Abstract
The role of the gut microbiome in human health, and non-invasive measurement of gut dysbiosis are of increasing clinical interest. New high-throughput methods are required for the rapid measurement of gut microbial metabolites and to establish reference ranges in human populations. We used ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) -- positive and negative electrospray ionization modes, multiple reaction monitoring transitions -- to simultaneously measure three urinary metabolites (phenylacetylglutamine, 4-cresyl sulphate and hippurate) that are potential biomarkers of gut function, among multi-ethnic US men and women aged 40–59 from the INTERMAP epidemiologic study (n = 2000, two timed 24-hr urine collections/person). Metabolite concentrations were quantified via stable isotope labeled internal standards. The assay was linear in the ranges 1ng/mL (lower limit of quantification) to 1000ng/mL (phenylacetylglutamine and 4-cresyl sulfate) and 3ng/mL to 3000ng/mL (hippurate). These quantitative data provide new urinary reference ranges for population-based human samples: mean (standard deviation) 24-hr urinary excretion for phenylacetylglutamine was: 1283.0 (751.7) μmol/24-hr (men), 1145.9 (635.5) μmol/24-hr (women); for 4-cresyl sulphate, 1002.5 (737.1) μmol/24-hr (men), 1031.8 (687.9) μmol/24-hr (women); for hippurate, 6284.6 (4008.1) μmol/24-hr (men), 4793.0 (3293.3) μmol/24-hr (women). Metabolic profiling by UPLC-MS/MS in a large sample of free-living individuals has provided new data on urinary reference ranges for three urinary microbial co-metabolites, and demonstrates the applicability of this approach to epidemiological investigations.
Introduction
Investigations of the mammalian-microbial symbiotic axis have reinforced the wide involvement of the gut microbiome in human health and disease1,2. Contribution of the intestinal microbiota includes influences on human drug metabolism3 and in the etiology of several acute and chronic conditions including Clostridium difficile-associated disease4, gut dysbiosis5, obesity6,7, diabetes8 and cancer9. Metabolic profiling has emerged as a valuable platform for investigating metabolic regulation and the modulation of gut microfloral composition in response to physiological, pathological or genetic stimuli10,11, and can be expressed as a fingerprint of biochemical perturbations unique to the nature, site or mechanism of a particular biological process12,13. An important focus of metabolic profiling is on untargeted screening of the metabolome (the sum of the metabolites present in a given biological compartment such as a cell, biofluid or tissue) to generate multi-parametric profiles of biological samples14–16. This can be used both to classify samples according to presence or absence of a specific condition or disease, and to generate a list of candidate biomarkers associated with a given condition. However, these untargeted methods are seldom suited for the quantification of candidate biomarkers, and hence there is also a need for the parallel development of suitable high throughput quantitative assays for predetermined classes of molecule.
Recent advances in metabolic profiling include the Metabolome Wide Association Study (MWAS) screening approach, to detect novel metabolite-disease risk factor connections. In MWAS, associations between thousands of metabolic variables and a measure of a phenotype, e.g., blood pressure, are assessed to identify metabolites and pathways that may be involved in disease etiopathogenesis17–19. The INTERMAP (INTERnational collaborative study of MAcronutrients, micronutrients and blood Pressure) epidemiologic study20 demonstrated proof of principle of the MWAS approach, with discovery of novel urinary metabolites associated with blood pressure19. Several metabolites of gut microbial origin were identified. Given the emerging role of the gut microbiome in human health and disease, we followed-up the MWAS by developing an Ultra Performance Liquid Chromatography (UPLC) tandem triple quadrupole (TQ) mass spectrometry (MS/MS) strategy to quantify the most commonly observed urinary metabolites of gut microbial origin: phenylacetylglutamine, 4-cresyl sulphate and hippurate in the 24-hr urine specimens of the US participants of the INTERMAP Study.
Here, we describe the targeted UPLC-MS/MS assay we developed and validated for the detection and quantification of these three human urinary metabolites, and provide reference ranges for their 24-hr urinary excretion in free-living individuals.
Results and discussion
Our goal was to develop and validate a targeted high-throughput UPLC-TQ-MS/MS method for simultaneous detection and quantification of phenylacetylglutamine, 4-cresyl sulphate and hippurate, and to quantify concentrations of these gut microbial co-metabolites in 4,000 human urine specimens obtained from the INTERMAP Study. We used a Waters ACQUITY™ UPLC system coupled with a Waters Xevo™ triple quadrupole mass spectrometer (Waters Corporation, Manchester, UK). LC-MS was selected over other analytical platforms such as LC-UV and NMR, since it is a more appropriate, faster and convenient analytical platform for targeted quantitative analysis21.
