Abstract
Older adults are not only at higher risk of experiencing stroke, but also have multiple co-morbidities that make treatment for secondary stroke prevention challenging. Very few clinical trials specifically related to secondary stroke prevention treatment efficacy have focused on the oldest-old (≥85 years) and, therefore, evidence-based recommendations for treatment specific to this population are not available. Some of the special considerations for stroke prevention treatments in older patients include careful titration of blood-pressure-lowering drugs to avoid hypotension, the risk of haemorrhagic stroke with HMG-CoA reductase inhibitors (statins) and weighing the risk of recurrent ischaemia versus bleeding in patients taking antiplatelet or anticoagulant therapy. The risk of peri-procedural complications appears to be high with both carotid angioplasty and stenting and carotid endarterectomy in older patients with carotid stenosis. Other common issues in older patients include adverse drug events, recognizing the risk of dementia, depression and osteoporosis and deciding when to discontinue secondary stroke prevention. In this review, we provide the practitioner with the evidence related to specific approaches to secondary stroke prevention in older patients, and identify the knowledge gaps that currently limit our ability to appropriately treat this vulnerable population.
1. Prevalence and Incidence of Stroke in Older Adults: the Need for Secondary Prevention
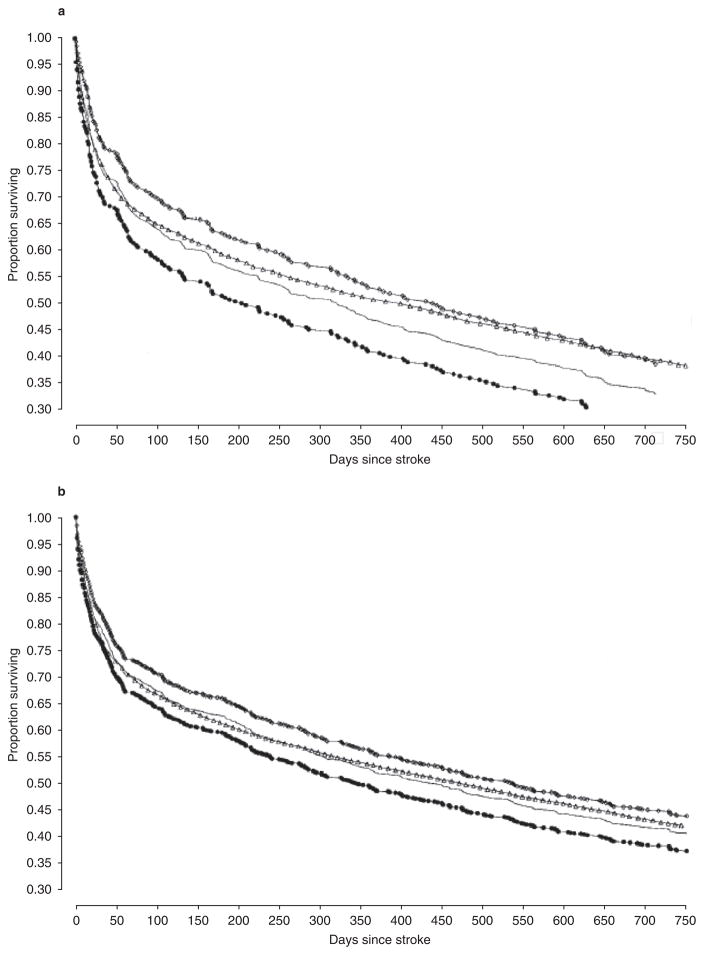
Stroke is a major cause of morbidity and mortality in older adults (figure 1), with the incidence increasing dramatically with age.[1–3] Previous occurrence of stroke is a major risk factor for recurrent ischaemic stroke,[4–7] with a cumulative 10-year risk of 43 % (95% CI 34, 51) after an initial event.[8] Survival after recurrent stroke is also significantly lower than after first stroke, especially with increasing age (figure 1).[9] The risk of secondary stroke is greatest in the first 6 months (9% ; 95% CI 5, 14) with an average annual risk of 4% thereafter. Previous occurrence of stroke is also a marker for increased risk of ischaemic events in other vascular beds.[4–7] Thus, even patients with limited life expectancies due to advanced age or co-morbidities may benefit from secondary prevention after a stroke.
Fig. 1.
Survival in patients aged ≥85 years with first and recurrent stroke: (a) males and (b) females. Open triangles represent the Kaplan-Meier estimate of the survival curve in patients with first stroke; lines represent the Kaplan-Meier estimate of the survival curve for patients with recurrent stroke. Open squares and closed circles represent the upper and lower 95% confidence limits (reproduced from Samsa et al.,[9] with permission).
Because ischaemic stroke is such a strong predictor of secondary ischaemic events, clinicians caring for older adults with a history of ischaemic stroke need to carefully consider secondary prevention strategies in these patients. Recent US studies suggest that older stroke patients receive less aggressive secondary prevention strategies than younger patients.[3,10] Of note, recurrent strokes are associated with higher rates of morbidity and mortality than incident events.[11] For example, in the Perth Community Stroke Study, the 30-day mortality rate for recurrent stroke was almost twice as great as that of an initial stroke (41% vs 22% ; p = 0.003).[8] Of the 637 patients enrolled in the MATCH (Management of Atherothrombosis with Clopidogrel in High Risk Patients) trial who had a recurrent event during the study period, 345 (54%) were disabled after the recurrent event; of these, only 33% were disabled prior to the event, [12] again emphasizing the need for secondary prevention. Unfortunately, although the burden of stroke is highest in the oldest subset of the population, much of the research on secondary prevention has been performed in relatively younger and healthier subjects. The purpose of this paper is to review the evidence for secondary prevention strategies after ischaemic stroke, with particular attention to its generalizability to frail, older populations.
2. Guideline Recommendations
The American Heart Association (AHA)/ American Stroke Association (ASA)[13,14] and the American College of Chest Physicians (ACCP)[15] have compiled evidence-based guidelines that contain recommendations for the secondary prevention of ischaemic stroke. The AHA/ASA guidelines cover the full range of modifiable risk factors (table I), whereas the ACCP guidelines pertain only to antithrombotic/thrombolytic therapy (table II).
Table I.
American Heart Association/American Stroke Association recommendations for risk factor modification in secondary stroke prevention[13,14]
| Recommendation | Class/level of evidencea |
|---|---|
| Hypertension | |
| Antihypertensive treatment is recommended for all ischaemic stroke or TIA patients who are beyond the hyperacute period | I/A |
| Drug choices should be individualized based on the available data and specific patient characteristics | I/A |
| Absolute BP levels are uncertain and should be individualized | IIa/B |
| Comprehensive therapy should include proven lifestyle modifications | IIb/C |
| Diabetes mellitus | |
| ACE inhibitors and ARBs should be prescribed as they reduce renal disease progression | I/A |
| Glucose levels should be as near to normoglycaemia as possible in patients with ischaemic stroke or TIA | I/A |
| HbA1c should be ≤7% | IIa/B |
| BP and lipids should be more rigorously controlled | IIa/B |
| Cholesterol | |
| Ischaemic stroke or TIA patients with elevated cholesterol, co-morbid CAD or evidence of an atherosclerotic origin should be managed according to NCEP III guidelines | I/A |
| HMG-CoA reductase inhibitors (statins) are recommended with a target LDL-C goal of <100 mg/dL (<70 mg/dL for high-risk patients) | I/A |
| Ischaemic stroke or TIA patients without known CHD should receive statin therapy to reduce the risk of stroke and cardiovascular events | I/B |
| Ischaemic stroke or TIA patients with low HDL-C may be treated with niacin or gemfibrozil | IIb/B |
| Smoking | |
| All patients who smoked in the past year should be encouraged to quit | I/C |
| A combination of counselling, nicotine products and oral smoking cessation products should be considered | IIa/B |
| Environmental smoke should be avoided | IIa/C |
| Alcohol | |
| Heavy drinkers should eliminate or reduce their alcohol consumption | I/A |
| ≤2 drinks/day for men and 1 drink/day for nonpregnant women may be considered | IIb/C |
| Obesity | |
| A goal BMI of 18.5–24.9 kg /m2 and a waist circumference <35 cm for women and <40 cm for men should be encouraged | IIb/C |
| Physical activity | |
| At least 30 minutes of moderate-intensity physical activity per day should be considered for all capable patients | IIb/C |
Class I: conditions for which there is evidence for and/or general agreement that it is useful and effective; class IIa: weight of evidence is in favour; class IIb: usefulness/efficacy is less well established; level of evidence A: data derived from multiple randomized clinical trials; level of evidence B: data derived from a single randomized trial or nonrandomized studies; level of evidence C: expert opinion or case studies.
ARB = angiotensin II type 1 receptor antagonist (angiotensin receptor blocker); BMI = body mass index; BP = blood pressure; CAD = coronary artery disease; CHD = coronary heart disease; HbA1c = glycosylated haemoglobin; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; NCEP = National Cholesterol Education Program; TIA = transient ischaemic attack.
Table II.
