Abstract
Purpose
To investigate the effect of posterior cruciate ligament (PCL) deficiency on the kinematics and the cartilage contact characteristics of the patellofemoral joint during an in vivo single-leg lunge.
Methods
Ten patients with an isolated PCL injury in one knee and the contralateral side intact participated in the study. Magnetic resonance and dual fluoroscopic imaging techniques were used to analyze the patellofemoral kinematics and cartilage contact of the intact and the PCL-deficient knee during a quasi-static single-leg lunge from 0° to 120° of flexion.
Results
PCL deficiency significantly changed the patellofemoral kinematics between 90° and 120° of knee flexion (P < 0.007): an increased patellar flexion angle by 10.7< on average and a decreased lateral shift (on average −1.9 mm), patellar tilt (approximately −2.7°), and valgus rotation (approximately −1.8°) were observed in the PCL-deficient knee compared with the intact contralateral joint. The changes in patellofemoral kinematics resulted in significant changes in patellofemoral cartilage contact (P < 0.007). PCL deficiency caused a distal (approximately −3.3 mm) and medial (approximately + 2.7 mm) shift of cartilage contact from 75° to 120° of flexion.
Conclusion
The altered tibiofemoral kinematics that were previously described in PCL deficiency resulted in changes in patellofemoral joint function at flexion angles greater than 75°. This abnormal loading of the patellofemoral joint might predispose the patellofemoral cartilage to degenerative changes. Because we did not detect differences in the patellofemoral joint behavior of the intact and the PCL-deficient knee between 0° and 60° of flexion, rehabilitation exercises might be safely performed in this range of flexion. On the other hand, repetitive deep knee squats should be avoided in PCL-deficient patients, so as not to excessively disturb the patellofemoral cartilage contact kinematics.
Keywords: DUAL FLUOROSCOPIC IMAGING, PATELLOFEMORAL COMPLICATIONS, PATELLAR TRACKING, PATELLOFEMORAL CARTILAGE CONTACT, SINGLE-LEG LUNGE
The posterior displacement of the tibia that occurs after rupture of the posterior cruciate ligament (PCL) is associated with degeneration of both the patello-femoral and the medial tibiofemoral joint compartments (7,28,35). Parolie and Bergfeld (33) reported on 25 patients with PCL injury that were treated conservatively at 6 yr of follow-up. Patellofemoral symptoms were present in 12 patients (48%), medial joint line tenderness in 3 patients (12%), and radiographic evidence of osteoarthritis in 9 patients (36%) total, eight patients (32%) of the medial and four patients (16%) of the patellofemoral compartment. Clancy et al. (6) reported that 90% of patients with PCL injuries for longer than 4 yr had grade 3 or 4 osteoarthritis of the medial femoral condyle, whereas only 31% of patients had preoperative radiographic changes. Dandy and Pusey (8) reported on 20 PCL-deficient patients treated nonoperatively after approximately 7 yr. Fourteen patients (70%) continued to have pain while walking, 11 patients (55%) had patellofemoral symptoms, whereas 9 patients (45%) had episodic giving-way. Similarly, Boynton et al. (1) reported that 81% of patients with isolated PCL-deficient knees had at least occasional pain and 17 patients (56%) had at least occasional swelling at a mean follow-up of 13.4 yr. Cross and Powell (7) evaluated 116 patients at a mean duration of follow-up of 5 yr: 40% of their patients had patellofemoral symptoms, whereas 20% had osteoarthritis of the medial compartment.
For the last three decades, most research on PCL deficiency has been directed predominantly at the tibiofemoral joint. This focus is comprehensible because restraining posterior tibial translation is the primary function of the PCL in the intact knee (3). Both in vitro and in vivo studies have documented an increased posterior tibial translation (3,5,12,15,16,25,27,31,32,34) as well as an increased external tibial rotation (16,21,24) and lateral translation (25) of the tibia after rupture of the PCL. In our recent in vivo study of 14 PCL-deficient patients, we found that these altered tibiofemoral kinematics in PCL deficiency resulted in a shift of the normal tibiofemoral contact location with a subsequent increase in cartilage deformation in the medial compartment, providing a possible explanation for the medial joint compartment cartilage degeneration (38).
In contrast, little is know about the patellofemoral joint in PCL deficiency. In vitro studies by Skyhar et al. (37) and Gill et al. (13) found that sectioning of the PCL in cadaveric studies resulted in elevated patellofemoral contact pressures. However, to our knowledge, no data have been reported on the patellofemoral joint function in PCL deficiency under in vivo weight-bearing conditions. This knowledge could be used to provide a scientific insight in the possible pathogenesis of patellofemoral complications after injury to the PCL and to formulate concrete guidelines for the development of potentially safer rehabilitation regimens for PCL-deficient patients after surgical or conservative treatment.
In this study, we hypothesized that PCL deficiency changes the patellofemoral kinematics and, subsequently, the cartilage contact point location of the patellofemoral joint. The objective of this study was to investigate the effects of PCL deficiency on the kinematics (patellar flexion, shift, tilt, and rotation) and the contact characteristics of the patellofemoral joint during an in vivo weight-bearing activity using the combined dual-orthogonal fluoroscopic and magnetic resonance (MR) imaging technique (30,39).
METHODS
Subject recruitment and exclusion criteria
Ten patients (average age = 34 ± 14 yr, age range = 19–51 yr; average weight = 80 ± 12 kg, weight range = 54–97 kg; average height = 173 ± 10 cm, height range = 165–180 cm; six males, four females; seven right injured knees, three left injured knees; active on a minimal to moderate athletic level before injury) with a PCL rupture documented by clinical examination (positive posterior drawer test measured by the senior author (TJG) and MR imaging were included in this study). The average time between injury and analysis was 4.9 ± 3 months. All subjects had healthy contralateral knees. Injury to other ligaments or capsule, noticeable cartilage lesions, meniscal injury requiring partial meniscectomy, and injury to the underlying bone were reasons for exclusion from the study. The 10 included patients were studied previously as part of a larger sample of 14 PCL-deficient patients for the analysis of tibiofemoral cartilage deformation in PCL deficiency (38).
The purpose of the present study was explained in detail to all of the patients at the time of recruitment. Each patient signed a consent form that had been approved by our institutional review board.