Development of the targeted UPLC-MS/MS method
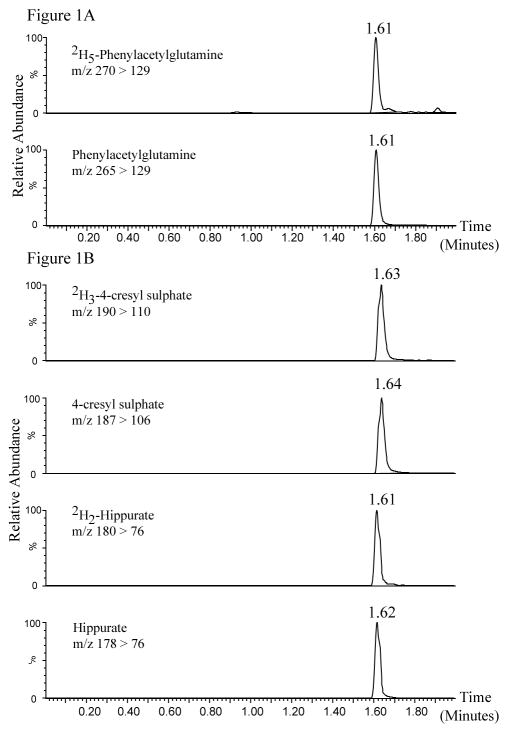
An initial method development process showed that the selected metabolites required detection in different ionization modes: phenylacetylglutamine was detected in positive electrospray ionization (ESI) mode (Figure 1A), while 4-cresyl sulphate and hippurate were detected in negative ESI mode (Figure 1B). Hippurate was also detected in positive ESI mode, but potential for interferences were apparent, so for this analysis negative mode acquisition was used. Consequently each urine specimen was injected twice in order to accommodate detection in both ESI modes.
Fig. 1.
UPLC/MS MRM chromatograms showing the retention times of the three human urinary microbial co-metabolites multiple transitions. (Figure 1A) Analytes and 2H internal standards detected in ESI(+) mode, (Figure 1B) analytes and 2H internal standards detected in ESI(−) mode. The x-axis shows the time in minutes and the y-axis the relative response (0–100%).
For detection of each analyte and deuterated internal standard, multiple reaction monitoring (MRM) transitions of a precursor ion [M+H]+ (for molecules ionizing in positive ion mode) or [M−H]− (for molecules ionizing in negative ion mode) into a characteristic fragment ion were recorded. The MRM transitions used for integration and quantification were: m/z 265>129 for phenylacetylglutamine, m/z 270>129 for 2H5-phenylacetylglutamine in positive ESI mode, and m/z 187>106 for 4-cresyl sulphate, m/z 190>110 for 2H3-4-cresyl sulphate, m/z 178>76 for hippurate, m/z 180>76 for 2H2-hippurate in negative ESI mode. ESI parameters, selection of MS/MS transitions, and conditions were established by direct infusion of each standard, followed by optimization utilizing in-built automated Waters software (IntelliStart™). Using the reported MRM transitions, no significant endogenous interfering peaks were observed in the blank wells for the analytes or the internal standards, indicating that there was no carry over or contamination.
The initial phase of the study also involved the optimization of analytical conditions. This involved measuring 1mg/mL stock solutions of each reference standard using the UPLC-MS/MS system, to achieve a short gradient and separation time. As a result, the total experimental time necessary to acquire the full set of concentration data was 5 minutes per urine specimen (including two injections per specimen run in both positive and negative ESI modes). Parameters such as gas flow rates, temperature and capillary voltages were investigated for each individual metabolite. However, the only parameters specific to each metabolite were cone voltage and the collision energy (Table 1).
Table 1.
UPLC-MS/MS conditions and retention times for the three analysed urinary metabolites and associated labelled standards. Separations were performed under isocratic conditions (95:5) of water plus 0.1% formic acid:acetonitrile plus 0.1% formic acid. A mobile phase gradient program was employed followed by re-equilibration to isocratic conditions giving a total run time of 2 minutes per injection, two injections per sample.
| Metabolite | Retention Time Window (Minutes) | ESIa Mode | MRMb Transition (m/z) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| Phenylacetylglutamine | 1.61–1.62 | Positive | 265>129 | 22.0 | 20.0 |
| 2H5-Phenylacetylglutamine | 1.61–1.62 | Positive | 270>129 | 24.0 | 20.0 |
| 4-cresyl sulfate | 1.63–1.64 | Negative | 187>106 | 34.0 | 22.0 |
| 2H3-4-cresyl sulfate | 1.63–1.64 | Negative | 190>110 | 34.0 | 22.0 |
| Hippurate | 1.61–1.62 | Negative | 178>76 | 30.0 | 20.0 |
| 2H2-Hippurate | 1.61–1.62 | Negative | 180>76 | 30.0 | 20.0 |
ESI = Electrospray Ionisation
MRM = Multiple Reaction Monitoring
Calibration Curve
We validated linearity using calibrators at concentrations 1, 3, 10, 30, 100, 300 and 1000ng/mL for phenylacetylglutamine and 4-cresyl sulphate, and 3, 10, 30, 100, 300, 1000 and 3000ng/mL for hippurate made up in water. It was not possible to make up the standard curves by spiking into the analytical matrix (urine), since the natural urinary abundances of these endogenous metabolites were high (especially hippurate). Thus a very high concentration of internal standard would have had to be spiked into the matrix, which would have saturated the source. Calibration curves were established for each analyte based on the ratio of integrated area under the peak of each calibrator divided by that of the spiked-in deuterated internal standard used to quantify each metabolite in each specimen. We found that for the best fit of the calibration curve a weighted (1/x) linear regression gave R2 > 0.997 for phenylacetylglutamine and 4-cresyl sulphate, whereas R2 > 0.997 was obtained for hippurate by fitting to a weighted (1/x2) second order regression (calculated by Waters TargetLynx™ 4.1 software). Acceptance criteria were <15% deviation of calibrators from nominal concentrations (<20% at the LLOQ).