American College of Chest Physicians recommendations for antiplatelet and anticoagulant therapy in secondary stroke prevention[15]
| Therapy | Patient population | Gradea | Dosage |
|---|---|---|---|
| Antiplatelet | Patients who have experienced a cryptogenic stroke and have a PFO | 1C+ | Dependent on therapy chosenb |
| Patients who have aortic atherosclerotic lesions or mitral valve strands or prolapse | 1C+ | Dependent on therapy chosenb | |
| Option for patients who have experienced a cryptogenic stroke associated with mobile aortic arch thrombi | 2C | Dependent on therapy chosenb | |
| Aspirin (acetylsalicylic acid) | Option for all patients who have experienced a noncardioembolic stroke or TIA | 1A | 50–100 mg/day |
| Patients undergoing CEA (treatment should start prior to CEA and continue thereafter) | 1A | 50–100 mg/day | |
| Patients with a cardioembolic stroke who have contraindications to anticoagulant therapy | 1A | 75–325 mg/day | |
| Patients with a moderate to high risk of bleeding complications | 1C+ | 50–100 mg/day | |
| Aspirin + dipyridamolec | Option for all patients who have experienced a noncardioembolic stroke or TIA | 1A | 25 mg aspirin + 200 mg dipyridamole twice daily |
| Clopidogreld | Option for all patients who have experienced a noncardioembolic stroke or TIA | 1A | 75 mg/day |
| Oral anticoagulant, vitamin K antagonists (e.g. warfarin) | Patients with AF | 1A | Target INR, 2.5; INR range, 2–3 |
| Patients with cerebral venous sinus thrombosis | 1B | Target INR, 2.5; INR range, 2–3 | |
| Patients with well documented prothrombotic disorders | 2C | Target INR, 2.5; INR range, 2–3 | |
| Option for patients who have experienced a cryptogenic stroke associated with mobile aortic arch thrombi | 2C | Target INR, 2.5; INR range, 2–3 |
Grade 1 recommendations are strong and indicate that the ratio of benefit to risk, cost and burden is favourable. Grade 2 recommendations indicate patients’ values may lead to different choices. A full description of the grading system can be found in Guyatt et al.[16]
Aspirin 50–325 mg/day, aspirin 25 mg + dipyridamole 200 mg twice daily and clopidogrel 75 mg/day are equivalent.
Recommended over aspirin alone (grade 2A).
Recommended over aspirin alone (grade 2B).
AF = atrial fibrillation; CEA = carotid endarterectomy; INR = international normalized ratio; PFO = patent foramen ovale; TIA = transient ischaemic attack.
2.1 Literature Search Methods
Articles for this review were identified by searching the MEDLINE database (1950 to September 2008) using the following terms, either singly or in combination: ‘adverse drug events’, ‘arthritis’, ‘dementia’, ‘depression’, ‘elderly’, ‘ischemic stroke’, ‘osteoporosis’, ‘quality improvement initiative’, ‘recurrent stroke’, ‘secondary prevention’ and ‘transient ischaemic attack’. Additional terms, which were identified using the current AHA/ASA recommendations for secondary stroke prevention as a guide, included ‘anti-platelet’, ‘antithrombotic’, ‘diabetes’, ‘dyslipidemia’, ‘carotid stenosis’, ‘hyperhomocysteinemia’, ‘hypertension’ and ‘smoking’. Relevant articles published in the English language were identified by a review of their abstracts. Additional articles were identified by manually searching the reference lists of the initially identified articles. Articles were included in this review if they were (i) randomized controlled trials of secondary prevention of stroke; (ii) randomized controlled trials of primary prevention if focused on the elderly or those aged ≥75 years and stroke was a primary or secondary outcome; or (iii) cohort studies of strokes in the elderly or those aged ≥75 years. No other specific exclusion criteria or formal assessments of article quality were employed.
Based on the results of this search, we review the evidence for the major recommendations, highlighting results of clinical trials targeted to older adults, as well as the generalizability of the results of other trials to patients aged ≥75 years. Summaries of clinical trials included in this review can be found in tables III and IV.
Table III.
Clinical trials relevant to the secondary prevention of stroke at any age, or prevention of stroke in the oldest patients with cardiovascular risk factor modification
| Name | Population | Treatment | Key clinical findings |
|---|---|---|---|
| Hypertension | |||
| PROGRESS[17] | 6105 hypertensive and non-hypertensive patients with a history of stroke of any cause or TIA (mean age 64 years; SD 10 years) | β-Adrenoceptor antagonist ± diuretic vs placebo | Antihypertensive therapy resulted in an ARR of 3.7% for stroke (10.1% vs 13.8% ; p < 0.0001) and 4.8% for vascular events (15.0% vs 19.8% ; p < 0.0001) compared with placebo; dual therapy with a β-adrenoceptor antagonist and diuretic was superior to monotherapy with a β-adrenoceptor antagonist |
| STOP- Hypertension[18] | 1627 hypertensive patients aged 70–84 years | 3 β-adrenoceptor antagonists + 1 diuretic vs placebo | Antihypertensive therapy reduced the number of primary endpoint eventsa by 36 (58 vs 94; p = 0.0031), fatal and non-fatal strokes by 24 (29 vs 53; p = 0.0081) and total deaths by 27 (36 vs 63; p = 0.0079) compared with placebo |
| SHEP[19] | 4736 patients aged ≥60 years (mean 72 years) with systolic hypertension | β-Adrenoceptor antagonist + diuretic vs placebo | Antihypertensive therapy resulted in an ARR of 2.0% (3.6% vs 5.6%) |
| HYVET (pilot study)[20] | 1283 hypertensive patients aged ≥80 years (mean age 83.8 years; range 79.5–96.1 years) | Diuretic vs β-adrenoceptor antagonist vs placebo | Antihypertensive therapy prevented 19 strokes but resulted in 20 additional non-stroke deaths per 1000 patients per year |
| HYVET[21] | 3845 hypertensive patients aged ≥80 years (mean age 83.6 ± 3.2 years for active treatment, 83.5 ± 3.1 years for placebo) | Indapamide vs placebo | Treating 1000 patients with indapamide for 2 years would prevent 11 strokes (95% CI 0, 21) |
| Dyslipidaemia | |||
| HPS[22] | 20 536 patients aged 40–80 years with a history of cerebrovascular disease (mean age 65.5 years; SD 7.8) or other arterial occlusive disease (mean age 63.7 years; SD 8.5) | Simvastatin vs placebo | In the 3280 patients with pre-existing cerebrovascular disease, simvastatin did not significantly reduce the absolute risk for stroke (10.3% vs 10.4%), but did reduce the absolute risk of having a major CVE by 5.1% (24.7% vs 29.9% ; p = 0.001) |
| SPARCL[23] | 4731 patients aged ≥18 years of age with hyperlipidaemia, no known CHD and a history of stroke or TIA in the previous 1–6 months (mean age 62.5 years; SD 0.2 years) | Atorvastatin vs placebo | Treatment with atorvastatin resulted in a 2.2% ARR for stroke (p = 0.03) and a 3.5% ARR for a major CVE (p = 0.002) |
| PROSPER[24] | 5804 patients aged 70–82 years with a history of, or risk factors for, vascular disease | Pravastatin vs placebo | Allocation to pravastatin resulted in an ARR of 2.1% (14.1% vs 16.2% ; p = 0.014) compared with placebo for the primary endpoint of CHD death, non-fatal MI, non-fatal stroke |
| Retrospective analysis of 50 clinical trials[25] | 5924 persons aged ≥65 years (mean age range 71–74 years) enrolled in the Pfizer Atorvastatin Clinical Program Database | Atorvastatin vs placebo | The rate of having at least one adverse event was similar among patients taking 4 different doses of atorvastatin (10.2–16.1%) and placebo (15.0%) and the number of serious adverse events was low (≤1.0%) |
Stroke, MI or cardiovascular death.
ARR = absolute risk reduction; CHD = coronary heart disease; CVE = cardiovascular event; MI = myocardial infarction; SD = standard deviation; TIA = transient ischaemic attack.
Table IV.
Relevant clinical trials of antiplatelet therapy in elderly patients with ischaemic stroke
| Name | Population | Treatment | Key clinical finding | Findings in subgroups by age |
|---|---|---|---|---|
| CAPRIE[26] | 19 185 patients with a history of ischaemic stroke, MI or PAD. Mean age 62.5 years |
ASA vs clopidogrel | Clopidogrel reduced the absolute risk of the primary outcomea by 0.51% compared with ASA (p = 0.043) | No published data |
| MATCH[27] | 7599 patients with TIA or ischaemic stroke within 3 months. Mean age 66.3 years |
Clopidogrel + placebo vs clopidogrel + ASA | Clopidogrel + ASA did not significantly reduce the absolute risk of the primary outcomeb (1.0%, p = 0.244) but did significantly increase the risk of life-threatening, major and minor bleeding (p < 0.0001) compared with clopidogrel alone | No significant differences between age <65 (ARR 2.3% ; HR 0.86; 95% CI 0.64, 1.02) and ≥65 years (ARR 0.3% ; HR 1.0; 95% CI 0.86, 1.12) |
| CHARISMA[28] | 15 603 patients with clinically evident cardiovascular disease or multiple risk factors. Mean age 64 years |
ASA + placebo vs ASA + clopidogrel | Clopidogrel + ASA had no effect on the primary outcomea (ARR 0.5% ; RR 0.93; 95% CI 0.83, 1.05) and increased the absolute risk of moderate bleeding events by 0.8% (p < 0.001) compared with ASA alone | No difference in age <75 (HR 0.9; 95% CI 0.8, 1.05) vs ≥75 years (HR 0.91; 95% CI 0.75, 1.2) |
| ESPS-2[29] | 6602 patients with TIA or ischaemic stroke within 3 months. Mean age 66.7 years |
Placebo vs ASA vs dipyridamole vs ASA/ER dipyridamole | ASA/ER dipyridamole reduced the absolute risk of the primary outcomec by 12.9% (p = 0.056) compared with ASA alone and by 10.7% (p = 0.073) compared with dipyridamole alone | No published data |
| ESPRIT[30] | 2739 patients with a TIA or minor stroke within 6 months. Mean age 63 years |
ASA + placebo vs ASA/ER dipyridamole | ASA/ER dipyridamole resulted in an ARR of 1.0% per year in the primary outcomed (95% CI 0.1, 1.8) compared with ASA alone | No significant difference between age ≤65 (HR 0.9; 95% CI 0.7, 1.1) and >65 years (HR 0.8; 95% CI 0.65, 1.0) |
| PRoFESS[31] | 20 332 patients with ischaemic stroke <90 days before randomization. Mean age 66.1 years |
ASA/ER dipyridamole vs clopidogrel | No significant difference in primary outcome (ASA/ER dipyridamole 9%, clopidogrel 8.8%). Did not meet statistical criteria for non-inferiority for ASA/ER dipyridamole | No significant difference between treatments among age <65 years, age ≥65 years to < 75 years or age ≥75 years |
Ischaemic stroke, MI, cardiovascular death.
Ischaemic stroke, MI, cardiovascular death, rehospitalization for acute ischaemia.
