Abstract
Fear-potentiated startle is defined as an increase in the magnitude of the startle reflex in the presence of a stimulus that was previously paired with an aversive event. It has been proposed that a subject’s awareness of the contingencies in the experiment may affect fear-potentiated startle. The authors adapted a conditional discrimination procedure (AX+/BX−), previously validated in animals, to a human fear-potentiated startle paradigm in 50 healthy volunteers. This paradigm allows for an assessment of fear-potentiated startle during threat conditions as well as inhibition of fear-potentiated startle during safety conditions. A response keypad was used to assess contingency awareness on a trial-by-trial basis. Both aware and unaware subjects showed fear-potentiated startle. However, awareness was related to stimulus discrimination and fear inhibition.
Keywords: human startle response, contingency awareness, discrimination learning, fear inhibition
Excessive fear and anxiety, along with an inability to overcome these emotions, are some of the defining characteristics of many psychiatric disorders such as phobias, panic disorder, and posttraumatic stress disorder. Animal models of fear conditioning and fear inhibition provide useful tools for the study of these phenomena; however, it is essential to ascertain the face validity of these models by translating them to human subjects. The goal of this study was to examine the relationship between inhibition of conditioned fear and the awareness of stimulus meaning during conditioning.
Fear-potentiated startle is defined as an increase in the magnitude of the startle reflex when it is elicited in the presence of a conditioned stimulus (CS + ) that was previously paired with an aversive stimulus (unconditioned stimulus, US) in contrast to when it is elicited in the absence of this CS. Fear-potentiated startle can be demonstrated in animals and humans (Ameli, Ip, & Grillon, 2001; Davis, 1992; Grillon & Davis, 1997; for a recent review see Grillon & Baas, 2003). As a result, it provides an objective measure of conditioned fear and is an ideal model for translational research (Davis, Falls, Campeau, & Kim, 1993). Grillon, Falls, Ameli, and Davis (1994) adapted the fear-potentiated startle paradigm using instructed rather than conditioned fear: The experimenter told the subject to expect electric shock when a particular light (threat cue) came on, and a different light signaled the absence of the US (safety cue). However, more recent studies have used conditioning experiments analogous to the animal models to elicit fear-potentiated startle (Ameli, et al., 2001; Grillon & Davis, 1997). In both experimental designs, startle amplitude is greater when elicited in the presence of the threat cue than when it is elicited in the absence of this cue or in the presence of a safety cue, which itself has little effect on startle magnitude. Fear conditioning paradigms differ from instructed fear because subjects learn to fear an explicit, predictable cue. When a cue does not reliably signal the aversive event, fear becomes less predictable, a situation that produces more generalized anxiety rather than fear (Grillon, 2002). Fear is distinct from anxiety in that it relates to a known threat, whereas anxiety is a more diffuse fear not linked with a specific threat. Davis (1998) have recently argued for a double dissociation between fear and anxiety as mediated by different neural mechanisms, fear by the central nucleus of the amygdala and anxiety by the bed nucleus of the stria terminalis (Davis, 1998).
Given that predictability of the US may contribute to the distinction between fear and anxiety, the extent to which a subject is aware of the CS–US contingency may elicit one or the other emotion. The term contingency awareness is defined as the subject’s knowledge of the reinforcement contingencies in the experiment (Lovibond & Shanks, 2002). Lovibond (2004) proposed a cognitive model of fear conditioning in which cognitive awareness of experimental contingencies is necessary for fear conditioning and extinction. He contrasted this view with the earlier arguments that fear conditioning may occur through unconscious, automatic mechanisms that are independent of awareness (Seligman, 1971). According to the cognitive model, a subject who is aware of the contingency in the experiment should show significant increases in startle when the CS+ is presented. On the other hand, a subject who is not aware of the contingency would not be able to predict when the US would occur and should therefore demonstrate a more anxiety-like response, that is, increased startle to all stimuli rather than to any particular stimulus (Lovibond, 2004). This is exactly what Grillon found when he compared fear-potentiated startle between subjects who were aware and those who were unaware of the reinforcement contingency (Grillon, 2002). The aware subjects potentiated from baseline startle by 70% during the presentation of the CS + , compared with 20% potentiation in the unaware subjects. However, the unaware subjects had higher baseline startle and higher startle during the presentations of CS – than the aware subjects, perhaps indicative of anxiety as opposed to stimulus-specific fear. On the other hand, other investigators have reported fear-potentiated startle in the absence of awareness, whereas changes in skin conductance were associated with awareness (Hamm & Vaitl, 1996; Hamm & Weike, 2005). On the other hand, Purkis and Lipp (2001) found that fear conditioning was dependent on awareness using both startle and skin conductance as the measure of conditioning.
The issue of whether conditioning can occur without awareness is controversial. Most of the literature examining the relationship between awareness and conditioning in humans has compared trace and delay conditioning paradigms (Clark & Squire, 1998; Manns, Clark, & Squire, 2000). In trace conditioning, the US is not temporally contiguous with the CS; that is, there is a period of time between the offset of the CS and the start of the US. This task appears to be hippocampally driven and dependent on awareness (Clark & Squire, 1998). On the other hand, delay conditioning, in which the CS and US overlap and coterminate may be observed in the absence of contingency awareness (Knight, Nguyen, & Bandettini, 2003; LaBar & Disterhoft, 1998). However, some of the earlier studies used postexperimental questionnaires to assess awareness; in several studies the contingency between the CS and the US changed during the experimental session, and thus the awareness information was very problematic (Hannula, Simons, & Cohen, 2005; LaBar & Disterhoft, 1998). Current technology allows for use of trial-by-trial measures of awareness such as keypads or joysticks (Hannula et al., 2005). Although it is possible that these tasks detract some of the attention away from conditioning stimuli, and thus slow down learning, they provide superior insight into the onset of explicit learning.
Contingency awareness also appears to be dependent on the age of the subjects in the experiment (LaBar, Cook, Torpey, & Welsh-Bohmer, 2004; LaBar & Disterhoft, 1998). LaBar and colleagues analyzed fear conditioning using skin conductance and found that younger subjects were more likely to be aware of stimulus contingencies as measured by a postexperimental interview. However, the effect of awareness on fear conditioning was most pronounced in the older subjects and did not appear to impact the skin conductance data in the younger subjects.
We recently developed an experimental paradigm that allows for the independent evaluation of excitation and inhibition of fear conditioning in humans (Jovanovic et al., 2005). The procedure, referred to as a conditional discrimination (abbreviated as AX+/BX−), was translated from a rodent model of fear inhibition (Myers & Davis, 2004) that was based on a paradigm used in earlier learning theory experiments (Wagner, Logan, Haberlandt, & Price, 1968; Wagner & Rescorla, 1972). In this experiment, reinforcement of X is conditional upon the presence of either A or B. A becomes excitatory with training as the subject learns that A and X presented together predict the unconditioned stimulus (US). B becomes inhibitory in that B presented with X predicts the absence of the US (i.e., B is a safety signal). The presentation of A and B together (AB) results in a reduced fear response to A because B transfers its inhibitory property to A. Thus the AB trials are referred to as conditioned inhibition test trials and indicate the ability to transfer safety to a danger cue. Consistent with these predictions, we have found greater startle magnitude in the presence of AX vs. BX and in the presence of AX vs. AB (Jovanovic et al., 2005).