MR imaging scan and three-dimensional knee model
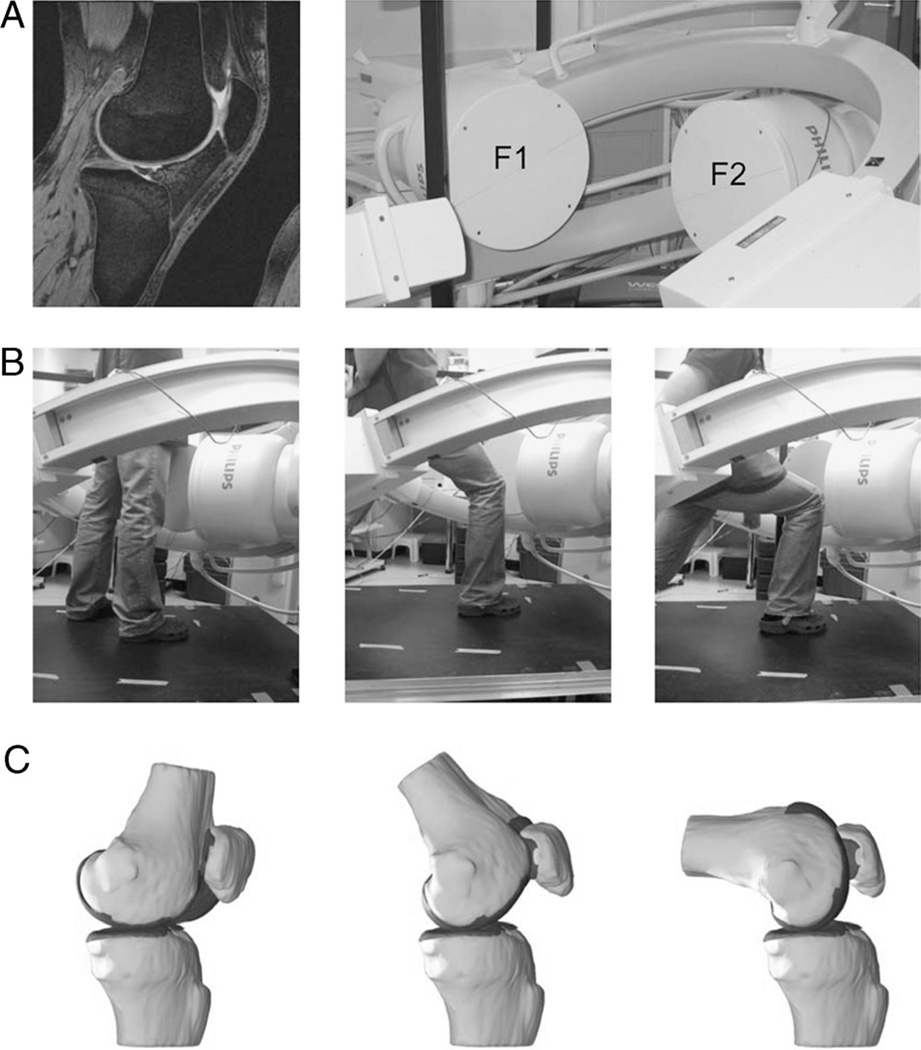
With the patients supine and the knee in a relaxed, extended position, both the left and the right knee were imaged with an MR scanner using a 3-T magnet (Magnetom Trio®; Siemens, Erlangen, Germany) and a fat-suppressed three-dimensional (3D) spoiled gradient-recalled echo sequence. The MR scans spanned the medial and the lateral boundaries of the knee. Parallel sagittal and coronal plane images (resolution = 512 × 512 pixels) with a field of view of 16 × 16 cm and a spacing of 1 mm were taken. For each knee, the MR scanning time was approximately 12 min. These MR images were used to create 3D meshed models of the knees using a protocol established in our laboratory (10). Each anatomic knee model included the bony geometry of the femur, the tibia, the fibula, and the patella as well as the patellar and the femoral cartilage layers (Fig. 1A).
FIGURE 1.
A, The combined MR and dual fluoroscopic imaging technique, in which 3D knee models are created from a series of sagittal MR images (image left), and the motion of the patient’s tested knee is recorded using two orthogonally placed fluoroscopes (image right). B, In the present study, the tested activity was a single-leg quasi-static lunge at 0°, 30°, 60°, 75°, 90°, 105°, and 120° of flexion while the upper body remained upright (only three flexion angles are shown for illustrative purposes). C, The 3D meshed knee models and the series of dual fluoroscopic images were combined to reproduce the knee positions. F1, fluoroscope 1; F2, fluoroscope 2.
Dual fluoroscopic imaging of the knee during a weight-bearing activity
Both knees of each patient were simultaneously imaged using two orthogonally placed fluoroscopes (Fig. 1A; BV Pulsera; Philips, Eindhoven, The Netherlands) set to generate an 8-ms width x-ray pulses with a dose rate of 13 µGy per scanning as the patient performed a single-leg quasi-static lunge at 0°, 30°, 60°, 75°, 90°, 105°, and 120° of flexion while their upper body remained upright. Flexion angle of the knee was monitored using a handheld goniometer. The patient kept the knee stable for one second at each target flexion angle so that the fluoroscopes captured the knee position and then flexed the knee to the next target position. At each selected flexion angle, the patient supported his or her body weight on the leg being scanned while the other leg was used to help balance the body (Fig. 1B).
Measurement of in vivo knee kinematics using image-matching technique
The fluoroscopic images were imported into a solid modeling software (Rhinoceros; Robert McNeel and Associates, Seattle, WA) and placed in the orthogonal planes based on the position of the fluoroscopes during the imaging of the patient. In the following step, the 3D MR image-based knee model of the patient was imported into the same software, viewed from the two orthogonal directions corresponding to the orthogonal fluoroscopic setup used to acquire the images, and manually manipulated in six degrees of freedom inside the software until the projections of the model matched the outlines of the fluoroscopic images. When the projections matched the outlines of the images taken during in vivo knee flexion, the model reproduced the in vivo position of the knee (Fig. 1C). MR and dual fluoroscopic imaging techniques have been described in detail in previous publications. This system has an error of less than 0.1 mm and 0.3° in measuring tibiofemoral joint translations and rotations, respectively (10). The procedure was further validated for measuring the patellofemoral kinematics. The methodology has an error of less than 0.1 ± 0.2 mm in measuring patellar shift and 0.1° ± 0.2° in patellar tilt (30).