Ischaemic stroke or cardiovascular death.
Ischaemic stroke, MI, cardiovascular death, major bleeding.
ARR = absolute risk reduction; ASA = aspirin (acetylsalicylic acid); ER = extended-release; HR = hazard ratio; MI = myocardial infarction; PAD = peripheral arterial disease; RR = relative risk; TIA = transient ischaemic attack.
2.2 Blood Pressure Lowering
Hypertension is one of the most prevalent modifiable risk factors for stroke. Antihypertensive treatments have been shown to reduce the relative risk of incident stroke by 28–39%.[32] Therefore, pharmacological therapy and lifestyle modifications are recommended for all patients who have had an ischaemic stroke or transient ischaemic attack (TIA) and who are beyond the hyperacute period, with a goal systolic blood pressure (BP) of <140 mmHg.[14] The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recommends that a combination of diuretics and ACE inhibitors be used for secondary prevention of all patients, regardless of their BP levels.[33] These recommendations are primarily based on results from PROGRESS (Perindopril Protection Against Recurrent Stroke Study), in which patients with a recent stroke regardless of a history of hypertension were enrolled and randomized to perindopril or placebo (plus indapamide if additional BP control was necessary).[17,34] PROGRESS is the only trial of secondary prevention with BP-lowering medications, and it demonstrated that patients taking ACE inhibitors and diuretics in combination had a reduced risk of recurrent stroke, regardless of BP levels or a history of hypertension. However, it is important to recognize that the mean age of subjects in PROGRESS was 64 years and that older patients may be at higher risk of adverse drug reactions from these agents.
We also included primary prevention trials in this review when stroke was a primary outcome and the trial was focused on older patients. A number of trials have shown that antihypertensive treatment can lower the risk of incident stroke in older patients with known hypertension. These include the STOP-Hypertension (Swedish Trial in Old Patients with Hypertension)[18] and the SHEP (Systolic Hypertension in the Elderly Program).[19,35] Interestingly, in the per-protocol analysis of the SYST-EUR (Systolic Hypertension in Europe) trial,[36] the benefit of mortality reduction with anti-hypertensive treatment was attenuated in patients aged 75–80 years, but the protection against cardiovascular events was not. Therefore, the totality of current evidence favours control of BP in all older patients.[18,19,36–38]
Recently, results from the HYVET (Hypertension in the Very Elderly Trial) were reported.[21] In this placebo-controlled trial of 3845 patients aged ≥80 years, antihypertensive treatment with an indapamide-based regimen reduced the number of fatal and non-fatal strokes from 17.7 per 1000 patient-years to 12.4 per 1000 patient-years (unadjusted hazard ratio [HR], 0.70; 95% CI 0.49, 1.01; p = 0.06); the number of fatal strokes from 42 per 1000 patient-years to 27 per 1000 patient-years (HR 0.61; 95% CI 0.38, 0.99; p = 0.046); and the number of deaths from any cause from 235 per 1000 patient-years to 196 per 1000 patient-years (HR 0.79; 95% CI 0.65, 0.95; p = 0.02). This reduction in mortality is somewhat surprising because results from the HYVET pilot study reported that antihypertensive therapy increased the number of non-stroke deaths by 20 for every 1000 patients treated for 1 year.[20] A recent study of elderly veterans found a higher mortality rate among patients aged >80 years with lower BP, raising concern about the advisability of aggressive BP targets in the oldest-old, although the cohort design may have led to unmeasured confounders that influenced the result.[39] Given the preponderance of evidence from randomized trials such as SHEP, SYST-EUR, STOP-Hypertension and HYVET, which included subjects aged >80 years, we believe that current BP targets are appropriate with careful titration to avoid orthostatic hypotension.
2.3 Dyslipidaemia
Hyperlipidaemia is a stronger risk factor for coronary artery disease than for stroke. [40,41] However, treatment with HMG-CoA reductase inhibitors (statins) in patients with coronary disease significantly reduced the risk of stroke in multiple randomized controlled trials.[42–49] Current guidelines recommend that patients with cerebrovascular and/or coronary vascular events should have cholesterol levels managed according to guidelines established by the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Cholesterol in Adults (NCEP III), with a goal low-density lipoprotein cholesterol (LDL-C) level of <100 mg/dL.[13,14,49]
Although it is very important to assess secondary prevention with statin therapy in patients with cerebrovascular disease but no evidence of coronary disease, very few trials have directly addressed this question or that of use of statins for stroke prevention in the elderly. However, the Medical Research Council/British Heart Foundation Heart Protection Study (HPS)[42] was sufficiently large and included a suitably wide variety of patients with vascular disease to allow assessment of these issues. In the subgroup of patients with a history of cerebrovascular disease but no coronary disease in this study, there was a significant reduction in major cardiovascular events with statin treatment, although rates of stroke were unaffected. The PROSPER (Pravastatin in Elderly Individuals at Risk of Vascular Disease) study, conducted specifically in the 70-to 82-year-old population with a history of, or risk factors for, vascular disease, also showed no significant reduction in stroke events, although there was a modest reduction in TIAs.[24] The only trial to address secondary prevention for stroke was the SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) study.[23] This was a randomized, controlled trial of high-dose atorvastatin versus placebo in patients with a history of recent stroke or TIA and LDL-C levels between 100 and 190 mg/dL. A significant treatment benefit in favour of atorvastatin was observed, with an absolute risk reduction of 2.2% and an adjusted HR of 0.84 (95% CI 0.71, 0.99; p = 0.03) for the primary outcome of non-fatal or fatal stroke. However, similar to the results of the HPS,[42] there was even greater protection against cardiovascular events (e.g. HR for any coronary event 0.65; 95% CI 0.46, 0.73; p < 0.001).[23]
An additional important result of SPARCL was a haemorrhagic stroke rate of 2.3 % in the atorvastatin group versus 1.4% in the placebo group (adjusted HR 1.66; 95% CI 10.8, 2.55).[23] Aside from statin therapy, other independent predictors of haemorrhagic stroke in SPARCL included male sex, stage 2 hypertension at the last visit preceding the event and increased age (HR 1.42; 95% CI 1.16, 1.74 for 10-year increments).[50] Because the mean age of subjects in SPARCL was 63 years and there was a significant age-related risk of haemorrhagic stroke, caution is required when administering high-dose atorvastatin to the oldest population of stroke patients. Thus, the available evidence suggests that statin therapy after stroke yields only a small reduction in recurrent strokes and a small but significantly increased risk of haemorrhagic stroke in the elderly. While randomized trials are not available for the oldest-old, a matched cohort study of 2626 nursing home residents with vascular disease, 1313 of whom were taking statins, showed a 31% relative decrease in the hazard of hospitalization and mortality from secondary vascular events associated with statin use, with the number needed to treat to prevent one additional event being 5–7 patients.[51] However, it should be emphasized that this cohort was treated with conventional doses rather than the high doses of statins used in SPARCL. Although selection bias may well have impacted on these results, this study supports the use of statins in appropriately selected frail patients, including those in nursing facilities. In general, statin therapy is a reasonable strategy for reducing recurrent stroke and other cardiovascular events in this high-risk population.
Other concerns associated with statin use in older adults include cognitive decline, muscular dysfunction, myotoxicity, hepatotoxicity, rhabdomyolysis and drug-drug interactions. The evidence regarding the impact of statins on cognitive decline is inconsistent as clinical trials have shown negative,[52,53] neutral[24,54–56] and beneficial[57,58] effects, although none of these studies included patients with prior stroke. Although the incidence of severe or fatal rhabdomyolysis is rare (0.15 deaths per 1 million prescriptions), the incidence of myalgias with or without elevation of creatine kinase is poorly defined.[59] Hepatotoxicity manifested by 3-fold increases in amino-transferases occurs in approximately 1–3% of patients, although there is little evidence to suggest that mild to moderate elevations correlate with histological liver injury, and regular monitoring of liver function tests is of questionable benefit.[60] Therefore, the decision to treat older adults with statins for secondary stroke prevention should take into account the risks for coronary disease and intracerebral haemorrhage and the possibility of myotoxicity and hepatotoxicity. There is no clear evidence to suggest that these severe adverse effects are more likely to occur with advancing age.
2.4 Hyperhomocysteinaemia
In observational studies, elevated levels of homocysteine have been shown to increase the risk of stroke by as much as 3-fold.[61–64] While hyperhomocysteinaemia can be easily treated with B vitamins, primary prevention trials[65,66] have not shown a reduced risk of cardiovascular events or stroke with vitamin supplementation. Similarly, a large secondary prevention trial in patients with prior ischaemic stroke, the VISP (Vitamin Intervention for Stroke Prevention),[64] showed no impact on stroke, coronary heart disease (CHD), severe stroke or death with high-dose compared with low-dose B vitamins over 2 years. A post hoc subgroup analysis reported a significant benefit on a combined stroke and CHD outcome for patients with a baseline vitamin B12 level above the median;[67] however, this somewhat counter-intuitive finding may be a chance result and requires further confirmation. While others have expressed concerns that the trial may have been underpowered in the setting of folic-acid fortified cereals,[68] any true treatment effect is likely to be small, and current evidence is insufficient to recommend routine vitamin supplementation after stroke.
Interestingly, a study of Japanese patients aged ≥65 years with residual hemiplegia at least 1 year after a stroke who were prescribed combined folate and vitamin B12 treatment reported a significant 7.1% absolute risk reduction in sustaining a hip fracture compared with those prescribed placebo.[69] The mechanism for this reduction is unclear, and the generalizability of the finding to non-Japanese populations needs to be established before vitamin B12 supplementation can be routinely advised.
2.5 Cigarette Smoking
The majority of studies linking cigarette smoking to ischaemic stroke have been focused on incident events. However, the South London Stroke Register reported that patients aged >75 years who smoked at the time of stroke were more likely than younger patients to have either tried or succeeded with smoking cessation in the first 3 years after incident stroke.[70]
2.6 Diabetes Mellitus
Only one published study has identified diabetes mellitus as an independent predictor of recurrent stroke in the elderly. In the Cardiovascular Health Study cohort, patients aged >65 years (80% were aged >75 years) were followed for death, recurrent stroke and cardiovascular disease event.[3] Diabetes was independently associated with a nearly 60% increased risk of recurrent stroke (HR 1.59; 95% CI 1.07, 2.37; p = 0.022).