A unique feature of this study is the use of the response keypad during training and testing to assess online contingency awareness (Lovibond & Shanks, 2002). This was accomplished by having the subjects rate each light as reinforced or nonreinforced by pressing different buttons on the keypad. An additional advantage of using the response pad was that we believe it forced the subjects to process each light in the experiment as separate elements. One of the difficulties in translating animal paradigms to humans is that humans tend to perceive compound stimuli as a unique single stimulus rather than separate stimuli (Williams, Sagness, & McPhee, 1995). Such configural processing would result in AB being perceived as a single, novel compound stimulus rather than a combination of danger (A) and safety (B). In that case, there would be no transfer of safety to the danger cue in the AB test trials. Hence, we believe that instructing subjects to label each light separately with the keypad encouraged them to consider the independent reinforcement value of each stimulus. Prior to the startle session we conducted neuropsychological testing in the subjects, to measure intelligence, memory, and attention span to see whether any of these measures were related to the subjects’ contingency awareness. Given the data from LaBar et al. (2004) on the relationship between age and awareness, we purposely chose a wide range of age in our subjects (20–74 years).
On the basis of Grillon’s work on contingency awareness (Grillon, 2002), we hypothesized that subjects who were aware of the experimental contingencies would startle more in the presence of AX than in the presence of BX. On the other hand, subjects who were unaware of the contingency would show increased baseline startle as a marker of heightened anxiety if the airblast was perceived as an aversive enough stimulus (Grillon et al., 2005). These subjects should also demonstrate a lack of discrimination between AX+ and BX−. We also hypothesized that subjects who were aware of the contingencies would be better at inhibiting fear-potentiated startle during AB trials.
Method and Materials
Subjects
Fifty healthy subjects participated in the study after signing a consent form approved by the Emory University institutional review board and the Atlanta Veteran’s Affairs Medical Center research and development committee. The sample included 20 women and 30 men ranging in age from 20 to 74 years old. The subjects had no current or lifetime Axis I disorders, including substance abuse and dependence, as ascertained by the Structured Clinical Interview for DSM–IV Axis I Disorders (First, Spitzer, Gibbon, & Williams, 1997). All subjects were screened for auditory or visual impairment. Using an audiometer (MA27, Maico Diagnostics, Eden Prairie, MN), the subjects had to be able to detect tones at 30 dB(A) sound pressure level (SPL) at frequencies ranging from 250 to 4,000 Hz. The subjects were not color blind and had at least 20/40 vision in both eyes (using correction, if necessary) at day of testing. In addition, all subjects had negative urine toxicology screens.
Startle Procedure
The acoustic startle response (eyeblink component) was measured through electromyography (EMG) of the right orbicularis oculi muscle. Two 5-mm Ag/AgCl electrodes filled with electrolyte gel were positioned approximately 1 cm under the pupil and 1 cm below the lateral canthus, and a ground electrode was placed behind the right ear over the mastoid. The impedances for all subjects were less than 6 kilo-ohms. EMG activity was amplified and digitized using a computerized EMG startle response monitoring system (SR-LAB, DOS version, San Diego Instruments, San Diego, CA). The EMG signal was filtered with low- and high-frequency cutoffs at 30 and 1,000 Hz, respectively. The system was set to record 250 readings of 1 ms starting at the onset of the startle stimulus. Subjects were seated and asked to look at the set of four lights mounted on the wall approximately 5 ft from their seat. All acoustic stimuli were delivered binaurally through headphones (TDH-39-P, Maico Diagnostics, Eden Prairie, MN).
The startle data were used from a paradigm designed to test fear inhibition (see Jovanovic et al., 2005) and used more stimuli combinations than will be discussed here. The session began with a 1-min acclimation period consisting of 70-dB(A) SPL broadband noise, which continued as the background noise throughout the session. The startle probe (noise burst) was either a 104- or 108-dB(A) SPL, 40-ms burst of broadband noise with a near instantaneous rise time. According to methods established by Grillon and Ameli (1998), the aversive stimulus (US) was a 250-ms airblast with an intensity of 140 psi directed to the larynx, emitted by a compressed air tank attached to polyethylene tubing and controlled by a solenoid valve. A, B, and X were green, purple, or blue lights ranging in light transmission from 4.0% to 4.2% (counterbalanced color assignment across subjects).
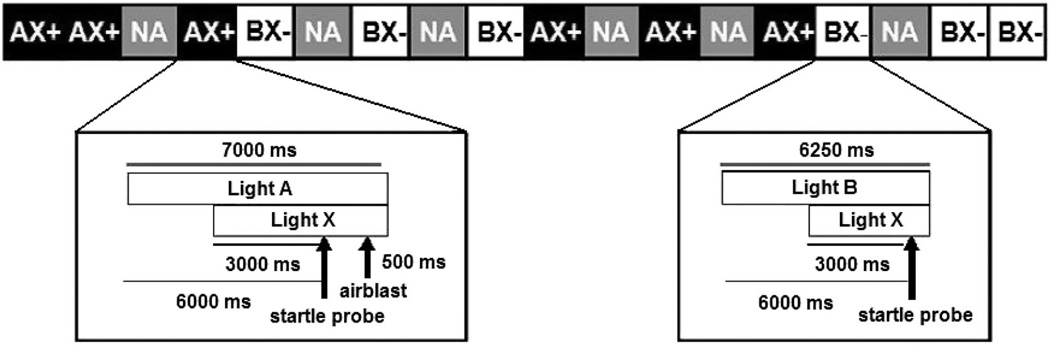
The test session began with a habituation phase consisting of six startle probes (three at 104 dB[A] and three at 108 dB[A]) to reduce initial startle reactivity and rule out nonstartlers. To minimize individual variability in baseline startle, subjects were either assigned to the 104-dB(A) session or the 108-dB(A) session on the basis of startle level in the habituation phase (if startle was below 100 machine units, subjects were assigned to 108 dB[A]). The conditioning phase included six startle probes presented alone, six trials in which stimuli A and X were paired with the US (AX+), and six trials in which stimuli B and X were not paired with the US (BX−). The AX+ stimuli were presented serially within a trial, and the order of A and X alternated randomly across trials. Figure 1 shows a diagram of the trials in the session. In the AX+ trials, the first light came on and stayed on for 7,000 ms; after 3,000 ms, the second light came on, so that for the last 4,000 ms the two lights were presented together. The startle probe was presented at the end of 6,000 ms (when the two lights had been presented together for 3,000 ms) and was followed by the air blast 500 ms later. The airblast lasted for 250 ms, and the lights stayed on for another 250 ms after that, so that both lights were still on during both the startle probe and the airblast. In the BX− trials, as well as in the AB test trials, there was no airblast; therefore, the first light stayed on for 6,250 ms, and the second light was presented for the last 3,250 ms. In these trials as well, the startle probe was presented at the end of the first 6,000 ms, and the lights stayed on for another 250 ms after the startle probe. The testing phase consisted of two blocks; each block included six startle probes presented alone and six presentations of AB. In all phases of the experiment, intertrial intervals were of randomized duration ranging from 9 to 22 seconds. After the session, a subset of the subjects (n = 37) were asked to rate the aversiveness of the airblast and the startle probe on a scale from 1 to 5.
Figure 1.
Diagram of the AX+/BX− startle session. The airblast was the unconditioned stimulus, and the startle probe was a 40-ms noise burst. A = green light; B = purple light; X = blue light; + = reinforced; − = nonreinforced; NA = startle probe without the conditioned stimulus.
Response Keypad
A response keypad unit (SuperLab, Cedrus Corporation, San Pedro, CA) was incorporated into the startle session so that the EMG startle response monitoring system (SR-LAB, San Diego Instruments, San Diego, CA) signaled the onset of a light in the SuperLab software program. Each trial contained two light components (e.g., A and X). Subjects were instructed to respond to each light separately on each trial by pressing one of three buttons: one when they expected a light to be followed by the airblast, a second button when they did not expect the light to be followed by the airblast, and a third button when they were uncertain of what to expect. The exact instructions given to the subjects were
During this experiment you will hear some sudden tones and noises in addition to seeing several colored lights turn on. The tones are there to elicit startle and occur every time something happens. However, some of the lights will be followed by the blast of air while other lights will not. Throughout the experiment please press the button on the keypad to tell us whether you think a light will be followed by air (the plus sign), or will not be followed by air (the minus sign). If you do not know, press the 0 sign. You should press a button for each light.