Description of patellofemoral kinematics
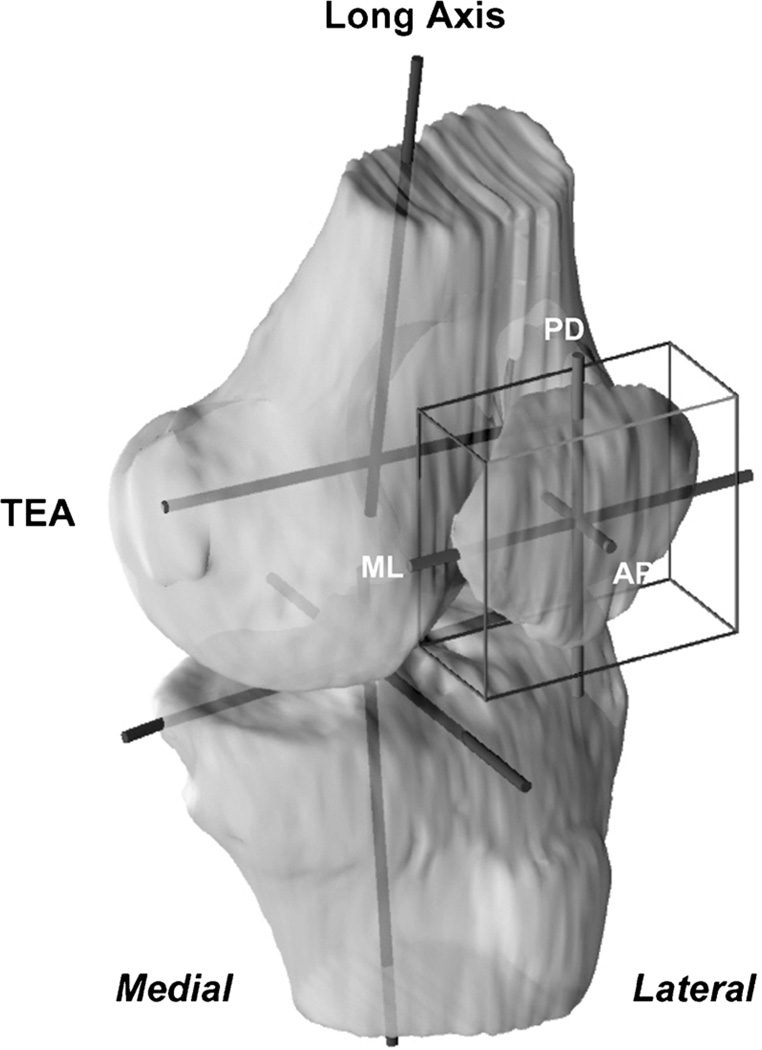
After reproducing the in vivo knee positions along the flexion path, the patellofemoral kinematics were measured from the series of knee models (30,39). A joint coordinate system (17) was established for each knee to describe the motion of the patella (Fig. 2). Two axes were drawn on the femur: the long axis along the posterior femoral shaft surface in sagittal plane and the transepicondylar axis (TEA) connecting the epicondyle extremes of the medial and the lateral femoral condyles (29). The knee center was defined as the midpoint of the TEA. An axis parallel to the posterior wall of the tibial shaft was defined as the long axis of the tibia. The flexion angle of the knee was defined as the angle between the long axes of the femur and the tibia in sagittal plane. To reduce the variability in creating patellar coordinate systems, a cuboid was used to enclose the patella so that it touched the proximodistal, the anteroposterior, and the mediolateral borders of the patella (26,30). The center of the cuboid was defined as origin of the patella. The long axis of the patella was defined as the line along the superior-inferior direction.
FIGURE 2.
Coordinate systems used to quantify the patellofemoral kinematics. The femoral coordinate system consisted of the trans-epicondylar axis (TEA) and the long axis intersecting at the center of the knee joint (midpoint of TEA). A cuboid was enclosed around the patella to determine the patellar center. The patellar coordinate system consisted of the proximodistal (PD), the anteroposterior (AP), and the mediolateral (ML) axes.
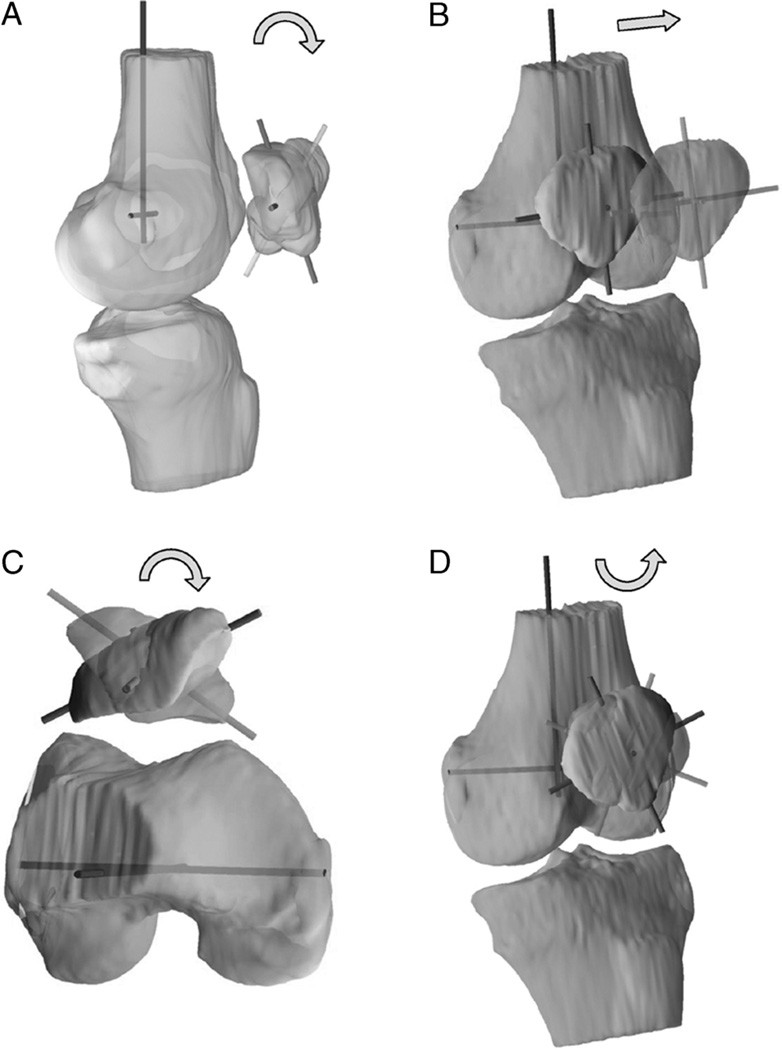
Patellar flexion was defined as the rotation of the patella about the TEA of the femur (Fig. 3A) (2). Patellar shift was defined as the medial or the lateral movement of the center of the patella along the TEA of the femur (Fig. 3B). A positive shift corresponded to the lateral movement of the patellar center with respect to the knee center along the TEA of the femur. Patellar tilt was defined as the rotation of the patella about the long axis of the femur, where lateral tilt followed the direction of external femoral rotation (Fig. 3C). Patellar rotation is the rotation of the patella about the anteroposterior axis of the femur, where valgus rotation follows the direction of valgus rotation in tibio-femoral motion (Fig. 3D), that is, an outward angulation of the distal segment of the patella. In this fashion, the patellofemoral kinematics were quantified for each subject as a function of flexion of the knee.