Method Validation
Additional experiments were carried out to validate the developed method according to published US Food and Drug Administration (FDA) guidelines22. To evaluate imprecision and recovery (inaccuracy), we analyzed multi-analyte QC samples at three concentration levels: low (3ng/ml for phenylacetylglutamine and 4-cresyl sulphate, 10ng/mL for hippurate), medium (30ng/ml for phenylacetylglutamine and 4-cresyl sulphate, 100ng/mL for hippurate) and high (300ng/ml for phenylacetylglutamine and 4-cresyl sulphate, 1000ng/mL for hippurate) using six replicates on three separate well plates. Imprecision (CV) was calculated as the standard deviation divided by the mean of the detected concentration and expressed as a percentage. Recovery (inaccuracy) was calculated as the agreement between measured analyte concentration and nominal (theoretical) target concentration. Intra-assay results (differences in measured concentration between the replicates on the same plate) and inter-assay results (differences in measured concentration between replicates on 3 different plates) were determined and are given in Table 2. The CVs determined at three concentrations were within ±15% of the theoretical values, except for 4-cresyl sulphate at 3ng/mL for the inter-assay assessment, which had a CV estimate of 16.7%. Thus, precision and recovery of the method were mostly acceptable according to FDA guidelines22.
Table 2.
Intra and inter-assay imprecision and recovery data of the three analytes, calculated for the validation of the UPLC-MS/MS method developed in this study.
| Phenylacetylglutamine | 4-cresyl sulphate | Hippurate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intra-assay (n = 6) | |||||||||
| Target concentration, ng/mL | 3.0 | 30.0 | 300.0 | 3.0 | 30.0 | 300.0 | 10.0 | 100.0 | 1000.0 |
| Mean concentration measured (Mean SD), ng/mL | 3.2 (0.3) | 31.7 (1.8) | 327.7 (20.8) | 3.3 (0.1) | 28.8 (2.2) | 316.4 (11.1) | 8.6 (0.3) | 96.7 (7.9) | 1020.0 (136.9) |
| Imprecision (CV)a, % | ±9.4 | ±5.7 | ±6.3 | ±3.0 | ±7.6 | ±3.5 | ±3.5 | ±8.2 | ±13.4 |
| Recoveryb, % | 106.7 | 105.6 | 109.2 | 110.0 | 96.0 | 105.5 | 86.0 | 96.7 | 102.0 |
| Inter-assay (n = 18) | |||||||||
| Target concentration, ng/mL | 3.0 | 30.0 | 300.0 | 3.0 | 30.0 | 300.0 | 10.0 | 100.0 | 1000.0 |
| Mean concentration measured (Mean SD), ng/mL | 3.2 (0.3) | 30.5 (2.1) | 314.4 (19.8) | 3.0 (0.5) | 31.2 (2.7) | 315.8 (17.3) | 9.4 (1.4) | 102.5 (9.4) | 1015.0 (87.9) |
| Imprecision (CV)a, % | ±9.4 | ±6.9 | ±6.1 | ±16.7 | ±8.6 | ±5.5 | ±14.9 | ±9.2 | ±8.7 |
| Recoveryb, % | 106.7 | 101.7 | 104.8 | 100.0 | 104.0 | 105.3 | 94.0 | 102.5 | 101.5 |
Imprecision, CV (%) = Mean SD/Mean Concentration Measured × 100
Recovery (%) = Mean Concentration Measured/Target Concentration × 100
As the assay used MS/MS detection, it was necessary to assess the likelihood of matrix effects23,24. A matrix effect is the suppression or enhancement of ionization of analytes by the presence of matrix components in biological samples. This can be especially detrimental in a quantitative assay, since a matrix effect may suppress or enhance analyte response, leading to underestimation or overestimation of the true concentration measurement24. Therefore, to check for the presence of matrix effects and to determine the selectivity and reliability of our quantification method, we performed a parallelism of dilution experiment, where analyte concentrations were measured following serial dilution of the matrix (human urine specimens). The results, given in Supplemental Data Table 1 show that after multiplying each measured concentration by its respective dilution factor, the concentrations were almost identical (with the exception of one, anomalous result for hippurate at a dilution of x200). These data confirmed that there were no interferences from other endogenous urinary metabolites, indicating the absence of matrix effects and demonstrating specificity and reliability.
Application of the developed method to large scale population samples
Using the UPLC-MS/MS method described here, we analyzed 4,000 urine specimens from 2,000 US INTERMAP participants (two specimens/person) in 500 hours. The targeted method developed here reliably measured three urinary metabolites at concentrations as low as 1ng/mL (phenylacetylglutamine and 4-cresyl sulphate) and 3ng/mL (hippurate). In order to obtain absolute quantification, metabolite concentrations were analyzed via addition of deuterated isotope internal standards. Stable isotope internal standards are an ideal choice for LC-MS quantification assays25, as their physicochemical properties are similar to those of the analyte. Therefore, in addition to co-eluting and undergoing the same ionization conditions, they also provide control over matrix effects26. Analytical specificity of the method was confirmed by the ability to differentiate and quantify the metabolites of interest in the presence of many other compounds in the urinary specimens.