2.7 Antithrombotic Therapy for Transient Ischaemic Attack and Noncardioembolic Stroke
The rationale for antithrombotic therapy in ischaemic stroke patients is predicated on the underlying atherosclerotic and atherothrombotic mechanisms that account for the majority of these events.[15] Most ischaemic stroke patients should be treated with some form of antithrombotic therapy, especially in light of the likelihood of polyvascular disease, and the overlap of treatment strategies for CHD and peripheral arterial disease. However, for ischaemic stroke, the optimal therapy differs depending on the underlying mechanism of ischaemia. For patients with noncardioembolic stroke or TIA, antiplatelet therapy is recommended rather than anticoagulants to reduce the risk of recurrent stroke and other cardiovascular events (tables I and II).[14,71] This recommendation is based on the results of the WARSS (Warfarin Aspirin Recurrent Stroke Study), which showed no increased efficacy of warfarin (adjusted dose to target international normalized ratio [INR] 1.4–2.8) over aspirin (acetylsalicylic acid) 325 mg for non-cardioembolic stroke or TIA.[72] Currently available antiplatelet drugs include aspirin, aspirin/ extended-release (ER), dipyridamole and clopidogrel. Aspirin is the most widely studied anti-platelet therapy, and is a standard of care for secondary prevention of all atherothrombotic events. In patients with prior TIA or stroke, aspirin reduces the relative risk of major cardiovascular events by 22%.[73]
Unlike aspirin, which inhibits platelet aggregation promoted by formation of thromboxane A2, the thienopyridine clopidogrel selectively inhibits adenosine diphosphate-induced platelet aggregation. The CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events) trial was a large, randomized, controlled trial of clopidogrel 75 mg versus aspirin 325 mg in patients with a history of ischaemic stroke, myocardial infarction (MI) or symptomatic peripheral arterial disease.[26] In the overall trial, there was a 0.5% absolute reduction in the composite endpoint of ischaemic stroke, MI or vascular death associated with clopidogrel and no significant difference in the risk of bleeding. However, subgroup analysis of patients who entered the study because of ischaemic stroke showed no significant difference between aspirin and clopidogrel. Current recommendations state that clopidogrel is acceptable for secondary prevention in patients with a history of ischaemic events, including those with stroke,[13,14,71] although its benefit over aspirin in ischaemic stroke patients may be modest. Because the mean age of subjects in the CAPRIE trial was 62.5 years, it is unclear how these results can be applied to the older population.
The effects of the combination of aspirin and clopidogrel for stroke prevention have also been studied in patients with prior stroke or TIA. The MATCH trial, which enrolled only patients with a history of ischaemic stroke or TIA, showed no benefit for the combination of aspirin and clopidogrel versus clopidogrel alone in preventing recurrent ischaemic stroke, MI, cardiovascular death or rehospitalization for acute ischaemia,[27] and the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilisation, Management, and Avoidance) trial failed to show a benefit for the combination of aspirin and clopidogrel versus aspirin alone on a similar composite endpoint in the overall study population of symptomatic (documented coronary artery disease, cerebrovascular disease or peripheral arterial disease) and asymptomatic (three or more atherothrombotic risk factors) patients.[28] In addition, the MATCH trial showed a significant increase in haemorrhagic strokes with aspirin and clopidogrel compared with clopidogrel alone. [27] Based on these results, the combination of aspirin plus clopidogrel is not routinely recommended for secondary stroke prevention.[13,14]
Dipyridamole, a phosphodiesterase inhibitor and nitric oxide carrier, represents another class of antiplatelet agent which, when used in its ER form in combination with low-dose aspirin, is an acceptable option for secondary prevention.[13,14,71] Results from the ESPS-2 (Second European Stroke Prevention Study)[29] and the ESPRIT (European/Australasian Stroke Prevention in Reversible Ischemia Trial)[30] showed that aspirin plus ER dipyridamole (ASA-ERDP) significantly reduced the absolute risk of the composite outcome (incidence of stroke or death in ESPS-2; incidence of death from all vascular causes, non-fatal stroke, non-fatal MI or major bleeding complication in ESPRIT) by 19% and 3%, respectively, compared with aspirin alone after TIA or stroke of presumed arterial origin. Subgroup analysis showed that patients aged >65 years had a similar or greater benefit from ASA-ERDP over aspirin compared with those aged ≤65 years in ESPRIT.[30] The recently completed PRoFESS (Prevention Regimen For Effectively avoiding Second Strokes) trial was designed to compare the efficacy of aspirin plus ER dipyridamole with that of clopidogrel monotherapy in patients with stroke.[31] The rates of recurrent stroke were similar in both groups (ASA-ERDP 9.0% vs clopidogrel 8.8% ; HR 1.01; 95% CI 0.92, 1.11), and therefore the trial did not meet the prespecified criteria for noninferiority for ASA-ERDP. The subgroup analysis based on age revealed no benefit in favour of either drug in patients aged ≥75 years. However, there was a significant increase in intracranial haemorrhages in the ASA-ERDP group (4.1 % vs 3.6% in the clopidogrel group; HR 1.15; 95% CI 1.00, 1.32). Whether the subjects with intracranial haemorrhages were older than those without was not reported.
The oldest subsets of the population appear to have a comparable relative risk reduction to that observed in younger patients and given their high risk of secondary vascular events, may derive a greater absolute risk reduction. Unfortunately, CAPRIE, ESPS-2 and ESPRIT did not provide data related to bleeding risk in this age group. However, even if we assume that the benefit of stroke risk reduction is partially offset by an increased bleeding risk in the oldest-old, the risk-benefit ratio favours antiplatelet therapy for most patients.
2.8 Antithrombotic Therapy for Cardioembolic Stroke
The most important cause of cardioembolic stroke in older adults is atrial fibrillation (AF), which accounts for about 50% of all cardioemboli.[71] AF increases the risk for stroke by about 5-fold[74] and is associated with poor outcomes[75] and higher costs;[76,77] therefore, aggressive treatment of AF is essential for both primary and secondary stroke prevention.
Clinical trials of warfarin versus aspirin for AF have determined that the risk of stroke in individuals with this condition differs depending on the presence of specific risk factors, which can be measured using several different validated scores.[78,79] Because oral anticoagulant therapy with warfarin (target INR 2.5; range 2.0–3.0) decreases the relative risk of stroke by about 62% versus 22% reduction with aspirin,[80] these risk scores can be used to determine the absolute benefit of warfarin treatment and aid the decision-making process in the setting of primary or secondary prevention.
For secondary prevention in AF patients with a history of stroke or TIA, treatment with oral anticoagulation is recommended unless major contraindications are present.[14] Most AF prevention trials have measured ischaemic stroke as a primary outcome, with the only clinical trial focusing solely on secondary prevention in AF being the EAFT (European Atrial Fibrillation Trial).[81] In this trial, there was a significant absolute risk reduction in stroke events with oral anticoagulation versus aspirin or placebo (anticoagulation 4 % vs placebo 12% ; HR 0.34; 95% CI 0.36, 0.79). Combining these results with those of other AF trials, individual patient meta-analyses have shown consistently better prevention of ischaemic stroke with oral anticoagulation over aspirin or placebo (table V). [82,83]
Table V.
Meta-analyses of antithrombotic therapies in patients with atrial fibrillation: pooled data from randomized trials (reproduced from Singer et al.,[82] with permission)
| Treatment comparison | RRR for ischaemic stroke (95% CI) |
|---|---|
| Adjusted-dose OAC vs no antithrombotic therapy[84] | 68% (50, 79) |
| ASA vs no antithrombotic therapy[85] | 21% (0, 38) |
| Adjusted-dose OAC vs ASA[83] | 52% (37, 63) |
ASA = aspirin (acetylsalicylic acid); OAC = oral anticoagulation; RRR = relative risk reduction.
Once warfarin treatment is initiated, it is critical to maintain INRs in the therapeutic range to achieve the maximum benefit; in one study, patients who were receiving subtherapeutic doses of warfarin (INR 1.5–1.9) at the time of admission for stroke had a 3.4-fold higher 30-day mortality than patients who were in the therapeutic range (INR 2.0–3.0).[86] The timing of initiation or reinitiation of warfarin for secondary prevention in patients with AF and a recent stroke is difficult because of the risk of haemorrhage. Although there are no studies to guide this decision, the EAFT compared haemorrhage rates in patients with warfarin initiated within 2 weeks and after 2 weeks of the acute stroke and found no difference.[81] Therefore, the ACCP Guidelines suggest 2 weeks is safe, although it is reasonable to wait longer for patients with large strokes.[15]
Alternative anticoagulants are also currently being tested in randomized controlled trials. In the ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial, rivaroxaban, a direct factor Xa inhibitor is being compared with adjusted-dose warfarin for prevention of stroke and thromboembolic events in 14 000 patients with non-valvular AF.[87] In addition, the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial is a double blind non-inferiority trial of apixaban versus adjusted dose warfarin in 15 000 patients with AF and at least one additional risk factor for stroke.[88]
For patients who are not candidates for warfarin, newer antiplatelet therapies are being tested to determine their benefit in reducing stroke risk. Although the warfarin arm of the ACTIVE (Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events) trial, ACTIVE-W, was discontinued because of the superiority of warfarin,[89] the aspirin arm (ACTIVE-A) is ongoing.[90] This trial is designed to assess whether dual antiplatelet therapy with clopidogrel 75 mg/day and aspirin 100 mg/day is superior to aspirin 100 mg/day monotherapy in preventing stroke, non-CNS systemic embolism, MI or vascular death in high-risk patients with AF during 3 years of follow-up.[89] The irbesartan arm (ACTIVE-I), in which patients from ACTIVE-W and ACTIVE-A were randomized in a factorial manner to receive either the angiotensin II type 1 receptor antagonist irbesartan or placebo, is also ongoing.[90]
While the overall AF population is under-treated with warfarin, this is especially true for older adults,[91–93] for whom therapy is often withheld because of a history or risk of falling.[92] A study of Medicare beneficiaries who were at high risk for falls and were anticoagulated for AF at hospital discharge showed that the rate of traumatic intracranial haemorrhage was more than twice as high as in those not at high risk for falls (2.0/100 patient-years; 95% CI 1.3, 3.1 vs 0.34 /100 patient-years in other patients; 95% CI 0.27, 0.45; p < 0.0001).[94] The CHADS2 risk score was used to assess future stroke risk, which assigns 1 point each for the presence of congestive heart failure, hypertension, age >75 years, and diabetes, and 2 points for history of stroke or TIA.[78] In patients discharged on anticoagulation with a CHADS2 risk score of 2–6, and therefore at high risk of stroke, there was still a protective benefit with anticoagulation for a composite outcome of hospitalization for stroke, any haemorrhage, MI or out-of-hospital death.[94] Therefore, many patients at risk for falls would benefit from warfarin therapy, and it is important for clinicians to carefully discuss the risks and benefits of anticoagulation with their older patients.