FIGURE 3.
The definition of patellofemoral kinematics, illustrated on a left knee model. Patellar flexion in sagittal view (A), patellar shift in coronal view (B), patellar tilt in axial view (C), and patellar rotation in coronal view (D). The arrows indicate the direction of positive value.
Description of patellofemoral cartilage contact points
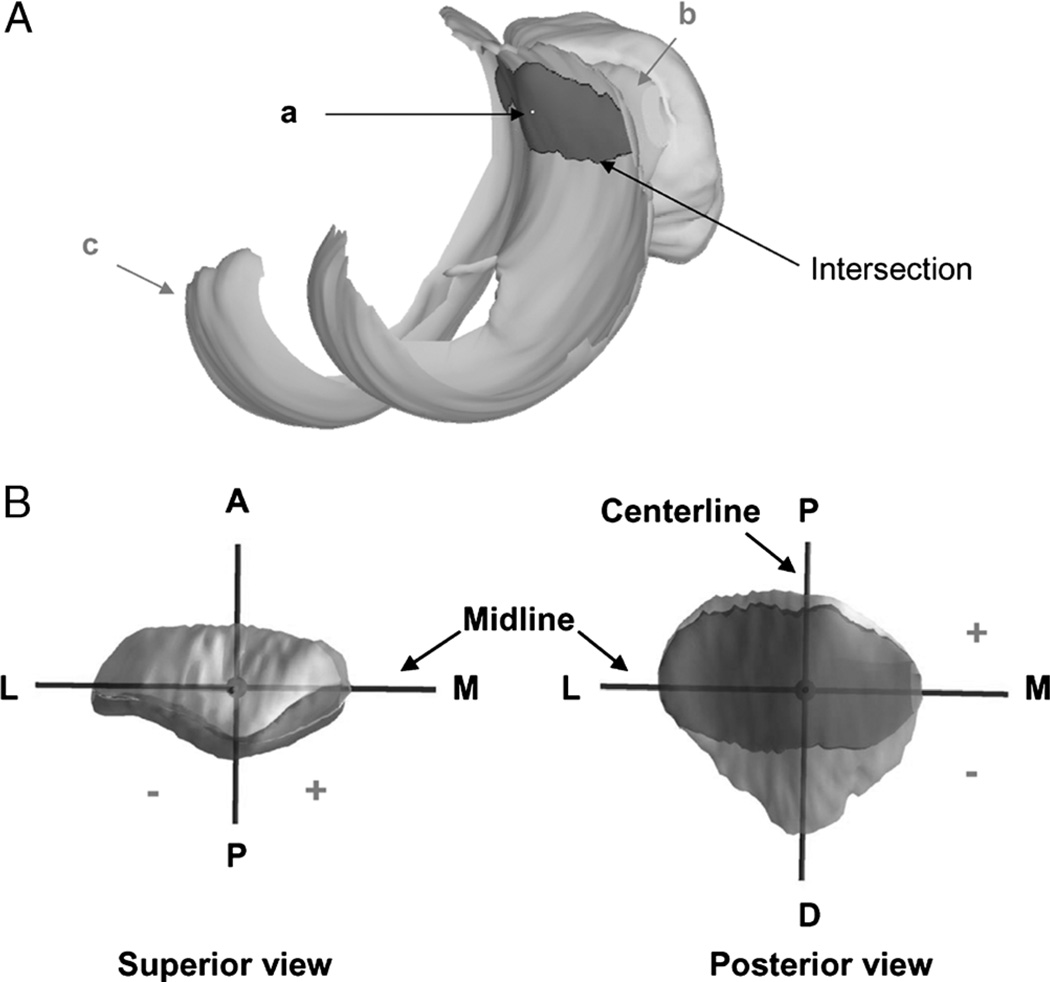
The contact points on the patellar cartilage were calculated by finding the centroid of the intersection of the patellar and the femoral cartilage layers (11,20,23,39). From the series of models used to reproduce knee motion, the relative positions of the cartilage layers on the femur and patella were determined. The overlap of the two cartilage layers was used to approximate the cartilage contact area (Fig. 4A). The solid modeling software automatically outlined the intersection of the patellar and the femoral cartilage layers and calculated the centroid of the enclosed area. The centroid of this contact area was defined as the contact point. To describe the motion of the cartilage contact points, a coordinate system was created on the surface of the patella (Fig. 4B). The center of the vertical ridge of the patella was the origin of the coordinate system. In this coordinate system, the proximodistal axis was called the centerline, and the mediolateral axis was called the midline. In the proximodistal direction, the contact point was positive if it was proximal to the midline and negative if it was distal to the midline. In the mediolateral direction, a contact point was positive if it was on the medial side of the centerline and negative if it was on the lateral side of the centerline.
FIGURE 4.
A, The centroid (a) of the intersection of the patellar (b) and femoral (c) cartilage was used to determine the patellofemoral contact locations. B, The coordinate system on the patellar cartilage surface for patellofemoral cartilage contact analysis. The proximal (P)–distal (D) axis was called the centerline. The medial (M)–lateral (L) axis was called the midline. Contact proximal to the midline and medial to the centerline was positive. Reprinted with permission from Van de Velde et al. Am J Sports Med. 2008 Jun;36(6):1150–9.