An earlier study by Grillon and Davis (1997) found that pressing a button per se did not potentiate startle in a fear conditioning study.
Awareness
We assessed awareness of the experimental contingency on the basis of the subjects’ keypad responses. To assess the effects of awareness on both fear potentiation and fear inhibition, we classified the subjects as either aware or unaware of the AX contingency (the reinforced stimulus) and as either aware or unaware of the BX contingency (the nonreinforced stimulus). To be classified as AX aware, the subjects needed to have two consecutive correct responses to the reinforced training trials (AX). The same was true of the subjects classified as BX aware. We operationally defined correct responses to AX+ trials as expectations of airblast either when presented with the A light or during an X light when it followed A, and the correct responses to BX− were expectations of no airblast either on the B light or on an X light when it followed B. For instance, if a subject indicated on two consecutive AX+ trials that they expected an airblast during either of the lights, they would be classified as AX aware. The unaware subjects were those that continued to change their responses or else consistently pressed the wrong buttons on the keypad.
Neuropsychological Assessments
Attention and distractibility were assessed by means of the Continuous Performance Test (Conners, 1995). Memory was assessed with the Logical Memory I and II subtests of the Wechsler Memory Scale (3rd ed.; Wechsler, 1997). Finally, general IQ was assessed with the Matrix Reasoning and Vocabulary subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Motor performance was also assessed using the Finger Tapping Test (Raitan & Wolfson, 1985) to control for motor deficits as a possible confound for tests using keypad responses.
Statistical Analyses
One-way analyses of variance (ANOVAs) were used to assess the association between demographic and neuropsychological variables and contingency awareness. Categorical data, such as sex, race, and recruitment status (Emory University or Atlanta Veteran’s Affairs Medical Center hospital) were analyzed with chi-square analyses. The development of contingency awareness, as assessed by the subjects’ responses (US expected, US not expected, and uncertain) across the six training trials were also analyzed for the aware and unaware subjects by use of chi-square analyses.
Because of the variable nature of the startle response, we averaged the first three occurrences and the last three occurrences of each trial type to form two conditioning blocks of AX, BX, and noise alone (NA). NA refers to trials in which the startle probe was delivered without the CS. Fear potentiation was tested with a three-way mixed ANOVA model (within-subject factors of trial type with two levels, NA vs. AX, and block, Block 1 and Block 2 of conditioning, to assess learning of the fear response) with the between-groups factor of awareness (two levels: AX aware and AX unaware). The dependent variable for these analyses was startle amplitude.
Discrimination between danger and safety cues was tested using a three-way mixed ANOVA with trial type (within-subjects factor with two levels, AX vs. BX, and block, Block 1 and Block 2 of conditioning, to assess learning of the discrimination) with the between-groups factor of awareness (two levels: BX aware and BX unaware). The dependent variable was the percent potentiation from noise alone calculated with the following formula:
where the startle amplitude during the presentation of the CS is subtracted from the startle amplitudes on noise alone trials, and this difference is divided by the NA value and multiplied by 100. Transfer of fear inhibition was tested by use of a two-way mixed ANOVA of Trial Type (withinsubjects factor with two levels: AX vs. AB) × Awareness (between-groups factor with two levels: BX aware and BX unaware). AX was the average of the three AX+ trials closest in the session to the AB test trials, and AB was the average of the first three trials of the testing phase, to capture transfer of safety without learning effects. Thus the within-subjects factor of block was not part of this analysis. We used BX awareness as the between-groups factor of awareness because the ability to transfer safety should be dependant on the initial cognitive understanding of the safety cue. The dependent variable for these analyses was percent potentiation calculated as described above. We followed up the mixed ANOVAs by analyzing the effect of trial type with one-way repeated measures ANOVAs separately in the aware and unaware subjects. Given our hypotheses regarding the effects of awareness on startle data, the follow-up analyses were performed regardless of whether there was a significant interaction effect with awareness. Effect sizes of the individual effects are reported as partial eta square (η2). Missing values were replaced with series means. All analyses were conducted using SPSS 12.0 for Windows with an alpha of .05.
Results
Demographics
Of the 50 subjects, 39 were aware and 11 were unaware of the AX contingency; 34 were aware and 16 were unaware of the BX contingency. For the purposes of describing the demographic and neuropsychological data, we collapsed the two classifications of awareness into a single category of aware (n = 30) and unaware (n = 20) subjects. There was a significant association between age and awareness, F(1, 49) = 9.93, p < .01, with the aware subjects being significantly younger (M = 36.2, SD = 12.7 years) than the unaware subjects (M = 47.8, SD = 12.7 years). The aware subjects also had more years of education (M = 16.4, SD = 1.91) than the unaware subjects (M = 14.7, SD = 2.39), F(1, 46) = 6.76, p < .05. The majority of subjects was recruited from the Atlanta Veterans Affairs Medical Center (68%), and there was a significant relationship between awareness and recruitment, with subjects recruited from the Atlanta Veterans Affairs Medical Center more likely to be unaware of the experimental contingencies, χ2(2, N = 50) = 8.75, p < .05. The distribution of sex and race was not significantly different between the awareness categories.
Neuropsychological Data
There was a significant relationship between awareness and intelligence, F(1, 45) = 11.86, p < .01, with the aware subjects having a higher IQ (M = 118.0, SD = 14.4) than the unaware subjects (M = 102.6, SD = 15.4), as measured by the Matrix Reasoning and Vocabulary subtests of the WASI. There was no association between awareness and memory, as measured by the immediate Logical Memory and delayed Logical Memory subtests, or by percent retention on these two subtests of the WMS. Furthermore, neither attention span, as measured by the hit rate or attentiveness score on the CPT, nor motor performance, as measured by the Finger Tapping Test, was related to awareness.
Aversiveness Ratings
The subjects rated the airblast as more aversive (M = 3.3, SE = 0.2) than the startle probe (M = 2.4, SE = 0.2), F(1, 36) = 26.83, p < .001. There was no interaction of Aversiveness × Awareness, and the unaware subjects found the airblast equally aversive as did the aware subjects, F(1, 36) < 1, ns.
