9 and other signaling cascades. Mol Biol Cell. 2007;18 (4):1421–9. doi: 10.1091/mbc.E06-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussai D, Mitsui H, Pettersen JS, Pierson KC, Shah KR, Suarez-Farinas M, et al. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J Invest Dermatol. 2011;131 (1):229–36. doi: 10.1038/jid.2010.266. [DOI] [PubMed] [Google Scholar]
- Neiva KG, Zhang Z, Miyazawa M, Warner KA, Karl E, Nor JE. Cross talk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia. 2009;11 (6):583–93. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu YN, Xia SJ. Stroma-epithelium crosstalk in prostate cancer. Asian J Androl. 2009;11 (1):28–35. doi: 10.1038/aja.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nor JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61 (5):2183–8. [PubMed] [Google Scholar]
- Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65 (10):3967–79. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- Onimaru M, Yonemitsu Y. Angiogenic and lymphangiogenic cascades in the tumor microenvironment. Front Biosci (Schol Ed) 2011;1(3):216–25. doi: 10.2741/s146. [DOI] [PubMed] [Google Scholar]
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5 (15):1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- Paupert J, Sounni NE, Noel A. Lymphangiogenesis in post-natal tissue remodeling: lymphatic endothelial cell connection with its environment. Mol Aspects Med. 2011;32 (2):146–58. doi: 10.1016/j.mam.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Peters KG, Coogan A, Berry D, Marks J, Iglehart JD, Kontos CD, et al. Expression of Tie2/Tek in breast tumour vasculature provides a new marker for evaluation of tumour angiogenesis. Br J Cancer. 1998;77 (1):51–6. doi: 10.1038/bjc.1998.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25 (1):30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316 (17):2713–22. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, et al. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15 (7):1239–41. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- Scavelli C, Vacca A, Di Pietro G, Dammacco F, Ribatti D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia. 2004;18 (6):1054–8. doi: 10.1038/sj.leu.2403355. [DOI] [PubMed] [Google Scholar]
- Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161 (3):947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XJ, Lu WQ, Liu C. Different effects of angiogenesis inhibitors IFN-alpha and TIMP-1 on lymphangiogenesis. Lymphology. 2008;41 (2):64–74. [PubMed] [Google Scholar]
- Shao XJ, Xie FM. Influence of angiogenesis inhibitors, endostatin and PF-4, on lymphangiogenesis. Lymphology. 2005;38 (1):1–8. [PubMed] [Google Scholar]
- Shin JY, Yoon IH, Kim JS, Kim B, Park CG. Vascular endothelial growth factor-induced chemotaxis and IL-10 from T cells. Cell Immunol. 2009;256 (1–2):72–8. doi: 10.1016/j.cellimm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159 (3):893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28 (9):385–92. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Achen MG. From anti-angiogenesis to anti-lymphangiogenesis: emerging trends in cancer therapy. Lymphat Res Biol. 2008;6 (3–4):165–72. doi: 10.1089/lrb.2008.1015. [DOI] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65 (3):550–63. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tarquinio SB, Zhang Z, Neiva KG, Polverini PJ, Nor JE. Endothelial cell Bcl-2 and lymph node metastasis in patients with oral squamous cell carcinoma. J Oral Pathol Med. 2011;41 (2):124–30. doi: 10.1111/j.1600-0714.2011.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25 (5):235–41. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis--impact on cancer metastasis. J Leukoc Biol. 2006;80(4):691–96. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- Warner KA, Miyazawa M, Cordeiro MM, Love WJ, Pinsky MS, Neiva KG, et al. Endothelial cells enhance tumor cell invasion through a crosstalk mediated by CXC chemokine signaling. Neoplasia. 2008;10 (2):131–9. doi: 10.1593/neo.07815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Li S, Chepeha DB, Giordano TJ, Li J, Zhang H, et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8 (1):13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol. 2010 doi: 10.1007/s12032-010-9638-5. [DOI] [PubMed] [Google Scholar]