Statistical methods
At each flexion angle, the Wilcoxon signed rank test was used to compare the patellofemoral joint function (patellar flexion, shift, tilt, rotation, and position of the patellofemoral contact points on the patellar cartilage) of the PCL-deficient and intact (contralateral) knees. A Bonferroni correction factor (1/7) was used to account for multiple comparisons (at seven flexion angles). Differences at each flexion angle between the PCL-deficient and the intact knees were considered significant for P < 0.007.
RESULTS
Patellofemoral kinematics
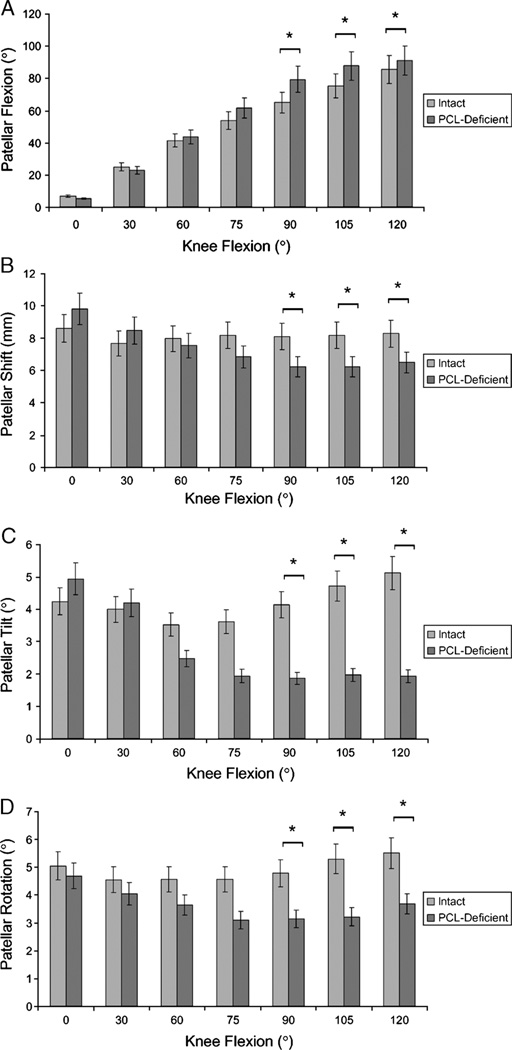
Between 90° and 120° of flexion, PCL deficiency increased the patellar flexion angle by 10.7° on average (Fig. 5A). The maximum difference occurred at 90° (intact knee = 65.2° ± 8.1°, PCL-deficient knee = 79.5° ± 6.1°, P < 0.001).
FIGURE 5.
Patellofemoral kinematics. Patellar flexion (A), patellar shift (B), patellar tilt (C), and patellar rotation (D) as a function of knee flexion angle (mean ± SD; *P values <0.007 determined with the Wilcoxon signed rank test).
The patella in PCL-deficient knees shifted significantly less laterally between 90° and 120° of knee flexion (Fig. 5B; P < 0.007). In the PCL-deficient knee, the patella was on average 1.9 mm less lateral to the knee center along the TEA compared with the healthy knee between 90° and 120° of knee flexion, with a maximum difference occurring at 105° (intact knee = 8.2 ± 3.9 mm, PCL-deficient knee = 6.2 ± 2.4 mm, P < 0.007).
Between 90° and 120° of knee flexion, PCL deficiency significantly decreased the lateral tilt of the patella by nearly 2.7°] (Fig. 5C; P < 0.007). The maximum effect of PCL deficiency occurred at 120° of knee flexion: the lateral tilt decreased from 5.1° ± 2.7° to 1.9° ± 2.6° after PCL deficiency (P < 0.001).
PCL deficiency significantly changed the patellar rotation between 90° and 120° of knee flexion (Fig. 5D; P < 0.007). In the PCL-deficient knee, the patella was approximately 1.8° less valgusly rotated between 90° and 120° of knee flexion.
Patellofemoral cartilage contact point location
We did not observe any contact between the femoral and the patellar cartilage in the healthy and the PCL-deficient knees at 0° of knee flexion. At 30° of knee flexion, the patellofemoral cartilage contact point on the patellar cartilage surface was 2.1 ± 1.0 and 2.2 ± 1.4 mm proximal to the midline in the healthy and the PCL-deficient knee, respectively (P = 0.75), and was 0.6 ± 1.4 and 0.4 ± 1.3 mm lateral from the centerline in the healthy and the PCL-deficient knee, respectively (P = 0.52). At 60° of knee flexion, the patellofemoral cartilage contact point on the patellar cartilage surface was 5.3 ± 1.6 and 4.1 ± 1.3 mm proximal to the midline in the healthy and the PCL-deficient knee, respectively (P = 0.04), and was 0.6 ± 1.0 and 1.3 ± 1.1 mm medial from the centerline in the healthy and the PCL-deficient knee, respectively (P = 0.02).