While potential sources of error have been reported previously for UPLC-MS/MS quantification methods such as ionization inaccuracy, non-specificity, imprecision and inaccuracy relating to instrument and handling (e.g. sample preparation)27, 28, we took steps to mitigate these potential limitations in our study. This included using the most appropriate isotope labeled internal standards (deuterated and non-deuterated 4-cresol suphate was custom synthesised in-house), performing a method validation study prior to analyzing the INTERMAP urine specimens to ensure there were no matrix effects, and including QC samples on each analytical plate to monitor imprecision and inaccuracy. Supplemental Data Table 2 gives method validation data following analysis of the 4,000 urine specimens: mean concentration, % deviation of the mean concentration from nominal concentrations, imprecision and recovery (inaccuracy) of the six QC samples included in each batch. The quantification data from the QC samples distributed throughout the 50 batch run demonstrate that the method and instrument were robust and reproducible over multiple batches. Precision and recovery values for the QC samples did not decline over the duration of the study, attributable to the analysis of 13L injections of predominantly aqueous urine specimens. Sample preparation and chromatographic separation times were minimized, making the assay suitable for high-throughput applications, such as large-scale epidemiologic and biobank studies.
Reference ranges for Phenylacetylglutamine, 4-Cresyl Sulphate and Hippurate in Human Urine
Our analysis provides reference ranges for urinary concentrations and 24-hr urinary output of phenylacetylglutamine, 4-cresyl sulphate and hippurate for free-living multi-ethnic US men and women aged 40–59 (Table 3). Mean concentrations, (standard deviation) based on the mean of two specimens/person were: for urinary phenylacetylglutamine, 81.0 (48.9) μmol/mmol creatinine and 1283.0 (751.7) μmol/24-hr (men), 113.9 (64.3) μmol/mmol creatinine and 1145.9 (635.5) μmol/24-hr (women); for 4-cresyl sulphate, 63.0 (47.4) μmol/mmol creatinine and 1002.5 (737.1) μmol/24-hr (men), 103.1 (71.2) μmol/mmol creatinine and 1031.8 (687.9) μmol/24-hr (women); for hippurate, 398.0 (265.4) μmol/mmol creatinine and 6284.6 (4008.1) μmol/24-hr (men), 476.8 (340.1) μmol/mmol creatinine and 4793.0 (3293.3) μmol/24-hr (women).
Table 3.
Reference ranges for human urinary concentrations of phenylacetylglutamine (PAG), 4-cresyl sulphate (4CS) and hippurate, given in ng/mL, μmol/mmol creatinine (Cr.), and μmol/24-hr.
| Metabolite, units | PAG, ng/mL | PAG, μmol/mmol Cr | PAG, μmol/24-hr | 4-CS, ng/mL | 4-CS, μmol/mmol Cr | 4-CS, μmol/24-hr | Hippurate, ng/mL | Hippurate, μmol/mmol Cr | Hippurate, μmol/24-hr | |
|---|---|---|---|---|---|---|---|---|---|---|
| Men (n=1,019) | Mean | 201.43 | 80.95 | 1283.03 | 113.11 | 63.04 | 1002.49 | 644.00 | 398.00 | 6284.57 |
| SD | 135.33 | 48.85 | 751.71 | 92.17 | 47.41 | 737.14 | 442.15 | 265.35 | 4008.10 | |
| Median | 166.50 | 68.66 | 1117.44 | 91.70 | 54.30 | 871.53 | 555.70 | 334.86 | 5395.18 | |
| 2.5%a | 41.80 | 22.84 | 343.54 | 6.30 | 3.91 | 59.51 | 95.20 | 66.61 | 1021.34 | |
| 97.5%b | 559.05 | 197.51 | 3283.82 | 350.50 | 180.50 | 2848.59 | 1802.20 | 1100.45 | 16320.69 | |
| Women (n=981) | Mean | 187.74 | 113.94 | 1145.93 | 120.93 | 103.12 | 1031.84 | 504.39 | 476.75 | 4793.04 |
| SD | 120.98 | 64.30 | 635.49 | 92.26 | 71.19 | 687.94 | 341.53 | 340.11 | 3293.31 | |
| Median | 155.60 | 99.19 | 1018.95 | 100.65 | 89.65 | 908.31 | 426.15 | 391.43 | 4116.60 | |