Responses to Danger
Contingency awareness of the reinforced CS (AX)
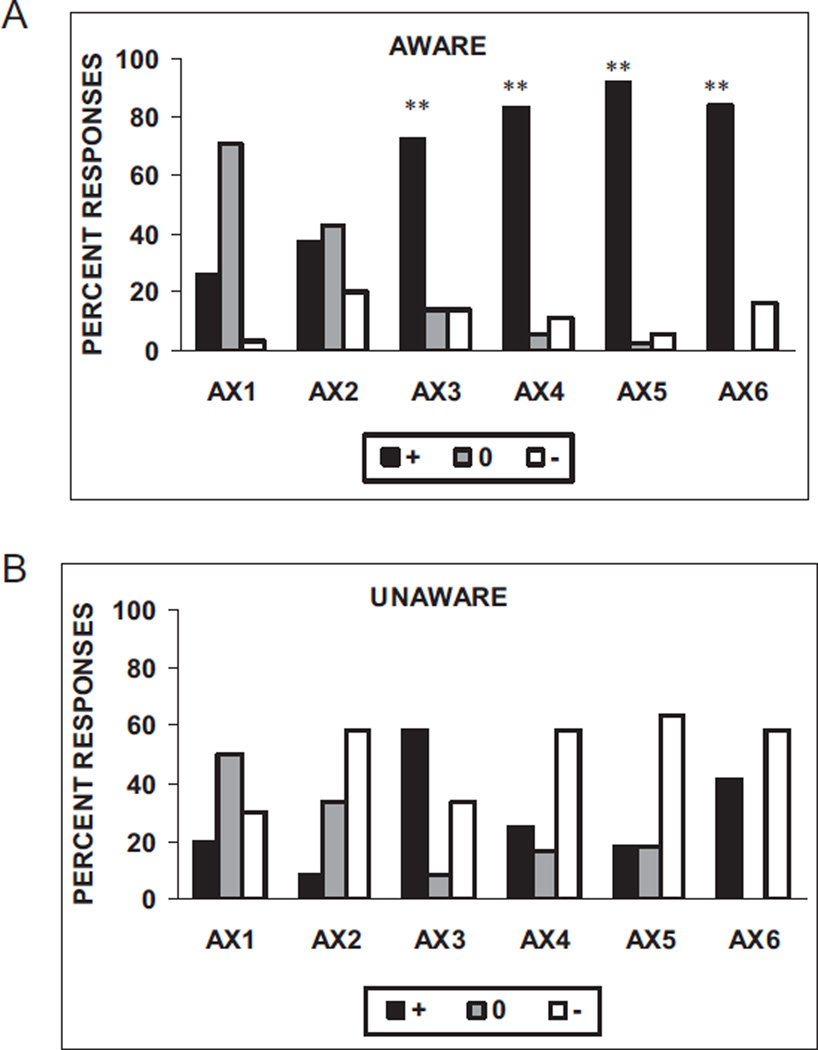
As expected, aware subjects more often expected an airblast when presented with the CS + than did the unaware subjects. Figure 2A shows the distribution of subjects’ keypad responses on the reinforced conditioning trials (AX + ). Chi-square analyses of the distribution of the subjects’ keypad responses on each AX+ trial (US expected, US unexpected, and uncertain) indicate that the AX unaware subjects were more likely to label the first two AX+ trials as nonreinforced, χ2(2, N = 41) = 6.16, p < .05, on the first trial, and χ2(2, N = 47) = 7.10, p < .05, on the second trial. There was no difference between the aware and unaware subjects’ responses on the third AX+ trial; on the fourth, fifth, and sixth presentations of AX + , the aware subjects were much more likely to label the trials as reinforced χ2(2, N = 48) = 14.54, p < .01; χ2(2, N = 48) = 24.05, p < .01; and χ2(2, N = 47) = 8.25, p < .01, respectively (see Figures 2A and 2B).
Figure 2.
Subjects’ keypad responses to the reinforced conditioning trials (AX+) in (A) subjects aware of the AX+ contingency (n = 39) and (B) subjects unaware of the AX+ contingency (n = 11). The stimulus was rated as “+” when the subject believed that stimulus was paired with the airblast, “−” when the subject believed that stimulus was not paired with the airblast, or “0” when the subject did not know. **p < .01 compared with “+” response in unaware subjects.
Fear-potentiated startle
The fear-potentiated startle data were analyzed with a three-way mixed ANOVA using trial type (startle magnitude in the presence of AX, relative to the startle magnitude to NA) and block (Blocks 1 and 2) as within-subjects factors and awareness (aware or unaware of the AX contingency) as betweensubjects factors. We found a significant two-way interaction effect of trial type and block, F(1, 48) = 5.29, p < .05, η2 = 0.10, indicating a growth in the magnitude of fear-potentiated startle from the first half to the second half of the training session, but no effect of awareness and no interactions involving awareness.
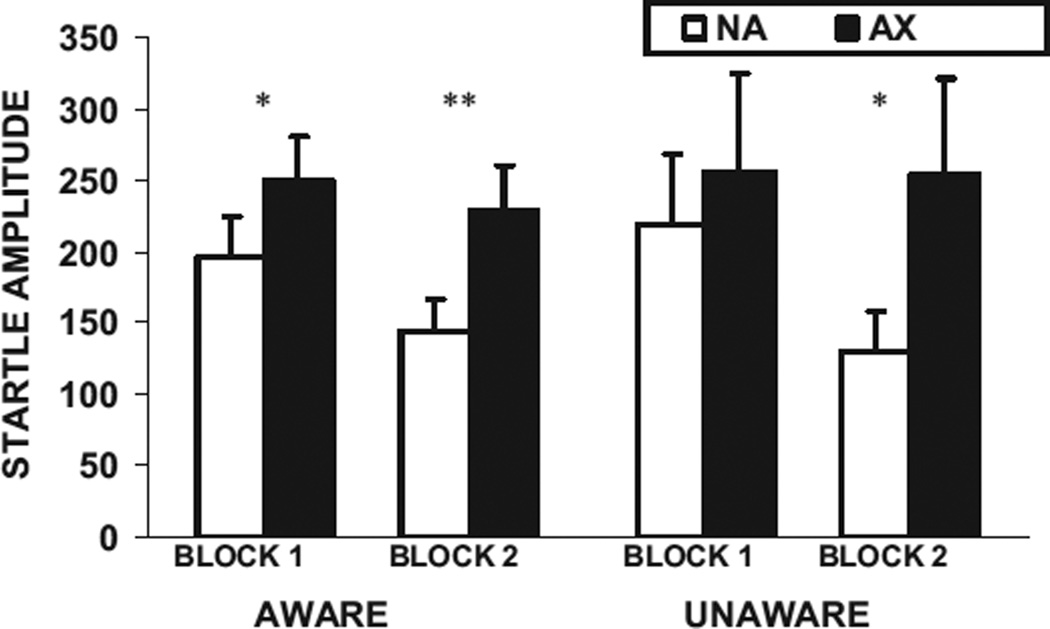
For better illustration of the increase in fear-potentiated startle over training trials, Figure 3 shows startle magnitude in the presence (AX) or absence (NA) of the CS + on each of the 2 conditioning blocks for the AX aware and AX unaware subjects. In the aware subjects, startle was potentiated on the first block, F(1, 38) = 8.75, p < .01, η2 = 0.19, as well as the second block, F(1, 38) = 15.17,p < .01, η2 = 0.29, and the unaware subjects showed significant fear potentiation to AX only in the second block, F(1, 10) = 7.77, p < .05, η2 = 0.44.
Figure 3.
Mean (+ SE) startle amplitude to noise alone (NA) and in the presence of the reinforced conditioned stimulus (AX+) in the first and second block of conditioning for the AX aware (n = 39) and AX unaware subjects (n = 11). *p < .05, **p < .01.