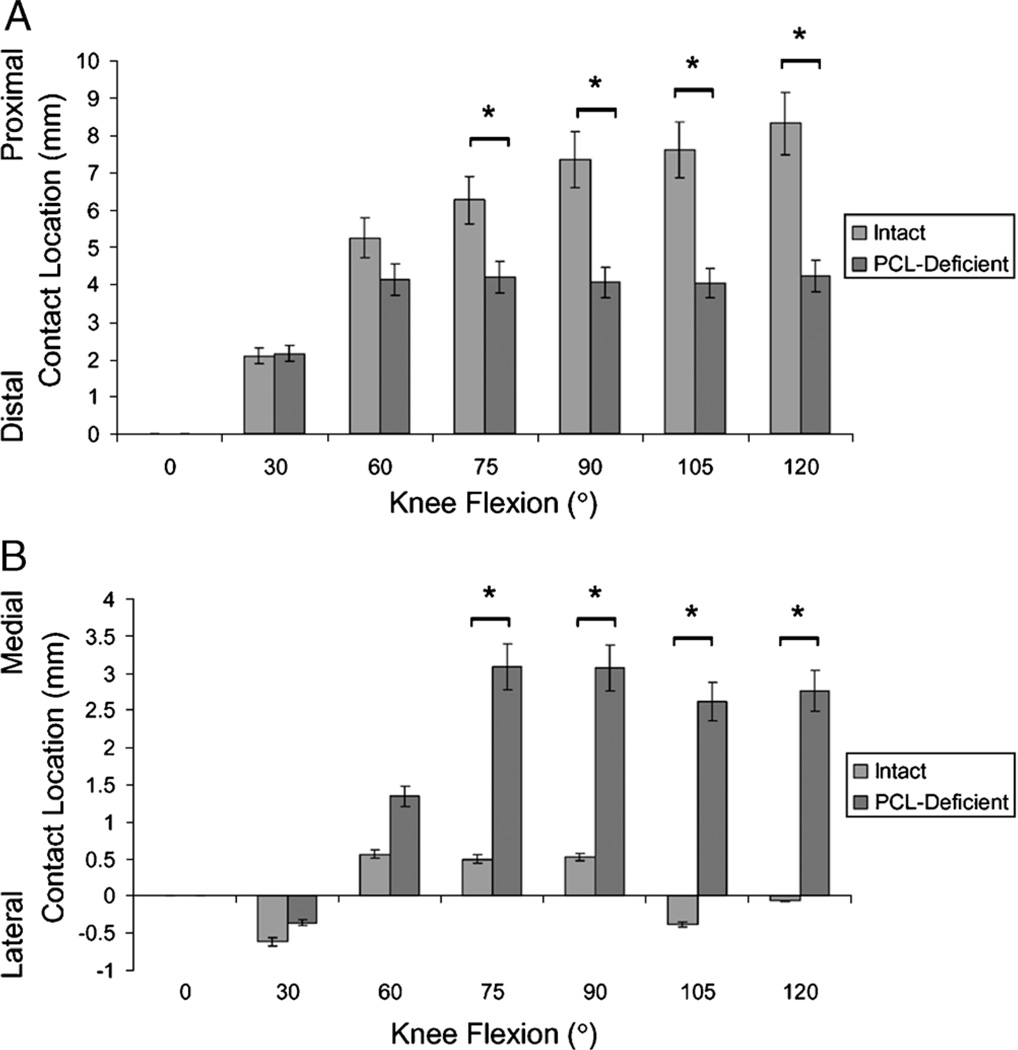
Between 75° and 120° of knee flexion, PCL deficiency caused a significant distal and medial shift of patellofemoral cartilage contact point location (Fig. 6; P < 0.007). Articular cartilage contact in the healthy knee moved along the centerline from 6.3 ± 1.7 mm proximal to the midline at 75° of knee flexion to 8.3± 1.3 mm proximal to the midline at 120° of knee flexion. In PCL deficiency, the patellofemoral contact point on the patellar cartilage surface remained in the same region, approximately 2.9 mm medial to the centerline and 4.1 mm proximal to the midline between 60° and 120° of knee flexion. The maximum difference between the healthy and the PCL-deficient knee was observed at 120° of knee flexion in the proximodistal direction (intact knee = 8.3 ± 1.3 mm, PCL-deficient knee = 4.2 ± 1.7 mm, P < 0.001; Fig. 6A) and at 105° of knee flexion in the mediolateral direction (intact knee = −0.4 ± 0.7 mm, PCL-deficient knee = 2.6 ± 1.7 mm, P < 0.001; Fig. 6B).
FIGURE 6.
Location of patellofemoral cartilage contact on the patellar surface. Cartilage contact in the proximodistal (A) and the mediolateral (B) direction as a function of knee flexion angle (mean ± SD; *P values <0.007 determined with the Wilcoxon signed rank test).
DISCUSSION
An increased prevalence of quadriceps weakness and atrophy, patellofemoral crepitus, and degeneration of the patellofemoral joint cartilage has been described in PCL-deficient patients (1,19,36). In the present study, we found that PCL deficiency increased the patellar flexion angle and decreased the lateral shift, tilt, and valgus rotation of the patella at flexion angles 90° and greater. Due to the congruency of the patellofemoral joint, these changes in patellofemoral kinematics resulted in changes in the location of the patellofemoral cartilage contact point. PCL deficiency caused a distal and medial shift of cartilage contact point from 75° to 120° of flexion.
It is challenging to formulate an explanation for the observed changes in patellofemoral kinematics after rupture of the PCL due to the complex interaction of muscle loading patterns, ligament and capsule deformation, and contact stress distribution on articular cartilage that occurs during of in vivo weight-bearing knee flexion to stabilize the patella within the femoral trochlea. It was brought to our attention in response to our study of the patellofemoral joint in anterior cruciate ligament deficiency (39) that the abnormal patellofemoral kinematics could in theory be attributed to changes in neuromuscular stabilization resulting in atrophy of the quadriceps, hereby altering the normal patellar tracking (Daniel Walz, M.D., personal communication, University Clinic München). Others have suggested that the degeneration of the patellofemoral joint cartilage is the result of an increased quadriceps activity and concomitant increased patellofemoral pressures as a compensation for the increased posterior tibial translation (4,7). On the basis our own analysis of the in vivo knee joints (26), we found that a kinematic coupling exist between the tibio-femoral and the patellofemoral joint, and consequently that tibiofemoral changes should be considered when investigating patellar pathologies (26). In the tibiofemoral joint of PCL-deficient patients, an increased posterior tibial translation as well as an increased external tibial rotation (16,21,24) and lateral translation (25) has been documented. Because the effect of tibiofemoral kinematic changes on patellofemoral kinematics is likely combined, isolating a particular tibial translation or rotation to a corresponding patellofemoral degree-of-freedom is difficult. Nevertheless, the increased posterior tibial translation could potentially explain the increased patellar flexion angle and subsequent distal shift in patellofemoral cartilage contact by moving the tibial tubercle more posteriorly and increasing the angle between the patellar tendon and the tibia in the sagittal plane. Conversely, the increased external tibial rotation observed in PCL deficiency would theoretically decrease the normal patellar tendon twist (9), explaining the decreased patellar tilt observed in the present study. The increased lateral translation of the tibia after PCL injury would move the tibial attachment of the patellar tendon more laterally relative to its patellar attachment, effectively increasing the coronal plane angle of the patellar tendon (9) and pushing the patella medially. The combination of decreased lateral shift and tilt could explain the medial shift of cartilage contact point from 75° to 120° of flexion that was observed in PCL deficiency. The kinetic coupling between the tibiofemoral and the patellofemoral joints implies that the optimal treatment of PCL injury, either surgical or conservative, should ideally restore the normal tibiofemoral kinematics, hereby possibly normalizing the patellofemoral function and potentially preventing long-term patellofemoral complications.