| 2.5%3a | 46.15 | 36.33 | 351.73 | 8.60 | 7.88 | 79.19 | 88.60 | 94.62 | 854.02 | |
| 97.5%b | 494.75 | 287.43 | 2869.62 | 366.65 | 270.80 | 2557.63 | 1372.55 | 1377.14 | 13208.34 | |
| Aged 40–493 (n=982) | Mean | 196.95 | 89.57 | 1151.95 | 117.89 | 76.22 | 963.19 | 578.88 | 402.84 | 5307.28 |
| SD | 128.65 | 53.61 | 620.36 | 94.14 | 59.57 | 671.79 | 412.65 | 285.19 | 3682.91 | |
| Median | 166.33 | 77.39 | 1038.07 | 99.48 | 62.79 | 864.35 | 472.85 | 332.86 | 4346.11 | |
| 2.5%3a | 43.00 | 24.21 | 322.75 | 7.35 | 4.23 | 59.70 | 103.55 | 76.87 | 912.64 | |
| 97.5%b | 513.05 | 217.67 | 2748.66 | 353.55 | 216.57 | 2503.79 | 1714.35 | 1129.42 | 15491.91 | |
| Aged 50–593 (n=1018) | Mean | 192.56 | 104.43 | 1277.35 | 116.03 | 88.95 | 1068.68 | 572.28 | 469.21 | 5789.98 |
| SD | 128.67 | 63.45 | 764.90 | 90.47 | 66.48 | 748.04 | 391.65 | 323.07 | 3798.30 | |
| Median | 156.48 | 90.41 | 1098.15 | 92.78 | 72.77 | 920.95 | 492.70 | 385.67 | 4912.95 | |
| 2.5%3a | 43.85 | 29.09 | 366.20 | 7.95 | 6.26 | 74.26 | 86.20 | 84.06 | 930.31 | |
| 97.5%b | 541.80 | 255.88 | 3326.98 | 366.65 | 260.41 | 2848.59 | 1477.45 | 1295.98 | 15749.19 |
2.5% = 2.5th percentile
97.5% = 97.5th percentile
Phenylacetylglutamine and 4-cresyl sulphate are derived from biotransformation of tryptophan and tyrosine respectively, whereas hippurate is the glycine conjugate of benzoic acid which is introduced via a range of plant and other dietary sources. Mean urinary excretion of phenylacetylglutamine was previously found to be 1080.0 μmol/24-hr in seven normal adults (gender unspecified)29 via isotope dilution GC-MS. Our results are consistent with these previous data, as we observed mean excretion of 1283.0 μmol/24-hr in men and 1145.9 μmol/24-hr in women. To our knowledge the present study is the first to report a reference range for human urinary 4-cresyl sulphate excretion, and so provides a unique resource for clinical chemistry measurements.
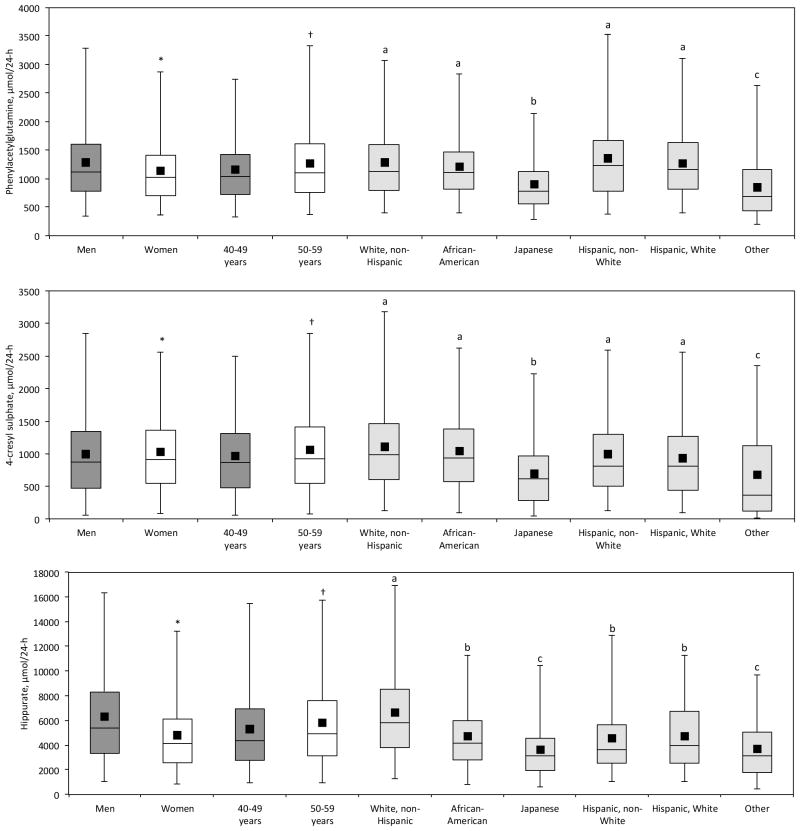
Mean urinary hippurate concentrations were previously reported to be 175.9 (SD 124.3) μmol/mmol creatinine in men and 207.3 (SD 118.8) μmol/mmol creatinine in women in a Greek population study30 compared with 398.0 μmol/mmol creatinine and 476.8 μmol/mmol creatinine respectively in our study. Since hippurate precursors are present in plant and other dietary sources31, this difference may be attributable to dietary differences between the Greek and multi-ethnic US population samples in our study. Furthermore, the previous study was based on a smaller sample than our own (N=122 vs. N=2000). Our newly generated reference ranges shown in Table 3, indicate that total 24-hr urinary excretion of phenylacetylglutamine and 4-cresyl sulphate are similar in men and women, whereas 24-hr hippurate excretion is considerably higher in men compared to women. Extant literature reported higher hippurate excretion in women compared with men30, based on μmol/mmol creatinine concentrations. We also observed this μmol/mmol creatinine concentration gender difference; however, since our study included replicate measurements (second 24-hr urine collection) on each individual, we are able to report 24-hr urinary excretion values. These may be more informative than concentration data since 24-hr urinary excretion measurements take into account diurnal variation and intra-individual differences in creatinine excretion relating to body mass and the responsiveness to meat in the diet. Our results also show an age related trend with higher 24-hr excretion of all three microbial metabolites, at ages 50–59 compared with 40–49 years (Figure 2, Table 3).