If AF is not the cause of an embolic-appearing stroke, other cardioembolic causes of stroke should also be considered, especially if no large vessel source has been identified. Causes with a high risk of recurrence include mitral stenosis, prosthetic mechanical valves, recent MI, left ventricular thrombus, infective endocarditis and dilated cardiomyopathies.[71] The risks and benefits of anticoagulation are likely to vary substantially in these settings, and the guideline recommendations cannot replace individualized clinical judgement.
2.9 Extracranial Carotid Stenosis
Symptomatic carotid stenosis carries a high rate of recurrent stroke. As shown in the NASCET (North American Symptomatic Carotid Endarterectomy Trial) study, the risk of ipsilateral ischaemic stroke in patients with symptomatic carotid stenosis >70% was 26% over 2 years, whereas surgical treatment with carotid endarterectomy (CEA) was associated with a stroke rate of 9% (absolute risk reduction, 17% ; number needed to treat to prevent one stroke = 6).[95] This trial, along with the ECST (European Carotid Surgery Trial)[96] and the Veterans Affairs Cooperative Studies Program, [97] has clearly defined our current practice of recommending CEA for patients with symptomatic, severe stenosis (70–99%).[14]
While the overall population of patients in NASCET who had moderate carotid stenosis (50–69%) did not benefit from CEA, patients aged ≥75 years did. Similarly, a pooled analysis of both NASCET and ECST showed that patients aged ≥75 years and with ≥50% symptomatic stenosis benefited from CEA, with an absolute risk reduction over 5 years of 19.2% (95% CI 10.2, 28.2).[98] CEA is therefore a consideration for symptomatic patients aged ≥75 years with moderate stenosis, provided there is an exceptionally low surgical complication rate of <4%.[95] However, an analysis of a large Medicare database from 1992 to 1993 revealed an increased 30-day mortality risk following CEA with each 5-year increase in age; 1.2% for those aged 65–69 years, 2.46% for those aged 80–84 years and 3.6% for those aged ≥85 years (p = 0.001).[99] These mortality rates were also lowest in the institutions that enrolled patients in NASCET and ACAS (Asymptomatic Carotid Artery Study) [100] compared with non-trial institutions, and in high-volume versus low-volume centres. In addition, rates of 30-day mortality risk following CEA were higher in trial institutions according to the ‘real-world’ database than rates reported in the randomized trials for these same institutions, indicating careful selection of subjects for trials.[99]
CEA for asymptomatic carotid stenosis has been a topic of intense debate. The Veterans Affairs Cooperative Study Group[101] and ACAS,[100] as well as the more recently completed European ACST (Asymptomatic Carotid Surgery Trial),[102] all showed a significant but small benefit from CEA for asymptomatic carotid stenosis. It is important to remember that to participate in these trials, surgeons must have demonstrated a very low complication rate, such that the overall benefit in reducing the rate of stroke during follow-up was not overshadowed by the risk of catheter angiography plus the perioperative complication rate. Therefore, CEA for asymptomatic carotid artery stenosis between 60% and 100% is recommended when performed by a surgeon with a complication rate of <3%.[103]
A systematic review of age- and gender-associated risks associated with CEA showed that women had a higher rate of operative stroke and death than men (odds ratio [OR] 1.31; 95% CI 1.17, 1.47). [104] Although operative mortality was increased in older patients (OR 1.50; 95% CI 1.26, 1.78), the risk of non-fatal stroke was not significantly increased. Unfortunately, this review did not include any other co-morbidities that could guide decision making for surgery. Therefore, based on well established operative risks, for the oldest subset of the population whose life expectancy, and therefore cumulative risk for stroke, is lower because of advanced age or co-morbidities, the operative risks of CEA for asymptomatic carotid stenosis may not outweigh the potential benefit.
An alternative to CEA for patients with symptomatic or asymptomatic carotid stenosis is carotid angioplasty and stenting (CAS). Unfortunately, despite its popularity, data from clinical trials concerning the efficacy and safety of CAS are lacking or contradictory. For example, two randomized clinical trials failed to demonstrate the noninferiority of CAS compared with CEA in treating patients with symptomatic carotid stenosis,[105,106] and one of these trials was stopped prematurely because of safety and futility issues associated with CAS.[105] In contrast, the SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) trial showed that in patients deemed to be high-risk candidates for CEA, regardless of whether or not the stenosis was symptomatic, CAS was not inferior to CEA.[107] Even though the endpoint for this trial was a combination of stroke, death or MI in 30 days, death from neurological causes or ipsilateral stroke between 31 days and 1 year, there was no evidence of any benefit for stroke as an outcome in any of the subgroups in this trial.
The results of the SAPPHIRE trial introduce a major evidence gap in the oldest-old who need treatment for carotid stenosis. Specifically, if patients are excluded from CEA because of high risk (oldest-old age groups) and are only referred for CAS, then the complication rates would be higher in this population by definition. Other studies have shown that in patients aged ≥75 years, CAS was associated with a significant increase in the 30-day stroke risk compared with CEA.[108] Also, in the ongoing CREST (Carotid Revascularisation Endarterectomy vs Stent Trial), the risk of stroke or death increased significantly with increasing age, especially in those aged ≥80 years.[109] Several anatomical factors have been hypothesized to cause the increased number of adverse effects related to CAS procedures in the elderly, including aortic arch elongation and calcification, as well as carotid tortuosity.[110] Therefore, based on high risk for early mortality with either procedure in patients aged >80 years, there are currently no data to support CEA over CAS (or vice versa), and more studies in this population are needed.
3. Special Challenges in Elderly Patients
3.1 Adverse Drug Events
Older adults are particularly susceptible to adverse drug events because of a higher risk for drug-drug and drug-disease interactions and toxicity arising from altered pharmacokinetics. Cardiovascular medications are consistently identified as one of the leading causes of adverse drug events in older adults, and are a frequent cause of emergency department visits.[111,112] While patient-reported adverse effects of anti-hypertensive agents such as β-adrenoceptor antagonists are actually lower in older adults compared with younger patients,[113] conduction system abnormalities and use of multiple cardiovascular agents are more prevalent and can lead to symptomatic bradyarrhythmias.[114] Less well recognized is that withdrawal of cardiovascular medications is also associated with adverse drug events, occurring approximately 26% of the time.[115] Although results are inconsistent as to whether age itself increases the risk of bleeding with warfarin therapy,[116,117] older patients are more likely to be taking other medications that may interact with warfarin, or to have underlying disease processes that increase bleeding risk. Thus, it is important to adjust all medications slowly and monitor for adverse effects diligently when either initiating or stopping secondary prevention therapies.
3.2 Dementia
Stroke is a major risk factor for cognitive decline. In a prospectively followed cohort of patients with a mean age of 75 years determined to be cognitively and neurologically normal at baseline, stroke increased the odds of dementia by 5.6-fold (95% CI 2.76, 11.4) and the odds of conversion of a mild cognitive impairment to dementia by 12-fold (95% CI 1.5, 99).[118,119] Moreover, cognitive and functional outcomes are substantially worse for patients with underlying Alzheimer’s type dementia who also experience a stroke.[120] Therefore, ongoing screening for worsening cognitive and functional impairment is warranted after stroke.
Cerebral amyloid angiopathy (CAA), a condition caused by deposition of β-amyloid protein in the vasculature, predisposes persons to both dementia and intracranial haemorrhage. The prevalence of this condition increases dramatically in older adults from virtually nonexistent in persons <50 years of age to >50% in persons aged >90 years.[121,122] Intracerebral haemorrhage due to CAA typically occurs in a lobar distribution, as opposed to the basal ganglia distribution that occurs with hypertensive intracerebral haemorrhage. In addition, anticoagulant and antiplatelet therapies increase haemorrhage risk in these patients[123–126] and should therefore be avoided. Detection of subclinical cerebral microbleeds is suggestive of underlying CAA, which can be diagnosed with the use of T2*-weighted gradient-refocused magnetic resonance imaging.[127] Although there is no evidence to support this approach, this type of imaging could be used to guide decision making for antithrombotic use in patients who may have evidence of microbleeds and therefore are at high risk of haemorrhagic stroke with these drugs.
3.3 Osteoporosis
Osteoporosis has been shown both to increase the risk of stroke and to become worse after stroke.[128] Loss of bone density is common after stroke, particularly on the ipsilateral side, and increases the risk of fracture, especially that of the hip.[129] Treatment of post-menopausal women with the bisphosphonate risedronic acid during the acute period following stroke reduced the odds of hip fracture in post-menopausal women by 7-fold[130] and in men aged ≥65 years by 5-fold,[131] and vitamin B12 and folate supplementation may decrease the risk of hip fracture. The results of these studies draw attention to the need to assess bone mineral density in all stroke patients and to aggressively treat osteoporosis both before and after stroke.[132,133]
3.4 Arthritis
Many older patients suffer from arthritis and require NSAIDs or cyclooxygenase (COX)-2 inhibitors to maintain their quality of life. There is evidence to suggest that ibuprofen, but not rofecoxib or diclofenac, interferes with the anti-platelet activities of aspirin, and therefore, its ability to protect against stroke and MI.[134,135] In older patients with stroke and arthritis, the choice of anti-inflammatory and antiplatelet agents should be made carefully. Caution is also required when NSAIDs and COX-2 inhibitors are used in conjunction with aspirin and warfarin because of an increased risk of gastrointestinal bleeding and with antihypertensive agents because of an increased chance of losing BP control. Overall, the relative risks and benefits of NSAIDs and COX-2 inhibitors should be carefully considered before they are prescribed to older stroke patients.