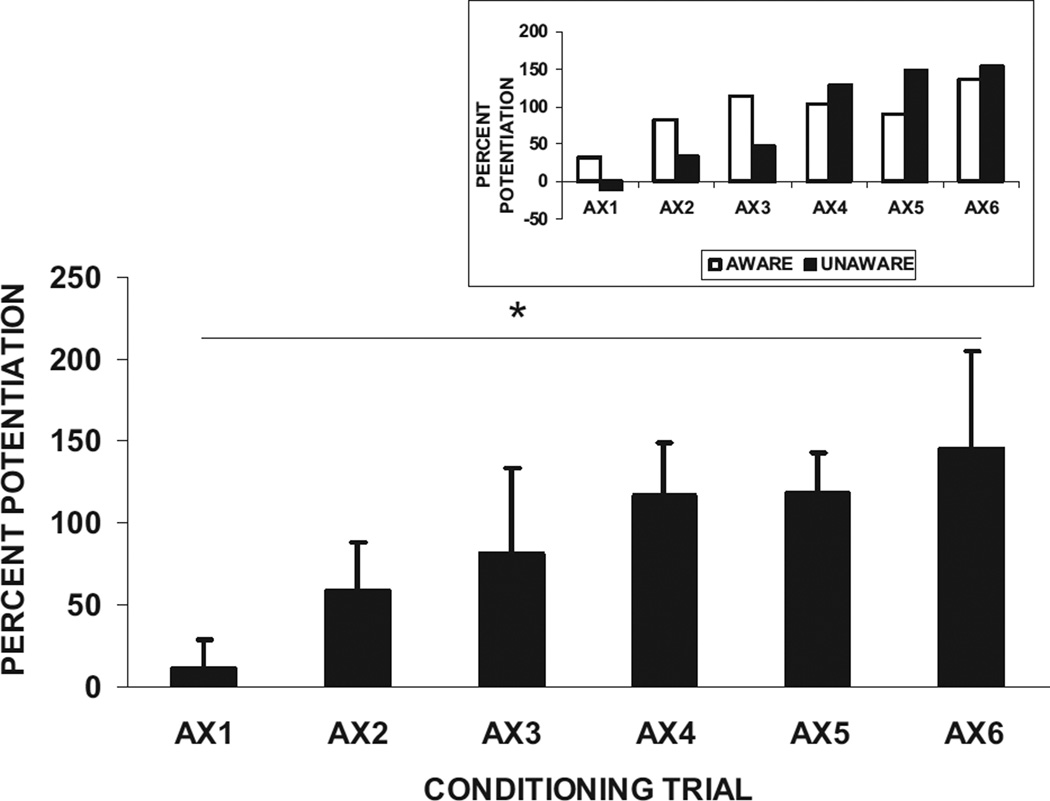
Given that startle was potentiated in both the aware and unaware subjects, and that the aware subjects showed potentiation on the first block, we wanted to determine whether the fear-potentiated startle was the result of conditioning, rather than due to general arousal associated with the presentation of a CS. Therefore, we analyzed the effect of trial type on the very first presentation of AX and found that there was no significant difference between AX and NA, F(1, 48) < 1, p > .1, η2= 0.00. This finding was the same in aware subjects, NA = 187.31 ± 27.84 (mean ± SE), AX = 222.59 ± 30.40, F(1, 38) = 2.40, p > .1, η2 = 0.06, and unaware subjects (NA = 235.55 ± 58.78, AX = 230.00 ± 77.51), F(1, 10) <1,p> .1, η2= 0.00. However, by the second trial there was a significant effect of trial type in the aware subjects (NA = 176.39 ± 26.44, AX = 239.82 ± 28.87), F(1, 38) = 12.01, p < .01, η2= 0.24, indicating very rapid conditioning. As indicated above, by the second block of conditioning (Trials 4, 5, and 6), there was a strong effect of trial type on startle amplitude in both aware and unaware subjects (see Figure 3). Breaking up the blocks by all six training trials revealed a significant linear trend, F(1, 32) = 5.66, p < .05, η2= 0.15, with no interaction with awareness, F(1, 32) = 0.90, p > .10, η2= 0.03, demonstrating a learning curve for both groups. Figure 4 shows the percent potentiation from baseline for all six conditioning trials collapsed over awareness.
Figure 4.
Mean (+ SE) percent potentiation from noise alone in the presence of the reinforced conditioned stimulus (AX+), on the six conditioning trials, collapsed over awareness. Insert: Percent potentiation from noise alone in each awareness group. *p < .05.
Another prediction was that baseline startle would be elevated in unaware subjects. However, Figure 3 shows that although both the aware and unaware subjects had significant fear potentiation there were no differences in startle amplitude to noise alone, F(1, 48) < 1, p > .10, η2= 0.00.
Discrimination Between Danger and Safety
Contingency awareness of the nonreinforced CS (BX)
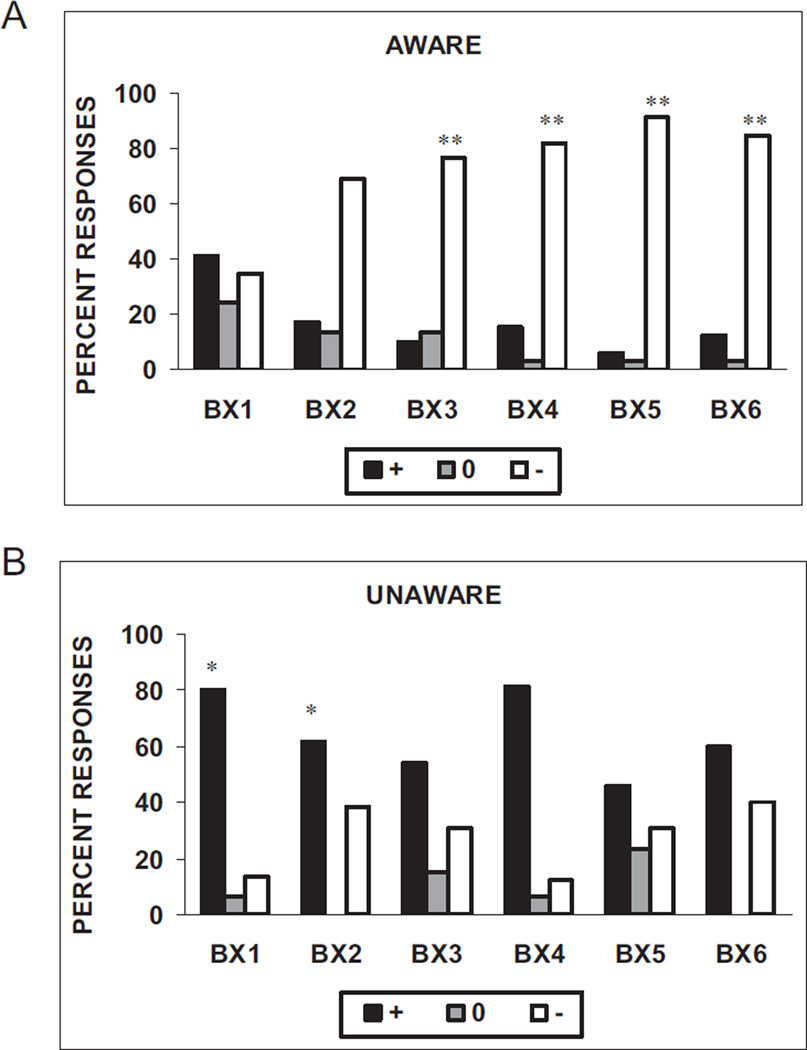
Figure 5a shows the distribution of subjects’ keypad responses on the nonreinforced conditioning trials (BX−). Chi-square analyses of the distribution of the subjects’ keypad responses on each BX− trial (US expected, US unexpected, and uncertain) indicate that the BX unaware subjects were more likely to label all the BX− trials as reinforced, χ2(2, N = 44) = 5.99, χ2(2, N = 42) = 0.89, χ2(2, N = 43) = 10.57, χ2(2, N = 49) = 21.84, χ2(2, N = 46) = 17.49, and χ2(2, N = 48) = 12.11, respectively, for the six BX− trials (all ps < .05). As opposed to the AX+ trials, the distribution of responses to BX− trials differed on every trial between the aware and unaware subjects. However, whereas the aware subjects were less likely to label the BX− trials as reinforced on the first two trials, beginning with the third presentation of BX−, the aware subjects were much more likely to label the trials as nonreinforced relative to the unaware subjects (see Figures 5A and 5B).
Figure 5.
Subjects’ keypad responses to the nonreinforced conditioning trials (BX−) in (A) subjects aware of the BX− contingency (n = 34) and (B) subjects unaware of the BX− contingency (n = 16). The stimulus was rated as “+” when the subject believed that stimulus was paired with the airblast, or “−” when the subject believed that stimulus was not paired with the airblast, or “0” when the subject did not know. *p < .05 compared with “+” response in aware subjects. **p < .01 compared with “−” response in unaware subjects.
Startle discrimination
Percent potentiation between the reinforced (AX + ) and nonreinforced stimuli (BX−) was analyzed with a three-way mixed ANOVA using trial type (startle magnitude in the presence of AX, relative to the startle magnitude in the presence of BX) and block (Blocks 1 and 2) as within-subjects factors and awareness (aware or unaware of the BX contingency) as a between-groups factor.