Fig. 2.
Box and whisker plots for phenylacetylglutamine, 4-cresyl sulphate, and hippurate (μmol/24-h), by gender, age and ethnic group. Corresponding statistics are given in Table 3 and Supplemental Data Table 3. Diamond=mean; central horizontal line=median; box=25th and 75th percentiles; whiskers=2.5th and 97.5th percentiles. Student’s t (gender, age) and Student-Newman-Keuls (ethnicity) tests were done on log-transformed metabolite data. * significantly different from men, p<0.05; † significantly different from 40–49 years, p<0.05; ethnic groups annotated with letters a, b or c are significantly different from each other, p<0.05.
The 2,000 INTERMAP US individuals in this study are from several ethnic groups. When urinary excretion differences were compared between ethnic groups (Figure 2, Supplemental Data Table 3), we found that urinary excretion of all three metabolites were significantly (p<0.05) lower in the Japanese subpopulation compared with other ethnic groups; most Japanese participants were from Hawaii with perhaps a more traditional lifestyle than on mainland USA. 24-hr phenylacetylglutamine and 4-cresyl sulphate excretion were not significantly different between White Non Hispanic, African American, Hispanic Non White and Hispanic White ethnic groups. For 24-hr urinary hippurate excretion White Non Hispanic participants excreted significantly higher amounts of hippurate compared with the other subpopulations (twice the 24-hr excretion of the Japanese subgroup). African American, Hispanic Non White and Hispanic White had similar 24-hr hippurate excretions.
Experimental
Population Samples (INTERMAP Study)
The INTERMAP US participants comprise 2,195 men and women aged 40–59 from eight population samples20. 1,190 were non-Hispanic white, 288 Hispanic, 369 African American, 269 Japanese, and 79 of other ethnicities. Both general population and occupational samples were included. Individuals were selected randomly from population lists, stratified by age/gender. Each participant attended the research center four times: two visits on consecutive days, and a further two visits on consecutive days on average three weeks later. Two timed 24-hr urine collections were obtained for measurement of urinary electrolytes, minerals, creatinine, urea, albumin, amino acids, and multiple metabolites. Urine was collected in standard 1-L plastic jars containing boric acid (for preservation)32. The two timed collections were started at the research center (first and third visits), and completed at the research center the following day. Specimens were rejected if collection time fell outside the range 22–26hr, if the participant reported that collection was incomplete, if he/she had lost ‘more than a few drops’ of urine, or if total volume was <250ml. Urine volume was measured and corrected to 24-hours. Urinary aliquots were obtained and stored locally at −20°C, then air-freighted on dry ice to the Central Laboratory (Leuven, Belgium) for urine biochemistry and amino acid analysis. Frozen aliquots were also sent from Leuven to Biomolecular Medicine, Imperial College London and from there to Pfizer, Kent, UK for the present study. INTERMAP received institutional review board approval, and all participants gave informed consent to participate.
Chemicals And Reagents
All chemicals and solvents used were of the highest purity grade available. Non-deuterated phenylacetylglutamine reference standard was purchased from Bachem (Weil am Rhein, Germany), hippurate from Sigma-Aldrich (Gillingham, UK). 2H5-phenylacetylglutamine and 2H2-hippurate deuterated internal standards were purchased from CDN Isotopes (Essex, UK). Deuterated 2H3-4-cresyl sulphate and non-deuterated 4-cresyl sulphate were synthesized in-house (Pfizer). LC-grade acetonitrile and formic acid were purchased from Sigma-Aldrich (Gillingham, UK), and deionized water produced by a Millipore Corporation Milli-Q water purifying system (Bedford, MA, USA) was used.
Preparation Of Solutions And Calibrators
Stock solutions of each analyte and deuterated internal standard were prepared at a concentration of 1mg/mL in acetonitrile/aqueous solution of formic acid (0.1%) (50:50). A multi-analyte (phenylacetylglutamine, 4-cresyl sulphate) working stock solution of non-deuterated reference standard was prepared by transferring 100μL of each 1mg/mL analyte stock solution with 700μL aqueous solution of formic acid (0.1%). Working stock solution of hippurate was made up separately (300μL of 1mg/ml hippurate stock solution plus 700μL aqueous solution of formic acid (0.1%), owing to the higher urinary analytical range of hippurate in human urine. Serial dilutions of these working stock solutions with aqueous solution of formic acid (0.1%) were done to obtain calibrators at concentrations of 1, 3, 10, 30, 100, 300 and 1000ng/mL for phenylacetylglutamine and 4-cresyl sulphate; and 3, 10, 30, 100, 300, 1000 and 3000ng/mL for hippurate. Working stock solutions of the deuterated internal standards were also prepared as above, followed by dilution to a fixed concentration of 30ng/mL for 2H5-phenylacetylglutamine and 2H3-4-cresyl sulphate and 100ng/mL for 2H2-hippurate, with aqueous solution of formic acid (0.1%) diluents.