3.5 Depression
Up to 34% of stroke patients, regardless of age or sex, experience depression, a serious problem that requires treatment.[136] Although selective serotonin reuptake inhibitors (SSRIs) are commonly used to treat post-stroke depression, their use has been linked to an excess risk of upper gastrointestinal and perioperative bleeding, especially in patients taking aspirin and SSRIs.[137] There is also a recognized interaction between SSRIs and warfarin that can prolong the effect of warfarin and increase bleeding risk.[138] When NSAIDs cannot be avoided, other options include use of non-SSRI medications for depression,[139] use of an SSRI that has a lower receptor affinity that may decrease the bleeding risk,[140] or use of proton pump inhibitors or high-dose histamine H2 receptor antagonists to reduce the risk of NSAID-related peptic ulcer disease. [141]
3.6 Deciding When to Discontinue Secondary Prevention Strategies
Although studies suggest that older stroke patients are often not offered secondary prevention strategies when indicated,[3,10] it is also clear that secondary prevention is inappropriate for some older patients. For patients who have had a recent stroke, the very high risk of additional events in the next 6 months makes use of secondary prevention strategies reasonable, at least acutely. In addition, there is evidence that withdrawal of lipid-lowering therapy in patients with acute coronary syndromes can precipitate vascular events,[142] and withdrawal of statins in the acute stroke period significantly worsens outcome at 90 days.[143] However, for patients with a remote history of stroke, most of the studies cited in table III reported a reduction in secondary events only after ≥2 years of therapy, suggesting that patients with more limited life expectancies as a result of other co-morbidities or very advanced age may not benefit once they have passed the immediate post-stroke period. Although objective data are lacking, it is reasonable to withdraw secondary prevention therapies when transitioning to a palliative care approach unless there has been a recent acute vascular event or therapy cessation would cause psychological distress to the patient.[144,145] Specific clinical situations such as gastrointestinal bleeding or frequent falls require careful, ongoing discussion about the competing risks and benefits of secondary prevention therapies.
4. Secondary Prevention Quality Improvement Initiatives
One of the greatest barriers to effective implementation of secondary preventive strategies after an atherothrombotic event appears to be related to the absence of a systemized approach to discharge planning.[146] The PROTECT (Preventing Recurrence of Thromboembolic Events through Coordinated Treatment) programme, conducted in a single academic hospital with a primary stroke service, sought to promote initiation of certain guideline-directed secondary preventive measures during acute hospitalization for an ischaemic cerebrovascular event. Compared with a pre-PROTECT patient cohort, discharge utilization and 90-day adherence rates were significantly enhanced with statin and anti-hypertensive therapies, implementation of long-term antithrombotic therapy approached 100%, and lifestyle-intervention goals were discussed with all patients.[147] A multicentre, ongoing study of secondary prevention adherence is the Adherence Evaluation After Ischemic Stroke-Longitudinal (AVAIL) registry.[148] AVAIL was designed to determine barriers to adherence following hospitalization for acute stroke, taking into account age, sex, socioeconomic status, disability, depression and quality of life. Another impediment to effective implementation is a lack of knowledge concerning the guidelines. The AHA’s Get With the Guidelines (GWTG)-Stroke initiative is designed to educate in-hospital healthcare providers about available treatments and prevention guidelines and provide tools to increase and streamline their implementation.[149]
5. Conclusion
Although more direct evidence for secondary prevention after ischaemic stroke in the oldest subset of the population is needed, such patients are potential candidates for the full range of strategies recommended for younger patients. Factors such as stroke severity, functional status, competing co-morbidities and the patient’s goals of care should take precedence over age when planning treatment and rehabilitation after stroke. The oldest patients are at higher risk for adverse drug events, drug-drug interactions and drug-disease interactions, and require a more thoughtful approach to prescribing and monitoring than younger stroke patients. There is substantial room for improvement in the use of medications that may improve prognosis after stroke in older adults; ongoing quality improvement initiatives such as AVAIL and GWTG-Stroke will hopefully improve treatment, and therefore outcomes, of stroke survivors.
Acknowledgments
This manuscript was written and edited by the authors, who take full responsibility for its content. The authors wish to thank Susan Abulhawa and Melanie Leiby, PhD, for their editorial assistance in coordinating revisions and creating tables. This editorial assistance was funded by the Bristol-Myers Squibb/Sanofi Pharmaceutical Partnership. The authors did not receive any remuneration for this work. Cheryl Bushnell receives research salary support from Bristol-Myers Squibb for work as an investigator on the AVAIL stroke registry.
Footnotes
Cathleen Colón-Emeric has no conflicts of interest that are directly relevant to the content of this review.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117 (4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Garrett E, Klag MJ, et al. Trends in stroke prevalence between 1973 and 1991 in the US population 25 to 74 years of age. Stroke. 2002;33 (5):1209–13. doi: 10.1161/01.str.0000015031.57955.d1. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan RC, Tirschwell DL, Longstreth WT, Jr, et al. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology. 2005;65 (6):835–42. doi: 10.1212/01.wnl.0000176058.09848.bb. [DOI] [PubMed] [Google Scholar]
- 4.Clark TG, Murphy MF, Rothwell PM. Long term risks of stroke, myocardial infarction, and vascular death in “low risk” patients with a non-recent transient ischaemic attack. J Neurol Neurosurg Psychiatry. 2003;74 (5):577–80. doi: 10.1136/jnnp.74.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coull AJ, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services [abstract] BMJ. 2004;328 (7435):326. doi: 10.1136/bmj.37991.635266.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Wijk I, Kappelle LJ, van Gijn J, et al. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet. 2005;365 (9477):2098–104. doi: 10.1016/S0140-6736(05)66734-7. [DOI] [PubMed] [Google Scholar]
- 7.Touze E, Varenne O, Chatellier G, et al. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke. 2005;36 (12):2748–55. doi: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 8.Hardie K, Hankey GJ, Jamrozik K, et al. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth Community Stroke Study. Stroke. 2004;35 (3):731–5. doi: 10.1161/01.STR.0000116183.50167.D9. [DOI] [PubMed] [Google Scholar]
- 9.Samsa G, Bian J, Lipscomb J, et al. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–49. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Steg PG, Ohman EM, et al. International pre-valence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295 (2):180–9. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 11.Hankey GJ, Jamrozik K, Broadhurst RJ, et al. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke. 2002;33 (4):1034–40. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- 12.Hankey GJ, Spiesser J, Hakimi Z, et al. Time frame and predictors of recovery from disability following recurrent ischemic stroke. Neurology. 2007;68 (3):202–5. doi: 10.1212/01.wnl.0000250327.73031.54. [DOI] [PubMed] [Google Scholar]
- 13.Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA Recommendations for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack. Stroke. 2008;39:1647–52. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association /American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37 (2):577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Amarenco P, Easton JD, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8) 2008;133:630–69. doi: 10.1378/chest.08-0720. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt G, Schunemann HJ, Cook D, et al. Applying the grades of recommendation for antithrombotic and thrombolytic therapy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126 (3 Suppl):179S–87S. doi: 10.1378/chest.126.3_suppl.179S. [DOI] [PubMed] [Google Scholar]
- 17.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358 (9287):1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 18.Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension) Lancet. 1991;338 (8778):1281–5. doi: 10.1016/0140-6736(91)92589-t. [DOI] [PubMed] [Google Scholar]
- 19.Perry HM, Jr, Davis BR, Price TR, et al. Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 2000;284 (4):465–71. doi: 10.1001/jama.284.4.465. [DOI] [PubMed] [Google Scholar]
- 20.Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens. 2003;21 (12):2409–17. doi: 10.1097/00004872-200312000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358 (18):1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 22.Collins R, Armitage J, Parish S, et al. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363 (9411):757–67. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 23.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355 (6):549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360 (9346):1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 25.Hey-Hadavi JH, Kuntze E, Luo D, et al. Tolerability of atorvastatin in a population aged > or = 65 years: a retrospective pooled analysis of results from fifty randomized clinical trials. Am J Geriatr Pharmacother. 2006;4 (2):112–22. doi: 10.1016/j.amjopharm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 26.CAPRIE Study Group. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348 (9038):1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 27.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364 (9431):331–7. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354 (16):1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 29.Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study: II. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143 (1–2):1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 30.Halkes PH, van Gijn J, Kappelle LJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367 (9523):1665–73. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 31.Sacco RL, Diener H-C, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359 (12):1238–51. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawes CM, Bennett DA, Feigin VL, et al. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35 (3):776–85. doi: 10.1161/01.STR.0000116869.64771.5A. [DOI] [PubMed] [Google Scholar]