In our present study, cartilage contact area and deformation were not considered during the calculation of the cartilage contact point, hereby impeding the calculation of contact pressure changes within the patellofemoral joint. Nevertheless, the documented changes in patellofemoral joint kinematics and cartilage contact after isolated rupture of the PCL might provide an insight in the possible pathogenesis of the patellofemoral complications observed in PCL deficiency. In the healthy knee joint, the cartilage contact point location occurred along the centerline, that is, the vertical ridge of the patella. Previous study showed that the vertical ridge of the patella had thicker cartilage than the medial and the lateral articulating aspect of the patellar cartilage surface (39). The thicker cartilage within the normal cartilage-to-cartilage contact area may result in a reduced contact stress, as was demonstrated by a 3D finite element analysis, suggesting that thicker cartilage bears a lower peak contact stress than does thinner cartilage under the same loading conditions (22). The abnormal shift of the cartilage contact point to thinner regions of patellar cartilage would therefore theoretically increase the peak contact stress in the patellofemoral joint, which is consistent with the elevated patellofemoral contact pressures that were found after sectioning of the PCL in cadaveric studies (14,37).
When combining the present patellofemoral joint data with our previous analysis of the in vivo PCL function (32), the tibiofemoral kinematics (25), and the cartilage contact deformation in PCL deficiency (38), it becomes apparent that a target range of motion could be formulated for PCL-deficient patients in which knee motion might be safely performed. During the single-leg lunge between 0°and 60° of knee flexion, we did not detect differences in either tibiofemoral or patellofemoral joint biomechanics of the intact and the PCL-deficient knee. Therefore, rehabilitation exercises might be safely performed in this range of flexion. On the other hand, repetitive deep knee squats should be avoided in PCL-deficient subjects, so as not to excessively alter normal patellofemoral cartilage loading. We would like to emphasize that the present findings were obtained during a quasi-static lunge activity. As most daily activities are dynamic, other in vivo activities such as walking, running, and stair climbing (18) should be considered in future studies to define the definite safe range of motion for PCL-deficient patients.
Our study has several limitations. As mentioned above, data were acquired during only one functional activity, namely, a single-leg lunge. Another limitation is that the patients were investigated at different time intervals from injury. Future studies should also follow PCL-deficient patients who are treated conservatively for longer periods using a methodology similar to that used in this study. The patellofemoral cartilage contact behavior and the health of the cartilage could therefore be monitored with time to quantify any possible biomechanical relationships. This study did not measure the ground reaction force. Future studies should incorporate a load cell into the system as well as a whole body motion analysis system to ensure that the performed functional activities are fully uniform among the tested limbs. The cartilage contact position was determined as the centroid of the intersection area formed by the patellar and femoral cartilage surfaces. Cartilage contact area and deformation were not considered during calculation of the contact point, impeding the calculation of contact pressure changes. Finally, the present analysis compared the patellofemoral joint function of the PCL-deficient and intact knees at each flexion angle, hereby ignoring potential interactions among the patellar rotational and the translational degrees of freedom and various knee flexion angles. Future research involving a larger study sample needs to be performed to confirm the present findings. Nonetheless, we believe that the current findings provided a comprehensible insight in the patellofemoral changes after injury of the PCL and identified important directions for future research.
In summary, the altered tibiofemoral kinematics that were previously described in PCL deficiency resulted in changes in patellofemoral joint kinematics and cartilage contact at flexion angles greater than 60°. This abnormal loading of the patellofemoral joint might predispose the patellofemoral cartilage to degenerative changes associated with PCL injury.
Acknowledgments
The authors thank Dr. Louis DeFrate, Dr. Dain Allred, and Jeffrey Bingham, Ramprasad Papannagari, and Kartik Varadarajan for their technical assistance. The authors gratefully acknowledge the financial support of the National Institutes of Health (NIH R01AR052408-02) (GL), the National Football League Charities Foundation (TJG), and the Belgian American Educational Foundation (SKV). The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Conflict of interest: The authors do not have any conflict of interest to declare.
REFERENCES
- 1.Boynton MD, Tietjens BR. Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med. 1996;24(3):306–310. doi: 10.1177/036354659602400310. [DOI] [PubMed] [Google Scholar]
- 2.Bull AM, Katchburian MV, Shih YF, Amis AA. Standardisation of the description of patellofemoral motion and comparison between different techniques. Knee Surg Sports Traumatol Arthrosc. 2002;10(3):184–193. doi: 10.1007/s00167-001-0276-5. [DOI] [PubMed] [Google Scholar]
- 3.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 4.Cain TE, Schwab GH. Performance of an athlete with straight posterior knee instability. Am J Sports Med. 1981;9(4):203–208. doi: 10.1177/036354658100900402. [DOI] [PubMed] [Google Scholar]
- 5.Carlin GJ, Livesay GA, Harner CD, Ishibashi Y, Kim HS, Woo SL. In-situ forces in the human posterior cruciate ligament in response to posterior tibial loading. Ann Biomed Eng. 1996;24(2):193–197. doi: 10.1007/BF02667348. [DOI] [PubMed] [Google Scholar]
- 6.Clancy WG, Jr., Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65(3):310–322. [PubMed] [Google Scholar]
- 7.Cross MJ, Powell JF. Long-term followup of posterior cruciate ligament rupture: a study of 116 cases. Am J Sports Med. 1984;12(4):292–297. doi: 10.1177/036354658401200409. [DOI] [PubMed] [Google Scholar]
- 8.Dandy DJ, Pusey RJ. The long-term results of unrepaired tears of the posterior cruciate ligament. J Bone Joint Surg Br. 1982;64(1):92–94. doi: 10.1302/0301-620X.64B1.7068728. [DOI] [PubMed] [Google Scholar]