UPLC-MS/MS
UPLC was performed on a Waters ACQUITY™ UPLC system equipped with a binary solvent delivery manager and sample manager, coupled with a Waters Xevo™ triple quadrupole (TQ) mass spectrometer (Waters Corporation, Manchester, UK). Chromatographic separations were carried out with a Waters Acquity™ UPLC BEH C18 column (1.7μm, 2.1 × 100mm), using a mobile phase comprising an aqueous solution of formic acid (0.1%) (A) and acetonitrile plus 0.1% formic acid (B). Analysis commenced under isocratic conditions (95:5) of A and B, and after 90 seconds a mobile phase gradient program was employed, where B was increased to 95% over 50 seconds, followed by re-equilibration to isocratic conditions to give a total run time of 2 minutes, with high sensitivity and no loss of resolution, as illustrated by the UPLC-MS chromatograms in Figure 1. The mobile phase flow rate was 0.6mL/min and the column temperature was maintained at 60°C. Volumes of 1μL of sample were injected by the auto sampler.
The specific MS/MS parameters for each urinary metabolite are given in Table 1. Ionization source parameters were: capillary voltage 3.00kV, extractor voltage 3.00V, source temperature 150°C, desolvation temperature 600°C, cone gas flow off, desolvation gas flow 800L/h and collision gas flow 0.15mL/min.
Preparation Of Urine Specimens And Limits Of Quantification
Two 24-hr specimens were available for each of 2,000 US INTERMAP participants (4,000 specimens). Specimens were thawed and prepared onto analytical well plates. The lower and upper limits of quantification (LLOQ and ULOQ) were defined as 1ng/ml and 1000ng/ml respectively for phenylacetylglutamine and 4-cresyl sulphate. The LLOQ was established using published guidelines22 as the lowest concentration of analyte that could be quantified with a deviation from target <20%. An initial dilution study was performed to establish the analytical range of the microbial co-metabolites in human urine. The natural abundances of phenylacetylglutamine and 4-cresyl sulphate were within the limits of quantification at a dilution of 1:1000, therefore urine specimens were subjected to a two-step 1:1000 dilution (1:50 dilution followed by 1:20 dilution) to keep the urinary concentration levels of the analytes in the range of 1–1000ng/mL. Natural urinary levels of hippurate were considerably higher than the other analytes after a 1:1000 dilution, so the method was adapted so that the LLOQ and ULOQ for hippurate were 3ng/mL and 3000ng/mL.
Each well plate (or batch), also included a multi-analyte non-deuterated calibration series and six quality control (QC) standards at three concentrations (see below). The criteria of acceptance for a batch run dictated that a minimum of two-thirds of all QCs were within 15% of their nominal values, with at least one acceptable QC at each concentration. Two wells containing blanks (water) were included in each batch run to detect carryover of metabolites or contamination.
Quantification
To all wells containing calibrators, urine specimens or QCs, 10μL of each deuterated internal standard (at a fixed concentration of 30ng/mL for 2H5-phenylacetylglutamine and 2H3-4-cresyl sulphate and 100ng/mL for 2H2-hippurate) were spiked-in for absolute quantification. Since urinary specimens were diluted 1:1000, the internal standards were added after dilution to minimise the amount of standard necessary to spike-in. Quantification was performed using the TargetLynx™ function of Waters MassLynx™ 4.1 software, by integration of the area under the curve from the specific MRM chromatograms of the analytes and their deuterated internal standards. The response (analyte/internal standard integrated area ratio) was compared to the generated calibration curve to give urinary concentration values. We examined different weighting factors (1/x, 1/x2 and none) and polynomial type (linear and quadratic) and found that for the best fit of the calibration curve a weighted (1/x) linear regression gave R2 > 0.997 for phenylacetylglutamine and 4-cresyl sulphate, whereas R2 > 0.997 was obtained for hippurate by fitting to a weighted (1/x2) second order regression (calculated by Waters TargetLynx™ 4.1 software).
Conclusions
Our study demonstrates the feasibility of a UPLC-MS assay for rapid quantitative analysis of urinary gut microbial co-metabolites, which could be readily extended to other metabolite classes. Given the increasingly recognised role of the gut microbiome in disease aetiology and gut health, these new data supplement and greatly extend existing knowledge of the urinary excretion of gut microbial-mammalian co-metabolites in a multi-ethnic US human population. The application of such methods to large-scale epidemiological studies has the potential to further understanding of the role of the gut microbiome in health and disease.
Supplementary Material
Acknowledgments
We thank all the INTERMAP staff at local, national, and international centers for their invaluable efforts; a partial listing of these colleagues is given in reference 20 of this article. The INTERMAP Study in the USA is supported by grants R01-HL050490 and R01-HL084228 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, USA.