- 33.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42 (6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers J, MacMahon S. Perindopril pROtection aGainst REcurrent Stroke Study (PROGRESS): interpretation and implementation. J Hypertens Suppl. 2003;21 (5):S9–14. doi: 10.1097/00004872-200306005-00003. [DOI] [PubMed] [Google Scholar]
- 35.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265 (24):3255–64. [PubMed] [Google Scholar]
- 36.Staessen JA, Fagard R, Thijs L, et al. Subgroup and per-protocol analysis of the randomized European Trial on Isolated Systolic Hypertension in the Elderly. Arch Intern Med. 1998;158 (15):1681–91. doi: 10.1001/archinte.158.15.1681. [DOI] [PubMed] [Google Scholar]
- 37.Bulpitt C, Fletcher A, Beckett N, et al. Hypertension in the Very Elderly Trial (HYVET): protocol for the main trial. Drugs Aging. 2001;18 (3):151–64. doi: 10.2165/00002512-200118030-00001. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson PM. Reducing the risk of stroke in elderly patients with hypertension: a critical review of the efficacy of antihypertensive drugs. Drugs Aging. 2005;22 (6):517–24. doi: 10.2165/00002512-200522060-00005. [DOI] [PubMed] [Google Scholar]
- 39.Oates DJ, Berlowitz DR, Glickman ME, et al. Blood pressure and survival in the oldest old. J Am Geriatr Soc. 2007;55 (3):383–8. doi: 10.1111/j.1532-5415.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 40.Amarenco P, Tonkin AM. Statins for stroke prevention: disappointment and hope. Circulation. 2004;109(23 Suppl 1):III44–9. doi: 10.1161/01.CIR.0000131518.25959.8F. [DOI] [PubMed] [Google Scholar]
- 41.Gorelick PB. Stroke prevention therapy beyond anti-thrombotics: unifying mechanisms in ischemic stroke pathogenesis and implications for therapy: an invited review. Stroke. 2002;33 (3):862–75. doi: 10.1161/hs0302.103657. [DOI] [PubMed] [Google Scholar]
- 42.MRC/BHF Heart Protection Study of cholesterol lowering !with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360 (9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 43.4S Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994 Nov 19;344(8934):1383–9. [PubMed] [Google Scholar]
- 44.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339 (19):1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 45.Amarenco P, Labreuche J, Lavallee P, et al. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35 (12):2902–9. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 46.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364 (9435):685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 47.Plehn JF, Davis BR, Sacks FM, et al. Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. The Care Investigators. Circulation. 1999;99 (2):216–23. doi: 10.1161/01.cir.99.2.216. [DOI] [PubMed] [Google Scholar]
- 48.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361 (9364):1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 49.National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106 (25):3143–421. [PubMed] [Google Scholar]
- 50.Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70:2364–70. doi: 10.1212/01.wnl.0000296277.63350.77. [DOI] [PubMed] [Google Scholar]
- 51.Eaton CB, Lapane KL, Murphy JB, et al. Effect of statin (HMG-Co-A-Reductase Inhibitor) use on 1-year mortality and hospitalization rates in older patients with cardioascular disease living in nursing homes. J Am Geriatr Soc. 2002;50 (8):1389–95. doi: 10.1046/j.1532-5415.2002.50360.x. [DOI] [PubMed] [Google Scholar]
- 52.Muldoon MF, Ryan CM, Sereika SM, et al. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117 (11):823–9. doi: 10.1016/j.amjmed.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 53.Padala KP, Padala PR, Potter JF. Simvastatin-induced decline in cognition. Ann Pharmacother. 2006;40 (10):1880–3. doi: 10.1345/aph.1H014. [DOI] [PubMed] [Google Scholar]
- 54.Agostini JV, Tinetti ME, Han L, et al. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55 (3):420–5. doi: 10.1111/j.1532-5415.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 55.Bernick C, Katz R, Smith NL, et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology. 2005;65 (9):1388–94. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 56.Summers MJ, Oliver KR, Coombes JS, et al. Effect of atorvastatin on cognitive function in patients from the Lipid Lowering and Onset of Renal Disease (LORD) trial. Pharmacotherapy. 2007;27 (2):183–90. doi: 10.1592/phco.27.2.183. [DOI] [PubMed] [Google Scholar]
- 57.Parale GP, Baheti NN, Kulkarni PM, et al. Effects of atorvastatin on higher functions. Eur J Clin Pharmacol. 2006;62 (4):259–65. doi: 10.1007/s00228-005-0073-z. [DOI] [PubMed] [Google Scholar]
- 58.Winblad B, Jelic V, Kershaw P, et al. Effects of statins on cognitive function in patients with Alzheimer’s disease in galantamine clinical trials. Drugs Aging. 2007;24 (1):57–61. doi: 10.2165/00002512-200724010-00004. [DOI] [PubMed] [Google Scholar]
- 59.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289 (13):1681–90. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 60.Maddrey WC. Drug-induced hepatotoxicity: 2005. J Clin Gastroenterol. 2005;39 (4 Suppl 2):S83–9. doi: 10.1097/01.mcg.0000155548.91524.6e. [DOI] [PubMed] [Google Scholar]
- 61.Clarke R, Daly L, Robinson K, et al. Hyperhomocys- teinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324 (17):1149–55. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 62.Perry IJ, Refsum H, Morris RW, et al. Prospective study of serum total homocysteine concentration and risk of stroke in middle-aged British men. Lancet. 1995;346 (8987):1395–8. doi: 10.1016/s0140-6736(95)92407-8. [DOI] [PubMed] [Google Scholar]
- 63.Tanne D, Haim M, Goldbourt U, et al. Prospective study of serum homocysteine and risk of ischemic stroke among patients with preexisting coronary heart disease. Stroke. 2003;34 (3):632–6. doi: 10.1161/01.STR.0000060203.58958.35. [DOI] [PubMed] [Google Scholar]
- 64.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291 (5):565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 65.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354 (15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 66.Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47 (6):1108–16. doi: 10.1016/j.jacc.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 67.Spence D, Bang H, Chambless L, et al. Vitamin Intervention for Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36:2404–9. doi: 10.1161/01.STR.0000185929.38534.f3. [DOI] [PubMed] [Google Scholar]
- 68.Bostom A, Selhub J, Jacques P, et al. Power shortage: clinical trials testing the “homocysteine hypothesis” against a background of folic acid fortified cereal grain flour. Ann Intern Med. 2002;137:295–6. doi: 10.7326/0003-4819-135-2-200107170-00014. [DOI] [PubMed] [Google Scholar]
- 69.Sato Y, Honda Y, Iwamoto J, et al. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA. 2005;293 (9):1082–8. doi: 10.1001/jama.293.9.1082. [DOI] [PubMed] [Google Scholar]
- 70.Ives S, Heuschmann P, Wolfe C, et al. Patterns of smoking cessation in the first 3 years after stroke: the South London Stroke Register. Eur J Cardiovasc Prev Rehabil. 2008;15:329–35. doi: 10.1097/HJR.0b013e3282f37a58. [DOI] [PubMed] [Google Scholar]
- 71.Albers GW, Amarenco P, Easton JD, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126 (3 Suppl):483S–512S. doi: 10.1378/chest.126.3_suppl.483S. [DOI] [PubMed] [Google Scholar]
- 72.Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345 (20):1444–51. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 73.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324 (7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22 (8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 75.Kammersgaard LP, Jorgensen HS, Reith J, et al. Short- and long-term prognosis for very old stroke patients. The Co-penhagen Stroke Study. Age Ageing. 2004;33 (2):149–54. doi: 10.1093/ageing/afh052. [DOI] [PubMed] [Google Scholar]
- 76.Bruggenjurgen B, Rossnagel K, Roll S, et al. The impact of atrial fibrillation on the cost of stroke: the Berlin Acute Stroke Study. Value Health. 2007;10 (2):137–43. doi: 10.1111/j.1524-4733.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 77.Luengo-Fernandez R, Gray AM, Rothwell PM. Population-based study of determinants of initial secondary care costs of acute stroke in the United Kingdom. Stroke. 2006;37 (10):2579–87. doi: 10.1161/01.STR.0000240508.28625.2c. [DOI] [PubMed] [Google Scholar]
- 78.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285 (22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 79.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290 (8):1049–56. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 80.Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131 (7):492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 81.European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255–62. [PubMed] [Google Scholar]
- 82.Singer D, Albers GW, Dalen J, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8) 2008;133:546S–92S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 83.van Walraven C, Hart R, Singer D, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–8. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 84.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57. [PubMed] [Google Scholar]
- 85.Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation: analysis of pooled data from 3 randomized trials. Arch Intern Med. 1997;157:1237–40. [PubMed] [Google Scholar]
- 86.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349 (11):1019–26. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 87.Bayer. ClinicalTrials.gov. US National Institutes of Health; [Accessed 2008 Feb 24]. Randomized, double-blind study comparing once daily oral rivaroxaban with adjusted-dose oral warfarin for the prevention of stroke in subjects with non-valvular atrial fibrillation [ClinicalTrials.gov identifier NCT00403767] [online]. Available from URL: http://www.clinicaltrials.gov. [Google Scholar]
- 88.Bristol-Myers Squibb. ClinicalTrials.gov. US National Institutes of Health; [Accessed 2008 Feb 24]. Apixaban for the prevention of stroke in subjects with atrial fibrillation [ClinicalTrials.gov identifier NCT00412984] [online]. Available from URL: http://www.clinicaltrials.gov. [Google Scholar]
- 89.Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367 (9526):1903–12. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 90.Sanofi-aventis Bristol-Myers Squibb. [Accessed 2007 Dec 17];Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE) [online] Available from URL: http://clinicaltrials.gov/ct2/show/NCT00249795.