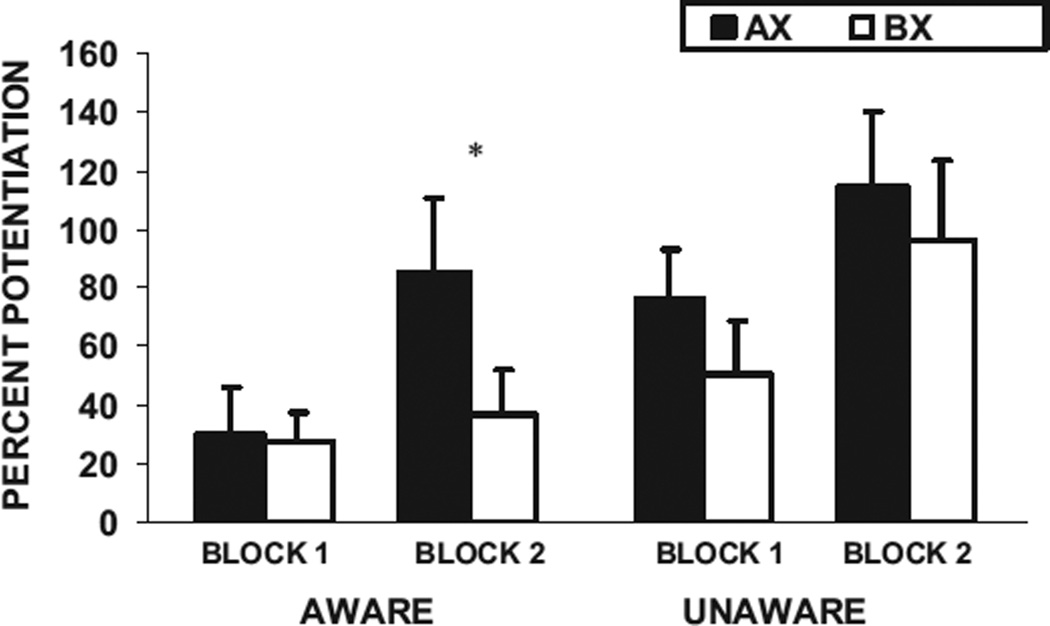
We found significant main effects of block, F(1, 48) = 12.83, p < .01, η2 = 0.21, and trial type, F(1, 48) = 5.63, p < .05, η2 = 0.11; however, there were no significant interaction effects with awareness. Figure 6 shows the percent startle potentiation in the presence of AX+ and BX− in the first and second block of conditioning for the BX aware and BX unaware subjects. Follow-up analyses indicated that neither the aware nor unaware subjects discriminated between AX+ and BX− on the first block; however, in the second block, only the aware subjects had greater potentiation to AX+ than BX−, F(1, 33) = 8.48, p < .01, η2 = 0.20, whereas the unaware subjects did not differentiate between AX+ and BX−, F(1, 15) = 0.02, p > .1, η2 = 0.001, see Figure 6.
Figure 6.
Mean (+ SE) percent potentiation from noise alone in the presence of AX+ and BX− in the first and second block of conditioning for the BX aware (n = 34) and BX unaware subjects (n = 16). *p < .05.
Transfer of Safety
Contingency awareness of the conditioned inhibition test trials (AB)
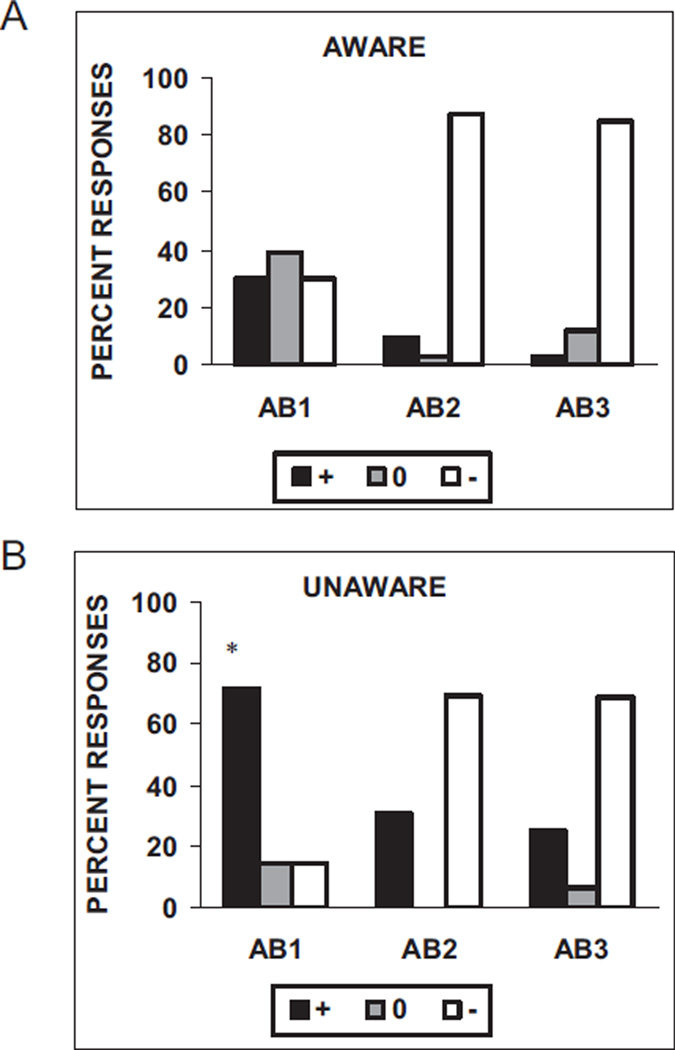
Figure 7a shows the distribution of subjects’ keypad responses on the first three conditioned inhibition test trials (AB). Chi-square analyses of the distribution of the subjects’ keypad responses on each AB trial (US expected, US unexpected, and uncertain) indicate that the unaware subjects were more likely than the aware subjects to expect an airblast on the first AB trial, χ2(2, N = 47) = 6.84, p < .05, indicating that they were not transferring the contingency from BX− to AB. There were no significant differences on the second and third AB trials between the aware and unaware subjects.
Figure 7.
Subjects’ keypad responses to the conditioned inhibition test trials (AB) in (A) subjects aware of the BX− contingency (n = 34) and (B) subjects unaware of the BX− contingency (n = 16). The stimulus was rated as “+” when the subject believed that stimulus was paired with the airblast, “−” when the subject believed that stimulus was not paired with the airblast, or “0” when the subject did not know. *p < .05 compared with “+” response in aware subjects.