Footnotes
Electronic Supplementary Information (ESI) available: Table. S1–S3. See DOI: 10.1039/b000000x/
Notes and references
- 1.Nicholson JK, Holmes E, Wilson ID. Nat Rev Microbiol. 2005;3:431–438. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 2.Othman M, Aguero R, Lin HC. Curr Opin Gastroenterol. 2008;24:11–16. doi: 10.1097/MOG.0b013e3282f2b0d7. [DOI] [PubMed] [Google Scholar]
- 3.Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Proc Natl Acad Sci U S A. 2009;106:14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley TV, Codde JP, Rouse IL. Lancet. 1995;345:455–456. doi: 10.1016/s0140-6736(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 5.Willing B, Halfvarson J, Dicksved J, Rosenquist M, Jarnerot G, Engstrand L, Tysk C, Jansson JK. Inflamm Bowel Dis. 2009;15:653–660. doi: 10.1002/ibd.20783. [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 7.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 8.Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, Bos NA. Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 9.Scanlan PD, Shanahan F, Clune Y, Collins JK, O’Sullivan GC, O’Riordan M, Holmes E, Wang Y, Marchesi JR. Environ Microbiol. 2008;10:789–798. doi: 10.1111/j.1462-2920.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin FP, Sprenger N, Montoliu I, Rezzi S, Kochhar S, Nicholson JK. J Proteome Res. 2010;9:5284–5295. doi: 10.1021/pr100554m. [DOI] [PubMed] [Google Scholar]
- 11.Swann J, Wang Y, Abecia L, Costabile A, Tuohy K, Gibson G, Roberts D, Sidaway J, Jones H, Wilson ID, Nicholson J, Holmes E. Mol Biosyst. 2009;5:351–355. doi: 10.1039/b811468d. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson J, Wilson ID. Progress in Nuclear Magnetic Resonance Spectroscopy. 1989;21:449–501. [Google Scholar]
- 13.Nicholson JK, Lindon JC, Holmes E. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson JK, Sadler PJ, Bales JR, Juul SM, MacLeod AF, Sonksen PH. Lancet. 1984;2:751–752. doi: 10.1016/s0140-6736(84)92656-4. [DOI] [PubMed] [Google Scholar]
- 15.Gika HG, Macpherson E, Theodoridis GA, Wilson ID. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2008;871:299–305. doi: 10.1016/j.jchromb.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 16.Jiye A, Huang Q, Wang GJ, Zha WB, Yan B, Ren HC, Gu SH, Zhang Y, Zhang Q, Shao F, Sheng LS, Sun JG. Analytical Biochemistry. 2008;379:20–26. doi: 10.1016/j.ab.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson JK, Holmes E, Elliott P. J Proteome Res. 2008;7:3637–3638. doi: 10.1021/pr8005099. [DOI] [PubMed] [Google Scholar]
- 18.Bictash M, Ebbels TM, Chan Q, Loo RL, Yap IK, Brown IJ, de Iorio M, Daviglus ML, Holmes E, Stamler J, Nicholson JK, Elliott P. J Clin Epidemiol. 2010;63:970–979. doi: 10.1016/j.jclinepi.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA, Daviglus ML, Kesteloot H, Ueshima H, Zhao L, Nicholson JK, Elliott P. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, Liu K, Ueshima H, Zhou BF. J Hum Hypertens. 2003;17:591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindon JC, Nicholson JK. Annu Rev of Anal Chem. 2008;1:45–69. doi: 10.1146/annurev.anchem.1.031207.113026. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration. Guidance for Industry, Bioanalytical Method Validation. US Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2001. [Google Scholar]
- 23.Annesley TM. Clin Chem. 2003;49:1041–1044. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 24.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 25.Stokvis E, Rosing H, Lopez-Lazaro L, Schellens JH, Beijnen JH. Biomed Chromatogr. 2004;18:400–402. doi: 10.1002/bmc.392. [DOI] [PubMed] [Google Scholar]
- 26.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 1998;70:882–889. doi: 10.1021/ac971078+. [DOI] [PubMed] [Google Scholar]
- 27.Vogeser M, Seger C. Clin Chem. 2010;56:1234–1244. doi: 10.1373/clinchem.2009.138602. [DOI] [PubMed] [Google Scholar]
- 28.Mortier KA, Clauwaert KM, Lambert WE, Van Bocxlaer JF, Van den Eeckhout EG, Van Peteghem CH, De Leenheer AP. Rapid Commun Mass Spectrom. 2001;15:1773–1775. doi: 10.1002/rcm.441. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Beylot M, Agarwal KC, Soloviev MV, Brunengraber H. Anal Biochem. 1993;212:277–282. doi: 10.1006/abio.1993.1323. [DOI] [PubMed] [Google Scholar]
- 30.Psihogios NG, Gazi IF, Elisaf MS, Seferiadis KI, Bairaktari ET. NMR Biomed. 2008;21:195–207. doi: 10.1002/nbm.1176. [DOI] [PubMed] [Google Scholar]
- 31.Mulder TP, Rietveld AG, van Amelsvoort JM. The American journal of clinical nutrition. 2005;81:256S–260S. doi: 10.1093/ajcn/81.1.256S. [DOI] [PubMed] [Google Scholar]
- 32.Smith LM, Maher AD, Want EJ, Elliott P, Stamler J, Hawkes GE, Holmes E, Lindon JC, Nicholson JK. Anal Chem. 2009;81:4847–4856. doi: 10.1021/ac9004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.