- 91.Andersen KK, Olsen TS. Reduced poststroke mortality in patients with stroke and atrial fibrillation treated with anticoagulants: results from a Danish quality-control registry of 22,179 patients with ischemic stroke. Stroke. 2007;38 (2):259–63. doi: 10.1161/01.STR.0000254622.52483.03. [DOI] [PubMed] [Google Scholar]
- 92.Fornari LS, Calderaro D, Nassar IB, et al. Misuse of antithrombotic therapy in atrial fibrillation patients: frequent, pervasive and persistent. J Thromb Thrombolysis. 2007;23 (1):65–71. doi: 10.1007/s11239-006-9012-9. [DOI] [PubMed] [Google Scholar]
- 93.Hylek EM, D’Antonio J, Evans-Molina C, et al. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37 (4):1075–80. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 94.Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118 (6):612–7. doi: 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 95.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339 (20):1415–25. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 96.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351(9113):1379–87. [PubMed] [Google Scholar]
- 97.Mayberg MR, Wilson SE, Yatsu F, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid Stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA. 1991;266 (23):3289–94. [PubMed] [Google Scholar]
- 98.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363 (9413):915–24. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 99.Wennberg D, Lucas F, Birkmeyer J, et al. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279:1278–81. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 100.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273 (18):1421–8. [PubMed] [Google Scholar]
- 101.Hobson RW, 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid Stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328 (4):221–7. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 102.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363 (9420):1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 103.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113 (24):e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 104.Bond R, Rerkasam K, Cuffe R, et al. A systematic review of the associations between age and sex and the operative risks of carotid endarterectomy. Cerebrovasc Dis. 2005;20:69–77. doi: 10.1159/000086509. [DOI] [PubMed] [Google Scholar]
- 105.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355 (16):1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 106.Ringleb PA, Allenberg J, Bruckmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368 (9543):1239–47. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 107.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotidartery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351 (15):1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 108.Kastrup A, Schulz JB, Raygrotzki S, et al. Comparison of angioplasty and stenting with cerebral protection versus endarterectomy for treatment of internal carotid artery stenosis in elderly patients. J Vasc Surg. 2004;40 (5):945–51. doi: 10.1016/j.jvs.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 109.Hobson RW, 2nd, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40 (6):1106–11. doi: 10.1016/j.jvs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 110.Lam RC, Lin SC, DeRubertis B, et al. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg. 2007;45 (5):875–80. doi: 10.1016/j.jvs.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 111.Handler SM, Wright RM, Ruby CM, et al. Epidemiology of medication-related adverse events in nursing homes. Am J Geriatr Pharmacother. 2006;4 (3):264–72. doi: 10.1016/j.amjopharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 112.Budnitz D, Shehab N, Kegler S, et al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147:755–65. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 113.Hoffler K, Morgenstern H. Age dependence of therapy result and risk in the treatment of arterial hypertension. J Cardiovasc Pharmacol. 1990;16 (Suppl 5):S184–8. [PubMed] [Google Scholar]
- 114.Edoute Y, Nagachandran P, Svirski B, et al. Cardiovascular adverse drug reaction associated with combined beta-adrenergic and calcium entry-blocking agents. J Cardiovasc Pharmacol. 2000;35:556–9. doi: 10.1097/00005344-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 115.Graves T, Hanlon JT, Schmader KE, et al. Adverse events after discontinuing medications in elderly outpatients. Arch Intern Med. 1997;157 (19):2205–10. [PubMed] [Google Scholar]
- 116.Lindh J, Holm L, Dahl M, et al. Incidence and predictors of severe bleeding during warfarin treatment. J Thromb Thrombolysis. 2008;25:151–9. doi: 10.1007/s11239-007-0048-2. [DOI] [PubMed] [Google Scholar]
- 117.Beyth R, Landefeld C. Anticoagulants in older patients: a safety perspective. Drugs Aging. 1995;6:45–54. doi: 10.2165/00002512-199506010-00004. [DOI] [PubMed] [Google Scholar]
- 118.Gamaldo A, Moghekar A, Kilada S, et al. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006;67 (8):1363–9. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- 119.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277 (10):813–7. [PubMed] [Google Scholar]
- 120.Regan C, Katona C, Walker Z, et al. Relationship of vascular risk to the progression of Alzheimer disease. Neurology. 2006;67 (8):1357–62. doi: 10.1212/01.wnl.0000240129.46080.53. [DOI] [PubMed] [Google Scholar]
- 121.McCarron MO, Nicoll JA. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 2004;3 (8):484–92. doi: 10.1016/S1474-4422(04)00825-7. [DOI] [PubMed] [Google Scholar]
- 122.Ritter MA, Droste DW, Hegedus K, et al. Role of cerebral amyloid angiopathy in intracerebral hemorrhage in hypertensive patients. Neurology. 2005;64 (7):1233–7. doi: 10.1212/01.WNL.0000156522.93403.C3. [DOI] [PubMed] [Google Scholar]
- 123.Kandzari DE, Granger CB, Simoons ML, et al. Risk factors for intracranial hemorrhage and nonhemorrhagic stroke after fibrinolytic therapy (from the GUSTO-i trial) Am J Cardiol. 2004;93 (4):458–61. doi: 10.1016/j.amjcard.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 124.Leblanc R, Haddad G, Robitaille Y. Cerebral hemorrhage from amyloid angiopathy and coronary thrombolysis. Neurosurgery. 1992;31 (3):586–90. doi: 10.1227/00006123-199209000-00025. [DOI] [PubMed] [Google Scholar]
- 125.McCarron MO, Nicoll JA, Ironside JW, et al. Cerebral amyloid angiopathy-related hemorrhage. Interaction of APOE epsilon2 with putative clinical risk factors. Stroke. 1999;30 (8):1643–6. doi: 10.1161/01.str.30.8.1643. [DOI] [PubMed] [Google Scholar]
- 126.Sloan MA, Price TR, Petito CK, et al. Clinical features and pathogenesis of intracerebral hemorrhage after rt-PA and heparin therapy for acute myocardial infarction: the Thrombolysis in Myocardial Infarction (TIMI) II Pilot and Randomized Clinical Trial combined experience. Neurology. 1995;45 (4):649–58. doi: 10.1212/wnl.45.4.649. [DOI] [PubMed] [Google Scholar]
- 127.Walker D, Broderick D, Kotsenas A, et al. Routine use of gradient-echo MRI to screen for cerebral amyloid angiopathy in elderly patients. AJR Am J Roentgenol. 2004;182:1547–50. doi: 10.2214/ajr.182.6.1821547. [DOI] [PubMed] [Google Scholar]
- 128.Tanko LB, Christiansen C, Cox DA, et al. Relationship between osteoporosis and cardiovascular disease in post-menopausal women. J Bone Miner Res. 2005;20 (11):1912–20. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 129.Yavuzer G, Ataman S, Suldur N, et al. Bone mineral density in patients with stroke. Int J Rehabil Res. 2002;25 (3):235–9. doi: 10.1097/00004356-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 130.Sato Y, Iwamoto J, Kanoko T, et al. Risedronate therapy for prevention of hip fracture after stroke in elderly women. Neurology. 2005;64 (5):811–6. doi: 10.1212/01.WNL.0000152871.65027.76. [DOI] [PubMed] [Google Scholar]
- 131.Sato Y, Iwamoto J, Kanoko T, et al. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Arch Intern Med. 2005;165 (15):1743–8. doi: 10.1001/archinte.165.15.1743. [DOI] [PubMed] [Google Scholar]
- 132.Beaupre GS, Lew HL. Bone-density changes after stroke. Am J Phys Med Rehabil. 2006;85 (5):464–72. doi: 10.1097/01.phm.0000214275.69286.7a. [DOI] [PubMed] [Google Scholar]
- 133.Poole KE, Reeve J, Warburton EA. Falls, fractures, and osteoporosis after stroke: time to think about protection? Stroke. 2002;33 (5):1432–6. doi: 10.1161/01.str.0000014510.48897.7d. [DOI] [PubMed] [Google Scholar]
- 134.Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345 (25):1809–17. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 135.Farkouh ME, Greenberg JD, Jeger RV, et al. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66 (6):764–70. doi: 10.1136/ard.2006.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Linden T, Blomstrand C, Skoog I. Depressive disorders after 20 months in elderly stroke patients: a case-control study. Stroke. 2007;38 (6):1860–3. doi: 10.1161/STROKEAHA.106.471805. [DOI] [PubMed] [Google Scholar]
- 137.Weinrieb RM, Auriacombe M, Lynch KG, et al. Selective serotonin re-uptake inhibitors and the risk of bleeding. Expert Opin Drug Saf. 2005;4 (2):337–44. doi: 10.1517/14740338.4.2.337. [DOI] [PubMed] [Google Scholar]
- 138.Ament P, Bertolino J, Liszewski J. Clinically significant drug interactions. Am Fam Physician. 2000;61:1745–54. [PubMed] [Google Scholar]
- 139.Bhogal SK, Teasell R, Foley N, et al. Heterocyclics and selective serotonin reuptake inhibitors in the treatment and prevention of poststroke depression. J Am Geriatr Soc. 2005;53 (6):1051–7. doi: 10.1111/j.1532-5415.2005.53310.x. [DOI] [PubMed] [Google Scholar]
- 140.van Walraven C, Mamdani MM, Wells PS, et al. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ. 2001;323 (7314):655–8. doi: 10.1136/bmj.323.7314.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singh G, Triadafilopoulos G. Appropriate choice of proton pump inhibitor therapy in the prevention and management of NSAID-related gastrointestinal damage. Int J Clin Pract. 2005;59 (10):1210–7. doi: 10.1111/j.1368-5031.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 142.Heeschen C, Hamm CW, Laufs U, et al. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105 (12):1446–52. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 143.Blanco M, Nombela F, Castellanos M, et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69 (9):904–10. doi: 10.1212/01.wnl.0000269789.09277.47. [DOI] [PubMed] [Google Scholar]
- 144.Vollrath AM, Sinclair C, Hallenbeck J. Discontinuing cardiovascular medications at the end of life: lipid-lowering agents. J Palliat Med. 2005;8 (4):876–81. doi: 10.1089/jpm.2005.8.876. [DOI] [PubMed] [Google Scholar]
- 145.Stevenson J, Abernethy AP, Miller C, et al. Managing comorbidities in patients at the end of life. BMJ. 2004;329 (7471):909–12. doi: 10.1136/bmj.329.7471.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Polk DM, Watson K. Hospital discharge treatments after an acute event: evidence for a systems approach to prevention. Coron Artery Dis. 2006;17 (3):239–41. doi: 10.1097/00019501-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 147.Ovbiagele B, Saver JL, Fredieu A, et al. PROTECT: a coordinated stroke treatment program to prevent recurrent thromboembolic events. Neurology. 2004;63 (7):1217–22. doi: 10.1212/01.wnl.0000140493.83607.f1. [DOI] [PubMed] [Google Scholar]
- 148.Bushnell CD, Zimmer L, Schwamm L, et al. The Adherence eValuation After Ischemic Stroke Longitudinal (AVAIL) Registry: design, rationale, and baseline patient characteristics. Am Heart J. doi: 10.1016/j.ahj.2008.11.002. In press. [DOI] [PubMed] [Google Scholar]
- 149.Smaha LA. The American Heart Association Get With Guidelines program. Am Heart J. 2004;148 (5 Suppl):S46–8. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]