Inhibition of fear-potentiated startle
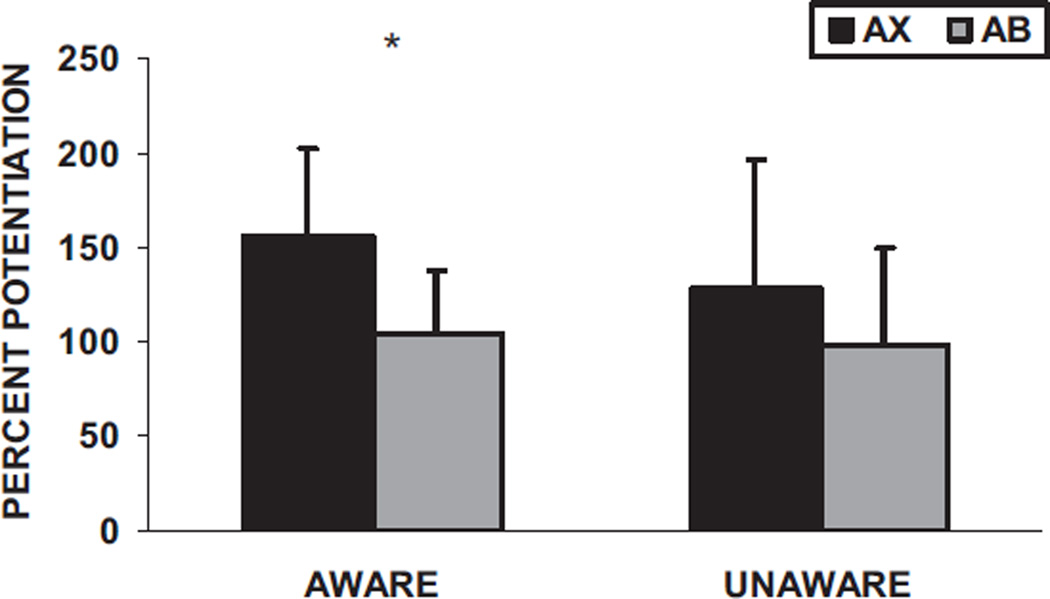
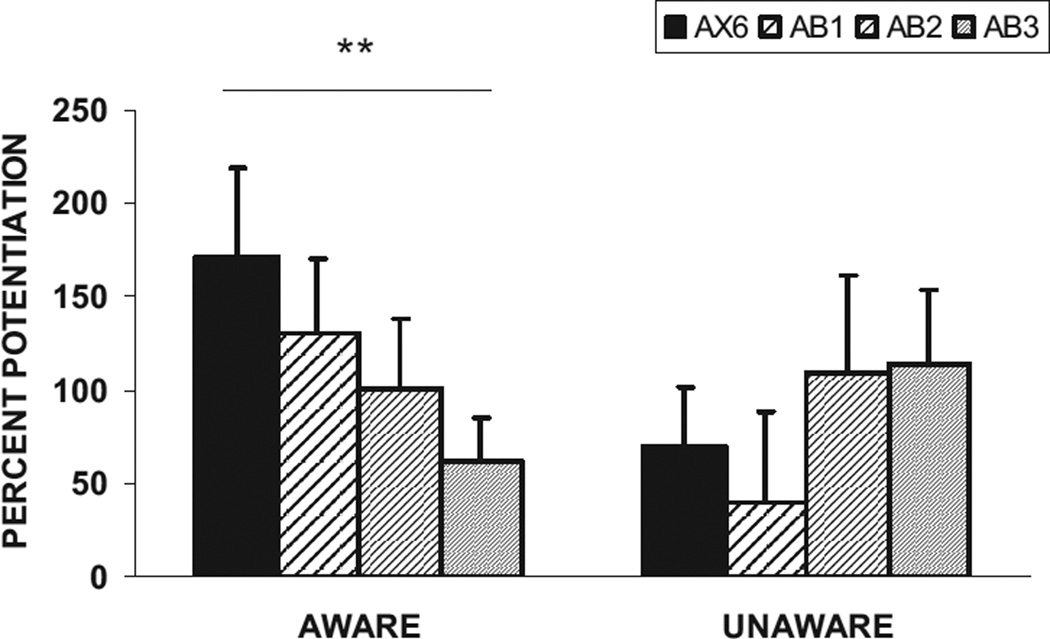
Percent potentiation between the reinforced (AX + ) and conditioned inhibition trials (AB) was analyzed with a two-way mixed ANOVA using trial type (startle magnitude in the presence of AX, relative to the startle magnitude in the presence of AB) as a within-subject factor and awareness (aware or unaware of the BX contingency) as a between-groups factor. We found a significant main effect of trial type, F(1, 48) = 5.54,p < .05, η2 = 0.10; however, there were no significant interaction effects with awareness. Figure 8 shows the percent startle potentiation in the presence of AX + and AB for the BX aware and BX unaware subjects. Follow-up analyses indicated that only the aware subjects had inhibited potentiation to AB trials compared to AX+ trials, F(1, 33) = 5.75, p < .05, η2 = 0.15. Although the unaware subjects startled less in the presence of AB than AX + , this difference did not approach significance, F(1, 15) = 2.07, p > .1, η2 = 0.12. Breaking up the block of AB trials into the last trial of AX and the first three trials of AB revealed an interaction effect of trial type and awareness, F(1, 48) = 6.04, p < .05, η2 = 0.11. Figure 9 shows the inhibition trials for the aware and unaware subjects. Only the aware subjects demonstrated a significant linear decrease in startle potentiation, F(1, 33) = 7.23, p = .01, η2 = 0.18.
Figure 8.
Mean (+ SE) percent potentiation from noise alone in the presence of AX+ and AB in the testing phase for the BX aware (n = 34) and BX unaware subjects (n = 16). *p < .05.
Figure 9.
Mean (+ SE) percent potentiation from noise alone in the presence of the last AX + trial and the first three AB trials for the BX aware (n = 34) and BX unaware subjects (n = 16). **p < .01.
Discussion
This study assessed the relationship between contingency awareness, defined as the knowledge of experimental contingencies, and fear potentiation and fear inhibition. Contingency aware ness was assessed on a trial-by-trial basis as the subjects used a response keypad to label the reinforcement contingencies to each light in a trial. This paradigm was a complex conditional discrimination task designed to assess inhibition of fear-potentiated startle and subsequently resulted in a large proportion of unaware subjects. It should be noted that the paradigm also contained very few training trials because of the subjects’ fast habituation to the US; that is, there were six trials of the AX+ and six trials of the BX−.
We found that subjects who were aware of the experimental contingencies showed fear potentiation to the reinforced stimulus (AX + ), discrimination between the reinforced (AX + ) and nonre-inforced stimulus (BX−), as well as inhibition of fear potentiation on the conditioned inhibition trials in which the nonreinforced and reinforced stimuli were combined (AB). Although the unaware subjects also potentiated startle to AX + , they did not discriminate between AX+ and BX−, and they did not show fear inhibition on AB trials. The aware subjects startled less on the first presentation of AB and continued to inhibit startle with each AB trial. The linear decrease over the first three AB trials could also suggest very rapid extinction, so that the aware subjects were rapidly learning that AB is a safety cue, rather than transferring safety from their prior learning of BX as a safety cue. However, data from our lab suggest that fear-potentiated startle does not extinguish so rapidly. In either case, whether the aware subjects were transferring safety or extinguishing, it is clear that only the aware subjects were showing fear inhibition.
These data suggest that different processes underlie fear acquisition and fear inhibition. Fear acquisition may occur through a low-level mechanism and may not require cognitive input. Such a mechanism would likely be adaptive and may allow an animal to be biologically prepared for danger-relevant cues (Seligman, 1971; see Ohman, 2005, for a recent review) and is supported by the large body of neuroanatomical evidence of amygdala-mediated fear-potentiated startle (Davis, 1992, 1998). Several studies have found that aversive learning can be observed in the absence of awareness (Hamm & Vaitl, 1996) and even in cases in which the CSs are not perceived by the subjects (Knight et al., 2003). Furthermore, patients with amygdala lesions do not show fearpotentiated startle regardless of contingency awareness (Weike et al., 2005). Purkis and Lipp (2001) argued that startle was modulated only in subjects who were aware of the contingency; however, their measure of startle potentiation compared startle with CS+ and CS−. This, in fact, is more like the discrimination task in which we also observed that awareness was necessary. It is interesting to note that startle potentiation may be less dependent on awareness than skin conductance response (Weike et al., 2005).
Fear inhibition processes, such as discrimination between reinforced and nonreinforced stimuli, extinction, conditioned inhibition, or even exposure therapy, in which safety cues are learned, may in humans be based on a cognitive model and rely on contingency awareness (Lovibond, 2004). One potential explanation for the diverging demands on awareness in fear acquisition and fear inhibition may be that inhibition involves the hippocampus, whereas acquisition involves the amygdala. Complex conditioning tasks that involve declarative memory, such as trace conditioning where there is temporal separation between the CS and US, appear to be dependent on an intact hippocampus (Clark & Squire, 1998) as well as contingency awareness (Carter, Hofstotter, Tsuchiya, & Koch, 2003; Clark & Squire, 1998). It is possible that the complexity of the conditional discrimination task used in the present study, in which the contingency of X was dependent on whether it is paired with A or B, required activation of the hippocampus. If the A and B lights served as a context for X, then the requirement for contingency awareness in AX+ versus BX− discrimination may be related to the involvement of the hippocampus in context conditioning.