- 9.Defrate LE, Nha KW, Papannagari R, Moses JM, Gill TJ, Li G. The biomechanical function of the patellar tendon during in-vivo weight-bearing flexion. J Biomech. 2007;40(8):1716–1722. doi: 10.1016/j.jbiomech.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Defrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34(8):1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- 11.DeFrate LE, Sun H, Gill TJ, Rubash HE, Li G. In vivo tibiofemoral contact analysis using 3D MRI-based knee models. J Biomech. 2004;37(10):1499–1504. doi: 10.1016/j.jbiomech.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Fox RJ, Harner CD, Sakane M, Carlin GJ, Woo SL. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology. A cadaveric study. Am J Sports Med. 1998;26(3):395–401. doi: 10.1177/03635465980260030901. [DOI] [PubMed] [Google Scholar]
- 13.Gill TJ, DeFrate LE, Wang C, et al. The biomechanical effect of posterior cruciate ligament reconstruction on knee joint function. Kinematic response to simulated muscle loads. Am J Sports Med. 2003;31(4):530–536. doi: 10.1177/03635465030310040901. [DOI] [PubMed] [Google Scholar]
- 14.Gill TJ, DeFrate LE, Wang C, et al. The effect of posterior cruciate ligament reconstruction on patellofemoral contact pressures in the knee joint under simulated muscle loads. Am J Sports Med. 2004;32(1):109–115. doi: 10.1177/0095399703258794. [DOI] [PubMed] [Google Scholar]
- 15.Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop. 1975;(106):216–231. doi: 10.1097/00003086-197501000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69(2):233–242. [PubMed] [Google Scholar]
- 17.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 18.Iwata S, Suda Y, Nagura T, et al. Clinical disability in posterior cruciate ligament deficient patients does not relate to knee laxity, but relates to dynamic knee function during stair descending. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):335–342. doi: 10.1007/s00167-006-0198-3. [DOI] [PubMed] [Google Scholar]
- 19.Keller PM, Shelbourne KD, McCarroll JR, Rettig AC. Nonoperatively treated isolated posterior cruciate ligament injuries. Am J Sports Med. 1993;21(1):132–136. doi: 10.1177/036354659302100122. [DOI] [PubMed] [Google Scholar]
- 20.Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med. 2005;33(1):102–107. doi: 10.1177/0363546504265577. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Gill TJ, DeFrate LE, Zayontz S, Glatt V, Zarins B. Biomechanical consequences of PCL deficiency in the knee under simulated muscle loads–an in vitro experimental study. J Orthop Res. 2002;20(4):887–892. doi: 10.1016/S0736-0266(01)00184-X. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Lopez O, Rubash H. Variability of a three-dimensional finite element model constructed using magnetic resonance images of a knee for joint contact stress analysis. J Biomech Eng. 2001;123(4):341–346. doi: 10.1115/1.1385841. [DOI] [PubMed] [Google Scholar]
- 23.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88(8):1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Most E, DeFrate LE, Suggs JF, Gill TJ, Rubash HE. Effect of the posterior cruciate ligament on posterior stability of the knee in high flexion. J Biomech. 2004;37(5):779–783. doi: 10.1016/j.jbiomech.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Papannagari R, Li M, et al. Effect of posterior cruciate ligament deficiency on in vivo translation and rotation of the knee during weightbearing flexion. Am J Sports Med. 2008;36(3):474–479. doi: 10.1177/0363546507310075. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Papannagari R, Nha KW, Defrate LE, Gill TJ, Rubash HE. The coupled motion of the femur and patella during in vivo weightbearing knee flexion. J Biomech Eng. 2007;129(6):937–943. doi: 10.1115/1.2803267. [DOI] [PubMed] [Google Scholar]
- 27.Markolf KL, Slauterbeck JR, Armstrong KL, Shapiro MS, Finerman GA. A biomechanical study of replacement of the posterior cruciate ligament with a graft. Part 1: isometry, pretension of the graft, and anterior-posterior laxity. J Bone Joint Surg Am. 1997;79(3):375–380. doi: 10.2106/00004623-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Moore HA, Larson RL. Posterior cruciate ligament injuries. Results of early surgical repair. Am J Sports Med. 1980;8(2):68–78. doi: 10.1177/036354658000800203. [DOI] [PubMed] [Google Scholar]
- 29.Most E, Axe J, Rubash H, Li G. Sensitivity of the knee joint kinematics calculation to selection of flexion axes. J Biomech. 2004;37(11):1743–1738. doi: 10.1016/j.jbiomech.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Nha KW, Papannagari R, Gill TJ, et al. In vivo patellar tracking: clinical motions and patellofemoral indices. J Orthop Res. 2008;26(8):1067–1074. doi: 10.1002/jor.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noyes FR, Stowers SF, Grood ES, Cummings J, VanGinkel LA. Posterior subluxations of the medial and lateral tibiofemoral compartments. An in vitro ligament sectioning study in cadaveric knees. Am J Sports Med. 1993;21(3):407–414. doi: 10.1177/036354659302100314. [DOI] [PubMed] [Google Scholar]
- 32.Papannagari R, DeFrate LE, Nha KW, et al. Function of posterior cruciate ligament bundles during in vivo knee flexion. Am J Sports Med. 2007;35(9):1507–1512. doi: 10.1177/0363546507300061. [DOI] [PubMed] [Google Scholar]
- 33.Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35–38. doi: 10.1177/036354658601400107. [DOI] [PubMed] [Google Scholar]
- 34.Race A, Amis AA. Loading of the two bundles of the posterior cruciate ligament: an analysis of bundle function in a-P drawer. J Biomech. 1996;29(7):873–879. doi: 10.1016/0021-9290(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 35.Schulte KR, Chu ET, Fu FH. Arthroscopic posterior cruciate ligament reconstruction. Clin Sports Med. 1997;16(1):145–156. doi: 10.1016/s0278-5919(05)70011-9. [DOI] [PubMed] [Google Scholar]
- 36.Shelbourne KD, Davis TJ, Patel DV. The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries. A prospective study. Am J Sports Med. 1999;27(3):276–283. doi: 10.1177/03635465990270030201. [DOI] [PubMed] [Google Scholar]
- 37.Skyhar MJ, Warren RF, Ortiz GJ, Schwartz E, Otis JC. The effects of sectioning of the posterior cruciate ligament andthe posterolateral complex on the articular contact pressures within the knee. J Bone Joint Surg Am. 1993;75(5):694–699. doi: 10.2106/00004623-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Van de Velde SK, Bingham JT, Gill TJ, Li G. Analysis of tibiofemoral cartilage deformation in posterior cruciate ligament-deficient knee. J Bone Joint Surg Am. 2009;91(1):167–175. doi: 10.2106/JBJS.H.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van de Velde SK, Gill TJ, DeFrate LE, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency and reconstruction on the patellofemoral joint. Am J Sports Med. 2008;36(6):1150–1159. doi: 10.1177/0363546508314404. [DOI] [PMC free article] [PubMed] [Google Scholar]