In this paradigm, fear potentiation is measured as the increase in startle amplitude in the presence of a CS that was previously paired with an aversive event. Although fear-potentiated startle was originally developed in animal studies, the human adaptation of this paradigm includes verbal instructions that indicate that the US will be delivered during the presentation of one of the lights. As a result of these instructions, we cannot discount the contribution of verbally mediated anticipatory anxiety to the potentiation of the startle response. However, the gradual increase in fear-potentiated startle over the training trials indicates that the fear response was acquired through conditioning.
A person who could learn that an aversive stimulus is linked to a specific, predictable cue should be less anxious throughout the session, but more fearful in the presence of the reinforced stimulus. Thus, an aware person should show increased fear potentiation, but also better discrimination. The hypothesis that aware subjects should show greater fear-potentiated startle on AX+ trials was supported, in that subjects who learned the contingency showed stronger fear potentiation to the reinforced stimulus and less fear potentiation to the nonreinforced stimuli. It is possible that the unaware subjects learned to associate the airblast with the onset of a light, but did not learn specifically which of the lights was associated with the US. This suggestion is supported by the finding that the unaware subjects did not discriminate between AX+ and BX−, and would explain why the subjects did not show increased baseline startle. It is also possible that the unaware subjects were too uncertain of the CS+ contingency to press the+ button, but were not entirely unaware of this relationship. However, the wording of the instructions to the subject (“if you think that a light will be followed by an airblast”) allows for a degree of uncertainty. Nevertheless, it is possible that uncertain subjects would show increased anxiety in this experiment, which might manifest in startle potentiation. A study in which the US is not paired to a particular stimulus may be a better way to compare fear and anxiety using startle (Grillon & Davis, 1997).
In the present study, we found that awareness was related to the subject’s age, level of education, and intelligence, in that the aware subjects were younger, more educated, and had higher IQs than the unaware subjects. Memory and attention span did not appear related to awareness. As mentioned above, the design of the experiment was rather complex and was thus very sensitive to differences in intelligence.
In conclusion, we found that awareness was not necessary for fear potentiation to danger cues and that both aware and unaware subjects showed robust fear-potentiated startle. On the other hand, only aware subjects inhibited fear-potentiated startle, suggesting that contingency awareness may be necessary for individuals to discriminate between danger and safety and to transfer safety to a danger cue.
Acknowledgments
This research was supported by the Mental Health Service, Atlanta Veterans Affairs Medical Center; the Science and Technology Center Program, Center for Behavioral Neuroscience, National Science Foundation under Agreement No. IBN-9876754 (Venture grant to Erica J. Duncan, principal investigator [PI]); the American Psychiatric Association and GlaxoSmithKline (Erica J. Duncan, PI); National Institute of Mental Health Grants 1R24MH067314-01A1 (B. Rothbaum, PI) and R37 MH47840 (Michael Davis, PI); Kirschstein National Research Service Award Individual Fellowship 1F32 MH070129-01A2 (Tanja Jovanovic, PI); and the Woodruff Foundation, Emory University School of Medicine. We thank Bram Vervliet for his thoughtful comments on a version of this article.
Contributor Information
Tanja Jovanovic, Department of Psychiatry, Emory University, and Veterans Affairs Medical Center, Atlanta, Georgia.
Seth D. Norrholm, Department of Psychiatry, Emory University, and Veterans Affairs Medical Center, Atlanta, Georgia
Ana Fiallos, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology.
Karyn M. Myers, Yerkes National Primate Center, Woodruff Health Sciences Center, Emory University
Michael Davis, Department of Psychiatry, Emory University.
Megan Keyes, Veterans Affairs Medical Center, Atlanta, Georgia.
Sasa Jovanovic, OKI Telecom, Atlanta, Georgia.
Erica J. Duncan, Department of Psychiatry, Emory University, and Veterans Affairs Medical Center, Atlanta, Georgia
References
- Ameli R, Ip C, Grillon C. Contextual fear-potentiated startle conditioning in humans: Replication and extension. Psychophysiology. 2001;38:383–390. [PubMed] [Google Scholar]
- Carter RM, Hofstotter C, Tsuchiya N, Koch C. Working memory and fear conditioning. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1399–1404. doi: 10.1073/pnas.0334049100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998 Apr 3;280:78–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test computer program. Toronto, Canada: Multi-Health Systems; 1995. [Computer program manual] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: A neural and pharmacological analysis. Behavioral Brain Research. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders (SCID-I)— Clinician version. Arlington, VA: American Psychiatric Publishing; 1997. [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Effects of threat and safety signals on startle during anticipation of aversive shocks, sounds, or airblasts. Journal of Psychophysiology. 1998;12:329–337. [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Effects of explicit and contextual cue conditioning following paired vs. unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Falls WA, Ameli R, Davis M. Safety signals and human anxiety: A fear-potentiated startle study. Anxiety. 1994;1:13–21. doi: 10.1002/anxi.3070010105. [DOI] [PubMed] [Google Scholar]
- Grillon C, Warner V, Hille J, Merikangas KR, Bruder GE, Tenke CE, et al. Families at high and low risk for depression: A three-generation startle study. Biological Psychiatry. 2005;57:953–960. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D. Affective learning: Awareness and aversion. Psychophysiology. 1996;33:698–710. doi: 10.1111/j.1469-8986.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Simons DJ, Cohen NJ. Imaging implicit perception: Promise and pitfalls. Nature Reviews Neuroscience. 2005;6:247–255. doi: 10.1038/nrn1630. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan E. Fear potentiation and fear inhibition in a human fearpotentiated startle paradigm. Biological Psychiatry. 2005;57:1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Knight D, Nguyen HT, Bandettini PA. Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15280–15283. doi: 10.1073/pnas.2535780100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cook CA, Torpey DC, Welsh-Bohmer KA. Impact of healthy aging on awareness and fear conditioning. Behavioral Neuroscience. 2004;118:905–915. doi: 10.1037/0735-7044.118.5.905. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Disterhoft JF. Conditioning, awareness, and the hippocampus. Hippocampus. 1998;8:620–626. doi: 10.1002/(SICI)1098-1063(1998)8:6<620::AID-HIPO4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Cognitive processes in extinction. Learning & Memory. 2004;11:495–500. doi: 10.1101/lm.79604. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Parallel acquisition of awareness and trace eyeblink classical conditioning. Learning & Memory. 2000;7:267–272. doi: 10.1101/lm.33400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. AX+, BX− discrimination learning in the fear-potentiated startle paradigm: Possible relevance to inhibitory fear learning in extinction. Learning & Memory. 2004;11:464–475. doi: 10.1101/lm.74704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Purkis HM, Lipp OV. Does affective learning exist in the absence of contingency awareness? Learning & Motivation. 2001;32:94–99. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead–Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Seligman MEP. Phobias and preparedness. Behavior Therapy. 1971;2:307–320. [Google Scholar]
- Wagner AR, Logan FA, Haberlandt K, Price T. Stimulus selection in animal discrimination learning. Journal of Experimental Psychology. 1968;76:177–186. doi: 10.1037/h0025414. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Rescorla RA. Inhibition in Pavlovian conditioning: Application of a theory. In: Boakes RA, Halliday MS, editors. Inhibition and learning. London: Academic Press; 1972. pp. 301–336. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Third Edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Weike AI, Hamm AO, Schupp HT, Runge U, Schroeder HWS, Kessler C. Fear conditioning following unilateral temporal lobectomy: Dissociation of conditioned startle potentiation and autonomic learning. Journal of Neuroscience. 2005;25:11117–11124. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Sagness KE, McPhee JE. Configural and elemental strategies in predictive learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;20:694–709. [Google Scholar]